Abstract
Purpose:
The Children’s Oncology Group protocol AHOD0831, for pediatric patients with high-risk classical Hodgkin lymphoma (cHL), used response-adapted radiation fields, rather than larger involved-field radiation therapy (IFRT) that were historically used. This retrospective analysis of patterns of relapse among patients enrolled in the study was conducted to study the potential effect of a reduction in RT exposure.
Methods and Materials:
From December 2009 to January 2012, 164 eligible patients under 22 years old with stage IIIB (43%) and stage IVB (57%) enrolled on AHOD0831. All patients received 4 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC). Those patients with a slow early response (SER) after the first 2 ABVE-PC courses were nonrandomly assigned to 2 intensification cycles with ifosfamide/vinorelbine before the final 2 ABVE-PC cycles. Response-adapted RT (21 Gy) was prescribed to initial areas of bulky disease and SER sites. Rapid early response (RER) sites without bulk were not targeted. Imaging studies at the time of progression or relapse were reviewed centrally for this retrospective analysis. Relapses were characterized with respect to site (initial, new, or both; and initial bulk or initial nonbulk), initial chemotherapy response, and radiation field (in-field, out-of-field, or both).
Results:
Of the entire cohort, 140 patients were evaluable for the patterns of failure analyses. To investigate the pattern of failure, this analysis focuses on 23 patients who followed protocol treatment and suffered relapses at a median 1.05 years with 7.97-year median follow-up time. These 23 patients (11 RER and 12 SER) experienced a relapse in 105 total sites (median, 4; range, 1–11). Of the 105 relapsed sites, 67 sites (64%) occurred within an initial site of involvement, with 12 of these 67 sites (18%) at an initial site of bulky disease and 63 of these 67 relapses (94%) occurring in sites that were not fluorodeoxyglucose (FDG)-avid after 2 cycles of ABVE-PC (PET2-negative). Of the 105 relapsed sites, 34 sites (32%) occurred in a new site of disease (that would not have been covered by RT); and, overall, only 4 of 140 patients (2.8%) (occurring in 3 RER and 1 SER) experienced isolated out-of-field relapses that would have been covered by historical IFRT.
Conclusions:
For a cohort of high-risk patients with cHL patients, most failures occurred in nonbulky, initially involved sites, largely due to response-based consolidation RT delivered to patients with bulky disease. In this analysis, we discovered low rates of failures outside of these modern risk-adapted radiation treatment volumes. Also, FDG uptake on PET2 did not identify most relapse sites.
Introduction
Combined modality therapy has been established as the standard of care treatment for patients with advanced stage pediatric Hodgkin lymphoma (HL).1–3 However, the radiation therapy field designs for these patients with stage III and IV disease were large and encompassed all sites of initial involvement and in some cases elective coverage of sites, often approximating a subtotal lymphoid irradiation field. Although the radiation therapy dose has generally been low, there has been much concern about the potential risk of late effects from such large radiation therapy fields, especially in pediatric patients.4,5
The historical involved field radiation therapy (IFRT) included all abnormally enlarged lymph nodes at the time of diagnosis and nearby nodal basins adjacent to the index site. These larger fields were necessary due to limitations in imaging. Various advances in imaging that have allowed for smaller, response-adapted radiation therapy fields included positron emission tomography (PET)/functional imaging, 3-dimensional computer tomography (CT)-based radiation planning, improved organ motion management, improved target delineation, improved immobilization during radiation therapy, and limiting setup uncertainty. These advances have allowed practitioners to decrease the large size of previous radiation fields and limit exposure to nearby normal tissues. Thus, modifying these previous IFRT fields to include the prechemotherapy nodal tissues and adding a “safety margin” of 1 to 2 cm to areas of potential lymphatic spread has been a reasonable approach in the management of lymphoma patients.6
The Children’s Oncology Group protocol AHOD0831, which enrolled pediatric patients with high-risk classical HL, tested a response-based approach.7 All patients were treated with 4 cycles of doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC). Response after the first 2 cycles was categorized as either a rapid early response (RER) or a slow early response (SER). Two cycles of intensification with ifosfamide/vinorelbine)8 was given to all those with a SER before the last 2 cycles of ABVE-PC.7 A risk-adapted approach to radiation therapy was delivered to sites of initial bulky disease or to slowly responding sites.7 This approach significantly reduced the radiation therapy field size that had been used in prior North American pediatric trials for advanced stage disease, specifically Pediatric Oncology Group 9425 and Children’s Cancer Group 5942.2,3
These smaller fields reduce exposure to nearby normal tissues, but prospective studies are still evaluating their ability to maximize tumor control. The purpose of this report is to describe the patterns of failure with this strategy of a rational reduction in radiation therapy volume in a cohort of pediatric patients with high-risk disease.
Methods and Materials
The Children’s Oncology Group protocol AHOD0831 was reviewed and approved by the National Cancer Institute, the Pediatric Central institutional review board, and the institutional review boards of the participating institutions. The planned secondary objective of Protocol AHOD0831 was to describe the patterns of relapse after chemotherapy and risk-adapted radiation therapy. Written informed consent was obtained from patients and parents or guardians in accordance with the Declaration of Helsinki as required by government regulations.
Patients
Children and adolescents aged ≤21 years, with classical HL were enrolled on AHOD0831, a prospective nonrandomized phase 3 multicenter study. Stages, based on clinical evaluation by history and physical examination, imaging studies and bilateral bone marrow biopsies, were defined by the Ann Arbor staging system.9 Patients with HL were eligible if they had stage III or IV, and B symptoms. Bulky disease was defined as any one of the following: (1) large mediastinal adenopathy (tumor diameter greater than one-third of the thoracic diameter on a posterior-anterior chest radiograph); (2) a continuous aggregate of nodal tissue that measures >6 cm in the longest transverse diameter in the axial plane in any nodal area; or (3) macroscopic splenic nodules (focal defects in the spleen seen on CT, PET or magnetic resonance imaging studies consistent with HL).
Treatment
Details of the treatment regimens and response assessment are listed in the initial report of this study.7 PET-CT was obtained as a baseline staging study (PET0) and after the first and/or second cycle of ABVE-PC chemotherapy. Imaging was submitted for central review to the Quality Assurance Review Center (QARC), part of the National Cancer Center’s National Clinical Trials Network. If PET-CT after first cycle (PET1) met criteria for a complete metabolic response (CMR) a PET-CT after the second cycle (PET2) was not required; but, PET2 was performed if PET1 was positive to guide protocol directed response-based treatment. End of chemotherapy PET-CT imaging before radiation therapy was obtained in patients with positive PET2 findings as recommended by the study. Patients were considered to have rapid early response to therapy if they had a CMR after 2 cycles of therapy. A CMR was consistent with fluorodeoxyglucose (FDG) avidity less than or equal to mediastinal blood pool, as per Modified Lugano criteria.10 These patients went on to receive consolidation therapy with 2 more cycles of ABVE-PC followed by radiation therapy to sites of initial bulky involvement only. Patients with a slow early response to induction chemotherapy were administered 2 cycles of ifosfamide and vinorelbine, followed by 2 more cycles of ABVE-PC, followed by response-adapted radiation therapy.
Radiation therapy
All treatment plans were reviewed by QARC and AHOD0831 (AHOD0831) and approved before the start of radiation therapy. The indications for radiation therapy include disease sites with the following characteristics: initial bulky disease defined as above, and slow early responding, nonbulky disease as determined by FDG-PET scan residual avidity after the first 2 cycles of chemotherapy. Also treated was disease with a residual cross-sectional diameter ≥2.5 cm on CT scan after the completion of all chemotherapy in patients defined as having a SER at other locations even if the particular site was FDG-PET negative after the first 2 cycles of chemotherapy.
The treating radiation oncologist was responsible for contouring gross tumor/target volume, clinical target volume, and planning target volume as well as organs at risk on treatment planning CT scans to facilitate adequate field construction, dosimetric analysis, and QARC oversight. This protocol adopted volumetric treatment planning for HL radiation therapy while modifying the historical involved field volumes to define anatomically defined lymphatic regions that need to be targeted. Figure 1 shows an example of treatment to a patient with typical response-adapted IFRT, where the total dose to the targeted volume was 2100 cGy in 14 fractions of 150 cGy each. Radiation therapy boosts (to total doses beyond 2100 cGy) were not allowed, even for patients with any sites of persistent FDG-PET uptake at the end of chemotherapy. Proton therapy was not allowed in this study. Radiation therapy plans were prospectively reviewed by QARC. Where there were protocol deviations, the treating radiation oncologist was given an opportunity to modify the treatment plan. A post hoc final review of treatment plans was performed by QARC staff and a radiation oncology principal investigator, grading any protocol deviations as minor or major using the criteria listed in Table E1.
Fig. 1.
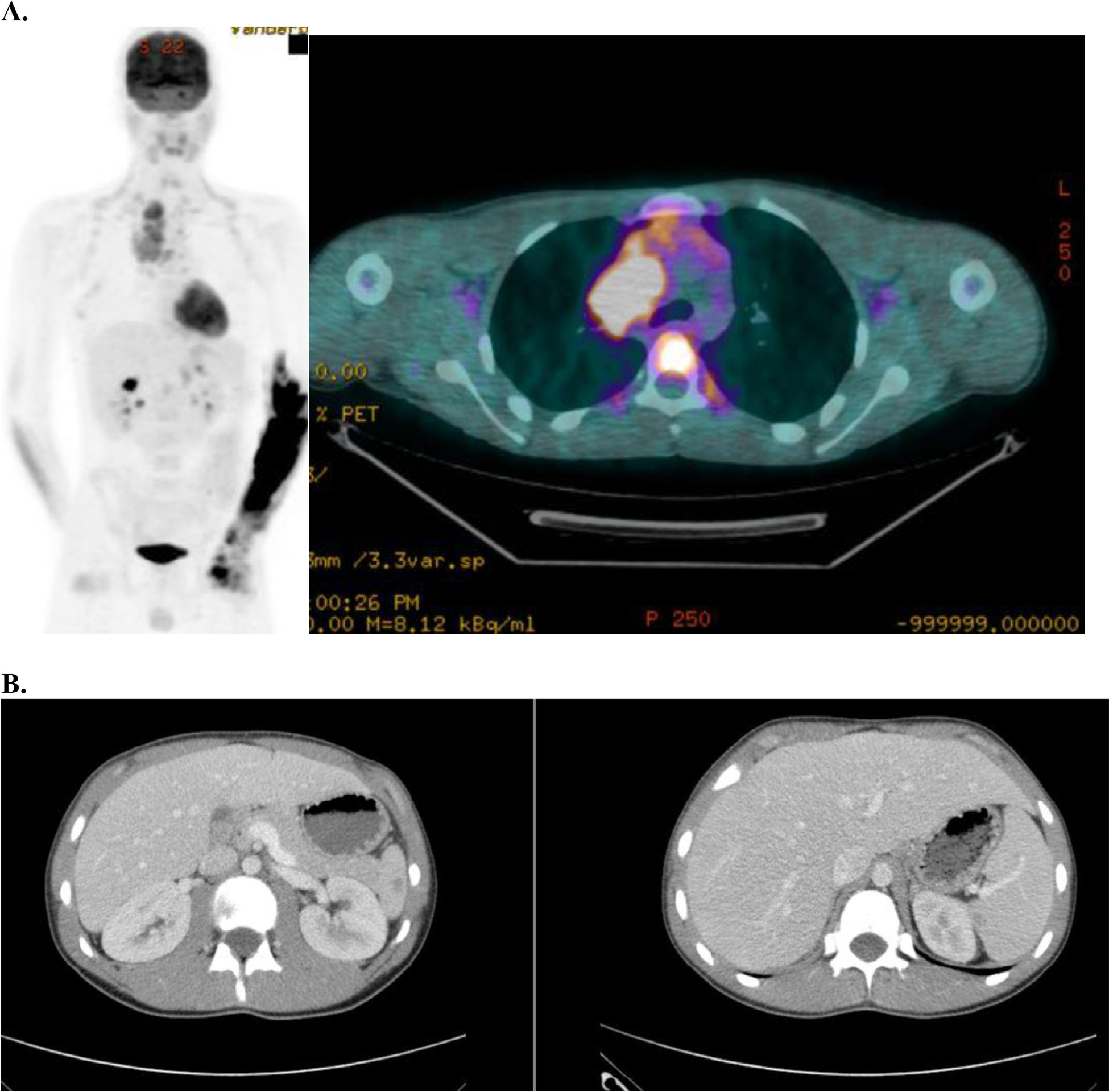
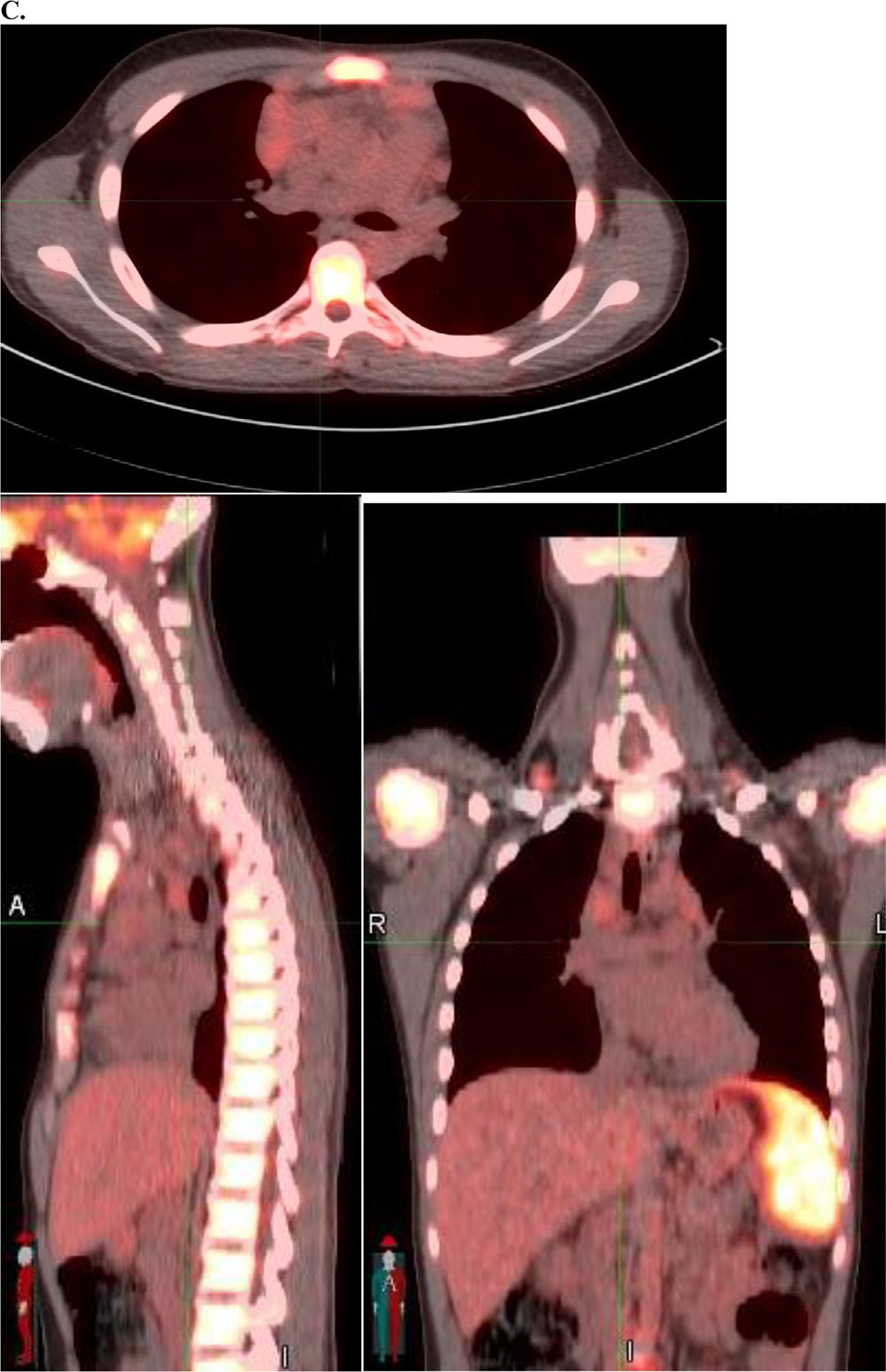
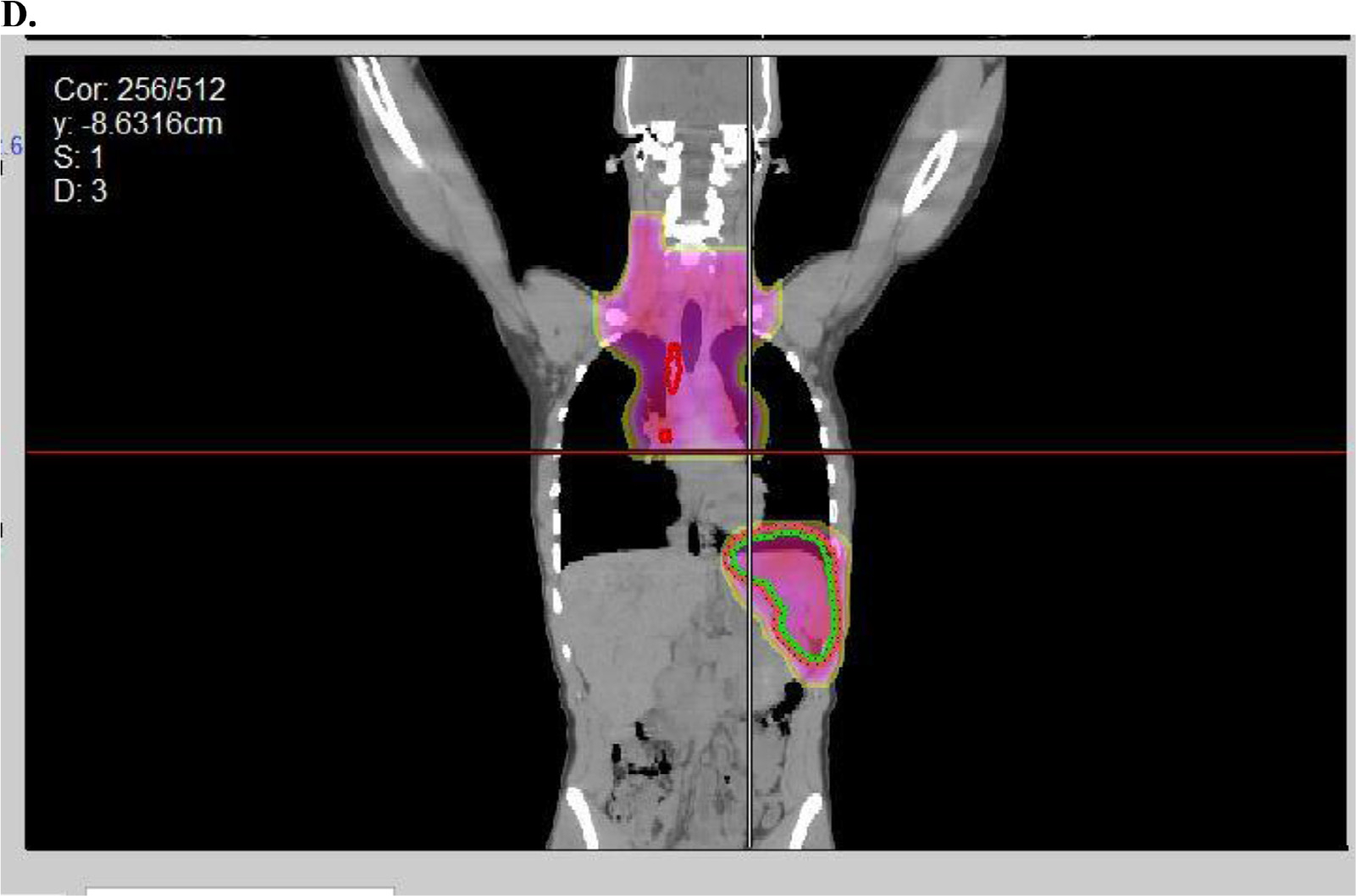
Example of radiation therapy fields used in AHOD0831. (A) Baseline positron emission tomography-computed tomography with maximum intensity projection image on left and axial slice in mediastinum showing bulky disease. (B) Spleen involvement at baseline was documented by several hypodensities that resolved with chemotherapy. (C) Postchemo positron emission tomography-computed tomography scan showing a rapid early response. (D) Radiation therapy (dose color-wash indicates 2100 cGy /14 fractions; 50% isodose line is depicted) was given in accordance to protocol with radiation therapy to bulk sites (mediastinum and spleen). Red indicates postchemo gross tumor volume; green indicates spleen clinical target volume.
Statistical analysis
The statistical analysis for the primary endpoint has been described in detail in the initial report of this study.7 A description of the patterns of relapse after ABVE-PC and risk-adapted radiation therapy was a secondary objective of this study, but a detailed statistical plan was not elaborated. Descriptive statistics were used to indicate proportions, and 95% confidence intervals were calculated.
Patterns of failure analysis
Protocol mandated follow-up included physical, examination, laboratory testing, and CT imaging of the neck, chest, abdomen, and pelvis over the subsequent 2 years according the schedule listed in Table E2. The first CT scan was obtained 6 to 8 weeks after radiation therapy was completed or after chemotherapy completed if no radiation therapy was given. Magnetic resonance imaging could be optionally substituted for CT in follow-up. PET-CT was recommended as the first follow-up imaging surveillance study only if the PET before radiation therapy administration was considered positive. Relapses were reported to QARC with submission of imaging that documented relapse sites. After any retrieval therapy, CT imaging was recommended every 4 months for the first year, then every 6 months for the second year, and then annually for years 3 and 4 after the last treatment for HL. Subsequent relapses were also reported. The sites of initial involvement and at first relapse were categorized by anatomic sites based on Figure E2 for patterns of failure analysis. At the conclusion of this study, central review was performed by the radiation oncologists and radiologists to determine the exact patterns of relapse. All of the sites of relapse were meticulously analyzed using segments as seen in Figure E1.
Results
Patients
From December 2009 to January 2012, 165 eligible patients were enrolled in the overall study, and 164 were evaluable (1 patient withdrew consent after enrollment onto protocol therapy). The following are the characteristics of the entire AHOD0831 cohort: bulk disease was seen in 138 patients (84%), with the distribution of patients with large anterior mediastinal mass, extramediastinal bulk, and with macronodular splenic involvement (Fig. 2). Among the 164 evaluable patients, 126 patients (86%) received radiation therapy in this study. Eleven of the 126 patients received intensity modulated radiation therapy, and the rest (115) received 3-dimensional conformal radiation therapy most often as anterior-posterior field pairs. Sixty of the 126 patients, 60 were RER, and 66 were SER.
Fig. 2.
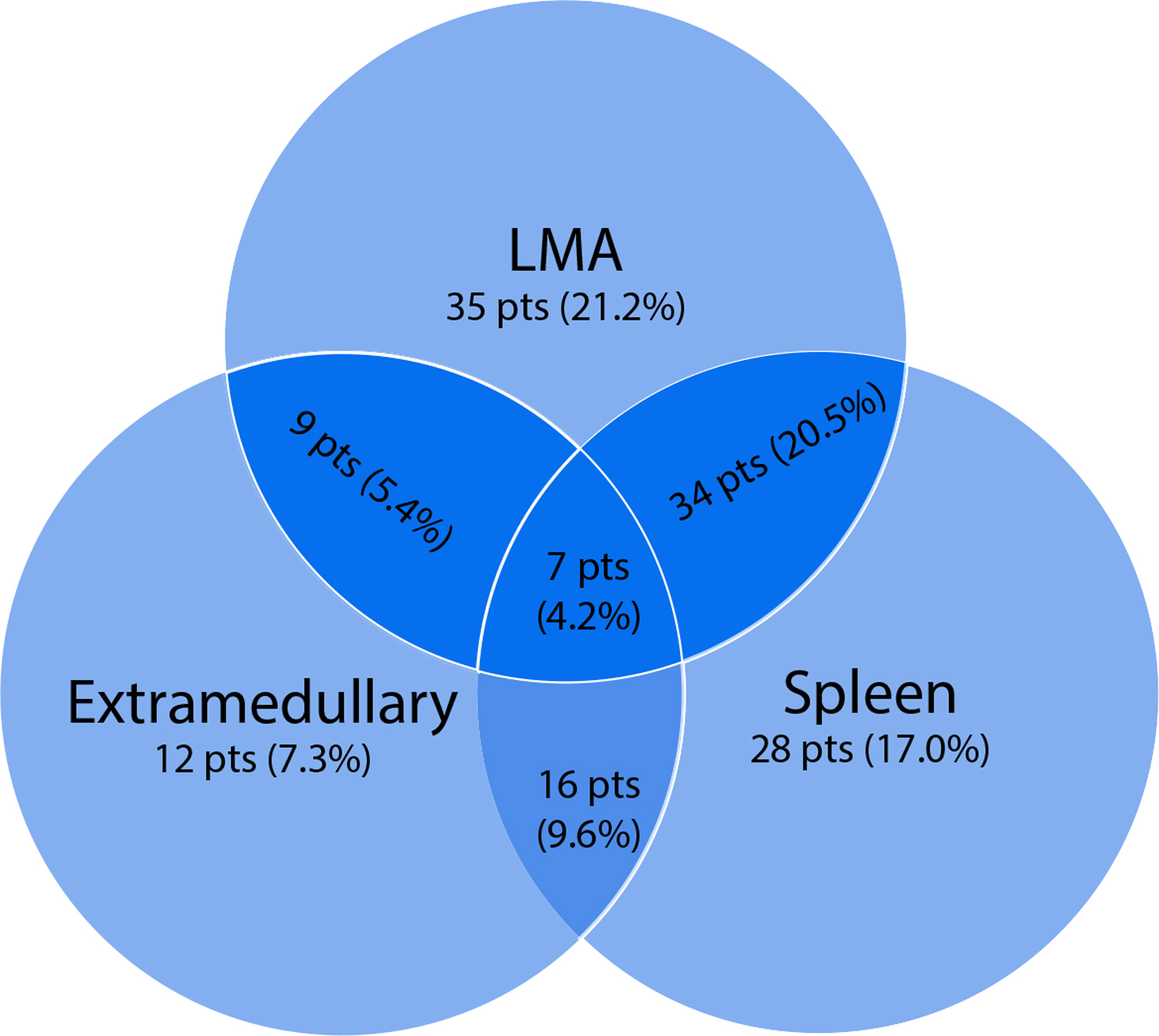
Distribution of bulky disease. Of 137 patients with bulky disease, 85 patients (51.5%) presented with a large anterior mediastinal mass, 44 patients (26.7%) presented with extramediastinal bulk, and 85 patients (51.5%) presented with macronodular splenic involvement. Abbreviation: LMA = large mediastinal adenopathy.
For this current, specific study examining the patterns of failure, 140 patients had imaging data available for analysis, and 28 patients experienced a relapse. Five of the 28 patents were not evaluable for the current analysis (see Fig. E1). Among the 23 evaluable patients for this pattern of relapse study, age ranged from 8 to 21 years (median, 15), and 61% were men. As per protocol specifics, no patients received a boost beyond the prescribed 21 Gy. Seventeen RER patients did not have bulk disease and thus did not require RT as per protocol. Two patients stopped radiation therapy early at a dose of 9 Gy, in 1 instance due to an acute fatality from sepsis, likely unrelated to radiation therapy. There were a variety of reasons that patients did not receive any protocol intended radiation therapy. Three RER patients had bulk disease and refused radiation therapy. Three SER patients with bulk disease withdrew from the study before completing protocol chemotherapy. Eight SER patients had FDG-avid disease at the end of chemotherapy and did not undergo biopsy confirmation of active lymphoma. Three additional SER patients with positive end of chemotherapy PET scans had biopsy proven refractory disease and did not receive radiation therapy, and instead went off protocol.
Few radiation therapy protocol violations were observed among those patients assigned to receive radiation therapy: 16 patients received no radiation therapy due to study withdrawals; 14 patients had minor deviations; and 3 patients had major deviations (Fig. 3). One of the major deviations was based on treating at 175 cGy rather than 150 cGy per fraction, but for the correct total dose of 2100 cGy. Potentially consequential major deviations apart from patients who did not receive protocol-mediated radiation therapy were related to inadequate coverage of mediastinal disease. None of these patients with major or minor deviations suffered a relapse.
Fig. 3.
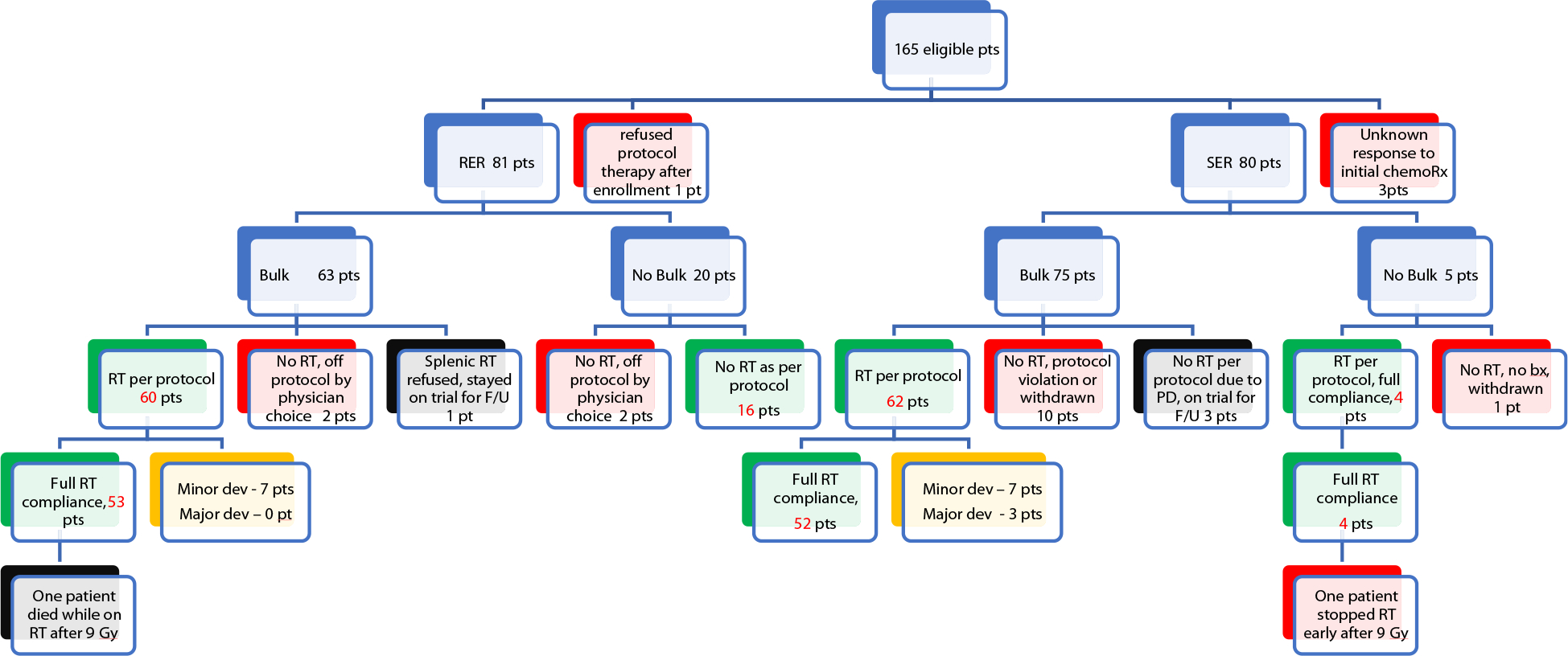
Categorization of patients on AHOD0831 by response to second cycle of chemotherapy, bulky disease, protocol exclusions, and radiation therapy protocol compliance. Abbreviation: RER = rapid early response; RT = radiation therapy; SER = slow early response.
As shown in Figure E1, 140 patients were evaluable for the patterns of relapse analyses by excluding 24 patients who had premature termination or deviation from protocol-directed therapy. Of these 24 patients, 19 had been excluded from the primary endpoint survival analysis. Due to circumstances violating protocol-directed therapy (eg, no radiation therapy delivered), 5 more patients were excluded from this analysis: 1 patient with bulky splenic disease should have had radiation therapy but did not (the patient eventually relapsed in spleen and multiple sites); 4 patients went off protocol (did not receive protocol radiation therapy). Thus, 117 patients did not relapse and were excluded from this pattern of failure analysis, and, overall, a total of 23 patients were analyzed for study.
Patterns of relapse (by patient)
As seen in Table 1, of the 23 evaluable patients with recurrences after protocol-specified radiation therapy, 11 patients were diagnosed with RER and 12 patients were diagnosed with SER. Five patients relapsed only at sites involved at diagnosis, 17 patients relapsed within initially involved and new sites, and only 1 patient relapsed in new sites not initially involved. Twelve patients had bulky splenic involvement (a similar rate to the overall incidence of splenic involvement in the whole treatment cohort), and only 1 of these patients relapsed in the spleen. The median time from enrollment to relapse was 1.05 years (range, 0.45–4.56); there was no difference in relapse time between RER and SER patients (t test, P = .74). Overall, only 2.8% of patients (4 out of 140 patients, 3 RER and 1 SER) experienced out-of-field relapses that would have been covered by historical IFRT.
Table 1.
Demographic, clinical, and relapse characteristics for all 23 evaluable patients with relapses
| Case number | Sex | Age | Stage | Site of bulk | Response (RER, SER) | Site of SER | No. of sites of relapse | Site(s) of relapse | No. of relapse sites involved at dx | No. of relapse sites initially bulky | No. of relapse sites PET2 negative | No. of relapse sites out-of-field | No. of out-of-field relapse sites would theoretically have been covered by IFRT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| 1 | M | 18 | IIIB | LMA | RER | 5 | L hilar, R paratracheal, pericardium, subcarinal, celiac | 4 out of 5 | 3 out of 4 | 4 out of 4 | 2 out of 5 | 1 out of 2 | |
| 2 | F | 21 | IVB | LMA; spleen | RER | 11 | L cervical, epicardial, L hilar, R hilar, mediastinum, R paratracheal, subcarinal, celiac, L paraortic, R paraortic, spleen | 10 out of 11 | 3 out of 10 | 10 out of 10 | 5 out of 11 | 5 out of 5 | |
| 3 | F | 14 | IVB | L neck | RER | 1 | L supraclavicular | 1 out of 1 | 1 out of 1 | 1 out of 1 | 0 out of 1 | n/a | |
| 4 | F | 14 | IVB | LMA | RER | 2 | Celiac, L paraortic | 1 out of 2 | 0 out of 1 | 1 out of 1 | 2 out of 2 | 2 out of 2 | |
| 5 | M | 17 | IVB | LMA; L axilla | RER | 11 | R cervical, R hilar, R infraclavicular, R paratracheal, subcarinal, celiac, L paraortic, portal, spleen, R axillary, R iliac | 5 out of 11 | 0 out of 5 | 5 out of 5 | 8 out of 11 | 4 out of 8 | |
| 6 | M | 16 | IIIB | L axilla | RER | 3 | R supraclavicular, R paratracheal, retrotracheal | 2 out of 3 | 0 out of 3 | 3 out of 3 | 3 out of 3 | 3 out of 3 | |
| 7 | M | 17 | LMA; spleen | RER | 3 | Mediastinum, L paraortic, R paraortic | 3 out of 3 | 1 out of 3 | 3 out of 3 | 2 out of 3 | 2 out of 2 | ||
| 8 | F | 16 | IIIB | Spleen | RER | 6 | R hilar, mediastinum, subcarinal, L paraortic nodes, portal, R pectoral | 4 out of 6 | 0 out of 4 | 4 out of 4 | 6 out of 6 | 5 out of 6 | |
| 9 | M | 16 | IVB | LMA | RER | 3 | L paraortic, R paraortic, portal | 1 out of 3 | 0 out of 1 | 1 out of 1 | 3 out of 3 | 3 out of 3 | |
| 10 | M | 15 | IVB | LMA | RER | 3 | L hilar, L lung, subcarinal | 1 out of 3 | 0 out of 1 | 1 out of 1 | 1 out of 3 | 0 out of 1 | |
| 11 | M | 14 | IVB | none | RER | 2 | R Iliac, skin/subcutaneous | 1 out of 2 | 0 out of 1 | 1 out of 1 | 2 out of 2 | 0 out of 2 | |
| 12 | M | 8 | IVB | LMA; spleen | SER | R supraclav; mediastinum | 5 | Supraclavicular (L), supraclavicular (R), mediastinum, paratracheal (R), paratracheal (L) | 5 out of 5 | 1 out of 5 | 4 out of 5 | 0 out of 5 | n/a |
| 13 | F | 15 | IVB | LMA; L supraclav | SER | L supraclav; mediastinum | 5 | Cervical (R), supraclavicular (L), supraclavicular (R), mediastinum, subcarinal | 5 out of 5 | 2 out of 5 | 3 out of 5 | 1 out of 5 | 1 out of 1 |
| 14 | F | 16 | IVB | LMA; spleen | SER | Spleen | 4 | Cervical (L), supraclavicular (L), axillary (L), pectoral (L) | 3 out of 4 | 0 out of 3 | 3 out of 3 | 3 out of 4 | 1 out of 3 |
| 15 | M | 16 | IVB | L paraortic; spleen | SER | Spleen, bone | 8 | R cervical, R paratracheal, pericardium, subcarinal, periaortic/esophageal, R axillary, L inguinal, L pectoral | 3 out of 8 | 0 out of 3 | 3 out of 3 | 8 out of 8 | 5 out of 8 |
| 16 | F | 13 | IVB | LMA | SER | Mediastinum | 3 | R cervical, epicardial, mediastinum | 3 out of 3 | 1 out of 3 | 2 out of 3 | 2 out of 3 | 2 out of 2 |
| 17 | M | 17 | IIIB | LMA; spleen | SER | Mediastinum; spleen | 2 | R supraclavicular, liver | 1 out of 2 | 0 out of 1 | 1 out of 1 | 2 out of 2 | 1 out of 2 |
| 18 | M | 15 | IVB | LMA | SER | L supraclav/axilla; mediastinum | 9 | Epicardial, subcarinal, celiac, R paraortic, portal, L retrocrural, R retrocrural, spleen, R iliac | 4 out of 9 | 0 out of 4 | 4 out of 4 | 7 out of 9 | 6 out of 7 |
| 19 | F | 15 | IIIB | Celiac; portal; spleen | SER | Spleen | 2 | Liver, mesenteric | 1 out of 2 | 0 out of 1 | 1 out of 1 | 2 out of 2 | 1 out of 2 |
| 20 | F | 17 | IVB | Spleen | SER | Spleen | 3 | L supraclavicular, L iliac, L inguinal | 3 out of 3 | 0 out of 3 | 3 out of 3 | 3 out of 3 | 3 out of 3 |
| 21 | M | 15 | IIIB | Spleen | SER | Bilat neck | 4 | L lung, R lung, R paratracheal, subcarinal | 2 out of 4 | 0 out of 2 | 2 out of 2 | 1 out of 4 | 0 out of 1 |
| 22 | M | 14 | IIIB | LMA; spleen | SER | Mediastinum | 5 | L lung, celiac, liver, L paraortic, portal | 0 out of 5 | n/a | n/a | 4 out of 5 | 0 out of 4 |
| 23 | M | 10 | IIIB | LMA; spleen | SER | Spleen | 5 | Celiac, L paraortic, R paraortic, portal, L axillary | 3 out of 5 | 0 out of 3 | 3 out of 3 | 4 out of 5 | 3 out of 4 |
Abbreviations: IFRT = involved-field radiation therapy; L = left; LMA = large mediastinal adenopathy; PET2 = second cycle of chemotherapy; R = right; RER = rapid early response; SER = slow early response.
Patterns of relapse (by site)
Within the 23 recurrent patients, there were 105 sites of relapse, with a median of 4 (range, 1–11) relapsed sites per patient. Only 1 patient had a single site of relapse, which was at the initially bulky neck mass, despite an RER at PET2. Out of 105 relapsed sites, 67 sites (64%) were at initial sites of involvement. Of these 67 sites, 12 sites (18%) were at initial sites of bulk. Of these 12 sites, 6 sites (50%) were mediastinal bulk and another 6 sites (50%) were nonmediastinal bulk. Of the 67 relapsed sites at the initial sites, 63 sites (94%) were PET2 negative. Out of the initial 105 relapsed sites, 34 of the relapses (32%) were at new sites of disease (that would not have been covered by IFRT).
Discussion
Pediatric HL is the third most common pediatric malignancy in the United States, and the overall survival rate has increased dramatically in the modern era,11,12 thus minimizing secondary treatment-related effects in survivorship is important. There is much interest in reducing the volume of irradiated tissues in young patients (ie, radiation fields) while preserving maximal tumor control, the focus of the current study. It has been demonstrated that combined modality therapy may be the preferred approach for patients with more aggressive disease (either initially bulky disease, or slow responding patients), although response adapted assignment of radiation therapy may be a useful approach to limit radiation therapy to those patients who are more likely to see benefit.2,3,5
In this report, we have analyzed the patterns of relapse in a cohort of high-risk pediatric patients with classical HL undergoing a dose intensification of systemic therapy and response-adapted consolidation RT. It appears that the planned use of response-adapted approach for these patients led to a reasonable event-free survival and overall survival, comparable to recent trials in both pediatric2,3 and adult patients with advanced disease.13–16 Of note, 2.7% of all patients experienced out-of-field relapses that may have been included within larger, traditional IFRT fields. Thus, the reduction in radiation therapy fields did not lead to a dramatically different relapse pattern than historical IFRT. The traditional IFRT in pediatric HL has entailed treating all regions of initial disease and using bony landmarks on plain radiographs to define the generous borders of the field.
Given the relatively small cohort in the current study and few number of events (relapses), it is difficult to draw conclusions to the overall behavior of relapse patterns and its relation to RT fields. However, the results are similar to those reported in the Children’s Oncology Group intermediate-risk Hodgkin study (AHOD0031),17 in which first relapses rarely occurred outside of the generous radiation fields (IFRT was used in that study).17,18 Relapses generally occurred in the initially involved nodal sites, both bulky and nonbulky. In that study, across all the different treatment cohorts the predominant pattern of relapse (87–98%) included an initial site of presentation and 31% to 53% occurring only in the initial presenting site.18 The authors hypothesized that 21 Gy may not be a sufficient dose for pediatric Hodgkin lymphoma, when radiation therapy is indicated.
Moreover, a large study of approximately 138 patients (42% pediatric, 58% adult) enrolled on 3 different registry studies with classical HL were treated with consolidation proton involved-site radiation therapy).19 They reported 10 recurrences (3-year recurrence-free survival percentage of 92%) in a cohort predominantly comprised of unfavorable stage I/II (73%) or stage III/IV (27%) patients. Of the recurrences, 6 were in-field, 1 in-field and out-of-field, and 3 out-of-field in immediately adjacent nodal regions. Six of the 7 recurrences (86%) with an in-field component developed in pediatric patients treated to <30 Gy, including 2 with a partial response treated to 21 Gy. Taken together, these reports suggest that local control in the initial sites of disease may require higher doses of radiation therapy to control.20
The inclusion of low-dose splenic irradiation for baseline macronodules was based on the understanding that imaging underestimates the burden of splenic disease as well as the adult Hodgkin experiences with the Stanford V protocol where reduction in radiation therapy volumes especially splenic coverage were detrimental.14,21 From the analysis of AHOD0031, splenic relapses are quite low (representing only 3% of relapses).3 Thus, one must weigh the potential risks of inclusion of the spleen within RT fields, including functional asplenia, infection, and hematologic toxicities, against the potential benefit of splenic irradiation.
Study limitations
There are limitations of the analysis of the current study. First, there is a relatively small number of events (relapses), thus precluding the ability to determine statistically meaningful differences within the patient cohort. For example, this “shift” from the traditional IFRT to response-adapted IFRT (as performed in this study) resulted in 23 patient relapses among 140 evaluable patients and a worst-case scenario of a 2.8% risk of excess relapse due to volume reduction (outside of the smaller IFRT field, but within the historical IFRT field). However, due to the small number of events, it is difficult to ascertain clinical factors that would have predicted for worse relapse-free survival. Second, there were 25 patients who could not be evaluated secondary to deviations to protocol-directed treatment (Figure 3 identifies unevaluable for patterns of failure in red and black boxes). Although 165 patients were enrolled on protocol, only 140 patients were included in the as-intended treatment analysis. The minor radiation therapy deviations were arguably inconsequential as these deviations related to dose inhomogeneities or variances in treatment volume. Of the 2 patients who were found to have major deviations with inadequate coverage margin of their mediastinal disease with radiation therapy, none suffered a relapse. Thus, it was difficult to determine whether these deviations contributed to local control rates. Third, the results do not provide data to determine optimal dose.13–16 Fourth, given the scope of this study, and the patterns of relapse as a secondary endpoint, we were not powered to determine the following radiation therapy–related issues: (1) relapse not predicted by sites of initial bulk; (2) persistence of PET2 abnormal activity at bulk sites may be unfavorable (or unknown); (3) non-large mediastinal adenopathy bulk not irradiated.
Conclusions
This AHOD0831 trial represents a successful incremental reduction in the volume of radiation therapy in advanced pediatric HL to try to better balance disease control and risk of late effects. Radiation therapy was delivered in a risk-adapted fashion with a volume reduction intended to reduce toxicity by targeting those regions at highest risk for relapse, using modern radiation therapy principles. The aforementioned findings from this study suggest that by assigned RT based on early response to therapy did not increase relapse rates in areas that would have been treated with historical IFRT. These findings should be confirmed in upcoming, larger-scale studies that incorporate novel agents that may transform the patterns of relapse in patients with high-risk disease.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the National Institutes of Health to the Children’s Oncology Group (U10CA098543), Children’s Oncology Group Statistics and Data Center (U10CA098413), National Clinical Trials Network Operations Center (U10CA180886), National Clinical Trials Network Statistics and Data Center (U10CA180899), Quality Assurance Review Center (CA29511) and Imaging and Radiation Oncology Core Group Rhode Island (U24CA180803), and St. Baldrick’s Foundation.
This study was based on the Children’s Oncology Group and Quality Assurance Review Center/Imaging and Radiation Oncology Core Group Database. The authors do not personally own these data and hence are not permitted to share them in the original form (only in aggregate form, eg, publications).
Footnotes
Disclosures: none.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2021.10.152.
References
- 1.Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: Final results of the multinational trial GPOH-HD95. J Clin Oncol 2013;31:1562–1568. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425. Blood 2009;114:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolden SL, Chen L, Kelly KM, et al. Long-term results of CCG 5942: A randomized comparison of chemotherapy with and without radiotherapy for children with Hodgkin’s lymphoma—a report from the Children’s Oncology Group. J Clin Oncol 2012;30:3174–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodgson DC, Hudson MM, Constine LS. Pediatric hodgkin lymphoma: Maximizing efficacy and minimizing toxicity. Semin Radiat Oncol 2007;17:230–242. [DOI] [PubMed] [Google Scholar]
- 5.Kelly KM. Hodgkin lymphoma in children and adolescents: Improving the therapeutic index. Blood 2015;126:2452–2458. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson DC, Dieckmann K, Terezakis S, Constine L. International Lymphoma Radiation Oncology Group. Implementation of contemporary radiation therapy planning concepts for pediatric Hodgkin lymphoma: Guidelines from the International Lymphoma Radiation Oncology Group. Pract Radiat Oncol 2015;5:85–92. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KM, Cole PD, Pei Q, et al. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children’s Oncology Group. Br J Haematol 2019;187:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trippett TM, Schwartz CL, Guillerman RP, et al. Ifosfamide and vinorelbine is an effective reinduction regimen in children with refractory/relapsed Hodgkin lymphoma, AHOD00P1: A Children’s Oncology Group report. Pediatr Blood Cancer 2015;62:60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol 1989;7:1630–1636. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castellino SM, Parsons SK, Kelly KM. Closing the survivorship gap in children and adolescents with Hodgkin lymphoma. Br J Haematol 2019;187:573–587. [DOI] [PubMed] [Google Scholar]
- 12.Kahn JM, Kelly KM, Pei Q, et al. Survival by race and ethnicity in pediatric and adolescent patients with hodgkin lymphoma: A Children’s Oncology Group study. J Clin Oncol 2019;37:3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson PW, Sydes MR, Hancock BW, Cullen M, Radford JA, Stenning SP. Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma: Survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519). J Clin Oncol 2010;28:3352–3359. [DOI] [PubMed] [Google Scholar]
- 14.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: An intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013;31:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017;390:2790–2802. [DOI] [PubMed] [Google Scholar]
- 16.Casasnovas RO, Bouabdallah R, Brice P, et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol 2019;20:202–215. [DOI] [PubMed] [Google Scholar]
- 17.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: A report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol 2014;32:3651–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dharmarajan KV, Friedman DL, Schwartz CL, et al. Patterns of relapse from a phase 3 Study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): A report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppe BS, Hill-Kayser CE, Tseng YD, et al. Consolidative proton therapy after chemotherapy for patients with Hodgkin lymphoma. Ann Oncol 2017;28:2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellino SM, Parsons SK, Pei Q, et al. A randomized phase III trial of brentuximab vedotin (Bv) for de novo high-risk classical hodgkin lymphoma (cHL) in children and adolescents - study design and incorporation of secondary endpoints in Children’s Oncology Group (COG) AHOD1331. Klin Padiatr 2020;232:82–83. [Google Scholar]
- 21.Gobbi PG, Levis A, Chisesi T, et al. ABVD versus modified stanford V versus MOPPEBVCAD with optional and limited radiotherapy in intermediate- and advanced-stage Hodgkin’s lymphoma: Final results of a multicenter randomized trial by the Intergruppo Italiano Linfomi. J Clin Oncol 2005;23:9198–9207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


