Abstract
The potency of polymeric micelle-based doxorubicin, SP1049C, against cancer stem cells (CSCs) in triple negative breast cancer (TNBC) is evaluated. CSCs with high epithelial specific antigen (ESA), high CD44 and low CD24 expression levels were derived from the TNBC cancer cells, MDA-MB-231 and MDA-MB-468. These CSCs were resistant to free doxorubicin (Dox) and displayed increased colony formation, migration, and invasion in vitro, along with higher tumorigenicity in vivo, compared to the parental and non-CSCs counterparts. SP1049C downregulated the expression and inhibited the functional activity of the breast cancer resistance protein (BCRP/ABCG2) in CSCs. The polymeric micelle drug had higher cytotoxicity and potency in reducing the colony formation of CSCs compared to the free drug. It was also more potent in inhibiting the tumor growth in the orthotopic animal tumor models derived from CSCs. These results indicate that SP1049C is active against CSCs and has potential in treating TNBC.
Background
In the United States, in 2019, it is estimated that 41,760 women will die of breast cancer. Most breast cancer deaths are due to the spread of the disease to other parts of the body, and as a consequence impairing the function of vital organs like lung, liver, and brain. Breast cancer is usually classified by the presence, or absence of three receptors: estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). The therapeutic approach to breast cancer is highly influenced by the ability of therapeutic agents to specifically target these receptors. The triple negative breast cancer (TNBC) that lacks all three receptors is a heterogeneous disease characterized by aggressive biology and poor prognosis [1]. The lack of tumor-specific markers, aggressive nature, and special propensity to recur and metastasize, make TNBC tumors more difficult to treat than other subtypes.
Many tumors contain phenotypically and functionally heterogeneous populations of cancer cells that differ in metastatic [2] and angiogenic [3] potential, and drug resistance [4, 5]. In some tumors that follow the cancer stem cell (CSCs) model, the heterogeneous cancer cells are progenies of small number of CSCs [6, 7]. Increasing body of evidence suggests that CSCs have distinct phenotypes and display high tumorigenic and self-renewing potential being the driving force of tumor initiation, metastasis and development of drug resistance in such tumors [8, 9]. Moreover, CSCs themselves are commonly drug resistant, and can evade classic chemotherapy and repopulate tumors leading to cancer recurrence [4] [10], which is one of the main reasons for mortality in cancer patients [11]. Therefore, successful chemotherapy of such tumors should effectively eradicate CSCs, thereby preventing the cancer relapse.
To overcome these challenges, we have proposed a strategy that employs a polymeric micelle formulation of Dox with Pluronic block copolymers. These amphiphilic copolymers have unique ability to chemosensitize multidrug resistant (MDR) cancers by several mechanisms: 1) inhibiting ABC transporter–mediated drug efflux [12], mitochondrial respiration, and ATP synthesis [13], and 2) enhancing pro-apoptotic signaling in MDR cancer cells [14, 15]. Specific Pluronics with optimal composition can also prevent the development of drug resistance in response to chemotherapy [16, 17], and decrease formation of metastases [18]. One polymeric micelle formulation of Dox with Pluronic L61 and F127 mixture, SP1049C (now code-named SKC1049), was evaluated in Phase I and Phase II clinical trials and showed high objective response rates (43%) and increased median survival (10 months) in patients with inoperable metastatic adenocarcinoma of the esophagus and gastro-esophageal junction [19].
Previously, we have demonstrated that SP1049C suppresses the tumorigenicity and aggressiveness of murine leukemia cells by targeting CSCs [20]. Therefore, in this work we evaluate ability of SP1049C eradicate CSCs in TNBC, the most difficult form of breast cancer to treat. Using human TNBC cell lines, claudin-low MDA-MB-231 and basal MDA-MB-468, we show that SP1049C effectively reduces the CSCs population and thus, decreases tumorigenesis in vitro and in vivo. Altogether, these findings demonstrate simple, yet effective treatment strategy that eliminates both more differentiated cancer cells, comprising the bulk of the tumor, as well as small and extremely hard to target drug resistant CSCs subpopulation.
Methods
Isolation of CSCs
Cells (1×106in 100 μL 2% FBS in PBS) were stained with fluorescently labeled antibodies (1μg/mL for each antibody) in dark on ice for 30 minutes with 100 rpm shaking. Cells were then washed with PBS/2.0% FBS and then filtered through 30 μm strainer and processed for flow cytometry sorting. Compensation for each fluorescent probe was confirmed before sorting and two subpopulations (CSCs and non-CSCs) were collected using Aria II Three Laser Special Order System (Becton Dickinson) for the subsequent studies. CellQuest (Becton Dickinson, Franklin Lakes, NJ, USA) software package was used. The CSCs (ESA+CD44highCD24low), and non-CSCs (ESA−CD44lowCD24high) cells were isolated in two steps: First, the highest and lowest 10% of ESA-expressing cell subpopulations were isolated. These ESA+ and ESA− cells were then immediately plotted by the expression of CD44 and CD24 (Supplementary Figure S1) to determine CSCs and non-CSCs cell subpopulations, which comprised about 1–2% of all cells. Cells were sorted twice with each indicated biomarker to obtain a purer cell population.
Migration and Invasion Assay
The sorted CSCs and non-CSCs were trypsinized, re-suspended in alpha-MEM medium, and seeded in top section of transwell chambers with 8 mm pore size filter at 2×104 cells/well (BioCoat chambers; BD Biosciences, San Jose, CA, USA). After 10 minutes, alpha-MEM medium was added to the lower chamber and incubated for 13 hours. The cells on top of the membrane (not migrated) were removed and the migrated cells at the bottom surface of the membrane were visualized by staining with Diff-Quik Stain Set kit (Siemens Healthcare Diagnostics Inc., Erlangen, Germany) and counted using an inverted tissue culture microscope. The invasion assay was done as above except the cells were seeded on top of Matrigel-coated chambers (BD invasion chambers; 8 mm pore size filter; BD Biosciences). The cells were incubated for 15 hours prior to counting cells at the bottom surface.
Tumorsphere Formation Assay
Single-cell suspension of cells was plated (2 × 104/mL) in 6-well ultra-low attachment plates (Corning, Durham, NC, USA) in MammoCult® basal serum-free medium (human) with hydrocortisone (StemCell Technologies, Vancouver, Canada), and cultured for 10 days. Images were acquired under a Nikon inverted microscope. Total number of tumorspheres was counted microscopically under a manually prepared ‘quadrant grid’.
Tumorigenicity assay
MDA-MB-231 and MDA-MB-468 TNBC cells were sorted by fluorescence-activated cell sorting (FACS) to obtain subpopulations of CSCs (ESA-CD44highCD24low), and non-CSCs (ESA-CD44lowCD24high). After sorting, the limiting dilutions of the cells were assessed by measuring the efficiency of tumor formation in the fourth pair of mammary fat pad of athymic nu/nu mice (6- to 8-weeks-old females). Cells at different doses (from 500 to 10,000 cells/ 50μL/mouse) in culture medium were mixed with a matrigel (1:1 ratio; BD Biosciences) and implanted to the mammary fat pads to generate two tumors per mouse (n = 10 mice, 20 injections/group). Tumor growth was recorded weekly and the frequencies of CSCs were calculated using web-based extreme limiting dilution assay (ELDA) software http://bioinf.wehi.edu.au/software/elda/index.html. The frequency of CSCs was calculated from semilogarithmic plot of the fraction of negative responses as a function of the cell dose.
Antitumor activity
To establish orthotopic TNBC mouse model, MDA-MB-231, or MDA-MB-468 cells (100,000 cells/50 μL/mouse) were implanted to the mammary fat pad (left side) in nude/nude mice (n = 5 – 7 mice/group) as described above. Cells at a dose of 100,000 in culture medium (50 μL) were mixed with a 1:1 ratio of matrigel (BD Biosciences) and then implanted to the mammary fat pad. When the tumor size reached a size of about 50 mm3, mice were randomly divided into groups and i.v. injected with following treatment solutions: 1) saline (100 μl), 2) SP polymers (1:8 mixture of Pluronics L61 and F127; 2.25 mg/kg body weight), 3) Dox (2.5 mg/kg body weight) or 4) SP1049C (SP Polymers alone, 2.25 mg/kg body weight, Dox, 2.5 mg/kg body weight) on days 1, 4 and 7. Tumor length (L) and width (W) were measured, and tumor volume (WR) was calculated as follows: WR=1/2 × L × W2. Animal weight and tumor volumes were measured every other day.
Results
Characterization of Sorted Cells for CSCs Properties
A number of biomarkers and their combinations including epithelial specific antigen (ESA, also known as “epithelial cell adhesion molecule” (EpCAM)), CD24−/CD44+, CD133, and CD49f are commonly used to identify CSCs phenotype in human breast cancer [21–24]. In addition, CSCs show certain functional properties, such anchorage independent growth, high migration and invasiveness, drug resistance, and in vivo tumorigenicity [25].
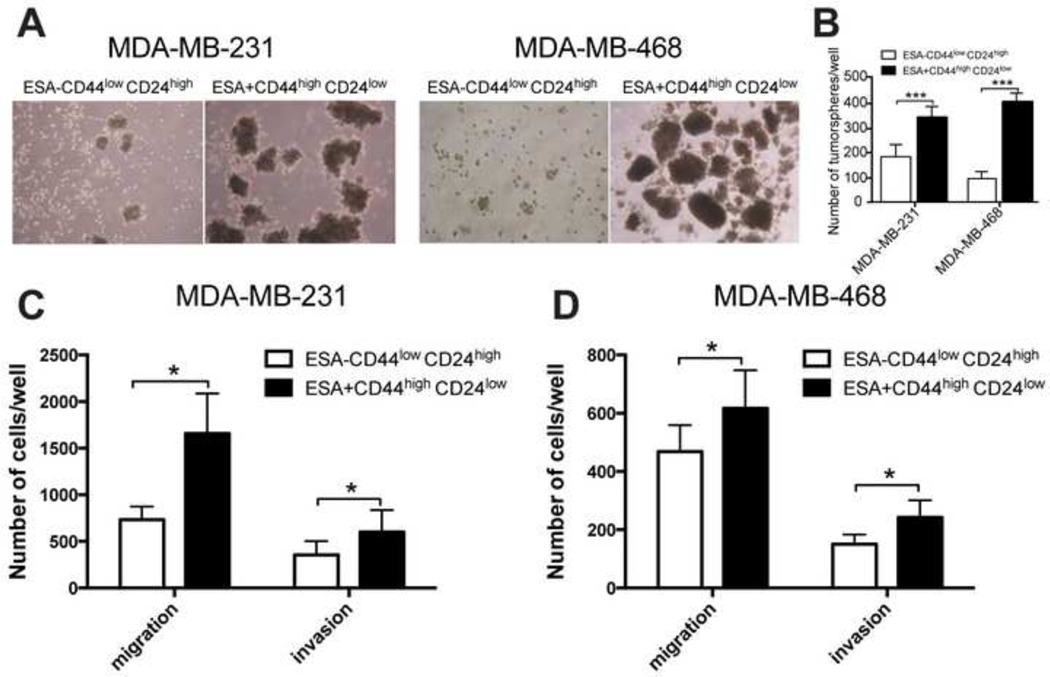
We first isolated the CSCs subpopulation with ESA+CD44highCD24low phenotype from TNBC MDA-MB-231 and MDA-MB-468 cells by FACS (Supplementary Figure S1), and then carried out functional assays of these cells to confirm their CSCs-like properties. The ESA−CD44lowCD24high cells and unsorted parental cells were used as the “non-CSCs” controls throughout the study. Then, as a part of the functional analysis we evaluated the ability of the isolated cells to form tumorspheres in serum-free growth conditions. Consistent with the studies reported elsewhere [26, 27] the CSCs formed significantly more tumorspheres (p<0.005), which were also larger than the tumorspheres formed by the respective non-CSCs cells (Figure A, 1B). Furthermore, the CSCs displayed greater ability to migrate through the polycarbonate membrane compared to non-CSCs cells, a process associated with cancer cell metastasis (p<0.05, Figure C, 1D). In addition, CSCs showed significantly higher invasion compared to non-CSCs cells suggesting ability to move through the extracellular matrix into neighboring tissues (p<0.05, Figure C, 1D). Finally, we evaluated Dox cytotoxicity in the sorted cells and found that cells with CSCs phenotype were indeed more drug resistant compared to non-CSCs cells (Error! Reference source not found., Supplementary Figure S2).
Figure 1. In vitro characterization of ESA+CD44highCD24low subpopulation sorted from MDA-MB-231 and MDA-MB-468 cell lines for CSCs properties.
(A) Phase contrast images and (B) quantification of tumorspheres formed by CSCs (ESA+CD44highCD24low) and non-CSCs (ESA-CD44lowCD24high) cells. (C, D) Invasion and migration assay for CSCs and non-CSCs sorted from (C) MDA-MB-231 and (D) MDA-MB-468 cell lines. (B-D) Statistical comparisons were made by unpaired t-test with Welch’s correction. *p < 0.05; **p < 0.01; ***p < 0.005; n.s. - not significant. Data are shown as mean ± SD, (B) n=4, or (C, D) n=6.
Next, the in vivo tumorigenicity of sorted cells was evaluated in a limiting dilution assay that is commonly used to determine the frequency of CSCs [28]. For this purpose, CSCs and non-CSCs cells were inoculated into mammary fat pad of nude mice (18 or 20 mice/group) at different doses, and tumor formation was monitored for two months. The estimated CSCs frequencies are presented in Table 2. Consistent with previously published data [6, 23], the tumorigenic potential of CSCs derived from both cell lines was higher than the respective potential of non-CSCs (Table 2). Furthermore, in MDA-MB-231 derived ESA+CD44highCD24low cells, the CSCs frequency was estimated as 1 in 1,576 cells, while in ESA−CD44lowCD24high cells it was 3.2 times lower, 1 in 5,116. Similar results were observed for MDA-MB-468 derived ESA+CD44highCD24low cells where the frequency of CSCs was 1 in 430, almost 6-fold higher than that for ESA-CD44lowCD24high cells, where it was 1 in 2,567.
Table 2.
Frequencies of tumor formation and calculated frequencies of CSCsa.
| Cells | MDA-MB-231 | MDA-MB-231 | MDA-MB-468 | MDA-MB-468 |
|---|---|---|---|---|
|
|
|
|||
| Dose | ESA-CD44lowCD24high | ESA+CD44highCD24low | ESA-CD44lowCD24high | ESA+CD44highCD24low |
|
| ||||
| 500 | 5/20 | 11/20 | - | - |
| 1000 | 8/20 | 15/20 | 10/20 | 18/20 |
| 3000 | - | - | 15/20 | 20/20 |
| 5000 | 5/20 | 13/18 | - | - |
| 10,000 | - | - | 18/20 | 20/20 |
|
| ||||
| CSCs frequency (95% CI) | 1/5116 (1/8380-1/3124) | 1/1576 (1/2385-1/1042) | 1/2567 (1/3799-1/1734) | 1/430 (1/746-1/248) |
|
| ||||
| p value | 3.15E-05 | 3.09E-08 | ||
After sorting by flow cytometry, cells at indicated dose in culture medium (50 μL) mixed with a 1:1 ratio of matrigel (BD Biosciences) were injected into the fourth mammary fat pads of 6- to 8-week-old female nude/nude mice. Mice were monitored daily to observe palpable tumors up to 2 months after tumor cells injection. CSCs frequencies were calculated using web-based ELDA statistical software at http://bioinf.wehi.edu.au/software/elda/index.html. It is noteworthy, that 90% of animals (18/20) injected with MDA-MB-468 derived ESA+CD44highCD24low cells formed tumors already at the lowest cell dose (1,000 cells/mouse), with the higher doses (3,000 and 10,000 cells/mouse) producing tumors in 100% cases.
Overall, we confirmed that the isolated breast cancer cells with ESA+CD44highCD24low phenotype display basic CSCs properties in vitro and in vivo, which provided basis for using these models in subsequent studies.
SP Polymers Sensitize CSCs to Dox and Decrease their Colony Formation Ability
We demonstrated earlier that Pluronic bock copolymers are potent chemosensitizers of MDR tumors [13, 29]. Here, we examined whether SP polymers (0.25% wt. Pluronic L61, and 2% wt. Pluronic F127) can sensitize CSCs with respect to Dox. Indeed, Dox/SP formulation showed greater cytotoxicity in CSCs than Dox alone (Table 1). The IC50 values for Dox/SP in these cells were comparable to those in the parental and non-CSCs for both studied cell lines. Furthermore, a formulation of Dox in SP polymers did not increase drug cytotoxicity in the parental and non-CSCs cells as the IC50 values of Dox/SP polymers and Dox were nearly the same. Noteworthy, the SP polymers themselves were not toxic to all cells in the absence of the drug (data not shown).
Table 1.
IC50 values of Dox and Dox/SP polymers in sorted CSCs, non-CSCs, and unsorted TNBC cells.
| MDA-MB-231 | MDA-MB-468 | |||
|---|---|---|---|---|
|
|
|
|||
| Dox (ng/mL) | Dox/SP polymers (ng/mL Dox) | Dox (ng/mL) | Dox/SP polymers (ng/mL Dox) | |
|
|
|
|||
| Unsorted cells | 6.366±0.662 | 4.732±0.381 | 4.307±0.221 | 3.825±0.259 |
|
|
|
|
|
|
| ESA-CD44lowCD24high | 5.413±0.899 | 4.814±0.555 | 5.193±0.889 | 4.781±0.369 |
|
|
|
|
|
|
| ESA+CD44highCD24low |
14.043±0.201**,## | 5.114±0.134n.s., n.s. |
9.077±0.551**,# |
5.051±0.428n.s., n.s. |
The treatment was the same as shown in Figure 3. Statistical comparisons were made by two-way ANOVA with Tukey’s correction for multiple comparisons between parental and ESA+CD44highCD24low group, or between ESA-CD44lowCD24high and ESA+CD44highCD24low group
p<0.05;
p<0.01,
p<0.005
p<0.05;
p<0.01,
p<0.005, n.s. not significant).
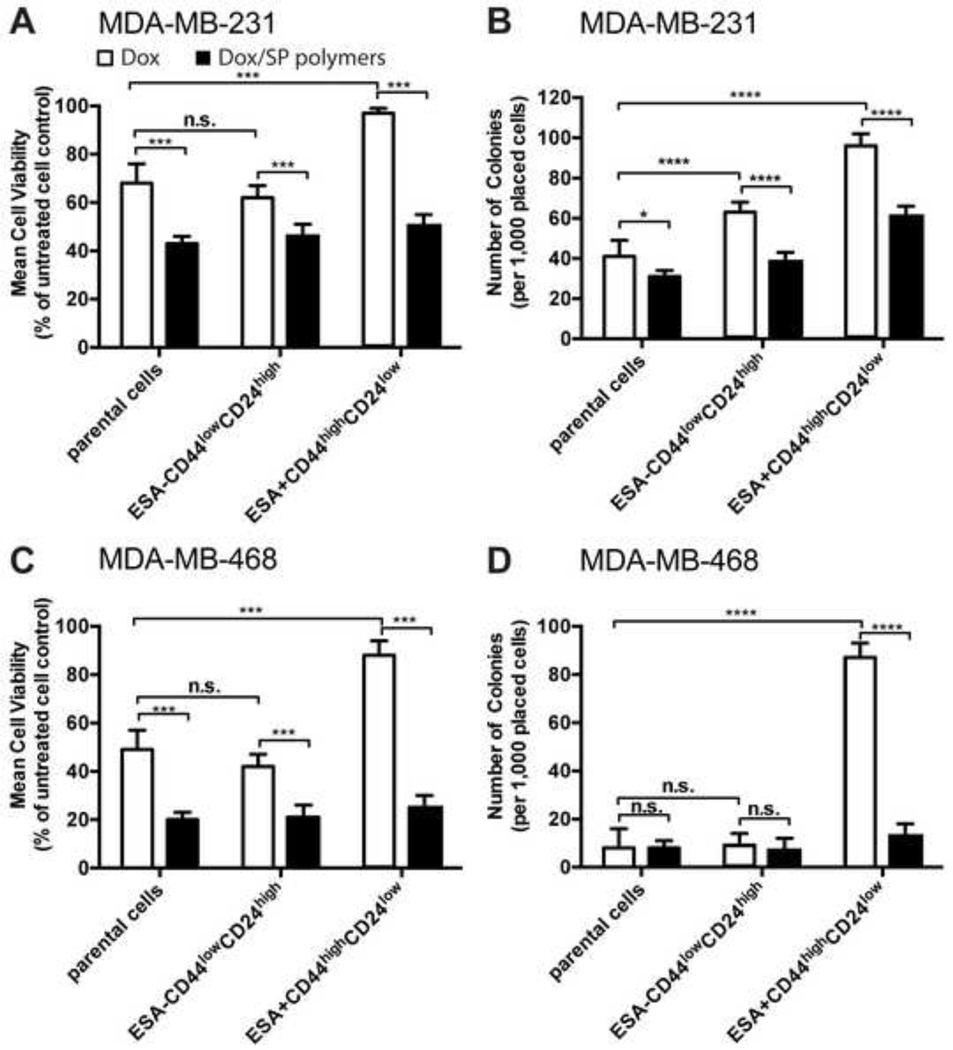
We further evaluated the effect of SP polymers on the colony formation potential of CSCs. In this set of experiments, the cells were treated with Dox (5 ng/ml, close to the IC50 of Dox in MDA-MB-231 and MDA-MB-468 unsorted parental cells) in the absence or presence of SP polymers (0.005% wt) for five days. As expected, in the absence of SP polymers the viability of CSCs upon Dox exposure was significantly higher than that of respective non-CSCs (Figure 2A, 2C). The addition of SP polymers increased the sensitivity of CSCs to Dox (Figure 2A, 2C). Next, as a functional measure of CSCs frequency, we also examined the ability of the cells to form colonies after this treatment. Thus, following the treatment 1,000 viable cells from each group were collected and seeded into new plates, cultured in drug-free media and counted following two weeks. The number of colonies formed by CSCs MDA-MB-231 and MDA-MB-468 cells treated with Dox/SP polymers decreased by ca. 1.5 and 6-fold, respectively, compared to the cells treated with the drug alone (Figure 2B, 2D). The formation of colonies was also inhibited in the parental and non-CSCs MDA-MB-468 cells. Overall, we conclude that Dox/SP formulation effectively sensitized CSCs that resulted in higher toxicity, and reduced colony formation potential of the survived cells.
Figure 2. SP polymers sensitize CSCs to Dox and reduce colony forming potential.
Sorted CSCs (ESA+CD44highCD24low), non-CSCs(ESA-CD44lowCD24high), and unsorted parental MDA-MB-231 (A, B) and MDA-MB-468 (C, D) cells were treated with Dox alone (white bars) or Dox formulated with SP copolymers (0.005% wt.) (empty bars) for five days. Cells were then harvested and viable cells were quantified using trypan blue exclusion (A, C). Survived cells (1,000/well) were placed into new 6-well plate and cultured in the drug-free media for two weeks. Colonies were fixed, stained with 2% methylene blue in 95% ethanol and counted (B, D). Only colonies comprising >50 cells were scored as survival colonies. Data are means ± SD from three independent experiments (n = 3). Statistical comparisons were made using two-way ANOVA with Tukey’s correction for multiple comparisons. *p < 0.05; **p < 0.01, ***p < 0.005. n.s. - not significant.
Effect of Dox/SP Formulation on Drug Efflux in Transporters in CSCs
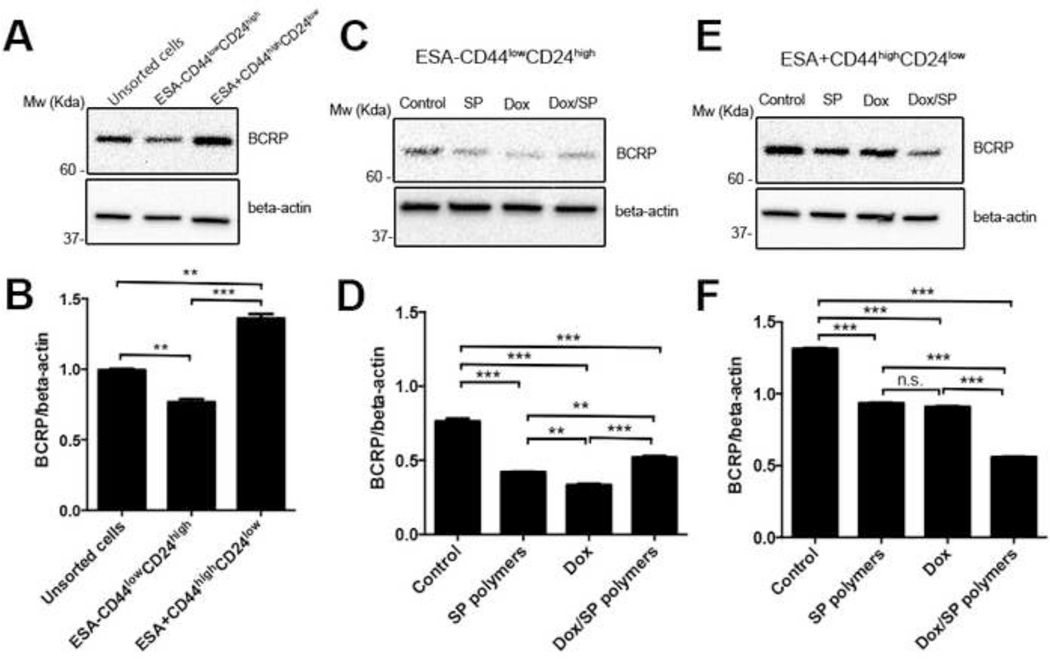
Sensitization of MDR cells by Pluronic copolymers was previously related to the expression of the ABC transporters in these cells [30, 31]. Both MDA-MB-231 and MDA-MB-468 parental cells lines express breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance protein 1 (MDR1/ABCB1), also known as P-glycoprotein (Pgp) (Supplementary Figure S3). It has also been reported that CSCs-like subpopulation of MDA-MB-231 cells have increased expression levels of BCRP/ABCG2 but not MDR1/ABCB1 [32]. Therefore, we examined the expression of drug efflux transporter, BCRP/ABCG2 in parental and sorted MDA-MB-231 cells. The data indicate that CSCs have higher expression levels of BCRP/ABCG2 than non-CSCs or parental cells (Figure 3A, 3B). Next, the cells were treated with 1) media, 2) SP polymers alone (0.005%), 3) Dox (5 ng/mL) or 4) Dox (5 ng/mL)/SP (0.005% wt.) for five days, and the BCRP/ABCG2 expression was assessed by western blot. The BCRP/ABCG2 expression levels were significantly reduced in both non-CSCs (Figure 3C, 3D) and CSCs (Figure 3E, 3F) subpopulations upon treatment with SP, or Dox, or Dox/SP compared to control cells in media. Noteworthy, Dox/SP treatment resulted in most pronounced depletion of BCRP/ABCG2 levels in CSCs subpopulation (Figure 3E, 3F).
Figure 3. Effect of Dox/SP polymers on BCRP/ABCG2 expression levels in MDA-MB-231 cells.
(A, B) BCRP/ABCG2 expression in CSCs, non-CSCs, and unsorted parental MDA-MB-231 cells was measured by western blot. BCRP/ABCG2 expression in (C, D) non-CSCs and (E, F) CSCs subpopulations was measured after treatment with media, SP polymers (0.005%) or Dox (5 ng/mL) with/without SP polymers (0.005% wt.). Beta-actin was used as protein loading control. Band intensities were measured with ImageJ software and presented as means ± SD. Statistical comparisons were made by one-way ANOVA with Tukey’s corrections for multiple comparisons. *p < 0.05; **p < 0.01, ***p < 0.005. n.s. - not significant.
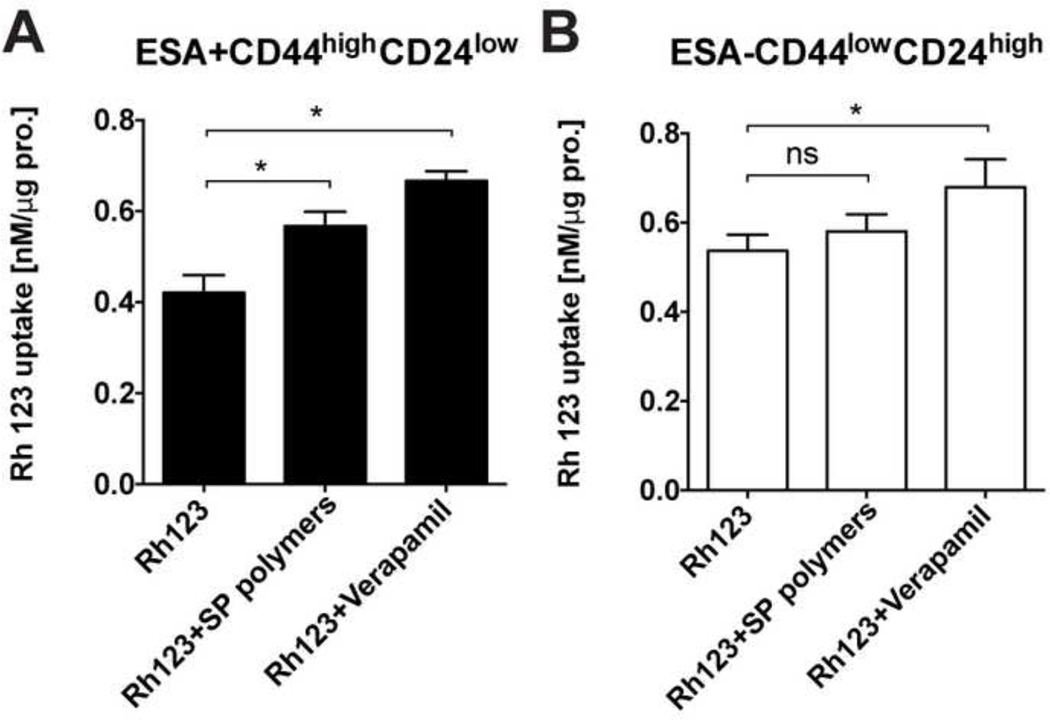
To assess effect of SP polymers on the functional activity of the drug efflux transporters we studied the uptake of a BCRP/ABCG2 and MDR1/ABCB1 substrate, rhodamine 123 (Rh123), in CSCs and non-CSCs subpopulations derived from MDA-MB-231 cells. We demonstrated here that Rh123 accumulation in CSCs increased significantly in the presence of SP polymers compared to untreated control (Figure 4A). This effect was similar to that of Verapamil, a competitive efflux transporter inhibitor [33]. In contrast, no significant Rh123 accumulation increases were recorded in non-CSCs subpopulation upon SP polymers treatment (Figure 4B).
Figure 4. Effect of SP polymers on Rh123 uptake in MDA-MB-231 cells.
Rh123 uptake in (A) CSCs and (B) non-CSCs subpopulations was examined after treatment with 1) Rh123 (3.2 μM) alone, 2) Rh123 (3.2 μM) plus SP polymers (0.005% w.) or 3) Rh123 (3.2 μM) plus Verapamil (50 μM) for two hours. Data are shown as mean ± SD (n = 8) and analyzed using one-way ANOVA with Tukey’s correction for multiple comparisons. *p < 0.05; **p < 0.01, ***p < 0.005. n.s. - not significant.
Effect of SP Polymers on Intracellular ATP Levels
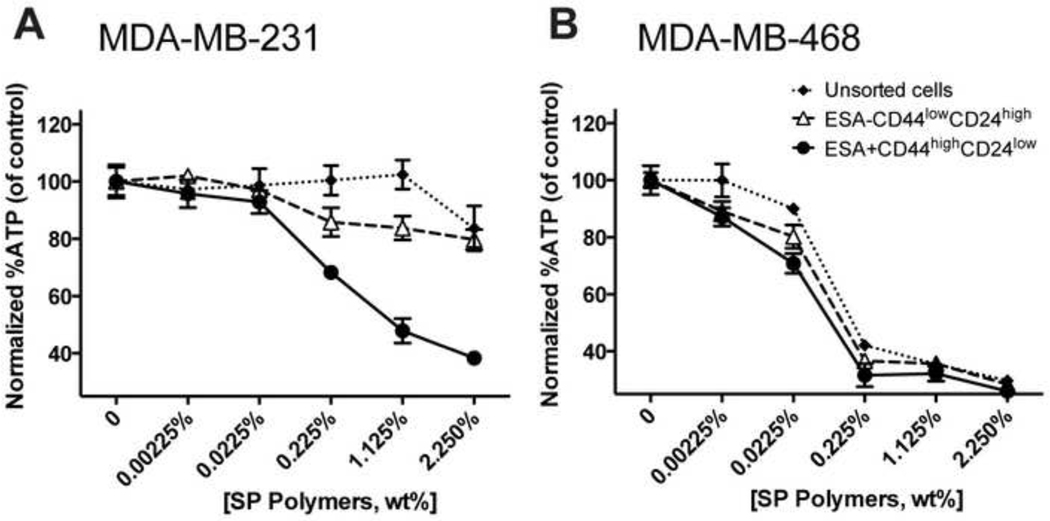
The sensitizing effects of Pluronic copolymers in MDR cancer cells were linked to the ability of these copolymers deplete to intracellular ATP [30, 31, 34]. In this regard, we examined whether SP polymers can also produce ATP depletion in TNBC cells subpopulations. In the CSCs derived from MDA-MB-231 cells the ATP levels were depleted at quite low concentrations of SP polymers of ca. 0.225% wt., while in the non-CSCs or unsorted parental MDA-MB-231 cells no ATP depletion was observed up to 2.25% wt. (Figure 5A). In MDA-MB-468 cells we did not observe significant differences between the sorted and unsorted cells, as they all appeared to be highly sensitive to SP polymers in terms of ATP depletion, exhibiting a considerable decrease in ATP levels at the copolymer concentration as low as 0.0225% to 0.225% wt. (Figure 5B).
Figure 5. Effect of SP polymers on intracellular ATP levels in TNBC cells.
Sorted non-CSCs (ESA-CD44lowCD24high), CSCs (ESA+CD44highCD24low),and unsorted parental cells derived from MDA-MB-231 (A) and MDA-MB-468 (B) were incubated with various concentrations (0–2.25 wt. %) of SP copolymers for 2 hours, washed twice with ice-cold PBS and lysed. ATP concentrations in the lysates were determined using a luciferin/luciferase assay. ATP levels were normalized for protein content. Data are mean ± SD (n = 4) of 3 individual replications.
Dox/SP Formulation Suppresses Tumor Growth and Depletes CSCs in vivo
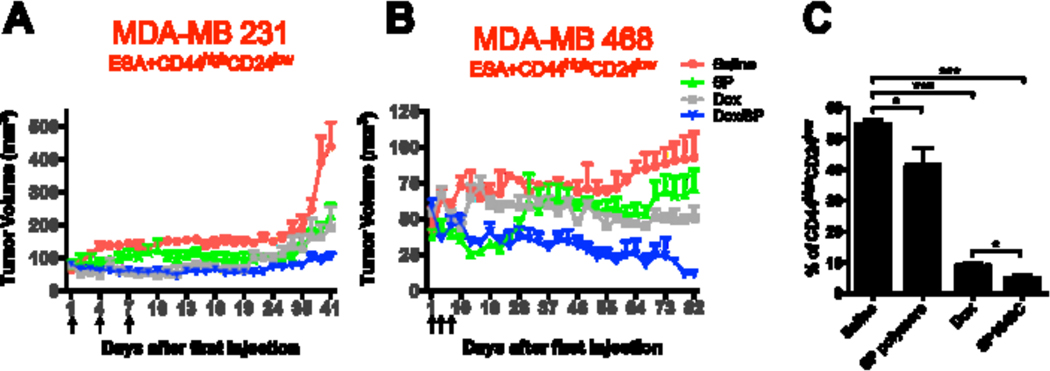
The antitumor activity of Dox/SP formulation was evaluated in vivo in mice with orthotopically inoculated ESA+CD44highCD24low CSCs derived from either MDA-MB-231 (Figure 6A) or MDA-MB-468 (Figure 6B) cells. Four weeks after inoculation, the mice were systemically treated with either: 1) saline; or 2) SP polymers (2.25 mg/kg); or 3) Dox (2.5 mg/kg); or 4) Dox/SP formulation (SP polymers, 2.25 mg/kg, Dox, 2.5 mg/kg) on days 1, 4 and 7 (the dose and schedule were consistent with our previous publications [13]). Forty days after the first treatment, the tumor size in the saline treatment group reached about 500 mm3, and the animals had to be sacrificed as guided by our IACUC regulations due to the necrotic tumors. Dox/SP formulation showed superior effect on the tumor growth inhibition compared to Dox alone for both cell lines (Figure 6A, 6B). Notably, SP polymers alone (without Dox) also delayed tumor growth compared to saline-treated group in CSCs MDA-MB-231 tumor bearing mice, although to a lesser extent (Figure 6A, see also Supplementary Figure S4). Clear inhibition of tumor growth was observed in MDA-MB-468 tumors as well. While these tumors developed very slowly requiring much longer observation time before the endpoint, Dox/SP combination suppressed the tumor growth almost completely (Figure 6B).
Figure 6. Dox/SP polymers (SP1049C) suppressed tumor growth and depleted CSCs in TNBC orthotopic model.
Nude mice bearing tumors from ESA+CD44highCD24low MDA-MB-231 (A) or MDA-MB-468 (B) CSCs subpopulation (n = 5–7/group) were i.v. injected with 1) saline, 2) SP polymers (2.25 mg/kg), 3) Dox (2.5 mg/kg) or 4) Dox/SP polymers (SP, 2.25 mg/kg, Dox, 2.5 mg/kg) on days 1, 4 and 7 (as indicated by arrowheads in the figures). The mean tumor volume ± SEM of each group was recorded over time. (C) Percent of CSCs in tumors from ESA+CD44highCD24low MDA-MB-231-bearing mice treated with different formulations was analyzed at the end point of the experiment shown in panel A (day 41). Data is means ± SD (n=4). Statistical comparisons are made using one-way ANOVA with Tukey’s correction for multiple comparisons. *p < 0.05; **p < 0.01, ***p < 0.005. n.s. - not significant.
We also analyzed the percentage of CSCs in tumors that were initiated by inoculation of MDA-MB-231 ESA+CD44highCD24low cells and then treated with saline, SP, Dox, or Dox/SP by flow cytometry. The treatments with saline and SP polymers alone resulted in high levels of CSCs (up to 55%), while treatments with Dox and Dox/SP polymers significantly depleted this cell subpopulation (Figure 6C). Consistent to the results described above, Dox/SP formulation depleted CSCs in tumors significantly stronger than Dox alone. Overall, the results indicate that Dox/SP formulation effectively suppressed tumor growth formed by CSCs depleting CD44highCD24low cells in mouse models.
Discussion
There is a growing focus on the development of cancer therapies that would target CSCs subpopulation [35, 36]. For example, previous work to eradicate chemoresistant CSCs reported the use for this purpose of the oncolytic herpes simplex virus type 1 (HSV1) [37], or polyplexes to deliver siRNA against AKT2, an oncogene involved in breast cancer tumorigenesis [38]. Other studies coupled a drug to gold nanoparticles that presumably bypassed the drug efflux transport system in CSCs [39] or co-delivered a drug with a second active pharmaceutical ingredient (API) capable of suppressing CSCs. For example, for this purpose Dox was co-formulated in multilamellar liposomes with salinomycin, an antibacterial compound that shows selective toxicity for the CSCs [40], in nanoparticles of poly(ethylene glycol)-block-polylactide with all-trans-retinoic acid, a CSCs differentiation agent [41], or in hyaluronic acid-decorated nanoparticles of Pluronic F127, poly(d,l-lactide-co-glycolide) (PLGA), and chitosan with irinotecan [42]. Contrary to these studies that use combination of the APIs incorporated in new, clinically untested delivery systems we demonstrate here that clinically evaluated SP1049C comprising a single chemotherapeutic agent, Dox and Generally Recognized as Safe (GRAS)excipients displays increased ability to kill TNBC CSCs.We have shown earlier that Dox formulated in Pluronic micelles (now code named SKC1049, formerly SP1049C) significantly decreased tumorigenic CD133+ cell subpopulation, and effectively suppressed the tumorigenicity and tumor aggressiveness in vivo in murine leukemia model [20]. In the present study, we evaluated the effect of SP1049C on the CSCs derived from two TNBC cell lines, a claudin-low MDA-MB-231 and basal MDA-MB-468. In each of these cell lines we isolated ESA+CD44highCD24low cells that were resistant to Dox and displayed CSCs properties, such as increased colony formation, migration, and invasion in vitro and high tumorigenicity in vivo compared to their parental or ESA-CD44lowCD24high (non-CSCs) counterparts. In vitro cytotoxicity studies demonstrated that SP1049C was more effective against CSCs compared to the drug alone, exhibiting essentially the same IC50 values as those in non-CSCs. We also observed that SP1049C decreases in vitro colony forming potential of CSCs derived from both MDA-MB-231 and MDA-MB-468 cells. In the latter TNBC cell line SP1049C also decreased the colony forming potential of parental and isolated non-CSCs counterparts. Furthermore, SP1049C showed superior effects compared to Dox alone in inhibition of the growth of orthotropic tumors derived from both TNBC CSCs in mouse models. Finally, SP1049C considerably depleted CSCs phenotype in the CSCs derived tumors. Notably, SP polymers themselves without Dox also exhibited the trend to decrease tumor growth and deplete CSCs.
Compared to the basal subtype TNBC such as MDA-MB-468 cells, the MDA-MB-231 cells belong to claudin-low subtype tumors and generally have lower response to chemotherapy. Such tumors are unique due to the additional downregulation of claudin-3 and claudinin-4, low expression of the proliferation marker Ki67, enrichment for markers associated with the epithelial-mesenchymal transition and expression of features associated with mammary CSCs (including the CD44+CD24-/low phenotype) [43]. These features are not shared with MDA-MB-468, so these two cell lines are different in molecular and phenotypic behavior. Moreover, MDA-MB-231 is a highly metastatic breast cancer cell, while MDA-MB-468 is not [44].
One very interesting difference between the two cell lines observed in this work is manifested by the response in ATP depletion induced in these cells by the SP polymers. The parent MDA-MB-468 cells and both the isolated CSCs (ESA+CD44highCD24low) and non-CSCs (ESA-CD44lowCD24high) subpopulations of MDA-MB-468 cells display profound ATP depletion in the presence of from ~ 0.02 % to ~ 0.2 % wt. SP-polymers. In contrast MDA-MB-231 parental cells and non-CSCs are not responsive to SP-polymers in terms of ATP depletion in the studied range of concentrations. Although there is no simple correlation between the ATP depletion and cytotoxicity of SP1049C in cancer cells, ATP depletion is one factor for sensitization of in MDR cancer cells by Pluronic copolymers [31]. It is remarkable therefore that in CSCs subpopulations isolated from MDA-MB-231 SP-polymers produce considerable ATP depletion along with a profound sensitization effect increasing Dox cytotoxicity by over two folds. A notable difference in the mitochondria of highly metastatic MDA-MB-231 cell line compared to less metastatic TNBC including MDA-MB-468 cells was reported [44]. In particular mitochondria from MDA-MB-231 cells has increased low conductance state of mitochondrial permeability transition (MPT) pores, which is believed to up-regulate autophagy, and may serve as a survival mechanism against stressful conditions such as hypoxia and nutrient deprivation in the poorly vascularized central areas of a solid tumor [44]. The relationship between the increased MPT and inability of Pluronic to deplete ATP in parental MDA-MB-231 cells is unknown. However, it is interesting that increased drug resistance in CSCs selected from this cell line comes along with increased “vulnerability” exhibited in ATP depletion by the copolymer. The responsiveness to Pluronic in MDR sublines of cancer cells was related to the elevated expression of the ABC transporters [30, 31]. In the mitochondria of such MDR cells Pluronic was shown to decrease of mitochondrial membrane potential, inhibit complex I and complex IV of the mitochondria respiratory chain, decrease oxygen consumption, increase production of ROS, and release cytochrome C, which all contribute to drug-induced cell death [13].
We have shown previously that Pluronics, and in particular SP polymers, inhibit functional activity of drug efflux transporters, such as MDR1/ABCB1 and BCRP/ABCG2, and increase accumulation of their substrates in MDR cells [20, 30, 45]. Notably, expression of BCRP/ABCG2 is a well-established feature of various stem/stem-like cells [46], and has been exploited to isolate the side population and non-side population cells using the Hoechst 33342 exclusion assay [47]. It was reported that BCRP/ABCG2 is overexpressed in CSCs in breast [48], ovarian [13], pancreatic [49], hematopoietic cancers [50] and others. The results of this work suggest that SP polymers not only inhibit the activity of drug efflux transporters in CSCs but also downregulate the expression of BCRP/ABCG2 in these cells. Dox alone also displayed ability to decrease BCRP/ABCG2 expression, yet this effect was the greatest when the drug and copolymers were combined in Dox/SP formulation. The effect can be attributed to the genetic and/or epigenetic changes that are induced by Pluronic treatment as we have shown earlier [16, 17, 20, 26]. For example, in vitro and in vivo selection of cancer cells with Dox/Pluronic formulation resulted in significant changes in gene expression profiles, and the affected genes in these treatment groups only partially overlap [16]. Moreover, treatment with Pluronic alone also caused changes in gene expression, although the set of the affected genes was completely different. Additionally, we have demonstrated earlier that SP polymers alone induced very distinct changes in DNA methylation profiles in murine leukemia cells upon continuous in vivo treatment [20].
Taken together, we present a novel property of SP1049C formulation regarding the potent inhibition of oncogenesis in TNBC in vitro and in vivo through the elimination of drug resistant CSCs. These findings advance our understanding of how Pluronic block copolymers may prevent the development of drug resistance. More importantly, SP1049C represents a promising treatment strategy for TNBC that eliminates both more differentiated cancer cells, comprising the bulk of the tumor, as well as the small and extremely hard to target drug resistant CSCs subpopulation.
Supplementary Material
Acknowledgements
This work was supported by the grants from the National Institutes of Health CA89225, CA151652, and CA184088 to AVK, CA144027 and Department of Defense grant W81XWH-11–1-0171 to VB. We would also like to thank the Confocal Microscopy and Flow Cytometry Core Facilities at UNMC.
Footnotes
Additional information
Competing interest statement
AVK is a co-inventor of the SP1049C technology as declares interest in commercial development of SP1049C for clinical applications.
References
- [1].Diana A, Franzese E, Centonze S, Carlino F, Della Corte CM, Ventriglia J, Petrillo A, De Vita F, Alfano R, Ciardiello F, Orditura M, Triple-Negative Breast Cancers: Systematic Review of the Literature on Molecular and Clinical Features with a Focus on Treatment with Innovative Drugs, Curr Oncol Rep, 20 (2018) 76. [DOI] [PubMed] [Google Scholar]
- [2].Wiech T, Nikolopoulos E, Hausmann M, Walch A, Werner M, Fisch P, A case of heterogeneous breast cancer with clonally expanded T-Cells in the HER2+ and metastasis of the HER2- tumor cells, Breast J, 14 (2008) 487–491. [DOI] [PubMed] [Google Scholar]
- [3].Salmaggi A, Boiardi A, Gelati M, Russo A, Calatozzolo C, Ciusani E, Sciacca FL, Ottolina A, Parati EA, La Porta C, Alessandri G, Marras C, Croci D, De Rossi M, Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype, Glia, 54 (2006) 850–860. [DOI] [PubMed] [Google Scholar]
- [4].Dean M, Fojo T, Bates S, Tumour stem cells and drug resistance, Nat Rev Cancer, 5 (2005) 275–284. [DOI] [PubMed] [Google Scholar]
- [5].Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, Louis DN, MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance, Clin Cancer Res, 15 (2009) 4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fillmore CM, Kuperwasser C, Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy, Breast Cancer Res, 10 (2008) R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang DG, Understanding cancer stem cell heterogeneity and plasticity, Cell Res, 22 (2012) 457–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singh A, Settleman J, EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer, Oncogene, 29 (2010) 4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cordon-Cardo C, Cancer stem cells, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO, 21 Suppl 7 (2010) vii93–94. [DOI] [PubMed] [Google Scholar]
- [10].Barrett HL, Gatford KL, Houda CM, De Blasio MJ, McIntyre HD, Callaway LK, Dekker Nitert M, Coat S, Owens JA, Hague WM, Rowan JA, Maternal and neonatal circulating markers of metabolic and cardiovascular risk in the metformin in gestational diabetes (MiG) trial: responses to maternal metformin versus insulin treatment, Diabetes Care, 36 (2013) 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].DeVita VT Jr., Rosenberg SA, Two hundred years of cancer research, N Engl J Med, 366 (2012) 2207–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alakhov V, Moskaleva E, Batrakova EV, Kabanov AV, Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer, Bioconjugate chemistry, 7 (1996) 209–216. [DOI] [PubMed] [Google Scholar]
- [13].Alakhova DY, Rapoport NY, Batrakova EV, Timoshin AA, Li S, Nicholls D, Alakhov VY, Kabanov AV, Differential metabolic responses to pluronic in MDR and non-MDR cells: a novel pathway for chemosensitization of drug resistant cancers, Journal of controlled release : official journal of the Controlled Release Society, 142 (2010) 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang Z, Sahay G, Sriadibhatla S, Kabanov AV, Amphiphilic Block Copolymers Enhance Cellular Uptake and Nuclear Entry of Polyplex-Delivered DNA, Bioconjugate chemistry, 19 (2008) 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alakhova DY, Kabanov AV, Pluronics and MDR reversal: an update, Mol Pharm, 11 (2014) 2566–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sharma AK, Zhang L, Li S, Kelly DL, Alakhov VY, Batrakova EV, Kabanov AV, Prevention of MDR development in leukemia cells by micelle-forming polymeric surfactant, Journal of controlled release : official journal of the Controlled Release Society, 131 (2008) 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Batrakova EV, Kelly DL, Li S, Li Y, Yang Z, Xiao L, Alakhova DY, Sherman S, Alakhov VY, Kabanov AV, Alteration of genomic responses to doxorubicin and prevention of MDR in breast cancer cells by a polymer excipient: pluronic P85, Mol Pharm, 3 (2006) 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alakhov V, Klinski E, Li S, Pietrzynski G, Venne A, Batrakova E, Bronitch T, Kabanov A, Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials, Colloids and Surfaces B: Biointerfaces, 16 (1999) 113–134. [Google Scholar]
- [19].Valle JW, Armstrong A, Newman C, Alakhov V, Pietrzynski G, Brewer J, Campbell S, Corrie P, Rowinsky EK, Ranson M, A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction, Investigational new drugs, 29 (2011) 1029–1037. [DOI] [PubMed] [Google Scholar]
- [20].Alakhova DY, Zhao Y, Li S, Kabanov AV, Effect of doxorubicin/pluronic SP1049C on tumorigenicity, aggressiveness, DNA methylation and stem cell markers in murine leukemia, PLoS One, 8 (2013) e72238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE, Kabanov AV, Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers, Journal of controlled release : official journal of the Controlled Release Society, 143 (2010) 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C, Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling, Proc Natl Acad Sci U S A, 107 (2010) 21737–21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF, Prospective identification of tumorigenic breast cancer cells, Proc Natl Acad Sci U S A, 100 (2003) 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guo C, Liu H, Zhang BH, Cadaneanu RM, Mayle AM, Garraway IP, Epcam, CD44, and CD49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability, PLoS One, 7 (2012) e34219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, Chadburn A, Murphy AJ, Valenzuela DM, Gale NW, Thurston G, Yancopoulos GD, D’Angelica M, Kemeny N, Lyden D, Rafii S, CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors, J Clin Invest, 118 (2008) 2111–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Blatt MEYNL, Shafigullina AK, Salafutdinov II, Kiyasov AP, Masgutov RE, Shtirlin YG, Kabanov AV, Rizvanov AA, Effect of Pluronic P85 on proliferation and osteogenic differentiation of human mesenchymal stem cells in vitro, Cellular Transplantation and Tissue Engineering V(2010) 66–70. [Google Scholar]
- [27].Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN, Integrin alpha 6 regulates glioblastoma stem cells, Cell Stem Cell, 6 (2010) 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O’Brien CA, Kreso A, Jamieson CH, Cancer stem cells and self-renewal, Clin Cancer Res, 16 (2010) 3113–3120. [DOI] [PubMed] [Google Scholar]
- [29].Batrakova EV, Kabanov AV, Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers, Journal of controlled release : official journal of the Controlled Release Society, 130 (2008) 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV, Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion, British journal of cancer, 85 (2001) 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kabanov AV, Batrakova EV, Alakhov VY, An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers, Journal of controlled release : official journal of the Controlled Release Society, 91 (2003) 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hiraga T, Ito S, Nakamura H, Side population in MDA-MB-231 human breast cancer cells exhibits cancer stem cell-like properties without higher bone-metastatic potential, Oncology reports, 25 (2011) 289–296. [PubMed] [Google Scholar]
- [33].Yusa K, Tsuruo T, Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells, Cancer Res, 49 (1989) 5002–5006. [PubMed] [Google Scholar]
- [34].Batrakova EV, Li S, Li Y, Alakhov VY, Kabanov AV, Effect of pluronic P85 on ATPase activity of drug efflux transporters, Pharmaceutical research, 21 (2004) 2226–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Medema JP, Cancer stem cells: the challenges ahead, Nature cell biology, 15 (2013) 338–344. [DOI] [PubMed] [Google Scholar]
- [36].Takebe N, Harris PJ, Warren RQ, Ivy SP, Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways, Nature reviews. Clinical oncology, 8 (2011) 97–106. [DOI] [PubMed] [Google Scholar]
- [37].Zhuang X, Zhang W, Chen Y, Han X, Li J, Zhang Y, Zhang Y, Zhang S, Liu B, Doxorubicin-enriched, ALDH(br) mouse breast cancer stem cells are treatable to oncolytic herpes simplex virus type 1, BMC Cancer, 12 (2012) 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rafael D, Gener P, Andrade F, Seras-Franzoso J, Montero S, Fernandez Y, Hidalgo M, Arango D, Sayos J, Florindo HF, Abasolo I, Schwartz S Jr., Videira M, AKT2 siRNA delivery with amphiphilic-based polymeric micelles show efficacy against cancer stem cells, Drug Deliv, 25 (2018) 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sun TM, Wang YC, Wang F, Du JZ, Mao CQ, Sun CY, Tang RZ, Liu Y, Zhu J, Zhu YH, Yang XZ, Wang J, Cancer stem cell therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds, Biomaterials, 35 (2014) 836–845. [DOI] [PubMed] [Google Scholar]
- [40].Kim YJ, Liu Y, Li S, Rohrs J, Zhang R, Zhang X, Wang P, Co-Eradication of Breast Cancer Cells and Cancer Stem Cells by Cross-Linked Multilamellar Liposomes Enhances Tumor Treatment, Mol Pharm, 12 (2015) 2811–2822. [DOI] [PubMed] [Google Scholar]
- [41].Muntimadugu E, Kumar R, Saladi S, Rafeeqi TA, Khan W, CD44 targeted chemotherapy for co-eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel, Colloids Surf B Biointerfaces, 143 (2016) 532–546. [DOI] [PubMed] [Google Scholar]
- [42].Wang H, Agarwal P, Zhao S, Xu RX, Yu J, Lu X, He X, Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells, Biomaterials, 72 (2015) 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM, Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer, Breast Cancer Res, 12 (2010) R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tu YF, Kaipparettu BA, Ma Y, Wong LJ, Mitochondria of highly metastatic breast cancer cell line MDA-MB-231 exhibits increased autophagic properties, Biochimica et biophysica acta, 1807 (2011) 1125–1132. [DOI] [PubMed] [Google Scholar]
- [45].Kabanov AV, Batrakova EV, Alakhov VY, Pluronic block copolymers for overcoming drug resistance in cancer, Advanced drug delivery reviews, 54 (2002) 759–779. [DOI] [PubMed] [Google Scholar]
- [46].Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP, The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype, Nature medicine, 7 (2001) 1028–1034. [DOI] [PubMed] [Google Scholar]
- [47].Scharenberg CW, Harkey MA, Torok-Storb B, The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors, Blood, 99 (2002) 507–512. [DOI] [PubMed] [Google Scholar]
- [48].Britton KM, Eyre R, Harvey IJ, Stemke-Hale K, Browell D, Lennard TW, Meeson AP, Breast cancer, side population cells and ABCG2 expression, Cancer letters, 323 (2012) 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shankar S, Nall D, Tang SN, Meeker D, Passarini J, Sharma J, Srivastava RK, Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition, PLoS One, 6 (2011) e16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bolster DR, Crozier SJ, Kimball SR, Jefferson LS, AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling, J Biol Chem, 277 (2002) 23977–23980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.