Abstract
Ro 63-9141 is a new member of the pyrrolidinone-3-ylidenemethyl cephem series of cephalosporins. Its antibacterial spectrum was evaluated against significant gram-positive and gram-negative pathogens in comparison with those of reference drugs, including cefotaxime, cefepime, meropenem, and ciprofloxacin. Ro 63-9141 showed high antibacterial in vitro activity against gram-positive bacteria except ampicillin-resistant enterococci, particularly vancomycin-resistant strains of Enterococcus faecium. Its MIC at which 90% of the isolates tested were inhibited (MIC90) for methicillin-resistant Staphylococcus aureus (MRSA) was 4 μg/ml. Ro 63-9141 was bactericidal against MRSA. Development of resistance to the new compound in MRSA was not observed. Ro 63-9141 was more potent than cefotaxime against penicillin-resistant Streptococcus pneumoniae (MIC90 = 2 μg/ml). It was active against ceftazidime-susceptible strains of Pseudomonas aeruginosa and against Enterobacteriaceae except Proteus vulgaris and some isolates producing extended-spectrum β-lactamases. The basis for the antibacterial spectrum of Ro 63-9141 lies in its affinity to essential penicillin-binding proteins, including PBP 2′ of MRSA, and its stability towards β-lactamases. The in vivo findings were in accordance with the in vitro susceptibilities of the pathogens. These data suggest the potential utility of Ro 63-9141 for the therapy of infections caused by susceptible pathogens, including MRSA. Since insufficient solubility of Ro 63-9141 itself precludes parenteral administration in humans, a water-soluble prodrug, Ro 65-5788, is considered for development.
Methicillin-resistant staphylococci (MRS) have become a serious problem in many parts of the world. Although the incidence of strains of methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), and other MRS varies from country to country and from hospital to hospital (3, 5), it has been steadily increasing worldwide in the last decade (10, 18, 19). MRS are resistant not only to the available β-lactam antibiotics, but also, in most instances, to structurally unrelated classes of antibacterials (for mainly unknown reasons) and thus represent a prime target in the current search for new antimicrobials.
MRS are characterized by the expression of a special penicillin-binding protein (PBP), PBP 2′, that is not present in methicillin-susceptible staphylococci. This PBP is causally connected with methicillin resistance, as it functions as a transpeptidase and is not efficiently inhibited by commercially available β-lactams, in contrast to the other transpeptidases in staphylococci, PBP 1, PBP 2, and PBP 3. However, the poor affinity of PBP 2′ for β-lactam antibiotics does not seem to be inherent in the β-lactam structure since new carbapenems and cephalosporins that are good inhibitors of PBP 2′ have recently been described (1, 6, 11, 12, 14, 20).
Ro 63-9141, the active principle of the water-soluble prodrug Ro 65-5788, is a novel parenteral cephalosporin with broad-spectrum activity against gram-positive and gram-negative pathogens. It differs from older, broad-spectrum cephalosporins in that it has antibacterial activity against MRS isolates. Its in vitro and in vivo antimicrobial properties and its mode of action are described below.
(Part of this work was presented previously [Abstr. 38th Conf. Antimicrob. Agents Chemother., abstr. F-22 to F-24, 1998].)
MATERIALS AND METHODS
Antimicrobial compounds.
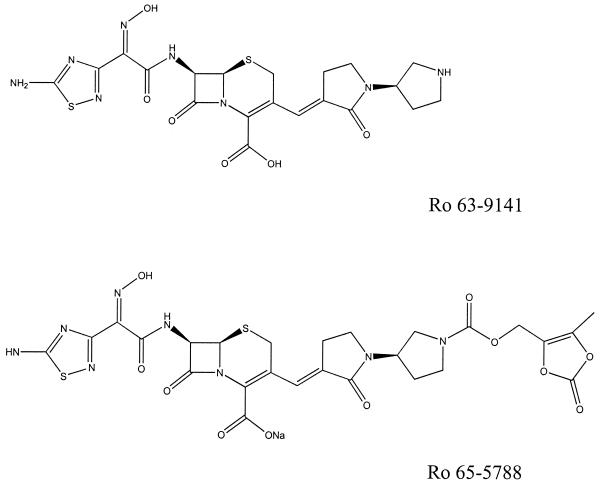
Ro 63-9141, Ro 65-5788, ceftriaxone, and linezolid were prepared for this study in the research laboratories of F. Hoffmann-La Roche Ltd., Basel, Switzerland. The other compounds were purchased from commercial sources. The chemical structures of Ro 63-9141 and its prodrug Ro 65-5788 are shown in Fig. 1.
FIG. 1.
Chemical structures of Ro 63-9141 and its prodrug Ro 65-5788.
Bacterial strains.
Except for some standard strains (American Type Culture Collection strains), the isolates used in the in vitro experiments were clinical isolates obtained from various, mainly European, Japanese, and American, hospitals over a 10-year period, except MRS, for which fresh clinical isolates from 1996 were employed. They were identified by standard methods and kept as stock cultures at −70°C or below. The strains used in the in vivo experiments were clinical isolates or standard strains that were adapted to the respective models through animal passage before use.
MIC determination.
MICs against aerobes were determined by an agar or broth dilution method as recommended by the NCCLS (15) and against Bacteroides fragilis by agar dilution on Wilkins-Chalgren medium (Difco Laboratories, Detroit, Mich.) supplemented with 5 mg of hemin chloride per liter and 0.5 mg of menadione per liter (16).
PBP affinity.
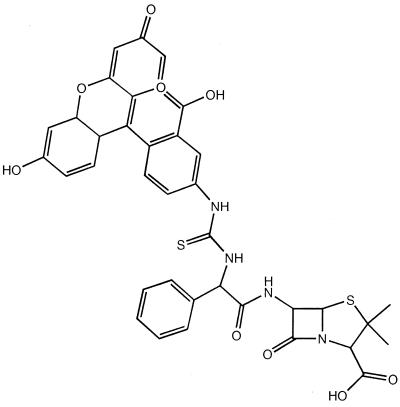
Penicillin binding was determined using fluorescein isothiocyanate-labeled ampicillin (FAMP) (Fig. 2).
FIG. 2.
Structure of FAMP.
FAMP was prepared according to established conjugation methods. Membrane fragments of S. aureus strains were prepared from frozen cell paste. Frozen cell paste (30 to 40 g) was suspended in 200 ml of 20 mM Tris-HCI, pH 7.8, containing 10 mM MgSO4, and the cells were pretreated with lysozyme (1 mg/liter) and lysostaphin (0.1 mg/liter) for 30 min before addition of DNase (1 mg/liter) and the following protease inhibitors: chymostatin (1 mg/liter), antipain (5 mg/liter), pepstatin (0.1 mg/liter), leupeptin (0.1 mg/liter), phenylmethanesulfonyl fluoride (10 μM), 5-aminocaproic acid (5 mM), and aprotinin (105 U/liter). The slurry was passed twice through a French press at about 2,500 MPa at 0 to 4°C and then centrifuged at 105 × g for 90 min at 4°C. After centrifugation, the pellets were collected and washed by resuspension in 20 mM Tris-HCl, pH 9.0, containing 10 mM sodium EDTA to remove extraneous proteins. The membranes were resuspended in 0.1 M sodium phosphate, pH 7.0, at around 50 mg of protein/ml and stored frozen at −20°C. After thawing, the membranes were sonicated briefly before use in labeling experiments. The membranes were used at a concentration of 10 mg of protein/ml, the fluorescent penicillin was added in 0.1 M sodium phosphate buffer, and the mixture was incubated at room temperature for 15 min before the addition of an equal volume (usually 0.02 ml) of sample buffer for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The sample was applied directly to a discontinuous sodium dodecyl sulfate-polyacrylamide gel (stacking gel, 2% polyacrylamide; separating gel, 7.6% polyacrylamide) for analysis. After electrophoresis, the unstained gels were placed in 70% ethanol to remove unbound fluorescent penicillin and then scanned in a Fluorimager (Molecular Devices) and the amount of fluorescence was determined using ImageQuant software from the manufacturer.
Labeling of soluble recombinant PBP 2′ (preparation described elsewhere [B. Mensch et al., submitted for publication]) was performed with 0.1 to 0.5 mg of protein per liter in 0.1 M sodium phosphate buffer. The protein solution (0.01 ml) was mixed with an equal volume of a solution of the test substance in the same buffer, and the mixture was incubated for 15 min. Then, 0.1 ml of 10 mM FAMP in 0.1 M sodium phosphate was added and the reaction was allowed to continue for a further 15 min. The reaction was stopped by the addition of 0.1 ml of ice-cold 10% aqueous trichloroacetic acid solution. The precipitate formed was collected by filtration through Whatman GF/F glass fiber filter paper. The filters were washed with 70% ethanol and air dried before reading of the fluorescence in a Cytofluor 2000 microtiter plate fluorimeter. For direct competition, the test substance and fluorescent penicillin were added in the same solution and the reaction mixture was incubated for 5 min before the trichloroacetic acid solution was added.
Stability towards β-lactamases.
β-Lactamases were purified to homogeneity, and their hydrolytic activities and inhibition were studied by standard methods (17).
Bactericidal activity.
Overnight cultures of the test strains (four MRSA strains and one MRSE, one methicillin-susceptible S. aureus [MSSA], and one Escherichia coli strain) grown in 30 ml of Mueller-Hinton broth (MHB; Remel, Lenexa, Kans.) were diluted into fresh medium to yield an inoculum of 106 CFU/ml or higher. Drug was added either with the inoculum or at intervals of 1.5 h (early log phase) and 3 h (log phase) after its addition. Drug concentrations of 0 (control), 0.5, 1, 2, or 5 times the MIC were used. Ten-microliter aliquots of appropriate dilutions were plated on Mueller-Hinton agar (MHA), and colonies were counted after 24 h of incubation. To check whether resistant clones had been selected, the MICs were determined for those cultures which showed growth after 24 h.
Population analysis.
The standard procedure described by Tomasz et al. (21) was followed. Single colonies from staphylococcal plates were inoculated into 30 ml of MHB in 100-ml Erlenmeyer flasks and incubated overnight at 35°C. Dilutions of the fully grown cultures were prepared in phosphate-buffered saline, and aliquots of 100 μl were plated out on MHA plates containing increasing concentrations of the compound to be tested, including controls with no antibiotic. Each experiment was run in triplicate, and the the numbers of CFU per plate were averaged. Plates were read after 2 days of incubation at 35°C.
Resistance development.
Development of resistance to Ro 63-9141, imipenem, linezolid, and ciprofloxacin was studied with three MRSA strains and one MSSA strain by serial passage over graded concentrations in 2 ml of MHB and application of an inoculum of 105 CFU/ml.
Since exposure to Ro 63-9141 at this inoculum did not lead to resistant mutants, a comparative study for Ro 63-9141, imipenem, ciprofloxacin, and linezolid was performed with an MRSA strain (S. aureus 745) using a much higher inoculum. MHA was used as the medium. The compounds were dissolved in water (imipenem) or in a small volume of dimethyl sulfoxide (Ro 63-9141, ciprofloxacin, and linezolid), diluted in water, and incorporated in twofold serial dilutions in the agar. An inoculum of about 2 × 108 CFU/plate was applied by plating aliquots of 0.1 ml of an overnight culture. The plates were incubated at 35°C for 48 h, and the MICs were recorded as the lowest concentrations that prevented visible growth of colonies. For the second and further passages, the inoculum was prepared from the highest concentration of each test compound that gave rise to growth. At least five colonies were suspended in 1 ml of MHB, and this suspension was adjusted to yield an inoculum of approximately 2 × 108 CFU/plate. The plates were incubated at 35°C for 48 h, and the MICs were read. A total of six passages were performed for Ro 63-9141, and a total of five each were performed for the comparator drugs.
Experimental septicemia in mice.
Septicemia was induced in outbred Swiss albino mice (Jbm MoRo; weight, 16 to 20 g). Mice were infected by intraperitoneal (i.p.) injection of diluted overnight cultures of the test organisms. All strains, with the exceptions of Streptococcus pyogenes β15, E. coli 25922, Klebsiella pneumoniae 418, Enterobacter cloacae MRW, Serratia marcescens 69438, and Pseudomonas aeruginosa BA, were injected as suspensions in 4% hog gastric mucin (Fluka Chemie AG, Buchs, Switzerland). Bacterial challenge doses were 4 to 10 times the number of organisms required to kill 50% of infected, but unmedicated, animals within 48 h. The test compounds were administered subcutaneously (s.c.) 1, 3, and 5 h after bacterial challenge in the P. aeruginosa BA infection and 1 and 3 h afterward in all other infections. The test compounds were used as solutions in saline, except for Ro 63-9141, which was administered as a suspension together with 2% (vol/vol) Tween 80 (Sigma Chemical Co., St. Louis, Mo.). Control and treatment groups at each dose were composed of five mice each. The 50% effective dose (ED50, in milligrams per kilogram of body weight) was calculated by probit analysis as described by Finney (4) from the survival rates on day 4 after infection.
Experimental s.c. abscesses in mice.
The test strains S. aureus I-6 and S. aureus Mu50 were grown for 18 h in semisolid brain heart infusion (BHI) medium (Difco Laboratories, Detroit, Mich.) (supplemented with 0.25% agarose) and subsequently diluted 103-fold in the same medium. Samples of 0.5 ml of this dilution were used as inocula for infection.
Female Swiss albino mice (Jbm MoRo; weight, 27 to 30 g) were injected beneath the loose skin of the left groin. The test compounds were administered i.p. 1 and 3 h after infection. Vancomycin was given as an aqueous solution, and linezolid and Ro 63-9141 were given as suspensions with 2% (vol/vol) Tween 80. Unmedicated mice developed visible abscesses at the site of infection by the third day following bacterial challenge.
Mice were killed on the third day after infection. The abscesses were excised, added to 2 ml of saline, and homogenized in a blender. Viable cell counts of the bacteria per abscess were determined in duplicate by a standard plate procedure on BHI agar.
RESULTS
MIC determination.
The antibacterial activity of Ro 63-9141 was compared with those of cefotaxime, cefepime, meropenem, and ciprofloxacin. For gram-positive organisms vancomycin was also used as a comparator, as were ampicillin for enterococci, ceftazidime for P. aeruginosa, and metronidazole for B. fragilis. The results are presented in Table 1.
TABLE 1.
In vitro activities of Ro 63-9141 and reference compounds
| MSSA (19) | |||
|---|---|---|---|
| Ro 63-9141 | 0.5 | 1 | 0.25–1 |
| Cefotaxime | 2 | 4 | 2–32 |
| Cefepime | 2 | 8 | 2–16 |
| Meropenem | 0.12 | 0.12 | 0.06–0.25 |
| Ciprofloxacin | 0.5 | 1 | 0.25–>8 |
| Vancomycin | 2 | 2 | 1–2 |
| MRSA (77) | |||
| Ro 63-9141 | 2 | 4 | 0.5–4 |
| Cefotaxime | >64 | >64 | >64 |
| Cefepime | >32 | >32 | >32 |
| Meropenem | 32 | >32 | >32 |
| Ciprofloxacin | 0.5 | >8 | 0.12–>8 |
| Vancomycin | 1 | 2 | 1–8 |
| MSSE (19) | |||
| Ro 63-9141 | 0.25 | 1 | 0.12–1 |
| Cefotaxime | 1 | 4 | 0.25–4 |
| Cefepime | 0.5 | 2 | 0.25–4 |
| Meropenem | 0.12 | 1 | 0.12–4 |
| Ciprofloxacin | 0.25 | 0.5 | 0.25–>8 |
| Vancomycin | 2 | 2 | 2–4 |
| MRSE (19) | |||
| Ro 63-9141 | 1 | 2 | 1–4 |
| Cefotaxime | 32 | >64 | 8–>64 |
| Cefepime | 16 | >32 | 4–>32 |
| Meropenem | 16 | >32 | 4–>32 |
| Ciprofloxacin | 0.25 | >8 | 0.25–>8 |
| Vancomycin | 2 | 4 | 2–4 |
| Staphylococcus haemolyticus (31) | |||
| Ro 63-9141 | 4 | 8 | 0.125–8 |
| Cefotaxime | >32 | >32 | 2–>32 |
| Cefepime | >32 | >32 | 1–>32 |
| Meropenem | >32 | >32 | 0.125–>32 |
| Ciprofloxacin | 0.5 | 32 | 0.25–>32 |
| Vancomycin | 2 | 4 | 1–4 |
| Staphylococcus hominis (28) | |||
| Ro 63-9141 | 4 | 4 | 0.25–4 |
| Cefotaxime | >32 | >32 | 1–>32 |
| Cefepime | >32 | >32 | 0.5–>32 |
| Meropenem | >32 | >32 | ≤0.06–>32 |
| Ciprofloxacin | 0.5 | 1 | 0.25–>32 |
| Vancomycin | 2 | 4 | 1–4 |
| Staphylococcus saprophyticus (55) | |||
| Ro 63-9141 | 0.25 | 0.5 | 0.06–4 |
| Cefotaxime | 4 | 8 | 0.5–32 |
| Cefepime | 2 | 4 | 0.5–>32 |
| Meropenem | 0.25 | 0.5 | 0.06–32 |
| Ciprofloxacin | 1 | 2 | 0.25–2 |
| Vancomycin | 2 | 2 | 0.5–2 |
| Staphylococcus sciuri (13) | |||
| Ro 63-9141 | 0.5 | 2 | 0.25–2 |
| Cefotaxime | 8 | >32 | 1–>32 |
| Cefepime | 4 | >32 | 1–>32 |
| Meropenem | 0.5 | 16 | 0.25–16 |
| Ciprofloxacin | 1 | 2 | 0.25–2 |
| Vancomycin | 2 | 2 | 1–2 |
| Staphylococcus warneri (10) | |||
| Ro 63-9141 | 0.25 | 2 | 0.25–4 |
| Cefotaxime | 2 | 32 | 1–>32 |
| Cefepime | 2 | 32 | 1–>32 |
| Meropenem | 0.25 | 8 | 0.25–>32 |
| Ciprofloxacin | 0.5 | 1 | 0.25–1 |
| Vancomycin | 2 | 2 | 1–4 |
| Penicillin-susceptible Streptococcus pneumoniae (19) | |||
| Ro 63-9141 | 0.015 | 0.03 | 0.015–0.03 |
| Penicillin G | ≤0.06 | ≤0.06 | ≤0.06 |
| Cefotaxime | ≤0.03 | 0.06 | ≤0.03–0.25 |
| Cefepime | 0.03 | 0.06 | 0.03–0.12 |
| Meropenem | 0.015 | 0.03 | 0.015–0.03 |
| Ciprofloxacin | 2 | 2 | 1–4 |
| Vancomycin | 0.5 | 1 | 0.5–1 |
| Penicillin-resistant Streptococcus pneumoniae (20) | |||
| Ro 63-9141 | 0.5 | 2 | 0.03–2 |
| Penicillin G | 1 | 16 | 1–>16 |
| Cefotaxime | 1 | 4 | 0.12–4 |
| Cefepime | 2 | 4 | 0.25–4 |
| Meropenem | 0.5 | 2 | 0.06–2 |
| Ciprofloxacin | 1 | 2 | 0.5–4 |
| Vancomycin | 0.5 | 0.5 | 0.5 |
| α-Hemolytic streptococci (21), Streptococcus salivarius (3), Streptococcus sanguis (4), Streptococcus bovis (3), Streptococcus intermedius (2), Streptococcus mitis (3), Streptococcus oralis (2), Streptococcus mutans (3), Streptococcus gordonii (1) | |||
| Ro 63-9141 | 0.04 | 0.25 | ≤0.008–2 |
| Cefotaxime | 0.12 | 2 | ≤0.03–8 |
| Cefepime | 0.12 | 2 | 0.03–8 |
| Meropenem | 0.06 | 1 | 0.015–4 |
| Ciprofloxacin | 2 | 4 | 1–>8 |
| Vancomycin | 1 | 1 | 0.5–2 |
| β-Hemolytic streptococci (20), Streptococcus pyogenes (10), Streptococcus agalactiae (10) | |||
| Ro 63-9141 | 0.03 | 0.06 | ≤0.008–0.12 |
| Cefotaxime | 0.03 | 0.12 | ≤0.03–0.12 |
| Cefepime | 0.03 | 0.12 | 0.03–0.25 |
| Meropenem | 0.03 | 0.12 | 0.015–0.25 |
| Ciprofloxacin | 1 | 2 | 0.5–4 |
| Vancomycin | 0.5 | 1 | 0.25–1 |
| Enterococcus faecalis (14) | |||
| Ro 63-9141 | 0.5 | 4 | 0.25–>32 |
| Ampicillin | 2 | 4 | 1–>32 |
| Cefotaxime | 8 | >32 | 4–>32 |
| Cefepime | >32 | >32 | 32–>32 |
| Meropenem | 8 | 32 | 4–>32 |
| Ciprofloxacin | 2 | >8 | 0.5–>8 |
| Vancomycin | 4 | >32 | 2–>32 |
| Enterococcus faecium (16) for which the ampicillin MIC is ≤8μg/ml | |||
| Ro 63-9141 | 4 | 8 | 1–8 |
| Ampicillin | 4 | 8 | 2–8 |
| Cefotaxime | >32 | >32 | 16–>32 |
| Cefepime | >32 | >32 | 32–>32 |
| Meropenem | 16 | >32 | 4–>32 |
| Ciprofloxacin | 4 | >8 | 2–>8 |
| Vancomycin | 4 | >32 | 4–>32 |
| Enterococcus faecium (20) for which the ampicillin MIC is ≥16 μg/ml | |||
| Ro 63-9141 | >32 | >32 | 8–>32 |
| Ampicillin | >32 | >32 | 16–>32 |
| Cefotaxime | >32 | >32 | >32 |
| Cefepime | >32 | >32 | >32 |
| Meropenem | >32 | >32 | >32 |
| Ciprofloxacin | >8 | >8 | 8–>8 |
| Vancomycin | 4 | >32 | 2–>32 |
| ESBL-negative Escherichia coli (24) | |||
| Ro 63-9141 | 0.06 | 0.06 | ≤0.03–0.12 |
| Cefotaxime | 0.06 | 0.12 | ≤0.03–0.12 |
| Cefepime | ≤0.03 | 0.06 | ≤0.03–0.5 |
| Meropenem | ≤0.03 | 0.06 | ≤0.03–0.06 |
| Ciprofloxacin | 0.015 | 0.12 | ≤0.008–0.12 |
| ESBL-positive Escherichia coli (17) | |||
| Ro 63-9141 | 4 | >32 | 0.06–>32 |
| Cefotaxime | 4 | 32 | 0.06–>64 |
| Cefepime | 2 | 8 | 0.06–16 |
| Meropenem | 0.06 | 0.06 | ≤0.03–0.12 |
| Ciprofloxacin | 0.06 | 0.25 | ≤0.008–0.25 |
| ESBL-negative Klebsiella pneumoniae (19) | |||
| Ro 63-9141 | ≤0.06 | 0.25 | ≤0.06–1 |
| Cefotaxime | ≤0.06 | ≤0.06 | ≤0.06 |
| Cefepime | ≤0.06 | 0.25 | ≤0.06–0.5 |
| Meropenem | ≤0.06 | ≤0.06 | ≤0.06–0.1 |
| Ciprofloxacin | ≤0.06 | ≤0.06 | ≤0.06–0.25 |
| ESBL-positive Klebsiella pneumoniae (22) | |||
| Ro 63-9141 | 4 | >32 | 0.12–>32 |
| Cefotaxime | 8 | 64 | 0.25–>32 |
| Cefepime | 2 | 16 | 0.12–32 |
| Meropenem | 0.12 | 0.25 | 0.06–16 |
| Ciprofloxacin | 1 | >8 | 0.03–>8 |
| Klebsiella oxytoca (9) | |||
| Ro 63-9141 | 0.5 | 0.06–>32 | |
| Cefotaxime | ≤0.03 | ≤0.03–1 | |
| Cefepime | ≤0.03 | ≤0.03–2 | |
| Meropenem | 0.06 | ≤0.03–0.12 | |
| Ciprofloxacin | 0.03 | 0.015–0.06 | |
| Citrobacter freundii (19) | |||
| Ro 63-9141 | 1 | 8 | 0.12–8 |
| Cefotaxime | 32 | 64 | 1–>64 |
| Cefepime | 0.5 | 2 | ≤0.03–4 |
| Meropenem | 0.12 | 0.25 | 0.06–0.5 |
| Ciprofloxacin | 0.03 | 0.25 | 0.015–0.25 |
| Enterobacter cloacae (25) | |||
| Ro 63-9141 | 4 | 8 | 0.12–8 |
| Cefotaxime | >64 | >64 | 16–>64 |
| Cefepime | 1 | 4 | 0.12–8 |
| Meropenem | 0.12 | 0.25 | 0.06–0.25 |
| Ciprofloxacin | 0.03 | 0.12 | 0.015–4 |
| Enterobacter aerogenes (10) | |||
| Ro 63-9141 | 0.25 | 4 | ≤0.03–8 |
| Cefotaxime | 32 | >32 | 0.12–>32 |
| Cefepime | 1 | 8 | 0.06–8 |
| Meropenem | 0.12 | 16 | 0.06–16 |
| Ciprofloxacin | 0.25 | 8 | ≤0.03–16 |
| Pantoea agglomerans (10) | |||
| Ro 63-9141 | 0.12 | 16 | 0.06–16 |
| Cefotaxime | 0.12 | 8 | ≤0.03–>32 |
| Cefepime | ≤0.03 | 0.5 | ≤0.03–4 |
| Meropenem | 0.06 | 0.12 | ≤0.03–0.5 |
| Ciprofloxacin | ≤0.03 | 2 | ≤0.03–32 |
| Proteus mirabilis (20) | |||
| Ro 63-9141 | ≤0.06 | ≤0.06 | ≤0.06–0.12 |
| Cefotaxime | ≤0.06 | ≤0.06 | ≤0.06–0.5 |
| Cefepime | 0.12 | 0.12 | ≤0.06–0.5 |
| Meropenem | ≤0.06 | ≤0.06 | ≤0.06–0.12 |
| Ciprofloxacin | ≤0.06 | 0.12 | ≤0.06–0.25 |
| Proteus vulgaris (10) | |||
| Ro 63-9141 | >32 | >32 | 0.25–>32 |
| Cefotaxime | ≤0.03 | 0.5 | ≤0.03–>32 |
| Cefepime | 0.06 | 0.25 | ≤0.03–16 |
| Meropenem | 0.25 | 0.5 | 0.25–2 |
| Ciprofloxacin | 0.06 | 0.12 | 0.015–>8 |
| Morganella morganii (15) | |||
| Ro 63-9141 | 0.06 | 0.12 | ≤0.03–0.12 |
| Cefotaxime | 0.06 | 8 | ≤0.03–16 |
| Cefepime | 0.06 | 0.12 | ≤0.03–0.12 |
| Meropenem | 0.25 | 0.5 | 0.25–0.5 |
| Ciprofloxacin | 0.06 | >8 | 0.06–>8 |
| Providencia spp. (11), Providencia rettgeri (6), Providentia stuartii (5) | |||
| Ro 63-9141 | ≤0.03 | 0.12 | ≤0.03–>32 |
| Cefotaxime | 0.12 | 4 | ≤0.03–>32 |
| Cefepime | 0.25 | 0.5 | ≤0.03–>32 |
| Meropenem | 0.25 | 1 | 0.06–>32 |
| Ciprofloxacin | 1 | >8 | 0.03–>8 |
| Enterobacter aerogenes (10) | |||
| Ro 63-9141 | 0.25 | 4 | ≤0.03–8 |
| Cefotaxime | 32 | >32 | 0.12–>32 |
| Cefepime | 1 | 8 | 0.06–8 |
| Meropenem | 0.12 | 16 | 0.06–16 |
| Ciprofloxacin | 0.25 | 8 | ≤0.03–16 |
| Serratia marcescens (19) | |||
| Ro 63-9141 | 1 | 4 | 0.06–>32 |
| Cefotaxime | 1 | 32 | 0.12–>64 |
| Cefepime | 0.5 | 1 | 0.06–16 |
| Meropenem | 0.12 | 0.25 | ≤0.03–0.25 |
| Ciprofloxacin | 0.25 | >8 | ≤0.03–>8 |
| Acinetobacter spp. (21), Acinetobacter junii (2), Acinetobacter lwoffii (4), Acinetobacter baumannii (15) | |||
| Ro 63-9141 | 8 | >8 | ≤0.03–>8 |
| Cefotaxime | 32 | >32 | 0.5–>32 |
| Cefepime | 32 | >32 | 0.12–>32 |
| Meropenem | 2 | 8 | 0.06–8 |
| Ciprofloxacin | 4 | >8 | 0.015–>8 |
| Ceftazidime-susceptible Pseudomonas aeruginosa (60) | |||
| Ro 63-9141 | 2 | 16 | 1–16 |
| Ceftazidime | 4 | 8 | 1–8 |
| Cefotaxime | 32 | >64 | 2–>64 |
| Cefepime | 4 | 16 | 1–16 |
| Meropenem | 0.5 | 2 | 0.25–8 |
| Ciprofloxacin | 0.25 | 1 | 0.12–>8 |
| Ceftazidime-resistant Pseudomonas aeruginosa (17) | |||
| Ro 63-9141 | 16 | >64 | 2–>64 |
| Ceftazidime | 16 | >64 | 16–>64 |
| Cefotaxime | >64 | >64 | 64–>64 |
| Cefepime | 32 | 32 | 4–64 |
| Meropenem | 2 | 16 | 0.5–16 |
| Ciprofloxacin | 2 | >8 | 0.5–>8 |
| Vibrionaceae (20), Aeromonas spp. (9), Plesiomonas shigelloides (3), Vibrio cholerae (4), Vibrio parahaemolyticus (4) | |||
| Ro 63-9141 | 0.12 | 0.5 | 0.06–1 |
| Cefotaxime | 0.03 | 0.25 | ≤0.008–>8 |
| Cefepime | 0.12 | 0.25 | ≤0.008–0.25 |
| Meropenem | 0.03 | 0.5 | ≤0.008–2 |
| Ciprofloxacin | 0.015 | 0.25 | 0.008–0.25 |
| Haemophilus influenzae (18) | |||
| Ro 63-9141 | 0.06 | 1 | 0.06–1 |
| Cefotaxime | 0.015 | 0.25 | 0.015–0.5 |
| Cefepime | 0.06 | 2 | 0.06–2 |
| Meropenem | 0.12 | 1 | 0.06–1 |
| Ciprofloxacin | 0.03 | 0.03 | 0.015–0.02 |
| Neisseria gonorrhoeae (19) | |||
| Ro 63-9141 | 0.03 | 0.12 | ≤0.015–0.12 |
| Cefotaxime | 0.06 | 0.25 | ≤0.015–0.25 |
| Moraxella catarrhalis (18) | |||
| Ro 63-9141 | 0.12 | 1 | ≤0.008–1 |
| Cefotaxime | 0.25 | 0.5 | 0.06–0.5 |
| Cefepime | 0.5 | 1 | 0.12–1 |
| Meropenem | ≤0.008 | ≤0.008 | ≤0.008 |
| Ciprofloxacin | 0.12 | 0.12 | 0.06–0.25 |
| Bacteroides fragilis (21) | |||
| Ro 63-9141 | 16 | 32 | 0.25–>64 |
| Cefotaxime | 16 | >64 | 0.5–>64 |
| Cefepime | 64 | >64 | 2–>64 |
| Meropenem | 0.12 | 0.5 | 0.06–2 |
| Metronidazole | 0.5 | 1 | 0.12–>32 |
Ro 63-9141 showed consistent activity against staphylococci. For MSSA, the MIC at which 90% of the isolates tested were inhibited (MIC90) of Ro 63-9141 was 1 μg/ml, which was lower than those of the reference cephalosporins. Ro 63-9141 inhibited all studied clinical isolates of MRSA and MRSE at ≤4 μg/ml. Slightly higher MICs were obtained for Staphylococcus haemolyticus (MIC90, 8 μg/ml). The MIC90s of cefotaxime, cefepime, meropenem, and ciprofloxacin against MRS were not in the range of susceptibility. For the MRSA strain S. aureus Mu50, which has decreased susceptibility to vancomycin (so-called vancomycin-intermediate S. aureus [VISA] strain) and was isolated after therapeutic failure with vancomycin (8), the MIC of Ro 63-9141 (2 μg/ml) was not higher than those for vancomycin-susceptible MRSA strains. For a β-lactamase-producing strain of S. aureus showing borderline oxacillin resistance with the same characteristics as those described by McDougal and Thornsberry in 1996 (e.g., lowering of oxacillin MICs into the susceptible range in the presence of β-lactamase inhibitors) (13), the Ro 63-9141 MIC was 1 μg/ml. Addition of 2% sodium chloride to MHA had little influence on the MIC of Ro 63-9141; it increased by at most one dilution step for some strains. Penicillin-susceptible strains of S. pneumoniae were highly susceptible to the new compound (MIC90, 0.03 μg/ml). The MIC90 of Ro 63-9141 for penicillin-resistant pneumococci was twofold lower than those of cefotaxime and cefepime and equal to that of meropenem. In contrast to the reference cephalosporins, which were inactive, Ro 63-9141 was equivalent to ampicillin in activity against ampicillin-susceptible isolates of Enterococcus faecalis and Enterococcus faecium. Ro 63-9141 did not inhibit ampicillin-resistant enterococci. In E. faecium these isolates are generally also resistant to vancomycin.
Since the formation of extended-spectrum β-lactamases (ESBLs) has a major influence on the susceptibility of Enterobacteriaceae to β-lactam antibiotics, MICs for ESBL-producing and ESBL-nonproducing isolates were determined separately for E. coli and K. pneumoniae. Likewise, isolates of P. aeruginosa were categorized according to susceptibility or resistance to ceftazidime before testing. Ro 63-9141 showed high and consistent activity against ESBL-negative isolates of E. coli and K. pneumoniae as well as against Proteus mirabilis, Morganella morganii, Providencia spp., Vibrionaceae, Haemophilus influenzae, Neisseria gonorrhoeae, and Moraxella catarrhalis. Comparable activities were obtained with the reference β-lactams. About 15% of the ESBL-positive E. coli and K. pneumoniae isolates were resistant to Ro 63-9141 (MIC50, 4 μg/ml; MIC90, >32 μg/ml). Cefepime and particularly meropenem were more potent against these strains. Ro 63-9141 was slightly more active than cefepime against Enterobacter aerogenes, of which 2 of the 10 strains studied were ESBL producers, but it was intermediate to cefepime and cefotaxime against Enterobacter cloacae and Citrobacter freundii. A concentration of 8 μg of Ro 63-9141 per ml was needed to prevent growth in 100% of these strains. Ro 63-9141 did not inhibit most isolates of Proteus vulgaris, in contrast to the comparator drugs. Ro 63-9141 was similar to ceftazidime and cefepime in activity against ceftazidime-susceptible P. aeruginosa (MIC90s, 16, 8, and 16 μg/ml, respectively), whereas cefotaxime was virtually inactive. No compound inhibited ceftazidime-resistant P. aeruginosa at low concentration, and cross-resistance between ceftazidime, cefepime, and Ro 63-9141 was noticed for most, but not all, strains. The activities of the tested cephalosporins, including Ro 63-9141, against B. fragilis varied widely, but for most isolates high MICs were exhibited.
Bactericidal activity.
Ro 63-9141 exhibited time-dependent bactericidal activity against MSSA, MRSA, E. coli, and other species. Killing rates were dependent on the inoculum and growth phase, as with other cell wall-active agents. For the surviving cells after 24 h of exposure to Ro 63-9141, MICs were not higher than those for the isolates before exposure.
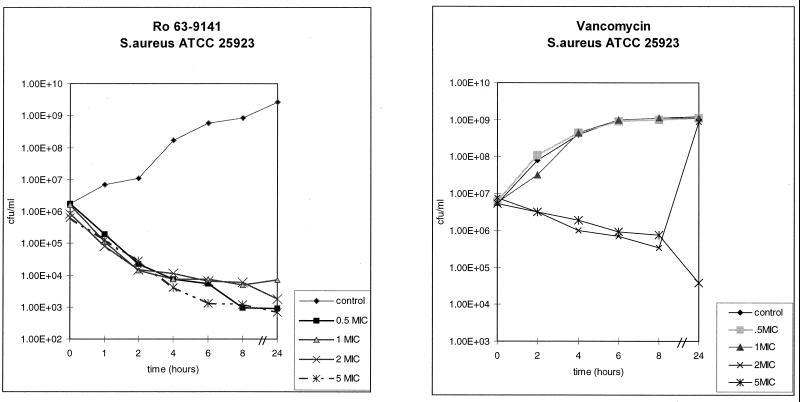
The rapid bactericidal activity of Ro 63-9141 against both MSSA and MRSA contrasted with the activity of vancomycin, which produced a modest drop in the viable cell count (Fig. 3).
FIG. 3.
Bactericidal activity against an MSSA isolate in comparison with that of vancomycin.
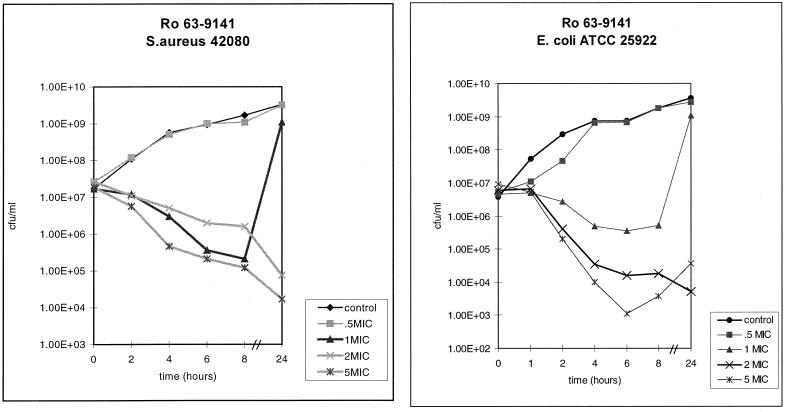
This rapid bactericidal effect was also achieved with resistant gram-positive strains and against E. coli (Fig. 4).
FIG. 4.
Rapid bactericidal action against MRSA (S. aureus 42080) and gram-negative E. coli ATCC 25922.
Inhibition of PBPs.
A number of PBPs have been cloned and purified after expression of the genes in E. coli in soluble form. These proteins were used to test Ro-63-9141 and reference compounds for inhibition (17).
Fifty percent inhibitory concentrations (IC50s), determined with a fluorescein-labeled penicillin, showed that Ro 63-9141 had a high affinity (IC50 = 0.87 μM) for S. epidermidis PBP 2′, in contrast to all other β-lactams measured (Table 2). The affinity for PBP 1b from C. freundii, a gram-negative organism, was comparable to that of ceftriaxone (IC50 of 0.16 versus 0.19 μM). It was also shown that Ro 63-9141 acylates PBP 2′ more rapidly than other β-lactam antibiotics do and forms a more stable acyl-enzyme complex through a unique mode of interaction with the protein. These effects lead to 100% inhibition of PBP 2′ and hence to potent antibacterial activity.
TABLE 2.
Affinity for PBPs
| Compound | IC50 for competition with fluorescein-labeled ampicillin (μM)
|
|||
|---|---|---|---|---|
| Staphylococcus epidermidis PBP 2′ | Enterococcus faecium PBP 5 | Streptococcus pneumoniae PBP 2x | Citrobacter freundii PBP 1b | |
| Ro 63-9141 | 0.87 | >500 | 0.27 | 0.16 |
| Ceftriaxone | 115 | >500 | 0.16 | 0.19 |
| Imipenem | >500 | >500 | 0.16 | 2.1 |
| Methicillin | >500 | >500 | 0.05 | Not tested |
β-Lactamase stability.
Like many third-generation cephalosporins (e.g., ceftriaxone), Ro 63-9141 is a poor substrate for class C β-lactamases and is hydrolyzed at very low rates compared to those of cephalothin or penicillin G (Table 3). This appears to be due to substrate inhibition induced by the 7-hydroxyiminoacetamido side chain (2, 17). Ro 63-9141 is also a poor substrate for class A enzymes, particularly penicillinases (e.g., S. aureus PC1). It is more readily hydrolyzed by the class A cephalosporinase from Proteus vulgaris 1028 and by ESBLs (TEM derivatives), but it is still relatively stable compared to good substrates.
TABLE 3.
Rates of hydrolysis by purified β-lactamases
| Compound | Mol of substrate hydrolyzed/mol of enzyme/min
|
||||||
|---|---|---|---|---|---|---|---|
| Class C
|
Class A
|
||||||
| Citrobacter freundii 1203 | Pseudomonas aeruginosa 18SH | Proteus vulgaris 1028 | Staphylococcus aureus PC 1 |
Escherichia coli
|
|||
| TEM1 | TEM3 | TEM4 | |||||
| Ro 63-9141 | 7.1 | 3.6 | 540 | 0.93 | 17 | 290 | 460 |
| Ceftriaxone | 4.1 | 6.8 | 900 | 19 | 900 | 4,900 | 640 |
| Cephalothin | 10,000 | 11,000 | 10,000 | 200 | 1,500 | 6,000 | 4,000 |
| Penicillin G | 900 | 760 | 1,000 | 10,000 | 12,000 | 9,000 | 15,000 |
Resistance development in MRSA.
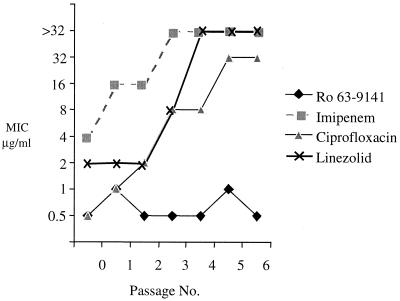
Serial passage on increasing concentrations of Ro 63-9141 was performed with three MRSA strains and one MSSA strain. In no case was there an increase in the MIC of more than one dilution step after six to eight passages, and the MICs remained below 4 μg/ml. In a more demanding approach, development of resistance to Ro 63-9141 was studied with a high inoculum of an MRSA strain (S. aureus 745) in parallel with that to imipenem, ciprofloxacin, and linezolid, a compound that is reported to show a very low incidence of resistance development (9). The size of the inoculum was increased to 2 × 108 CFU per plate. Resistance to imipenem, ciprofloxacin, and even linezolid emerged rapidly, resulting in final MICs of 32 μg/ml or higher, whereas an increase in MIC of only a factor of 2 was observed for Ro 63-9141 and was not sustained after reisolation (Fig. 5).
FIG. 5.
Increase of MIC upon exposure to Ro 63-9141 or reference compounds in S. aureus 745 (inoculum, 2 × 108 CFU/plate).
These data suggest that development of resistance to Ro 63-9141 due to chromosomal mutations seems to occur with low frequency, if ever, in MRSA strains.
Experimental septicemia in mice.
Ro 63-9141 was studied against septicemias caused by pathogens that differed in their susceptibilities to this compound. Cefepime, ceftriaxone, and meropenem, were used as comparators. Ro 63-9141 was highly effective upon s.c. administration (ED50s, <3 mg/kg) to animals with infections with strains for which MICs were ≤2 μg/ml (Table 4). This includes isolates of MSSA, MRSA, Streptococcus pyogenes, Streptococcus pneumoniae, E. coli, K. pneumoniae, C. freundii, Serratia marcescens, and Proteus mirabilis. In particular, Ro 63-9141 showed activity against three penicillin-resistant strains of Streptococcus pneumoniae, including a strain (Streptococcus pneumoniae 23 F-CTR) that showed reduced susceptibility to third-generation cephalosporins in vitro (MIC of ceftriaxone, 4 μg/ml; MIC of cefotaxime, 8 μg/ml versus that of Ro 63-9141 1 μg/ml) as well as in vivo (ED50 of ceftriaxone, 8.8 mg/kg; ED50 of cefotaxime, >12 mg/kg versus that of Ro 63-9141, 1.0 mg/kg). Against the MRSA strain S. aureus I-6, Ro 63-9141 (ED50, 2.4 mg/kg) was more effective than vancomycin (ED50, 6.7 mg/kg), in spite of demonstrating the same in vitro activity. Meropenem and cefepime were inactive at the highest dose tested (25 mg/kg). For a strain of P. aeruginosa for which the MIC was 8 μg/ml (P. aeruginosa BA) and one of Enterobacter cloacae for which the MIC was 4 μg/ml (Enterobacter cloacae MRW) ED50s of Ro 63-9141 were 4.0 and 3.8 mg/kg, respectively. The strain Proteus vulgaris 1028 with in vitro resistance to Ro 63-9141 was also resistant in vivo (ED50, >12 mg/kg). The activity of Ro 63-9141 after oral dosing was only marginal, as was found in MSSA septicemia (data not shown).
TABLE 4.
Activities of Ro 63-9141 and reference compounds against experimental septicaemias in mice
| Strain (CFU/mouse) and compound | MIC (μg/ml) | ED50a (mg/kg s.c.) |
|---|---|---|
| MSSA Smith (2 × 107) | ||
| Ro 63-9141 | 0.25 | <0.2 |
| Cefepime | 1 | 0.89 (0.55–1.5) |
| Ceftriaxone | 4 | 0.89 (0.55–1.5) |
| Meropenem | ≤0.12 | 0.22 (0.095–0.53) |
| Vancomycin | 1 | 0.73 (0.46–1.2) |
| MRSA I-6 (5 × 107) | ||
| Ro 63-9141 | 2 | 2.4 (1.4–4.0) |
| Cefepime | >16 | >25 |
| Ceftriaxone | >16 | >25 |
| Meropenem | >16 | >25 |
| Vancomycin | 2 | 6.7 (4.8–9.4) |
| Streptococcus pyogenes β15 (1 × 106) | ||
| Ro 63-9141 | ≤0.06 | <0.2 |
| Cefepime | ≤0.06 | <0.2 |
| Ceftriaxone | ≤0.06 | <0.2 |
| Meropenem | ≤0.06 | 0.27 (0.19–0.38) |
| Vancomycin | 0.5 | 0.70 (0.37–1.2) |
| Penicillin-susceptible Streptococcus pneumoniae 4241 (2 × 102) | ||
| Ro 63-9141 | ≤0.06 | <0.2 |
| Benzylpenicillin | ≤0.06 | <0.4 |
| Cefepime | ≤0.06 | <0.2 |
| Ceftriaxone | ≤0.06 | <0.2 |
| Meropenem | ≤0.06 | 0.34 (0.19–0.60) |
| Vancomycin | 0.5 | 0.69 (0.43–1.1) |
| Penicillin-resistant Streptococcus pneumoniae 1/37 (1 × 106) | ||
| Ro 63-9141 | 0.5 | 1.1 (0.80–1.5) |
| Benzylpenicillin | 1 | 1.1 (6.5–18) |
| Cefepime | 0.5 | 4.2 (3.0–5.9) |
| Ceftriaxone | 1 | 3.1 (1.7–5.1) |
| Meropenem | 0.25 | 1.8 (1.1–2.9) |
| Vancomycin | 0.5 | 0.44 (0.27–0.72) |
| Penicillin-resistant Streptococcus pneumoniae 536 (1 × 106) | ||
| Ro 63-9141 | 1 | 0.6 (0.35–1.0) |
| Benzylpenicillin | 2 | >12 |
| Ceftriaxone | 1 | 2.1 (1.5–3.0) |
| Meropenem | 1 | 1.8 (1.1–2.9) |
| Penicillin-resistant Streptococcus pneumoniae 23 F-CTR (5 × 104) | ||
| Ro 63-9141 | 1 | 1.0 (0.75–1.5) |
| Benzylpenicillin | 1 | >12 |
| Ceftriaxone | 4 | 8.8 (6.1–13) |
| Meropenem | 0.5 | 1.2 (0.70–2.0) |
| Escherichia coli 25922 (5 × 103) | ||
| Ro 63-9141 | ≤0.06 | <0.2 |
| Cefepime | ≤0.06 | <0.2 |
| Ceftriaxone | ≤0.06 | <0.2 |
| Meropenem | ≤0.06 | <0.2 |
| Klebsiella pneumoniae 418 (1 × 103) | ||
| Ro 63-9141 | ≤0.06 | <0.4 |
| Cefepime | ≤0.06 | <0.4 |
| Ceftriaxone | ≤0.06 | <0.4 |
| Meropenem | ≤0.06 | <0.4 |
| Enterobacter cloacae MRW (2 × 106) | ||
| Ro 63-9141 | 4 | 3.8 (2.2–6.0) |
| Cefepime | 2 | 1.1 (0.8–1.5) |
| Ceftriaxone | >32 | >25 |
| Meropenem | ≤0.06 | 0.22 (0.13–0.36) |
| Citrobacter freundii 1982 (1 × 107) | ||
| Ro 63-9141 | 1 | 1.5 (0.92–2.3) |
| Cefepime | 1 | 0.45 (0.19–1.1) |
| Ceftriaxone | >32 | >25 |
| Meropenem | ≤0.06 | <0.2 |
| Serratia marcescens 69438 (5 × 103) | ||
| Ro 63-9141 | 0.25 | 0.68 (0.41–1.2) |
| Cefepime | 0.12 | <0.2 |
| Ceftriaxone | 0.25 | <0.4 |
| Meropenem | ≤0.06 | 0.45 (0.27–0.72) |
| Proteus vulgaris 1028 (1 × 106) | ||
| Ro 63-9141 | >32 | >12 |
| Cefepime | 0.5 | 0.28 (0.09–0.84) |
| Ceftriaxone | 0.12 | <0.2 |
| Meropenem | ≤0.06 | <0.4 |
| Proteus mirabilis 2117 (2 × 106) | ||
| Ro 63-9141 | ≤0.06 | 0.35 (0.19–0.59) |
| Cefepime | 0.12 | <0.4 |
| Ceftriaxone | ≤0.06 | <0.2 |
| Meropenem | 0.12 | 4.4 (3.0–6.4) |
| Pseudomonas aeruginosa BA (1 × 105) | ||
| Ro 63-9141 | 8 | 4.0 (2.1–8.0) |
| Cefepime | 4 | 1.0 (0.73–1.5) |
| Ceftriaxone | 32 | 8.8 (6.1–13) |
| Meropenem | 2 | <0.4 |
ED50 after s.c. administration, with 95% confidence interval in brackets.
Experimental s.c. abscesses in mice.
Ro 63-9141 was tested against two MRSA strains in this model in comparison with linezolid and vancomycin. Ro 63-9141 (10 mg/kg i.p.) was more bactericidal than vancomycin (10 mg/kg i.p.) and linezolid (20 mg/kg i.p.) against the vancomycin-susceptible strain S. aureus I-6 (Table 5). The median viable count in the Ro 63-9141 group was 5.12 log units lower than in the untreated control group, compared to 3.42 log units lower in the vancomycin group and 0.80 log unit lower in the linezolid group. The same dose of Ro 63-9141 was also very effective against the VISA strain Mu50, as the pathogen was completely eliminated from most animals. Vancomycin (40 mg/kg i.p.) and linezolid (20 mg/kg i.p.) reduced the viable count only by a very minor extent, compared to the number of viable cells in the untreated control.
TABLE 5.
Activities of Ro 63-9141, vancomycin, and linezolid against experimental s.c. MRSA abscesses in mice
| Strain | Compound | MIC (mg/liter) | Dose (mg/kg i.p.) | No. of animals | Median log-unit no. of CFU at the site of infectiona | Median decrease in log-unit no. of CFU compared to that of the untreated control groupa |
|---|---|---|---|---|---|---|
| MRSA I-6 | No compound | 21 | 7.42 | |||
| Ro 63-9141 | 2 | 10 | 21 | 2.30 | 5.12 | |
| Vancomycin | 2 | 10 | 21 | 4.00 | 3.42 | |
| Linezolid | 2 | 20 | 5 | 6.62 | 0.80 | |
| S. aureus | No compound | 5 | 6.79 | |||
| Mu50 | Ro 63-9141 | 2 | 10 | 5 | <2 | >4.79 |
| MRSA | Vancomycin | 8 | 40 | 5 | 6.66 | 0.13 |
| VISA | Linezolid | 4 | 20 | 5 | 6.15 | 0.64 |
72 h after infection.
DISCUSSION
High affinity for PBP 2′ is crucial for the antibacterial activity of β-lactams against MRS. Unfortunately, none of the available β-lactam antibiotics sufficiently fulfils this requirement. This fact increasingly limits the therapeutic potential against staphylococcal infections of these otherwise very useful drugs.
However, the intensive search for new antistaphylococcal drugs has shown recently (1, 7, 11, 12, 14) that it is possible to achieve strong inhibition of PBP 2′ with selected β-lactam structures. Ro 63-9141 is a member of the class of pyrrolidinone-3-ylidenemethyl cephems, which show high potency against PBP 2′ of S. aureus but higher IC50s against normal, sensitive PBPs than other β-lactam antibiotics, e.g., methicillin and imipenem. This appears to be a common property of the pyrrolidinone-3-ylidenemethyl cephems, as it has been observed before for other members of this structural class (7). Ro 63-9141 shares features of cephalosporins with 7-aminothiazolylhydroxyimino side chains in that it is remarkably stable to hydrolysis by the S. aureus PC 1 enzyme.
Inhibition of PBP 2′ together with stability against the action of β-lactamase translates into bactericidal activity against MRS cells. Similar to those of other penicillins or cephalosporins, the bactericidal activity of Ro 63-9141 was time dependent. Ro 63-9141 inhibited all 77 tested clinical isolates of MRSA which stem from different parts of the world at a concentration of 4 μg/ml or below. These isolates also include strains highly resistant to imipenem and strains showing homogenous or heterogenous resistance to methicillin (21). Sodium chloride added at 2% hardly influenced the MICs of Ro 63-9141. Decreased susceptibility to vancomycin does not seem to affect the activity of Ro 63-9141 against MRSA, as the MIC of 2 μg/ml against the VISA strain S. aureus Mu50 is identical to that observed for many vancomycin-susceptible MRSA strains. It is vital for an antimicrobial agent that its antibacterial properties are not overcome easily by the rapid emergence of resistant strains. In our experiments, development of resistance to Ro 63-9141 could not be demonstrated during multipassage exposure to the compound, even when high inocula were used.
In contrast, resistant mutants could easily be selected for the reference compounds, including linezolid. This result differs from previously published findings (9), and it must be left open whether this is due to the experimental conditions used or a property of the particular strain used.
In keeping with the in vitro activities, Ro 63-9141 showed therapeutic efficacy in septicemia models of the mouse against both MSSA and MRSA after parenteral administration. The in vivo bactericidal properties of Ro 63-9141 could be demonstrated in a mouse abscess model. In this model the bactericidal effect is quantified by determination of the viable cell count after exposure to a test compound. Administration of 10 mg of Ro 63-9141 per kg i.p. reduced the viable count of a vancomycin-susceptible MRSA strain to a greater extent than the same dose of vancomycin, a slowly bactericidal drug in vitro. The cell count of a VISA strain fell below the limit of detection in this model upon administration of Ro 63-9141. The bactericidal properties of Ro 63-9141 (administered as the prodrug Ro 65-5788) against MRSA became obvious also in an endocarditis rat model, where it was more effective than vancomycin and amoxicillin-clavulanic acid (J. M. Entenza, P. Hohl, I. Heinze-Krauss, J. Vouillamoz, M. P. Glauser, and P. Moreillon, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-014, 1998).
Our findings suggest that Ro 63-9141 may have potential against infections caused by pneumococci. Its in vitro activity against both penicillin-susceptible and penicillin-resistant pneumococci was confirmed in vivo by its high therapeutic efficacy in mice. In particular, Ro 63-9141 is still active against a strain (Streptococcus pneumoniae 23 F-CTR) that had reduced in vitro and in vivo susceptibility to ceftriaxone (MIC of ceftriaxone, 4 μg/ml).
Ro 63-9141 was comparable to ampicillin in potency against penicillin-susceptible enterococci, which are not inhibited by available cephalosporins. However, Ro 63-9141 lacked affinity to PBP 5, which is overexpressed and mutated in penicillin-resistant enterococci, and thus lacked activity against such organisms. Ro 63-9141 also showed strong inhibition of essential PBPs in gram-negative organisms. The major targets in E. coli appear to be PBP 1b and PBP 2 and not PBP 3, the target for ceftriaxone and other third-generation cephalosporins. High affinity for PBP 2 was observed already with earlier pyrrolidinone-3-ylidenemethyl cephems (7) and again appears to be a general, novel property of this class of cephalosporins. PBP affinities to and stability with different classes of β-lactamases determine the antibacterial spectrum of Ro 63-9141 against gram-negative pathogens. Relatively high-level stability towards broad-spectrum class A β-lactamases allowed high antibacterial activity similar to those of cefotaxime and ceftriaxone to be obtained against Enterobacteriaceae producing such enzymes. On the other hand, the new cephalosporin was more labile than cefepime to some ESBLs, and as a consequence, its inhibitory activity against ESBL-producing isolates was weaker and inconsistent. Stability with class C chromosomal β-lactamases appears to be the determining factor for the activity of Ro 63-9141 against cefotaxime-resistant isolates of Enterobacter cloacae and C. freundii, which was, however, less pronounced than that of cefepime. The lack of activity against Proteus vulgaris relies on the efficient enzymatic hydrolysis of Ro 63-9141.
Unlike earlier pyrrolidinone-3-ylidenemethyl cephems, Ro 63-9141 showed substantial activity against strains of P. aeruginosa, at least those susceptible to ceftazidime. The mere presence of the 7-aminothiadiazolylhydroxyimino side chain cannot explain this activity because most cephalosporins bearing this substituent do not inhibit P. aeruginosa. This finding suggests that the positively charged 3′ substituent significantly contributes to Pseudomonas activity.
Susceptibility against many anaerobic isolates, including gram-positive cocci and a few strains of Clostridium difficile, has been demonstrated (K. E. Bowker, M. Wootton, H. A. Holt, and A. P. MacGowan, Abstr. 38th Intersci. Conf. Antimcrob. Agents Chemother., abstr. F-20, 1998), but members of the B. fragilis group were mostly resistant to the new compound. As it was the case for gram-positive pathogens, the studied gram-negative organisms responded to the therapy with Ro 63-9141 in animal models in line with their in vitro susceptibilities.
Ro 63-9141 is not sufficiently soluble in water to be used for parenteral administration in humans. Thus, a soluble prodrug, Ro 65-5788, has been prepared for development. Following parenteral dosing, Ro 65-5788 is rapidly cleaved in the blood to yield Ro 63-9141. The level of protein binding of Ro 63-9141 is relatively low, i.e., 38% for human plasma (personal communication of A. Schmitt-Hoffmann), which is characteristic for cephems with a basic 3′ side chain. A low level of protein binding, meaning a high fraction of freely available, active drug, is a favorable prerequisite for in vivo efficacy.
In conclusion, Ro 63-9141 is a new β-lactam antibiotic that combines activity against MRS with broad-spectrum activity against gram-negative bacteria and other gram-positive bacteria. The bases of its antimicrobial properties are inhibition of essential PBPs, including PBP 2′ of MRS, and stability against the hydrolytic actions of β-lactamases. In vivo experiments confirmed the potential utility of Ro 63-9141 against infections caused by susceptible pathogens. A water-soluble prodrug, Ro 65-5788, was selected for developmental studies.
ACKNOWLEDGMENTS
We thank Pia Aubry, Nicole Bingler, Pia Celesti, Gunther Gass, Margaret Kania, Barbara Mensch, Heinz Meyer, Véronique Schirmer, Heidi Schlunegger, Marie-Thérèse Traendlin, and Cäcilia Wolfgang for expert technical assistance.
REFERENCES
- 1.Chambers H F. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for penicillin-binding protein PBP 2a. Antimicrob Agents Chemother. 1995;39:462–466. doi: 10.1128/aac.39.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charnas R L, Then R L. Mechanism of inhibition of chromosomal β-lactamases by third-generation cephalosporins. Rev Infect Dis. 1988;10:752–760. doi: 10.1093/clinids/10.4.752. [DOI] [PubMed] [Google Scholar]
- 3.Emori T G, Gaynes R P. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finney D J. Statistical method in biological assay. 3rd ed. London, United Kingdom: Charles Griffin & Co., Ltd.; 1978. [Google Scholar]
- 5.Fluit A C, Jones M E, Schmitz F-J, Acar J, Gupta R, Verhoef J the SENTRY Participants Group. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from SENTRY Antimicrobial Surveillance Program, 1997 and 1998. Clin Infect Dis. 2000;30:454–460. doi: 10.1086/313710. [DOI] [PubMed] [Google Scholar]
- 6.Hecker S J, Cho I S, Glinka T, Zhang Z J, Price M E, Lee V J, Christensen B G, Boggs A, Chamberland S, Malouin F, Parr T R, Annamalaj T, Blais J, Bond E L, Case L, Chan C, Crase J, Frith R, Griffith D, Harford L, Liu N, Ludwikow M, Mathias K, Rea D, Williams R. Discovery of MC-02,331, a new cephalosporin exhibiting potent activity against methicillin-resistant Staphylococcus aureus. J Antibiot. 1998;51:722–734. doi: 10.7164/antibiotics.51.722. [DOI] [PubMed] [Google Scholar]
- 7.Heinze-Krauss L, Angehrn P, Guerry P, Hebeisen P, Hubschwerlen C, Kompis I, Page M G P, Richter H G F, Runtz V, Stalder H, Weiss U, Wei C-C. Synthesis and structure-activity relationship of (lactamylvinyl)cephalosporins exhibiting activity against staphylococci, pneumococci, and enterococci. J Med Chem. 1996;39:1864–1871. doi: 10.1021/jm950886v. [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 9.Kaatz G W, Seo S M. In vitro activities of oxazolidinone compounds U100592 and U100766 against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1996;40:799–801. doi: 10.1128/aac.40.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kresken M, Hafner D, Witte W, Reinert R R. Resistenzentwicklung bei Staphylokokken and anderen grampositiven Erregern gegenüber Chemotherapeutika im mitteleuropäischen Raum. Chemother J. 1999;8:136–145. [Google Scholar]
- 11.Lee V J, Hecker S J. Antibiotic resistance versus small molecules, the chemical evolution. Med Res Rev. 1999;19:521–542. doi: 10.1002/(sici)1098-1128(199911)19:6<521::aid-med4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto M, Tamaoka H, Ishikawa H, Kikuchi M. In vitro and in vivo antibacterial activities of OPC-20001 1, a novel broad-spectrum 2-oxaisocephem antibiotic. Antimicrob Agents Chemother. 1998;42:2943–2949. doi: 10.1128/aac.42.11.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDougal C K, Thornsberry C. The role of β-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1996;23:832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagano R, Shibata K, Adachi Y, Imamura H, Hashizume T, Morishima H. In vitro activities of novel trans-3,5-disubstituted pyrrolidinylthio-1β-methyl-carbapenems with potent activities against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2000;44:489–495. doi: 10.1128/aac.44.3.489-495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria. 4th ed. Approved standard M11–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 17.Page M G P. The kinetics of non-stoichiometric bursts of beta-lactam hydrolysis catalysed by class C beta-lactamases. Biochem J. 1993;295:295–304. doi: 10.1042/bj2950295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reacher M H, Shah A, Livermore D M, Wale M C J, Graham C, Johnson A P, Heine H, Monnickendam M A, Barker K F, James D, George R C. Bacteremia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. Br Med J. 2000;320:213–216. doi: 10.1136/bmj.320.7229.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh N, Paterson D L, Chang F Y, Gayowski T, Squier C, Wagener M M, Marino I R. Methicillin-resistant Staphylococcus aureus: the other emerging resistant gram-positive coccus among liver transplant recipients. Clin Infect Dis. 2000;30:322–327. doi: 10.1086/313658. [DOI] [PubMed] [Google Scholar]
- 20.Sumita Y, Nouda H, Kanazawa K, Fukasawa M. Antimicrobial activity of SM-17466, a novel carbapenem antibiotic with potent activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:910–916. doi: 10.1128/aac.39.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasz A, Nachman S, Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991;35:124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]