Abstract
Many xenobiotics are identified as potential thyroid disruptors due to their action to reduce circulating levels of thyroid hormone, most notably thyroxine (T4). Developmental neurotoxicity is a primary concern for thyroid disrupting chemicals yet correlating the impact of chemically-induced changes in serum T4 to perturbed brain development remains elusive. A number of thyroid-specific neurodevelopmental assays have been proposed, based largely on the model thyroid hormone synthesis inhibitor propylthiouracil (PTU). This study examined whether thyroid disrupting chemicals acting distinct from synthesis inhibition would result in the same alterations in brain as expected with PTU. The perfluoroalkyl substance perfluorohexane sulfonate (PFHxS, 50 mg/kg/day) and the antimicrobial Triclosan (300 mg/kg/day) were administered to pregnant rats from gestational day (GD) 6 to postnatal day (PN) 21, and a number of PTU-defined assays for neurotoxicity evaluated. Both chemicals reduced serum T4 but did not increase thyroid stimulating hormone. Both chemicals increased expression of hepatic metabolism genes, while thyroid hormone-responsive genes in the liver, thyroid gland, and brain were largely unchanged. Brain tissue T4 was reduced in newborns, but despite persistent T4 reductions in serum, had recovered in the PN6 pup brain. Neither treatment resulted in a low dose PTU-like phenotype in either brain morphology or neurobehavior, raising questions for the interpretation of serum biomarkers in regulatory toxicology. They further suggest that reliance on serum hormones as prescriptive of specific neurodevelopmental outcomes may be too simplistic and to understand thyroid-mediated neurotoxicity we must expand our thinking beyond that which follows thyroid hormone synthesis inhibition.
Introduction
Adequate supplies of thyroid hormone in the fetus and newborn are essential for normal brain development (Bernal 2015; Morreale de Escobar et al. 2000; Williams 2008; Zoeller and Rovet 2004). Severe hormone insufficiency, especially in pregnant women, can result in persistent neurological disorders, developmental delays, and mental retardation in their children (Delange 1996). It has become increasing apparent that even modest decreases in the thyroid hormone thyroxine (T4) during pregnancy or in early postnatal life can also result in alterations in brain structure and function; these more modest phenotypes often manifest as reduced scores on standard tests of intelligence (IQ) and memory function (Korevaar et al. 2016; Pop et al. 2003; Wheeler et al. 2015; Willoughby et al. 2014). Exposure to a number of environmental contaminants has been associated with altered thyroid hormones in pregnant women (Abdelouahab et al. 2013; Chevrier et al. 2008; Chevrier et al. 2013; Horton et al. 2015; Lee and Choi 2017; Leemans et al. 2019; Taylor et al. 2014), and these associations supported by decreased serum T4 following controlled exposures in experimental animals (Brucker-Davis 1998; US EPA 2014). These epidemiological and toxicology studies have raised concern that environmental exposures can affect thyroid hormone action, and thus brain development, in various populations.
Two pharmaceuticals, propylthiouracil (PTU) and methimazole (MMI), inhibit thyroid peroxidase activity and reduce serum T4 concentrations in humans and rodents; these drugs have been widely used to study how a xenobiotic exposure in animal models may impact the developing brain. When administered to pregnant rats and mice at high doses, both serum T4 and T3 are dramatically reduced and serum thyroid stimulating hormone (TSH) increased, which demonstrates the hypothalamic-pituitary-thyroid axis is disturbed. The resulting hypothyroidism induced by PTU and MMI can induce a number of structural and functional impairments in the rodent brain (Bernal 2015; Morreale de Escobar et al. 2004; Rami et al. 1986). Although some environmental contaminants inhibit hormone synthesis (e.g., ziram, mancozeb, mercaptobenzimidazole, ethylene thiourea, amitrole) (Nouh et al. 2013), others reduce serum T4 by interfering with targets distinct from the thyroid gland. Some of these potential extrathyroidal sites of chemical action include increases in hepatic metabolism and clearance, activation/inhibition of deiodinating enzymes, competition with hormone on distributor proteins and blockade of thyroid hormone transporters (Murk et al. 2013; Noyes et al. 2019; OECD 2014). Rodent models of thyroid deficiency induced by high doses of the pharmaceuticals, severe iodine deficiency, surgical or genetic ablation to obliterate the functionality of the thyroid gland all result in a common profile of severe reductions in serum hormones accompanied by exponential rises in serum TSH, and stark developmental defects (Christ et al. 2004; Friedman and Zeman 1979; Martinez-Galan et al. 1997; Rami et al. 1986; Rami and Rabie 1990; RIESCO et al. 1977). In contrast, exposure to environmental contaminants typically result in moderate disturbances in serum hormones, often restricted to reductions in serum T4, and these can occur in the absence of a corollary increase in TSH. This signature pattern in serum hormone profiles has been observed with perfluoroalkyl substances (PFAS), polychlorinated biphenyls (PCBs), polybrominated flame retardants (PBDEs), and many pesticides (Axelstad et al. 2011; Chang et al. 2008; Goldey et al. 1995a; Kodavanti et al. 2010; Louis et al. 2017; Morse et al. 1996; Paul et al. 2010b; Ramhoj et al. 2020; Rosiak et al. 1997). As such, environmental contaminants, even at relatively high doses, commonly induce a state of hypothyroxinemia (low T4) rather than overt hypothyroidism (low T4 with elevated TSH) characteristic of depraved iodine status or high dose MMI and PTU models. However, it is clear from recent findings that shifting to much lower doses of these pharmaceuticals or employing more marginal degrees of dietary iodine insufficiency can achieve a state of hypothyroxinemia that is also accompanied by subtle adverse neurological effects (Gilbert et al. 2013a; Gilbert 2011; O’Shaughnessy et al. 2018b).
One critical question with direct implications for regulatory toxicology is whether the magnitude of change in circulating levels of thyroid hormones typically achievable with environmental contaminants, is sufficient to induce developmental neurotoxicity (Li et al. 2019). A number of reliable thyroid-dependent brain-based markers of impaired neurodevelopment have been identified using low dose PTU and MMI models (Bastian et al. 2012; Bastian et al. 2014; Gilbert et al. 2016a; Gilbert et al. 2014; Liu et al. 2010; O’Shaughnessy et al. 2018b; Sharlin et al. 2008; Wei et al. 2015). In the present study we posed a simple question - would comparable reductions in circulating levels of serum T4 induced by chemical via ‘extrathyroidal’ action result in a neurotoxicity profile similar to that previously reported by our laboratory and others for PTU? To investigate this question, two chemicals were chosen for study. Pregnant rats were exposed to the perfluoroalkyl substance perfluorohexanesulfonic acid (PFHxS) and the antimicrobial Triclosan from early gestation and throughout lactation. Both chemicals were reported in other studies to induce hypothyroxinemia in adult and developing rats and the doses used in the present study were just below the maximally tolerated dose to avoid maternal toxicity. Importantly, the primary mechanisms of action of both PFHxS and Triclosan are hypothesized to be distinct from the synthesis inhibiting properties of PTU and MMI (Axelstad et al. 2013; Butenhoff et al. 2009; Chang et al. 2018; Paul et al. 2012; Paul et al. 2010b; Ramhoj et al. 2020). We conducted a more extensive evaluation of serum hormones and examined a suite of outcome measures reliably altered with doses of PTU that reduce circulating levels of T4 to greater or comparable degrees.
Methods
Animals
All experiments were conducted with prior approval from the United States Environmental Protection Agency’s Institutional Animal Care and Usage Committee (IACUC) and were carried out in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved facility. Pregnant Long-Evans (LE) rats were obtained from Charles River (Raleigh, NC) on gestational day (GD) 2 and housed individually in standard plastic hanging cages. All animal rooms were maintained on a 12:12 light:dark schedule, and animals were permitted free access to food (Purina 5008 rat chow) and purified tap water. PFHxS was prepared in a 25mg/ml suspension of 2% Tween-20 and deiodinzed water, Triclosan in a 150mg/ml suspension of corn oil. Dams were exposed to 50 mg/kg bw/day of PFHxS (N=9,), 300 mg/kg bw/day of triclosan (N=8;), or vehicle controls (N=6 2% Tween-20 and deionized water and N=6 corn oil) in 2ml/kg dosing solutions, by gavage beginning on GD6 until postnatal day (PN) 21. Pups received exposure in utero via the dam and by lactation afterbirth. Pups were not administered chemical directly. These high doses were chosen in an attempt to match as closely as possible serum T4 reductions attained in dams and pups following maternal exposure to a relatively low dose of PTU. Perfluorohexanesulfonic acid (PFHxS, potassium salt, CAS 3871996, ≥98% pure) was purchased from Sigma–Aldrich and Triclosan (CAS 3380–34-5, ≥98% pure) was obtained from Ciba Grenzach GmbH, Germany. Doses were chosen based on previous work to maximally reduce serum T4 but without inducing overt toxicity to the dam (Paul et al. 2012; Paul et al. 2010a; Ramhoj et al. 2018). The day of birth was denoted as PN0 and all litters were culled to 10 pups on PN2, balancing the sexes when possible. Euthanasia was achieved in pups and dams by decapitation in the absence of anesthesia and tissues collected from pups on PN0, PN0, PN6 and PN14. Dams were also euthanized on PN22 when pups were weaned. At weaning pups were transferred to plastic hanging cages (2/cage) with a same-sex littermate and permitted free access to food (Purina 5001) and filtered tap water until neurobehavioral assessments conducted between PN 60–80.
Serum Hormones.
Trunk blood was collected from pups on PN0, PN2, PN6 and PN14, pooling littermates regardless of sex. Samples were placed on ice and allowed to clot before centrifugation and serum isolated. Blood was collected from dams on GD20 via intravenous tail bleed as previously described (Hassan et al. 2017) and trunk blood was collected following decapitation on PN22, 24 hours following the last dose.
Total T4 and T3 were analyzed in serum by Liquid Chromatography Mass Spectrometry (LC/MS/MS) using an AB Sciex (Framingham, MA) Exion AC UHPLC-Qtrap 6500+ Linear Ion Trap LC/MS/MS system as previously described (Hassan et al. 2017; O’Shaughnessy et al. 2018a). Solvent-based calibration standards were used for quantitation over a range of 10 – 10,000 pg/mL in a 100uL standard curve volume. Each curve used a minimum of five sequential points and correlation coefficients of the curves were ≥ 0.99. Samples were diluted by a factor of 5 (20 μL) for adults and 2.5 (40μL) for pups younger than PN14. This dilution factor was applied after calculating the concentration against the standard curve corresponding to a quantification range of 0.025–50 ng/ml. Two ion transitions were monitored for each target analyte, qualitatively identified based on retention time relative to the internal standard and calibration standard, and the ratio of the peak areas of the monitored ion transitions. The lower limit of quantitation (LLOQ) for each analyte was set to the concentration of the lowest calibration standard that gave an acceptable ion ratio, and acceptable recovery of ±30% of the spike amount. Each sample batch consisted of a method blank, a laboratory control sample (blank spike), and a continuing calibration verification sample prepared in solvent. The lower limit of quantification (LLOQ) for both T4 and T3 was 0.1 ng/ml.
Thyroid-stimulating hormone (TSH) was measured by radioimmunoassay (RIA) using a standard double-antibody assay as described by (Louis et al. 2017; Stoker et al. 2010; Thibodeaux et al. 2003). All TSH samples were run in duplicate in single assay and the intra-assay CV was 2.2%. The limit of detection was 0.78 ng/ml.
Serum free T4 (fT4) concentration in dams on PN22 and pups on PN14 was examined by RIA (IVD, San Diego), samples run in duplicate, with limit of detection set to the lowest calibrator of 0.32 ng/dl and average intra-assay coefficient of variation was 6–12%.
Brain Tissue Thyroid Hormone Analysis.
Brain tissue thyroid hormones concentrations were analyzed by LC/MS/MS by methods previously described in O’Shaughnessy et al. (2019) with the following modifications. Hormone assays were optimized for a tissue mass ranging between 90–150 mg. To meet these requirements, whole brain from PN0 pups were excised, but the olfactory bulbs and the hindbrain and cerebellum were removed to more closely match tissue composition of older pups. For pups on PN2 and PN6, the forebrain was collected, while neocortex was selectively harvested from one hemisphere in PN14 pup brains to attain appropriate brain mass for hormone analyses. Tissue was rinsed in cold phosphate buffered saline, blotted dry, weighed, flash frozen in liquid nitrogen and subsequently stored at −80° C until analysis.
Frozen brain samples were weighed and transferred to a 15 ml centrifuge tube containing methanol 1 mM PTU and spiked with a mixed stable isotope solution containing 13C6-T2, 13C6-T3 and 13C6-T4. THs were extracted from the brain using methanol, chloroform and an aqueous solution of 0.05% CaCl2 followed by nitrogen evaporation. One ml of water was subsequently added to the tube and the total volume of the aqueous mixture was determined. Equal volumes of 80/20 (v/v) 4% formic acid (aq) and acetonitrile were added and the sample was vortexed then TH were extracted using an Evolute 50 mg, 3 ml CX solid phase extraction (SPE) cartridge (Biotage, Charlotte, NC, USA) on a vacuum manifold. The SPE cartridge was conditioned with methanol followed by 2% formic acid. After sample application, the cartridge was washed with 50 mM ammonium acetate (pH 6), 2% formic acid and then methanol. Thyroid hormones were eluted using 5% NH4OH in methanol, evaporated to dryness using nitrogen and the residue dissolved in 100 μl of 5% acetonitrile: 95% water with 0.1% formic acid for transfer to an amber liquid chromatography (LC) micro-vial. SPE reconstituted extracts were analyzed for TH by stable isotope dilution LC/MS/MS as described above for serum and expressed as ng/gm of brain tissue. The LLOQ for both T4 and T3 in the brain tissue was 0.1 ng/gm.
Gene Expression in Liver, Thyroid, Brain.
Animals were euthanized by decapitation and thyroid glands were removed from the trachea, weighed and flash frozen. The liver was removed and weighed, and a section from left lateral lobe from dams on PN22 and pups on PN0, PN2, PN6 and PN14 was excised and saved in RNAlater™ (Invitrogen) according to the manufacturer’s recommendation. An oblique slab of anterior neocortex from PN14 brain was collected and store in RNAlater™. Samples of anterior cortex were chosen for gene transcription analysis as this region is readily accessible and fairly homogeneous section can be obtained from animal to animal. This region was also chosen as our past studies had indicated a larger number of thyroid-hormone responsive genes were differentially expressed at this age in cortex relative to hippocampus or cerebellum (Royland et al. 2008). All samples subsequently stored at −80°C until analysis for gene expression.
RNA extraction and quantitative real time-PCR.
Total RNA was extracted via TRIzol® (Invitrogen) according to manufacturer’s protocol. RNA pellets were resuspended in nuclease-free H2O. RNA samples were treated with DNase I (Promega, M6101) and quantified using the Ribogreen Quantitation Kit (ThermoFisher, R11490). After measuring RNA by Ribogreen, the same DNased RNA samples were reverse transcribed using the ABI cDNA Archive Kit (ThermoFisher, 4322171) with random primers, and 25 ng of cDNA was amplified using ABI TaqMan Gene Expression Assays (and ABI Universal Master Mix (ThermoFisher, 4304437).
Quantitative real-time PCR (qRT-PCR) was performed on a 7600HT Fast Real-Time PCR System (Applied Biosystems), using TaqMan Universal PCR Master Mix and commercially purchased TaqMan probes (Applied Biosystems, see summary Table 1). The thermocycler program was as follows: 95° C for 5 min, followed by 40 cycles of 15 s at 95°C, and 1 min at 60° C. Each sample was ran in duplicate, and the data analyzed using 2−ΔΔCT method (Schmittgen and Livak 2008) with β2-microglobulin (β−2m) or beta actin (β-actin) for normalization procedures. These genes are stably expressed in the selected tissues, neither were altered by treatment as verified by one-way ANOVAs (p’s >0.10).
Table 1.
Gene transcript names and ID.
| Gene Name | ID | ||
|---|---|---|---|
| Liver Transcripts | |||
| Phase I Metabolism | Cypla2 | Cytochrome P-450 1a2 | Rn00561082_m1 |
| Cyp2bl | Cytochrome P-450 2B1 | Rn01457875_m1 | |
| Cyp3all | Cytochrome P-450 3A11 | Rn01412889_mH | |
| Cyp3a23 | Cytochrome P-450 3A23 | Rn01640761_gH | |
| Phase II Metabolism | Sultlc3 | Sulfotransferase 1C3 | Rn00669059_g1 |
| Ugtlal | Uridine 5'-diphospho- glucuronosyltransferase family 1 member | Rn00754947_m1 | |
| Phase III Metabolism | Car (Nrli3) | Constitutive androstane receptor 2, Nuclear receptor subfamily 1 group I | Rn04339043_m1 |
| Pxr (Nrli2) | Pregnone-X-receptor, Nuclear receptor subfamily 1 group 1 member 2 | Rn00583887_m1 | |
| Ppar 03b1 | Peroxisome proliferator-activated receptor alpha | Rn00566193_m1 | |
| TH-Responsive Genes | Diol | Iodothyronone Deiodinase 1 | Rn00572183_m1 |
| Mdrla | Multidrug resistance protein 1a | Rn01639253_m1 | |
| ME1 | Malic Enzyme 1 | Rn01755810_m1 | |
| Spotl4 (Thrsp) | Thyroid hormone Responsive | Rn01511034_m1 | |
| Thyroid Gland Transcripts | |||
| Slc5a5 (Nis) | Sodium iodine symporter | Rn00583900_m1 | |
| Tpo | Thyroid peroxidase | Rn00571159_m1 | |
| PVH Area Transcripts - PN6 | |||
| Direct TH Target | Hr | Hairless, HR Lysine Demethylase and Nuclear Receptor Corepressor | Rn00577605_m1 |
| Shh | Sonic hedge hog | Rn00568129_m1 | |
| Klf9 (Btebl) | Kruppel-like factor 9, Basic transcription element binding protein | Rn00589498_m1 | |
| TH-Responsive | Bdnf total | Brain-derived neurotropic factor | Rn02531967_s1 |
| Bmp7 | Bone morphogenetic protein 7 | Rn01528889_m1 | |
| PVH-Associated Transcripts | Casp3 | Caspase 3 | Rn00563902_m1 |
| Sox2 | Sex determining region Y box 2, SRY | Rn01286286_g1 | |
| Spred 1 | Sprouty-related, EVH1 domain-containing protein 1 | Rn01486390_m1 | |
| Pax6 | Paired box protein Pax-6 | Rn00689608_m1 | |
| Cortex Bioindicator | |||
| Transcripts PN14 | Agt | Angiotensinogen | Rn00593114_m1 |
| Col11a2 | Collagen type XI Alpha 2 Chain | Rn01428773_g1 | |
| Gjb6 | Gap junction beta-6 protein, Connexin 30 | Rn00584528_m1 | |
| Hopx | HOP Homeobox | Rn00592446_m1 | |
| Hr | Hairless, HR Lysine Demethylase and Nuclear Receptor Corepressor | Rn00577605_m1 | |
| Itih3 | Inter-alpha-trypsin inhibitor heavy chain 3 | Rn00569293_m1 | |
| Klf9 (Btebl) | Kruppel-like factor 9, Basic transcription element binding protein | Rn00589498_m1 | |
| Ngf | Nerve growth factor | Rn01533872_m1 | |
| Pnoc | Prepronociceptin | Rn01637101_m1 | |
| Pvalb | Parvalbumin | Rn00574541_m1 | |
| Sema7a | Semaphorin-7A | Rn01417778_m1 | |
| Reference Genes | |||
| B2m | Beta 2-microglobulin | Rn00560865_m1 | |
| Bactin | Beta actin | Rn00667869_m1 |
Thyroid Gland and Liver Gene Expression.
PFHxS and Triclosan were chosen for study because their primary in vivo purported modes of action are extrathyroidal. However, activity of these chemicals has been observed in two high-throughput in vitro assays targeting sites within the thyroid gland, i.e., inhibition of the synthesis enzyme thyroperoxidase (TPO) and blockade of the sodium-iodine symporter (NIS) (Paul Friedman et al. 2016; Wang et al. 2019; Zhang et al. 2018). The coupling of high-throughput testing with in vivo toxicogenomics has been used to inform and confirm potential in vivo hazard mode of action (Farmahin et al. 2017; Thomas et al. 2013). Therefore, we examined two intrathyroidal molecular targets (Tpo and Nis) of dams and pups treated with PFHxS and Triclosan to determine if activity within the gland may contribute to the serum hormone declines evident with these agents.
Proposed extrathyroidal targets of these agents include the distributer protein transthyretin (TTR) and a liver metabolism mode of action. Reductions in serum hormone induced by Triclosan have largely been attributed to an upregulation of hepatic catabolism (Paul et al. 2012; Paul et al. 2010b; Yueh and Tukey 2016), while hepatic clearance of serum T4 by perfluorinated compounds may be a secondary consequence of PFHxS outcompeting T4 for binding to TTR (Butenhoff et al. 2009; Chang et al. 2018). To determine if a pattern of hepatic gene expression consistent with declines in serum T4 was observed in animals exposed to PFHxS and Triclosan, gene transcripts tied to upregulation of liver catabolism were examined in livers of dams on PN22, and in pups on PN2, PN6 and PN14. A set of transcripts for Phase I (cytochrome P450 enzymes), Phase II (glucuronidation and sulfation enzymes), and Phase III (hepatic nuclear receptors) are summarized in Table 1. These targets were selected based on literature reports of correlations between increases in expression, augmented enzyme activity, and/or declines in serum thyroid hormones (Klaassen and Hood 2001; Paul et al. 2010b; van Raaij et al. 1993; Xu et al. 2019). A number of hepatic TH-responsive genes were also assessed as indicators of altered TH action in the liver that may result from reduced tissue hormone concentrations (Bansal et al. 2014).
Brain.
Two sets of thyroid hormone sensitive gene targets were investigated in brain at two different ages. On PN6 a set of 9 genes previously associated with the later appearance of a large set of ectopic neurons (periventricular heterotopia, PVH, as described below) were examined. The tissue was collected from the region where the PVH forms, the dorsal half of a ~0.5mm thick posterior section of brain as previously described (O’Shaughnessy et al. 2019). In the PN14 brain, a set of eleven gene markers of disrupted thyroid hormone action were selected from those previously identified to be altered by the greatest magnitude and at the lowest dose in a PTU model (Bastian et al. 2014; O’Shaughnessy et al. 2018b; Royland et al. 2008; Shiraki et al. 2014). All gene transcripts assessed in brain at these two ages are summarized in Table 1.
Periventricular Heterotopia Assessment
A periventricular heterotopia is characterized by the presence of a cluster of ectopic mature neurons directly medial to the lateral ventricular epithelium within the posterior region of the brain. This malformation is reliably produced in response to developmental TH insufficiency induced by PTU exposure (Gilbert et al. 2014; Goodman and Gilbert 2007; O’Shaughnessy et al. 2018a; O’Shaughnessy et al. 2019; Spring et al. 2016). Where possible, one male and one female from each litter was euthanized by decapitation on PN14, the brain removed from the skull, and whole brain immersion fixed in 4% paraformaldehyde for one week before transfer to a deOlmos cryogenic protective solution (de Olmos 1977) and thereafter maintained at 4° C. Brains were subsequently sectioned coronally on a vibratome at a thickness of 60 μM, and 30 consecutive sections were collected, spanning a region of ~Plates 29 to 40 in the brain atlas of Paxinos and Watson (1989). All 30 sections from each animal were then processed for Neuronal Nuclei (NeuN) immunohistochemistry according to previously published methods (Goodman and Gilbert 2007; O’Shaughnessy et al. 2018a). In brief, sections were incubated overnight at 4°C in NeuN primary antibody (Millipore, MAB377, 1:2500 dilution), followed by a biotinylated secondary antibody (1:400) in conjunction with avidin-biotin amplification (Vectastain Elite ABC, Vector, Burlingame, CA). Signal was detected by diaminobenzidine tetrahydrochloride (DAB) as the chromogen. Following immunohistochemistry, sections were mounted on gelatin coated glass slides, dried, and cover slipped. Slides were imaged using an Aperio AT2 slide scanner (Leica Biosystems, Buffalo Grove, IL). Each tissue section was scored for a periventricular heterotopia using Aperio ImageScope software. If a heterotopia was detected, the area of this region was calculated by tracing the perimeter of the NeuN-positive cell cluster. From these area measures, heterotopia volume was estimated according to standard stereological principles and mean volume estimates were compared across treatments. Incidence estimates were also calculated based on a nominal cutoff value of 0.006 mm3 determined from an evaluation of historical control data, and a more liberal value of 0.003 mm3 used in previous reports of heterotopia volume from our laboratory (Gilbert et al. 2014; O’Shaughnessy et al. 2018a; Spring et al. 2016).
Neurobehavioral Assessments
Two behavioral tests, trace fear conditioning and acoustic startle reflex, were performed in adult male offspring. Trace fear conditioning depends on circuitry in the amygdala, hippocampus and prefrontal cortex and is sensitive to thyroid disruption induced by PTU (Gilbert 2011; Gilbert et al. 2016b). The acoustic startle response can serve as a simple hearing test for rats, its habituation a measure of non-associative learning. Prepulse modification of the amplitude of the acoustic startle reflex serves as an index of sensory gating processes, impairments of which are correlated with deficiencies in cognitive processing (Fitch et al. 2008). These tests were chosen because they rely on brain regions that require adequate supplies of thyroid hormone for normal development, and both have been found to be sensitive to thyroid hormone disruption by chemical intervention including PTU (Crofton 2004; Gilbert et al. 2016a; Gilbert 2011; Gilbert et al. 2017; Goldey and Crofton 1998; Goldey et al. 1995a; Goldey et al. 1995b). One to two male offspring from 14 control, 7 PFHxS, and 6 Triclosan litters were examined on trace fear conditioning followed by acoustic startle reflex modification. Only males were sampled as a limited number of pups were available for adult testing following the number of distinct timepoints samples for other measures. Our previous work with PTU has shown greater sensitivity of males to disruption in fear conditioning (O’Shaughnessy et al. 2018a). No pharmacokinetic differences in male or female offspring have been demonstrated for PTU (O’Shaughnessy et al. 2018a), PFHxS (O’Shaughnessy et al, in preparation), or in adolescent male and adult females exposed to Triclosan (Louis et al. 2017; Zorrilla et al. 2009). Animals were pair-housed and gently handled by investigators to reduce stress for several days prior to trace fear acquisition training. Both behavioral tests followed procedures similar to those reported previously with some minor modifications as described below (Gilbert 2011; Gilbert et al. 2013b; O’Shaughnessy et al. 2018a).
Trace Fear Conditioning:
On the first day of trace fear conditioning animals were placed in a test chamber (Habitest, Coulbourn Instruments, Allentown, PA), equipped with a small animal shock generator (H13–16, Coulbourn Instruments). The chamber was sprayed with Windex™ cleaner to provide a distinctive olfactory cue during training and context testing. The training procedure consisted of a 2-min baseline period followed by onset of a 15-sec compound light/tone conditioned stimulus (CS). The unconditioned stimulus (US) was a scrambled foot shock (1 mA, 2 sec duration) delivered through a metal grid floor. Two CS-US pairings were delivered, two minutes apart. CS offset and US onset were separated by a 30-sec trace interval. A non-contingent distractor stimulus (a dim light on the wall opposite the cue light and flashing for 3 seconds) was randomly presented throughout the training session. This was introduced to increase the task difficulty and engage attentional brain circuitry in addition to amygdala (CS-US association) and hippocampus (context and trace element). Conditioning to context was assessed the following day by returning animals to the same test chamber for 5 min in presence of the olfactory Windex cue but the absence of explicit CS, UCS or distractor stimuli. Approximately one hour later, conditioning to cue was assessed by placing animals in a similarly sized test box, which was equipped with striped walls and a smooth floor and was located in a different room. In addition, the enclosure doors remained open, the room lights were on, and the distinct olfactory cue present during training was absent. A protocol similar to that used during training was employed with only a single CS presentation after the 2-minute baseline period, no shock US was delivered, or distractor stimuli were presented. Activity was recorded for three minutes after CS presentation.
Activity in each phase of testing was quantified using an infrared motion detector (Coulbourn Instruments) mounted on the ceiling of each test chamber. Reduced activity was taken as the measure of fear-induced learning, with greater suppression indicating better learning. Activity counts were recorded in 15-second bins for training, context and cue testing. Conditioning to context was measured as activity counts during the first 2 minutes of testing on Day 2, compared to the number of counts recorded during the 2 minutes before delivery of the CS on Day 1. Conditioning to cue was taken as the mean number of activity counts recorded in the novel test chamber on Day 2 in the 30-sec trace period after CS presentation compared to the mean counts/30 seconds during the 2-minute prestimulus baseline period activity before CS delivery.
Acoustic Startle and Pre-Pulse Inhibition:
Evaluation of the acoustic startle response was conducted a minimum of one week following completion of fear conditioning. Basal levels of the acoustic startle response (ASR), habituation of ASR, and inhibition of the ASR by prepulse noise (prepulse inhibition or PPI) were measured using the TSE Startle Response SystemTM (TSE Systems, Germany). Animals were placed into one of four TSE Systems test boxes housed within separate sound dampening acoustical chamber (IAC No. AC-361) located in a sound-attenuated test room. Loudspeaker calibrations were conducted following manufacturer’s instruction (Version 3.09, TSE Startle Response System, TSE Inc, Chesterfield, MO) and all noise levels were measured using a ¼ inch pressure field microphone, Model 4938, attached to a pre-amplifier, Model 2639, and a measuring amplifier, Model 2636-D (all equipment Bruel and Kjaer, Germany). Prior to the test, animals were gentled by handling for a minimum of 3 days and acclimated to the acoustic startle chambers for 10 min the day prior to testing. On the day of testing, after a 5 min baseline period, 30 ASR trials were delivered at a fixed intertrial interval (ITI) of 20 sec against a 65dB background noise. The startle stimulus was a 40 msec burst of white noise at 115 dB, SPL. A highly sensitive transducer (weight sensor) integrated into the measuring platform recorded dynamic changes from the animals’ reactions during this acquisition period (TSE startle response system software, version 3.09). The ASR was measured as the maximum amplitude in grams (MaxG) occurring within 100-msec of stimulus delivery. ASR habituation data were averaged across each 5-trial block for each animal, yielding 6 Blocks of ASR MaxG data for analysis of baseline ASR amplitudes and ASR habituation.
Prepulse inhibition of the ASR was assessed immediately following ASR habituation. Animals received a total 60 trials using a sensorimotor gating paradigm (Swerdlow et al. 2016) incorporating both prepulse and no-prepulse trials. These trials encompassed 6 trial types: blank trial where no stimulus was delivered on top of the 65 dB background noise, startle stimulus only with no prepulse, and 4 different 20 msec white noise prepulse trials consisting of stimuli 3, 6, 10, and 18 dB above the 65dB background noise. The onset of each prepulse was fixed, preceding the onset of the startle stimulus by 60 msec (interstimulus interval or ISI). Each trial type was delivered 10 times throughout the session in a pseudo-random order, programmed to occur once within each block of 6 trials to ensure equal distribution across the PPI assessment. Each PPI trial was preceded by a variable ITI between 20 and 30 sec in duration. Each of the 6 PPI trial types were averaged across the 10 trials to yield one MaxG measurement per PPI trial (Blank, AS stimulus alone, 3dB PP, 6dB PP, 10dB PP, and 18dB PP). PPI was defined as the decrease in the MaxG for each of the prepulse trials (PP) compared to MaxG on ASR trials. The percentage of startle MaxG for each dB was calculated as:
Statistical Analyses:
Analysis of variance (ANOVA) analyses were conducted using SAS (version 9.2, SAS Institute, Cary, NC). No differences were detected between corn oil and Tween controls in any measures taken, so these control groups were collapsed and treated as a single control for statistical and presentation purposes. Body weight and serum thyroid hormones were evaluated using a one-way repeated measures ANOVA with litter as the unit of analysis. Mean contrast test by treatment condition utilized Duncan’s t-test. With the exception of gene expression analyses, the alpha level was set at 0.05. Statistical analyses of gene expression were conducted on mean fold-change and assessed with one-way ANOVAs. To control false discovery, a minimum 2-fold change was required for liver and thyroid gland to be considered significant. A lower minimal fold-change of 1.25 was adopted for brain as the relative range of change typically observed in brain is quite small relative to liver and thyroid gland. To limit experiment-wise error for the large number of analyses performed, the alpha corrected by dividing 0.05 by the square root of the number of gene targets examined. As such, the alpha level for a significant change for thyroid gland and brain was set at p<0.02 and for liver p<0.01.
Behavioral tests were conducted on male offspring only and 1–2 animals were assessed from any given litter. In all analyses litter was considered the unit of analyses. Dependent variables were examined for normality using the Shapiro-Wilk test and if the data was judged to be non-normal, the dependent measure was transformed to achieve normality; all acoustic startle data were transformed to a log10 scale. Data were analyzed in separate mixed model ANOVAs with treatment as a between litter factor and either State (Trace Fear training, context and cue testing), Block (ASR habituation) or dB (prepulse inhibition) as repeated measures. Dam was treated as a random variable to control for more than 1 pup examined from each litter as per code described in Boyes et al. (2018). Various covariance structures were examined as appropriate for the type of repeated factor. The best fitting model was selected based on AICC values (Wagenmakers and Farrell 2004). For all measures, a first-order autoregressive covariance structure was applied.
Results
Toxicity Measures and Serum Thyroid Hormones.
Relative to pooled controls, dam body weights were slightly reduced in late gestation and throughout the postnatal period (Figure 1A). In repeated measures ANOVA, no effect of Treatment was found [F(2,24)=1.89, p>0.18], although a significant Treatment X Day interaction was revealed [F(76,912)=2.17, p<0.001]. Mean contrast tests, however, failed to detect significant differences between control and PFHxS or control and Triclosan dam weights at any gestational or postnatal day. Female and male pup body weight, dam or pup liver weights were not altered by developmental exposure to PFHxS or Triclosan (Figure 1B–1F, all p’s>0.05). Neither did liver-to-body weight ratios differ as a function of treatment in either dams or pups (data not shown).
Figure 1.
Mean (+/− SE) body and liver weights dams and pups. Dam body weights were slightly reduced by PFHxS and Triclosan relative to controls, a significant Treatment X Day interaction was seen [F(76,912)=2.17, p<0.001] (A). Mean litter-based body weights for male (B) and female (C) pup did not differ from control as no effect of Treatment or Treatment X Age interactions were observed (all p’s>0.10). Neither did dam liver weights at weaning (D) or male (E) or female (F) liver weights of neonates differ from controls. Statistical analyses conducted using repeated measures ANOVA, all p-values>0.18.
Serum total T4 was significantly reduced in dams when assessed in late gestation (GD20) and at the end of lactation (PN22), as evidenced by a significant main effect of Treatment [F(2,500=4.29, p<0.02] and a significant Age X Treatment interaction [F(2,49)=5.23, p<0.0087] (Figure 2A). At the doses chosen, Triclosan appeared to reduce serum T4 (~42% at GD20 and PN22) more than PFHxS (32 and 24% at GD20 and PN22, respectively). Both treatments decreased dam serum T3 to similar degrees at both ages as evidenced by a significant main effect of Treatment [F(2,50)=4.29, p<0.0190] but no Treatment X Day interaction [(p>0.88), Figure 2B]. No change in dam serum TSH was detected on PN22 [Figure 2C, Treatment F(2,25)=2.17, p>0.13]. Consistent with a lack of rise in TSH response, no difference in thyroid gland weights were observed [Mean±SE=10.8±1.24, 13.5± 1.0 and 13.15± 1.20 for Control, PFHxS and Triclosan dams, respectively; F(2,14)=1.70, P>0.20].
Figure 2.
Mean (+/− SE) thyroid hormones in serum are shown on the left for dams and the right panel for pups. (A) Dam serum total T4 and T3 were reduced by PFHxS and Triclosan relative to Controls. (B), but no significant effects were seen on dam serum TSH (C). (D) Reductions in serum free T4 followed the same pattern as observed with total T4 with significant declines in PFHxS (n=7), Triclosan (n=7) relative to Control (n=10). (E) In pups, both treatments reduced serum T4 at all ages tested, with PFHxS showing greater decreases than Triclosan. (F) Serum T3 was below the limit of quantification for most samples in control subjects at younger ages but both treatments reduced serum T3 at PN6 and PN14 (G). As with dam, no change was seen in TSH in PN14 pup serum (H), and fT4 followed a similar pattern as observed with total T4, significant reductions induced by both PFHxS (n=8) and Triclosan (n=6) relative to Control (n=10). Sample sizes are by litter. * p<0.05 significant difference based on mean contrast Dunnett’s T-test conducted following significant main effect of Treatment or Treatment X Age interaction.
More robust reductions in serum T4 were observed in the offspring (Figure 2E), and analyses revealed significant main effect of Treatment [F(2,38)=87.6, p<0.0001] and an Age X Treatment interaction [F(6,87)=211.02, p<0.0001]. In contrast to dam T4, PFHxS reduced pup serum T4 to a greater degree than did Triclosan. PFHxS and Triclosan also suppressed serum T3 in pups assessed on PN6 and PN14 (Figure 2F), and is supported by a significant main effect of Treatment [F(2,46)=15.5, p<0.0001]. In this case, the effects were similar at both ages and the Treatment X Age interaction was not significant (p>0.57). As with dams, serum TSH was similar across treatment conditions when measured on PN14 [F(2,21=0.34, P>0.71] (Figure 2G). Serum concentrations of fT4 were reduced in dams, Triclosan appearing slightly more effective in reducing fT4 than PFHxS, mirroring the pattern observed for total T4 [F(2,23)=26.95, p<0.0001] (Figure 2D). In pups, the pattern for fT4 also mirrored that seen with total T4, and PFHxS more sharply reduced fT4 than did Triclosan [F(2,21)=23.14, p<0.0001] (Figure 2H). Ratio of free T4/total T4 were not different from controls in dams or pups (data not shown) suggesting that total and free hormone levels fell in parallel. Thyroid weight was not significantly different between Control and PFHxS or Control and Triclosan offspring on PN14 [Mean±SE=3.13±0.29, 2.74±0.16 and 4.25± 0.67 for Control, PFHxS and Triclosan pups, respectively, despite a significant overall main effect of Treatment F(2,25)=3.75, P<0.04].
Gene Expression in the Thyroid Gland and Liver.
Increases in the relative expression of Nis and Tpo, genes whose products function to increase intrathyroidal iodine and synthesize thyroid hormones, were not observed in dams or in pups (Figure 3). Gene expression in liver examined as an indicator of potential upregulation of liver catabolism by these chemicals revealed a similar pattern of expression in in dams and pups. Of the 13 transcripts examined in liver, the Phase I metabolism gene, Cyp2b1, and the Phase II metabolism gene, Sult1c3, were significantly upregulated in the livers of treated dams [F(2,27)=24.7, p<0.0001 and F(2,27)=6.22, p<0.0001], and in treated pups of all ages (all p’s<0.0001) relative to pooled controls. The magnitude of change in these transcripts was generally greater in PN6 and PN14 pups than in dams and younger pups, and more marked in PFHxS pups than in those treated with Triclosan (Figure 4). Additional genes significantly upregulated in dams but not altered in pups included 3–6 fold increases in expression of Cyp3a11 [F(2,27)=6.02, p<0.007] and Cyp3a23 [F(2,27)=5.57, p<0.0094], and more modest 2-fold increases in the hepatic nuclear receptor Car [F(2,27)=12.7, p<0.0001]. No changes in TH-3responsive gene targets in the liver were detected in dams or pups.
Figure 3.
Mean (+/− SE) Percent Change in Thyroid Gland Gene Expression. Thyroid gland transcripts involved in hormone synthesis (Tpo, A) and responsive to TSH (Nis, B) were not altered in glands collected from Dams on PN22 or pups on PN0, PN6, or PN14. Data are expressed as percent change from mean fold change in controls. A minimum 2-fold change and p<0.01 was required to control for false discovery rate and experiment-wise error. Fold change data are summarized in Supplementary Figure 1.
Figure 4.
Mean (+/− SE) Percent Change in Relative Gene Expression Profiles in Dam and Pup Liver. Representative transcripts of Phase I, II, III Metabolism and thyroid hormone-responsive genes were examined in the liver of dams on PN22 (A), and in liver of pups on PN2 (B); PN6 (C), and PN14 (D). Relative expression of Phase I metabolism genes, most notably Cyb2b1 were increased by both PFHxS (n=6–9) and Triclosan (n=6–8) relative to Control (n=9–13) in dams and pups, with the largest increases seen in pups on PN2. Relative expression of Cyp2b1a was of greater magnitude in Triclosan-than PFHxS-treated dams (A), whereas the opposite was true in pups (B-D). Expression of Phase II metabolism genes was limited to small increase in Sult1c3 in Triclosan-exposed dams (A) whereas pups exposed to PFHxS or Triclosan exhibited more robust increases in relative expression and a higher magnitude of change seen in response to PFHxS (B-D). Increases in Phase III nuclear receptor expression was largely limited to modest increases in the expression of Car in both PFHxS and Triclosan treated dams (A). No change in the relative expression of TH-responsive genes were seen in dams or pups. * p<0.05 represents mean contrast by Dunnett’s t-test following significant main effect of Treatment in ANOVA. A two-fold change and p<0.01 were required for significance to control for false discovery and experiment-wise error. Fold-change data are summarized in Supplementary Figure 2.
Thyroid hormones in brain and expression of TH-responsive genes:
Thyroid hormones were reduced in the brains of treated pups for the first few days after birth (Figure 5). Significant effects of Treatment were evident in brain T4 [F(2,49)=5.64, p<0.0063] and brain T3 [F(2,50)=3.56, p<0.0357]. PFHxS and Triclosan reduced brain T4 on PN0 and PN2, and brain T3 was also significantly reduced in Triclosan exposed pups on PN2, with no difference from control levels in either hormone by PN6 (Figure 5 A,B). Consistent with a lack of effect on brain hormones on PN14, no changes in the relative expression of selected molecular readouts of thyroid hormone action were observed in anterior neocortex on PN14. Eleven candidate genes previously reported to be downregulated following low doses of maternal PTU exposure and reflective of altered TH action (Bastian et al. 2012; O’Shaughnessy et al. 2018b) remained unchanged in the neocortex of PFHxS or Triclosan treated pups on PN14 (Figure 5C).
Figure 5.
Mean (+/− SE) brain T3 and T4 and gene expression in neocortex. Pup brain T4 was reduced by both treatments on PN0 (A). Reductions in brain T4 were also present on PN2, but only Triclosan was significantly reduced from control based on mean contrast tests. (B) Reductions in brain T3 were evident only in Triclosan treated pups on PN2 (B). No differences in brain T4 or T3 were evident in older pups (all p-values>0.57). (C) No reductions in the relative expression of the 11 transcripts examined in cortex of Control (n=9), PFHxS (n=7) and Triclosan (n=6) pups on PN14 (all p-values>0.17). No change in cortical gene expression on PN14 is consistent with a lack of effect on brain hormone in cortex at this age in B and C. * p<0.05 represents mean contrast by Dunnett’s t-test following significant main effect of Treatment in ANOVA. A 1.25-fold change and p<0.015 were required for significance to control for false discovery and experiment-wise error. Mean fold-change in relative gene expression is summarized in Supplementary Figure 3A.
Periventricular heterotopia:
Following the examination of 1–2 pups per litter (n=12 controls, n=9 PFHxS, and n=8 Triclosan litters), we found no evidence of a periventricular heterotopia (Table 2 and Figure 6A). Consistent with the lack of a heterotopia in littermates assessed on PN14, no significant changes were seen in this brain region on PN6 in the relative expression of 9 gene transcripts previously associated with heterotopia (Figure 6B). It is of note however, that a trend for a modest reduction in relative expression of Pax6 [fold-change=0.78, F(2,18)=8.76, p<0.0022], Bmp7 [fold change=0.66, F(2,18)=4.73, p<0.02] and Sox2 [fold change=0.69, F(2,18)=2.53, p<0.05] were selectively present in the brains of PFHxS but not Triclosan-exposed pups. These transcripts are upregulated in the brains of PTU animals in which a heterotopia forms (O’Shaughnessy et al., 2019). If significant down regulation by maternal exposure to PFHxS is replicated in future studies, these alterations may suggest disruption in brain gene expression that is unrelated to heterotopia (Figure 6B).
Table 2.
Mean heterotopia volume in PN14 brain. PVH were not present in the brains of PFHxS or Triclosan treated pups. Incidence-1 based on nominal volume >0.003mm3., Incidence-2 based on nominal volume >0.006mm3
| # | Mean (+/− SE) Volume | Largest | Incidence-1 | Incidence-2 | |
|---|---|---|---|---|---|
| Litters | (mm3) | (mm3) | (>0.003mm3) | (>0.006mm3) | |
| Control | 12 | 0.0013 (±0.0004) | 0.0074 | 2 of 17 | 1 of 17 |
| PFHxS | 9 | 0.0006 (±0.0002) | 0.0026 | 0 of 17 | 0 of 17 |
| Triclosan | 8 | 0.0011 (±0.0004) | 0.0045 | 2 of 13 | 0 of 13 |
Figure 6.
Heterotopia were not observed in the brain of PFHxS or Triclosan exposed pups when assessed on PN14. (A) Representative coronal sections of NeuN-stained brain within the region where heterotopia typically appear. Some small clusters of NeuN+ cells in several animals from each group were seen in the thin strip of the lateral ventricle that infiltrates the corpus callosum, commonly observed in control animals at this age. Volume estimates were calculated and only 1 animal, a Control, presented with a volume that just exceeded the nominal cutoff value of 0.006 mm3 (see Table 2). (B) The relative expression of 9 genes previously shown to be altered in the heterotopia forming region of the PTU PN6 pup brain was not altered in the brains of PFHxS (n=6) or Triclosan (n=7) relative to Control (n=8) pups. Mean fold-change in relative gene expression is summarized in Supplementary Figure 3B.
Trace Fear Conditioning.
No evidence of impaired learning was seen in adult male offspring of PFHxS or Triclosan treated dams. All groups responded similarly to administration of foot shock by suppressing their activity indicating no differences in fear acquisition (Figure 7A) supported by results of ANOVA where no effects of Treatment [F(2,20.1)=0.45, P>0.52] or Treatment X State interaction [F(2,33.3)=0.04, p>0.96] were observed in contrasts of percent change from baseline counts 1 min after administration of the shock 1 and shock 2. On return to the same test chamber the following day, all groups showed a decrease in the activity relative to the baseline counts during training phase, indicating conditioning to context [Time F(1, 28.5)=79.3, p<0.0001]. No differences in the magnitude of suppression as a function of treatment group was observed [Treatment X Time F(1,28.5)=0.79, p>0.46] when data are expressed as mean activity counts (Figure 7B) or as a percent of baseline activity in the context box [F(2,21.6)=1.11, p=.34, Figure 7C]. In a novel box, presentation of the tone/light compound conditioning stimulus suppressed ongoing activity during the trace period but no differences between groups was observed (Figure 7D) [F(2,20.1)=0.76, p=0.48].
Figure 7.
Trace fear conditioning was not impaired in adult male offspring. (A) Mean (+/− SE) activity counts during training before and after delivery of mild foot shock were not different between Control, PFHxS and Triclosan groups. (B) Conditioning to Context, evidenced by a decrease in activity relative to training baseline when the animal is introduced to the chamber 24 hours after training was similar across groups. These data on conditioning to context are expressed as a percent of training baseline in (C) in order to compare to conditioning to cue (D). Conditioning to Cue was assessed by activity counts during the trace interval relative to baseline when animals were placed in a novel chamber and the tone/light conditioning stimulus delivered. No treatment differences were observed. Sample sizes of 22, 12 and 12 for Control, PFHxS and Triclosan from 14, 7 and 6 litters, respectively. Litter was the unit of analysis.
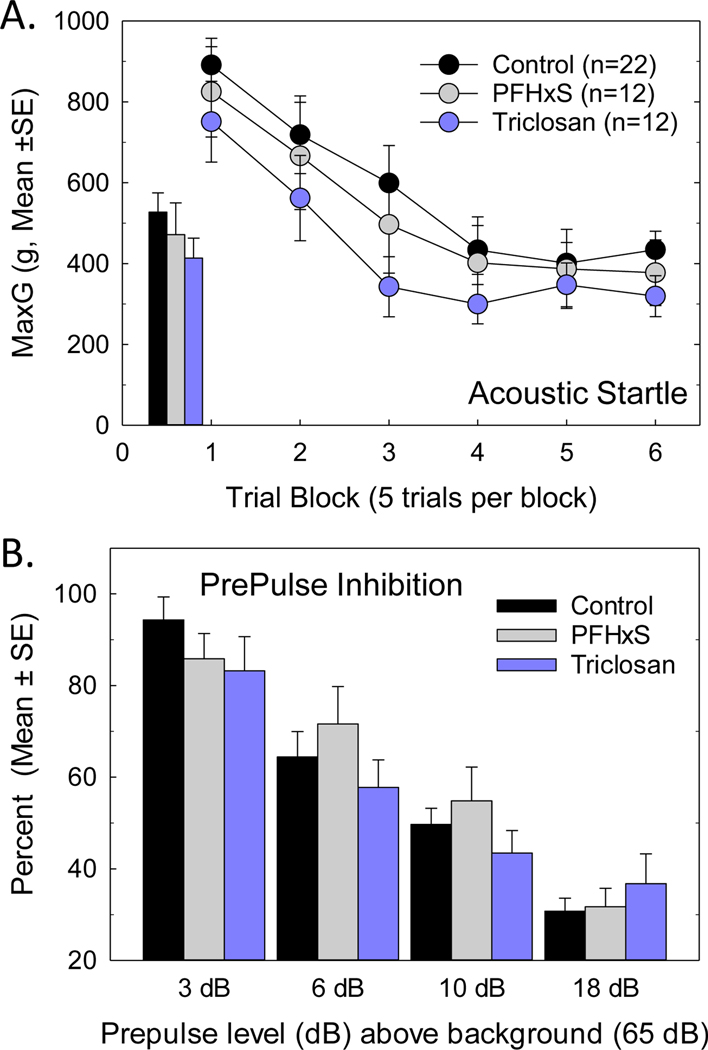
Acoustic Startle Response.
No effects on acoustic startle were observed. Baseline startle (Figure 8A inset) and its habituation (Figure 8A) were suggestive of lower startle responses in Triclosan exposed pups, but neither an effect of Treatment [F(2,27.4)=0.73, p=0.49] or Treatment by Trial Block [F(10,211)=0.87, p=0.57] were statistically significant. Neither was prepulse inhibition of the acoustic startle response affected by developmental treatment with PHFxS or Triclosan. The expected intensity-dependent reductions in startle amplitude were observed when low intensity acoustic stimuli precede delivery of the startle stimulus (Figure 8B, Prepulse dB F(3,121)=76.56, p<0.0001), but no Treatment X Prepulse dB interaction was seen [F(6,123)=1.54, p=0.18].
Figure 8.
Acoustic startle and Prepulse Inhibition were not altered in adult male offspring. (A) Mean (+/− SE) peak startle (MaxG) [inset] and startle habituation in PFHxS and Triclosan treated offspring did not differ from controls. (B) Preceding the startle eliciting stimulus with a lower intensity prepulse reduced the amplitude of the evoked startle, greater suppression was evident at higher prepulse intensities, but the degree of suppression in PFHxS- and Triclosan-treated offspring was similar to Controls at all prepulse intensities. Sample sizes of 22, 12 and 12 for Control, PFHxS and Triclosan from 14, 7 and 6 litters, respectively. Litter was the unit of analysis.
DISCUSSION
This study was conducted to determine if developmental neurotoxicity, based on metrics derived from studies using the model chemical PTU, would occur using environmental contaminants with presumed extrathyroidal sites of action. The effects of PFHxS and Triclosan on the developing brain were examined by measuring T4 and T3 concentrations in brains of neonates, differential gene expression presumably reflective of reduced TH action, and the presence of TH-dependent structural and behavioral phenotypes. Following a maternal exposure to PFHxS or Triclosan, reductions in pup serum T4 were associated with significant decreases in brain T4 on PN0 and PN2, which recovered by PN6, despite continued deficits in serum T4. Brain T3 was largely unaffected by either PFHxS or Triclosan. The lack of effect on brain T4 or T3 on PN6 is entirely consistent with the lack of gene expression changes previously associated with heterotopia formation at this age, and the absence of a heterotopia in the PN14 brain. Neither were brain hormone reductions or expression of TH-responsive genes altered in the cortex of PN14 pup, despite continued suppression of serum T4. Behavioral tests of learning and memory and sensory gaiting were unaltered in adult male offspring. As such, despite significant reductions in serum and brain T4 in the early neonatal period, the few effects that were observed for PFHxS and Triclosan were not consistent with what would be expected based on serum thyroid hormones.
Serum Hormone Profiles.
A more extensive characterization of the serum hormone profile than previously reported is provided for PFHxS and Triclosan in developing rats. Significant reductions in serum T4 were seen in the very early postnatal period and at PN14, consistent with previous reports at that age for Triclosan (Louis et al. 2017; Paul et al. 2010a; Zorrilla et al. 2009) and PFHxS (Ramhoj et al. 2018; Ramhoj et al. 2020). Reductions the newborn on PN0 and PN2 are consistent with declines of ~30% in serum T4 in the fetus on GD20 following maternal PFHxS (Ramhoj et al. 2020) and Triclosan (Gilbert, unpublished observations) exposure. Paralleling observations of total T4, serum free T4 was also reduced, and as in earlier reports, these serum hormone declines were not accompanied by increases in serum TSH in response to PFHxS (Ramhoj et al. 2018; Ramhoj et al. 2020) or to Triclosan (Louis et al. 2017; Paul et al. 2012; Paul et al. 2010a; Zorrilla et al. 2009). Although thyroid histopathology was not performed, other indicators of TSH-mediated thyroid gland activation (e.g., increases in thyroid gland weight, upregulation of thyroid gland gene transcripts) were not observed. This pattern of low T4 with little or no increase in TSH does not conform with the classic view of the hypothalamic-pituitary-thyroid (HPT) axis (Zoeller et al. 2007), the mechanism of which has yet to be elucidated. It is a commonly observed in response to exposure to environmental contaminants, notably with some but not all hepatic-enzyme inducers (Axelstad et al. 2011; Goldey et al. 1995a; Harry et al. 2014; Hood et al. 2003; Hood et al. 1999; Kodavanti et al. 2010; van Raaij et al. 1993; Visser et al. 1993). The neurodevelopmental consequences in rodent models of decreased T4 in the absence of a corresponding increases in TSH are presently unknown. The current data suggest they may well be distinct from those induced by PTU.
Hepatic catabolism was not assessed in the present study. Liver weights were not altered by PFHxS or Triclosan in dams or pups, but upregulation of hepatic metabolism genes was observed. These findings and are consistent with a previous report by Paul et al. (2012) where transcriptional readouts in the livers of Triclosan-treated rats were correlated with increased metabolic activity. Distinct from Triclosan, PFHxS competes with T4 for binding on the serum distributor protein, transthyretin (TTR) (Butenhoff et al. 2009; Chang et al. 2018; Weiss et al. 2009). This action may lower circulating levels of T4 by increasing tissue uptake or by increasing hormone elimination through hepatic mechanisms (Mariussen 2012). The general pattern of hepatic gene expression observed in PFHxS- and Triclosan-treated dams and pups was similar, characterized by a prominent upregulation of the Phase I metabolism gene, Cyb2b1 and the Phase II metabolizing enzyme transcript Sult1c3. The magnitude of change was greater in younger than in old neonates and was typically greater in response to PFHxS than to Triclosan. Little change was seen in the expression of Ugt1a1 or the hepatic nuclear receptors Car, Pxr and Pparα. Despite significant and persistent reductions in serum T4, differential expression of thyroid-responsive genes was not changed, findings paralleling the hepatic effects of developmental polybrominated flame retardants when compared to PTU (Bansal et al. 2014). These observations suggest that if hypothyroxinemia induced by PFHxS and Triclosan is partially reliant on upregulation of hepatic catabolism, differential expression of Cyp2b1 and Sult1c3 represent the most sensitive indicators of that action. As described above, interference at other target sites within the thyroid axis by these chemicals may also contribute to induced hypothyroxinemia (Paul Friedman et al. 2016; Wang et al. 2019; Weiss et al. 2009), although no transcriptional changes in Nis or Tpo were revealed in the thyroid gland at any age.
The developing brain appears unaffected by PFHxS and Triclosan at the Doses Administered:
Despite significant reductions in serum and brain tissue T4 in the early neonatal period, maternal exposure to PFHxS and Triclosan resulted in very few effects on the selected measures of thyroid-dependent developmental neurotoxicity. Brain tissue concentrations of T4 were reduced in PFHxS and Triclosan-treated pups in the late gestational/immediate postnatal period, but no change was seen in brain T3. Brain T4 recovered in PFHxS and Triclosan neonates by PN6 despite continued maternal exposure and maintained neonatal reductions in serum T4. Neither did PFHxS or Triclosan reduce the relative expression of cortical gene transcripts identified as bioindicators of thyroid hormone action in the neocortex. Although recovery of serum T4 was evident by PN14 in Triclosan-treated pups, a >50% decrement in serum T4 was maintained in PFHxS-exposed animals on PN14 yet no effect on cortical gene expression was observed. However, these findings in PN14 cortex are consistent with the lack of effect of brain tissue thyroid hormone concentrations with either treatment at the time of testing and support recent findings using a lower dose of PFHxS (Ramhoj et al. 2020). These findings stand in contrast to observations with a low dose of PTU (1ppm) where both T3 and T4 were reduced in serum and in brain of the fetus and the neonate and were accompanied by reductions in expression of a number of genes in the brain. In the PTU model, a 46% decrement in serum T4 on PN14 was associated with declines in brain T4 (73%) and T3 (12%) and significant reductions in the expression of TH-responsive genes (O’Shaughnessy et al. 2018b). A summary of serum and brain hormones for the 1ppm dose of PTU contrasted to the results of this study is available in Supplementary Table 1.
A heterotopia was not detected in the brains of PFHxS or Triclosan exposed pups, and this is consistent with the brain T3 and gene expression data presented herein. A periventricular heterotopia is a prominent and reproducible phenotype in rats exposed to PTU and MMI, and results from abnormal cell migration during postnatal development (Goodman and Gilbert 2007; O’Shaughnessy et al. 2019). It is a permanent structural defect, readily detected in the PN14 pup brain and persisting throughout life (Gilbert et al. 2014; O’Shaughnessy et al. 2018a; O’Shaughnessy et al. 2019). Recent work from our laboratory using a high dose but brief exposure to PTU (10 ppm for 5 days) indicates that this abnormality is dependent on perinatal TH insufficiency, and specifically, brain T3 reductions appear to be necessary from late gestation until PN2 for a heterotopia to form (O’Shaughnessy et al. 2019). Unfortunately, brain hormones have not been measured in the newborn and PN2 neonate in low dose PTU studies. Although a heterotopia was not detected in the brains of PFHxS or Triclosan exposed pups, it is important to consider that this defect represents a severe phenotype both clinically and in developmental biology studies, so more subtle alterations in brain morphology may still occur (Croquelois et al. 2009; Rosen et al. 2013).
Finally, neurobehavior assessed in adult male offspring also failed to reveal any differences from control animals. Decrements in serum T4 on PN14 induced by chemicals acting at extrathyroidal sites (i.e., polybrominated diphenyl ethers, polychlorinated biphenyls) are associated with elevated hearing thresholds and cochlear hair cell damage (Crofton (2004). Although hearing loss was not directly assessed in the current test procedure, no differences in acoustic startle response or in cortical sensory gaiting were revealed. Neither were deficits in hippocampal context conditioning or amygdala cue learning in the trace-fear conditioning induced by maternal exposure to PFHxS or Triclosan. Context learning is impaired in adult male offspring of PTU-treated dams at doses that produce serum hormone changes comparable in magnitude to those reported here (Gilbert 2011; Gilbert et al. 2016b). However, using a cross-fostering paradigm with maternal PTU exposure, we previously reported that pup serum (and presumably brain) hormone deficits must span both the pre- and post-natal periods (GD6-PN18) in order to result in impaired context learning (O’Shaughnessy et al. 2018a). PFHxS reduces fetal serum (Ramhoj et al. 2020) and brain hormones (Gilbert and Axelstad, unpublished observations) in late gestation and although suppression of serum T3 and T4 was maintained throughout the postnatal period in the present study, brain deficits were not, and no impairments in fear conditioning were observed. Similarly, Triclosan at the same dose used in this study reduced serum T4 in the late term fetus (Paul et al. 2012) (recently confirmed with a more sensitive LC/MS/MS assay, unpublished observations), and in the newborn and neonate as shown here. However, despite significant serum T4 reductions, the lack of effect on brain tissue thyroid hormones by either chemical beyond PN2 is consistent with intact context conditioning in the PTU model when exposure was primarily confined to the prenatal period (O’Shaughnessy et al. 2018a). These behavioral findings are limited by the fact that only a single dose of each chemical was examined, only male offspring were assessed, and the sample sizes were moderate. Although both tests are amenable to regulatory testing paradigms and are altered by developmental PTU exposure, neither is particularly sensitive to subtle alterations in sensory or cognitive processing.
Conclusions and Implications.
In this study, two chemicals with extrathyroidal sites of action were examined at concentrations that reduced serum T4 to a similar or greater degree than a low dose of PTU. Both treatments resulted in transient brain hormone deficits in the newborn, but unlike PTU, these were short-lived and were not accompanied by common measures of neurotoxicity previously reported for PTU by our laboratory and others (Axelstad et al. 2008; Bastian et al. 2012; Bastian et al. 2014; Gilbert et al. 2014; Goodman and Gilbert 2007; Hassan et al. 2017; O’Shaughnessy et al. 2018b). What can account for this conundrum? Clearly a chemical’s placental and lactational transfer in addition to life-stage-dependent hormone dosimetry are important determinants of neurodevelopmental outcome. For some of the biological endpoints examined here, brain T4 and T3 reductions established prenatally must be of sufficient magnitude and persist through the early perinatal period (i.e. heterotopia), while others require deficiencies be present in utero and be maintained up through the preweanling phase (i.e., behavioral deficits, gene expression). Limited exposure in the older neonate may partially contribute to the lack of effects of Triclosan (Axelstad et al. 2013; Paul et al. 2012), yet this seems less likely for PFHxS where reductions in serum T4 >50% remained at PN14.
Apart from kinetics, it is assumed that thyroid hormones synthesized and released from the gland are distributed to the organs in the body via the blood. How then can the same degree of serum hormone reduction brought about by different means lead to varying brain tissue concentrations and absence of a similar phenotypic signature? A number of possibilities can be proposed, serving only to underscore the incomplete state of our knowledge about the thyroid system. Is thyroid hormone in circulation the only source available to tissues? Is cellular uptake of hormone augmented under conditions of enhanced liver clearance but not hormone synthesis inhibition? Are tissues equipped with deconjugation processes that maintain T3 and T4 tissue levels despite increases in thyroid hormone metabolism and enhanced hepatic clearance? Does an interference with serum binding proteins still provide a sufficient pool of free hormone adequate to maintain tissue concentrations despite a drop in total serum T4? The present observations of minimal effects on brain hormones in the neonate and no change in readouts of thyroid hormone action in the brain or liver are consistent with such possibilities. The lack of augmented TSH response is the face of decreases in serum T4 may also reflect maintenance of hypothalamic or pituitary hormones by increased uptake or deconjugation mechanisms.
Another possibility is that treatments that negatively impact thyroid hormone synthesis (i.e., iodine deficiency, perchlorate, PTU and MMI even at low doses), place significantly higher demands on thyroid hormone tissue status than do actions of xenobiotics at extrathyroidal sites. The thyroid gland of the fetus/neonate, with a lower capacity for production and storage, fewer iodine stores, and facing higher physiological demands than the adult may be less able to counter xenobiotic actions that directly challenge hormone synthesis than xenobiotics acting on the maternal/fetal liver. As long as the central machinery of hormone production remains intact, thyroid hormones in serum may decline, but tissue concentrations are preserved. An alternative hypothesis is that a factor produced in the gland, independent of T4 or T3, plays a role in ameliorating the impact of low serum T4 following enhanced liver clearance. The thyronomines, a class of ‘novel thyroid hormones’ and ‘thyroid hormone metabolites’ may represent such a factor, possessing thyroid signaling properties and demonstrated physiological effects in heart, liver and in brain (Bellusci et al. 2017; Köhrle and Biebermann 2019; Zucchi et al. 2019). In such a scenario, compensatory mechanisms of thyroid hormone distribution, transport and deiodination may be sufficient when serum hormone declines stem from secondary peripheral perturbations but are inadequate when the central site of hormone production is compromised. However, evidence that thyronamine analytes are synthesized in the thyroid gland has not been conclusively demonstrated, and carboxylation and deiodination of T3 and T4 to form such metabolites can occur in the gut and liver (Köhrle and Biebermann 2019).
It is also important to consider that although brain hormone concentrations in the present study recovered within the early postnatal period, the declines evident in the fetus/newborn may be sufficient to alter neurodevelopment in a way that was not selectively probed by the tests we employed. Harry and colleagues (Harry et al. 2014) reported that hypothyroxinemia induced by the dioxin-like compound, 3–3’–4–4’ tetrachloroazobenzene, reduced cell number, altered astrocyte morphology, and decreased dendritic branching in principal neurons of hippocampus and cerebellum. Structural changes in brain were evident with serum T4 reductions in the dam (~25% on PN4) and pups (~60% on PN21) with no change in serum T3 or TSH. Conventional neuropathological examination and, as in our study, neurobehavioral assessments were negative. In congenital hypothyroidism, human genetic mutations, and various genetically altered mouse models (i.e., thyroid receptor, transporter, deiodinase modifications), distinct phenotypes arise as a function of the target site of disruption, many of which cannot be predicted from an evaluation of hormones in serum (Barez-Lopez et al. 2019; Galton et al. 2014; Mayerl et al. 2012). In a similar vein, is it not possible that neurological sequelae may also vary as a function of a chemical’s mode of action? Chemicals differing from each other in their primary target site within the ‘thyroid system’ and their kinetics may induce distinct timing and region-specific hormone deficits. These varying conditions are not likely to manifest as a uniform downstream neurotoxic outcome.
Finally, it is largely assumed that reductions in brain T3 concentrations following serum declines in T4 are responsible for the alterations in gene transcription that drive the neurodevelopmental consequences of thyroid hormone insufficiency. This may be true at the cellular level but even there, direct genomic and nongenomic actions of T4 and other thyroid analytes are well documented (Davis et al. 2016; Farwell et al. 2006). The present study is limited in that brain tissue hormones were assessed in large heterogeneous samples of brain tissue, constituting a very crude metric of brain hormone status. Numerous examples exist where reductions in brain tissue T3 in genetically modified animals are not accompanied by the expected gene transcription changes or phenotypic profile (Ferrara et al. 2013; Ferrara et al. 2015; Galton et al. 2014; Mayerl et al. 2014; Muller et al. 2014). Quantification of hormone concentrations in brain tissue can be technically challenging and estimates so derived are not likely to represent cellular hormone levels within specific neuronal populations. The coarseness of the estimate is particularly cogent when considered in the context of the mechanisms that have evolved to finely tune the control of transport, ligand presentation, receptor occupation, coregulators, and metabolic control at the cellular level that characterize thyroid hormone action in neurons. Nonetheless, we maintain that brain tissue concentrations of hormone still represent a more direct indicator of potential altered neurodevelopment than can be derived from serum hormones alone.
In conclusion, these findings raise some questions for the interpretation of serum biomarkers in regulatory toxicology and the pivotal position they occupy in Adverse Outcome Pathways developed for thyroid disruption (e.g., (Crofton et al. 2018; Rolaki et al. 2019), a framework embraced by regulatory agencies in the US and Europe. Although limited in scope, the data suggest that reliance on serum thyroid hormone measurements as prescriptive of specific neurodevelopmental outcomes may be too simplistic. They further reveal that our understanding of thyroid-mediated neurotoxicity based on high doses of thyroid hormone synthesis inhibitors is too narrow, highlighting significant gaps in our knowledge of how the thyroid system responds to chemical intervention (Gilbert et al. 2020; Kortenkamp et al. 2020; Li et al. 2019; O’Shaughnessy and Gilbert 2019; Ramhoj et al. 2020). Despite these limitations, serum hormones remain a critical tool for detection of potential thyroid disrupting chemicals. However, we propose that mapping the relationships between serum and brain tissue thyroid hormones in a temporal, quantitative, and preferably brain-region-specific manner may more accurately identify xenobiotics of neurotoxicological concern.
Supplementary Material
Acknowledgements-
The contributions of Angela Buckalew, Ashley Murr and Dr. Tammy Stoker for TSH and free T4 analysis is gratefully acknowledged. We also thank Drs. Louise Ramhoj and Marta Axelstad for input and inspiration to conduct this study, Drs. Christopher Lau and Marta Axelstad for review of an early version of this manuscript, and two anonymous reviewers who provided critical, provocative, and supportive feedback in the course of peer review.
Footnotes
This document has been subjected to review by the Center for Public Health and Environmental Assessment of the US Environmental Protection Agency and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Abdelouahab N, Langlois MF, Lavoie L, Corbin F, Pasquier JC, Takser L. 2013. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 178(5):701–713. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Boberg J, Hougaard KS, Christiansen S, Jacobsen PR, Mandrup KR, Nellemann C, Lund SP, Hass U. 2011. Effects of pre- and postnatal exposure to the uv-filter octyl methoxycinnamate (omc) on the reproductive, auditory and neurological development of rat offspring. Toxicol Appl Pharmacol. 250(3):278–290. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Boberg J, Vinggaard AM, Christiansen S, Hass U. 2013. Triclosan exposure reduces thyroxine levels in pregnant and lactating rat dams and in directly exposed offspring. Food Chem Toxicol. 59:534–540. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, U H. 2008. Developmental neurotoxicity of propylthiouracil (ptu) in rats: Relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol. 232(1):1–13. [DOI] [PubMed] [Google Scholar]
- Bansal R, Tighe D, Danai A, Rawn DF, Gaertner DW, Arnold DL, Gilbert ME, Zoeller RT. 2014. Polybrominated diphenyl ether (de-71) interferes with thyroid hormone action independent of effects on circulating levels of thyroid hormone in male rats. Endocrinology. 155(10):4104–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barez-Lopez S, Grijota-Martinez C, Auso E, Fernandez-de Frutos M, Montero-Pedrazuela A, Guadano-Ferraz A. 2019. Adult mice lacking mct8 and dio2 proteins present alterations in peripheral thyroid hormone levels and severe brain and motor skill impairments. PLoS One. 29(11):1669–1682. [DOI] [PubMed] [Google Scholar]
- Bastian TW, Anderson JA, Fretham SJ, Prohaska JR, Georgieff MK, Anderson GW. 2012. Fetal and neonatal iron deficiency reduces thyroid hormone-responsive gene mrna levels in the neonatal rat hippocampus and cerebral cortex. Endocrinology. 153(11):5668–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian TW, Prohaska JR, Georgieff MK, Anderson GW. 2014. Fetal and neonatal iron deficiency exacerbates mild thyroid hormone insufficiency effects on male thyroid hormone levels and brain thyroid hormone-responsive gene expression. Endocrinology. 155(3):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci L, Laurino A, Sabatini M, Sestito S, Lenzi P, Raimondi L, Rapposelli S, Biagioni F, Fornai F, Salvetti A et al. 2017. New insights into the potential roles of 3-iodothyronamine (t1am) and newly developed thyronamine-like taar1 agonists in neuroprotection. Front Pharmacol. 8(905). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal J. 2015. Thyroid hormones in brain development and function. . In: De Groot LJ, Beck-Peccoz GC P, Dungan K, Grossman A, Hershman JM, C. Koch R, McLachlan MN, Rebar R, Singer F, Vinik A, and Weickert MO,, editors. Endotext. South Dartmouth (MA). MDText.com, Inc. [Google Scholar]
- Boyes WK, Degn L, George BJ, Gilbert ME. 2018. Moderate perinatal thyroid hormone insufficiency alters visual system function in adult rats. Neurotoxicology. 67:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker-Davis F. 1998. Effects of environmental synthetic chemicals on thyroid function. Thyroid. 8(9):827–856. [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Chang SC, Ehresman DJ, York RG. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in sprague dawley rats. Reprod Toxicol. 27(3–4):331–341. [DOI] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB, Seed JG. 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in cd-1 mice. Reprod Toxicol. 78:150–168. [DOI] [PubMed] [Google Scholar]
- Chang SC, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork JA, Froehlich JW, Lau C, Singh RJ, Wallace KB, Butenhoff JL. 2008. Thyroid hormone status and pituitary function in adult rats given oral doses of perfluorooctanesulfonate (pfos). Toxicology. 243(3):330–339. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Eskenazi B, Holland N, Bradman A, Barr DB. 2008. Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Am J Epidemiol. 168(3):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. 2013. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the chamacos study. Environ Health Perspect. 121(1):138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ S, Biebel UW, Hoidis S, Friedrichsen S, Bauer K, Smolders JW. 2004. Hearing loss in athyroid pax8 knockout mice and effects of thyroxine substitution. Audiol Neurootol. 9(2):88–106. [DOI] [PubMed] [Google Scholar]
- Crofton KM. 2004. Developmental disruption of thyroid hormone: Correlations with hearing dysfunction in rats. Risk Anal. 24(6):1665–1671. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Gilbert ME, Paul-Friedman K, Demeneix B, Marty MS, Zoeller RT. 2018. Inhibition of thyroperoxidase and subsequent adverse neurodevelopmental outcomes in mammals. Aop no. 42. Aop wiki https://aopwiki.Org/aops/42 [Google Scholar]
- Croquelois A, Giuliani F, Savary C, Kielar M, Amiot C, Schenk F, Welker E. 2009. Characterization of the heco mutant mouse: A new model of subcortical band heterotopia associated with seizures and behavioral deficits. Cereb Cortex. 19(3):563–575. [DOI] [PubMed] [Google Scholar]
- Davis PJ, Goglia F, Leonard JL. 2016. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 12(2):111–121. [DOI] [PubMed] [Google Scholar]
- de Olmos JS. 1977. An improved hrp method for the study of central nervous connections. Exp Brain Res. 29(3–4):541–551. [DOI] [PubMed] [Google Scholar]
- Delange F. 1996. Endemic cretinism. In: Braverman LE, Utiger RD, editors. The thyroid: A fundamental and clinical text. Seventh ed. Philadelphia: Lippincott-Raven. p. 756–767. [Google Scholar]
- Farmahin R, Williams A, Kuo B, Chepelev NL, Thomas RS, Barton-Maclaren TS, Curran IH, Nong A, Wade MG, Yauk CL. 2017. Recommended approaches in the application of toxicogenomics to derive points of departure for chemical risk assessment. Arch Toxicol. 91(5):2045–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell AP, Dubord-Tomasetti SA, Pietrzykowski AZ, Leonard JL. 2006. Dynamic nongenomic actions of thyroid hormone in the developing rat brain. Endocrinology. 147(5):2567–2574. [DOI] [PubMed] [Google Scholar]
- Ferrara AM, Liao XH, Gil-Ibanez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. 2013. Changes in thyroid status during perinatal development of mct8-deficient male mice. Endocrinology. 154(7):2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara AM, Liao XH, Ye H, Weiss RE, Dumitrescu AM, Refetoff S. 2015. The thyroid hormone analog ditpa ameliorates metabolic parameters of male mice with mct8 deficiency. Endocrinology. 156(11):3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. 2008. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 76(1–2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DJ, Zeman FJ. 1979. Thyroid, pituitary, and hypothalamic hormone levels in neonatal rat pups following maternal protein deficiency. Proc Soc Exp Biol Med. 161(3):275–279. [DOI] [PubMed] [Google Scholar]
- Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. 2014. Life without the iodothyronine deiodinases. Endocrinology. 155(10):4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Hedge J, Valentin-Blasini L, Blount B, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, Fisher JW. 2013a. An animal model of marginal iodine deficiency during development: The thyroid axis and neurodevelopmental outcome. Toxicol Sci. 132(1):177–195. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Sanchez-Huerta K, Wood C. 2016a. Mild thyroid hormone insufficiency during development compromises activity-dependent neuroplasticity in the hippocampus of adult male rats. Endocrinology. 157(2):774–787. [DOI] [PubMed] [Google Scholar]
- Gilbert ME. 2011. Impact of low-level thyroid hormone disruption induced by propylthiouracil on brain development and function. Toxicol Sci. 124(2):432–445. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Goodman JH, Gomez J, Johnstone AFM, Ramos RL. 2017. Adult hippocampal neurogenesis is impaired by transient and moderate developmental thyroid hormone disruption. Neurotoxicology. 59:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Hedge JM, Valentin-Blasini L, Blount BC, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, Fisher JW. 2013b. An animal model of marginal iodine deficiency during development: The thyroid axis and neurodevelopmental outcome. Toxicol Sci. 132(1):177–195. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, O’Shaughnessy KL, Axelstad M. 2020. Regulation of thyroid-disrupting chemicals to protect the developing brain. Endocrinology. 161(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, Goodman JH. 2014. Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: Structural and functional characteristics. J Neuroendocrinol. 26(8):528–541. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sanchez-Huerta K, Wood C. 2016b. Mild thyroid hormone insufficiency during development compromises activity-dependent neuroplasticity in the hippocampus of adult male rats. Endocrinology. 157(2):774–787. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Crofton KM. 1998. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to aroclor 1254 in rats. Toxicol Sci. 45(1):94–105. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. 1995a. Developmental exposure to polychlorinated biphenyls (aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 135(1):77–88. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. 1995b. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol. 135(1):67–76. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Gilbert ME. 2007. Modest thyroid hormone insufficiency during development induces a cellular malformation in the corpus callosum: A model of cortical dysplasia. Endocrinology. 148(6):2593–2597. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Hooth MJ, Vallant M, Behl M, Travlos GS, Howard JL, Price CJ, McBride S, Mervis R, Mouton PR. 2014. Developmental neurotoxicity of 3,3’,4,4’-tetrachloroazobenzene with thyroxine deficit: Sensitivity of glia and dentate granule neurons in the absence of behavioral changes. Toxics. 2(3):496–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, Gilbert ME. 2017. Neurodevelopment and thyroid hormone synthesis inhibition in the rat: Quantitative understanding within the adverse outcome pathway framework. Toxicol Sci. 160(1):57–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood A, Allen ML, Liu Y, Liu J, Klaassen CD. 2003. Induction of t(4) udp-gt activity, serum thyroid stimulating hormone, and thyroid follicular cell proliferation in mice treated with microsomal enzyme inducers. Toxicol Appl Pharmacol. 188(1):6–13. [DOI] [PubMed] [Google Scholar]
- Hood A, Hashmi R, Klaassen CD. 1999. Effects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol Appl Pharmacol. 160(2):163–170. [DOI] [PubMed] [Google Scholar]
- Horton MK, Blount BC, Valentin-Blasini L, Wapner R, Whyatt R, Gennings C, Factor-Litvak P. 2015. Co-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ Res. 143(Pt A):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, Hood AM. 2001. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol. 29(1):34–40. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, Rayner JL, Kannan K, Birnbaum LS. 2010. Developmental exposure to a commercial pbde mixture, de-71: Neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 116(1):297–312. [DOI] [PubMed] [Google Scholar]
- Köhrle J, Biebermann H. 2019. 3-iodothyronamine—a thyroid hormone metabolite with distinct target profiles and mode of action. Endocr Rev. 40(2):602–630. [DOI] [PubMed] [Google Scholar]
- Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H et al. 2016. Association of maternal thyroid function during early pregnancy with offspring iq and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol. 4(1):35–43. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Axelstad M, Baig AH, Bergman Å, Bornehag CG, Cenijn P, Christiansen S, Demeneix B, Derakhshan A, Fini JB et al. 2020. Removing critical gaps in chemical test methods by developing new assays for the identification of thyroid hormone system-disrupting chemicals-the athena project. Int J Mol Sci. 21(9):3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Choi K. 2017. Perfluoroalkyl substances exposure and thyroid hormones in humans: Epidemiological observations and implications. Ann Pediatr Endocrinol Metab. 22(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans M, Couderq S, Demeneix B, Fini JB. 2019. Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front Endocrinol (Lausanne). 10:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Makris SL, Marty MS, Strauss V, Gilbert ME, Blacker A, Zorrilla LM, Coder PS, Hannas B, Lordi S et al. 2019. Practical considerations for developmental thyroid toxicity assessments: What’s working, what’s not, and how can we do better? Regul Toxicol Pharmacol. 106:111–136. [DOI] [PubMed] [Google Scholar]
- Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S, Fan C, Wang H, Zhang H. 2010. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid. 20(8):909–915. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE. 2017. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult wistar female rat. J Toxicol Environ Health A. 80(4):236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E. 2012. Neurotoxic effects of perfluoroalkylated compounds: Mechanisms of action and environmental relevance. Arch Toxicol. 86(9):1349–1367. [DOI] [PubMed] [Google Scholar]
- Martinez-Galan JR, Pedraza P, Santacana M, Escobar del Ray F, Morreale de Escobar G, Ruiz-Marcos A. 1997. Early effects of iodine deficiency on radial glial cells of the hippocampus of the rat fetus. A model of neurological cretinism. J Clin Invest. 99(11):2701–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S, Muller J, Bauer R, Richert S, Kassmann CM, Darras VM, Buder K, Boelen A, Visser TJ, Heuer H. 2014. Transporters mct8 and oatp1c1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 124(5):1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. 2012. Impact of oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 153(3):1528–1537. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2000. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 85(11):3975–3987. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2004. Role of thyroid hormone during early brain development. Eur J Endocrinol. 151 Suppl 3:U25–37. [DOI] [PubMed] [Google Scholar]
- Morse DC, Wehler EK, Wesseling W, Koeman JH, Brouwer A. 1996. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (aroclor 1254). Toxicol Appl Pharmacol. 136(2):269–279. [DOI] [PubMed] [Google Scholar]
- Muller J, Mayerl S, Visser TJ, Darras VM, Boelen A, Frappart L, Mariotta L, Verrey F, Heuer H. 2014. Tissue-specific alterations in thyroid hormone homeostasis in combined mct10 and mct8 deficiency. Endocrinology. 155(1):315–325. [DOI] [PubMed] [Google Scholar]
- Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MM, Furlow JD, Kavlock R, Kohrle J, Opitz R et al. 2013. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol In Vitro. 27(4):1320–1346. [DOI] [PubMed] [Google Scholar]
- Nouh WG, Selim AG, Sayed KF, Aly SM. 2013. Toxopathological studies on ethylene bisdithiocarbamates metabolite in albino rats. J Appl Environ Biol Sci. 3(5):1–16. [Google Scholar]
- Noyes PD, Friedman KP, Browne P, Haselman JT, Gilbert ME, Hornung MW, Barone S Jr., Crofton KM, Laws SC, Stoker TE et al. 2019. Evaluating chemicals for thyroid disruption: Opportunities and challenges with in vitro testing and adverse outcome pathway approaches. Environ Health Perspect. 127(9):95001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy KL, Gilbert ME. 2019. Thyroid disrupting chemicals and developmental neurotoxicity - new tools and approaches to evaluate hormone action. Mol Cell Endocrinol.110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy KL, Kosian PA, Ford JL, Oshiro WM, Degitz SJ, Gilbert ME. 2018a. Developmental thyroid hormone insufficiency induces a cortical brain malformation and learning impairments: A cross-fostering study. Toxicological sciences : an official journal of the Society of Toxicology. 163(1):101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy KL, Thomas SE, Spring SR, Ford JL, Ford RL, Gilbert ME. 2019. A transient window of hypothyroidism alters neural progenitor cells and results in abnormal brain development. Scientific reports. 9(1):4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy KL, Wood CR, Ford RL, Kosian PA, Hotchkiss MG, Degitz SJ, Gilbert ME. 2018b. Thyroid hormone disruption in the fetal and neonatal rat: Predictive hormone measures and bioindicators of hormone action in the developing cortex. Toxicol Sci. 166(1):163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. 2014. New scoping document on in vitro and ex vivo assays for the identification of modulators of thyroid hormone signaling. In: Oecd environment, health and safety publications series on testing and assessment. . [Google Scholar]
- Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO. 2016. Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the toxcast phase i and ii chemical libraries. Toxicol Sci. 151(1):160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, Crofton KM. 2012. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: A dynamic and kinetic evaluation of a putative mode-of-action. Toxicology. 300(1–2):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Devito MJ, Crofton KM. 2010a. Developmental triclosan exposure decreases maternal and neonatal thyroxine in rats. Environ Toxicol Chem. 29(12):2840–2844. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, DeVito MJ, Crofton KM. 2010b. Short-term exposure to triclosan decreases thyroxine in vivo via upregulation of hepatic catabolism in young long-evans rats. Toxicol Sci. 113(2):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1989. The rat brain in stereotaxic coordinates. 2nd edition New York: Academic Press. [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. 2003. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin Endocrinol (Oxf). 59(3):282–288. [DOI] [PubMed] [Google Scholar]
- Ramhoj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F, Axelstad M. 2018. Perfluorohexane sulfonate (pfhxs) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol Sci. 163(2):579–591. [DOI] [PubMed] [Google Scholar]
- Ramhoj L, Hass U, Gilbert ME, Wood C, Svingen T, Usai D, Vinggaard AM, Mandrup K, Axelstad M. 2020. Evaluating thyroid hormone disruption: Investigations of long-term neurodevelopmental effects in rats after perinatal exposure to perfluorohexane sulfonate (pfhxs). Sci Rep. 10(1):2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A, Patel AJ, Rabie A. 1986. Thyroid hormone and development of the rat hippocampus: Morphological alterations in granule and pyramidal cells. Neuroscience. 19(4):1217–1226. [DOI] [PubMed] [Google Scholar]
- Rami A, Rabie A. 1990. Delayed synaptogenesis in the dentate gyrus of the thyroid-deficient developing rat. Dev Neurosci. 12(6):398–405. [DOI] [PubMed] [Google Scholar]
- RIESCO G, TAUROG A, LARSEN PR, KRULICH L. 1977. Acute and chronic responses to iodine deficiency in rats1. Endocrinology. 100(2):303–313. [DOI] [PubMed] [Google Scholar]
- Rolaki A, Pistollato F, Munn S, Bal-Price A. 2019. Adverse outcome pathway on inhibition of na+/i symporter (nis) leads to learning and memory impairment https://aopwiki.org/aops/54.
- Rosen GD, Azoulay NG, Griffin EG, Newbury A, Koganti L, Fujisaki N, Takahashi E, Grant PE, Truong DT, Fitch RH et al. 2013. Bilateral subcortical heterotopia with partial callosal agenesis in a mouse mutant. Cereb Cortex. 23(4):859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosiak KL, Seo BW, Chu I, Francis BM. 1997. Effects of maternal exposure to chlorinated diphenyl ethers on thyroid hormone concentrations in maternal and juvenile rats. J Environ Sci Health B. 32(3):377–393. [DOI] [PubMed] [Google Scholar]
- Royland JE, Parker JS, Gilbert ME. 2008. A genomic analysis of subclinical hypothyroidism in hippocampus and neocortex of the developing rat brain. J Neuroendocrinol. 20(12):1319–1338. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time pcr data by the comparative c(t) method. Nat Protoc. 3(6):1101–1108. [DOI] [PubMed] [Google Scholar]
- Sharlin DS, Tighe D, Gilbert ME, Zoeller RT. 2008. The balance between oligodendrocyte and astrocyte production in major white matter tracts is linearly related to serum total thyroxine. Endocrinology. 149(5):2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki A, Saito F, Akane H, Takeyoshi M, Imatanaka N, Itahashi M, Yoshida T, Shibutani M. 2014. Expression alterations of genes on both neuronal and glial development in rats after developmental exposure to 6-propyl-2-thiouracil. Toxicol Lett. 228(3):225–234. [DOI] [PubMed] [Google Scholar]
- Spring SR, Bastian TW, Wang Y, Kosian P, Anderson GW, Gilbert ME. 2016. Thyroid hormone-dependent formation of a subcortical band heterotopia (sbh) in the neonatal brain is not exacerbated under conditions of low dietary iron (fed). Neurotoxicol Teratol. 56:41–46. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, Zorrilla LM. 2010. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicological sciences : an official journal of the Society of Toxicology. 117(1):45–53. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. 2016. Sensorimotor gating of the startle reflex: What we said 25 years ago, what has happened since then, and what comes next. J Psychopharmacol. 30(11):1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PN, Okosieme OE, Murphy R, Hales C, Chiusano E, Maina A, Joomun M, Bestwick JP, Smyth P, Paradice R et al. 2014. Maternal perchlorate levels in women with borderline thyroid function during pregnancy and the cognitive development of their offspring: Data from the controlled antenatal thyroid study. J Clin Endocrinol Metab. 99(11):4291–4298. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: Maternal and prenatal evaluations. Toxicological Sciences. 74(2):369–381. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Wesselkamper SC, Wang NC, Zhao QJ, Petersen DD, Lambert JC, Cote I, Yang L, Healy E, Black MB et al. 2013. Temporal concordance between apical and transcriptional points of departure for chemical risk assessment. Toxicol Sci. 134(1):180–194. [DOI] [PubMed] [Google Scholar]
- US EPA. 2014. Nonmonotonic dose response: State of the science. Appendix c: Mammalian studies describing the effects of chemicals that disrupt the thyroid signaling pathways.: https://www.epa.gov/sites/production/files/2016-01/documents/appendix_c_mammalian_thyroid_v_7.1.pdf; https://www.epa.gov/sites/production/files/2016-01/documents/nmdr.pdf. [Google Scholar]
- van Raaij JA, Kaptein E, Visser TJ, van den Berg KJ. 1993. Increased glucuronidation of thyroid hormone in hexachlorobenzene-treated rats. Biochem Pharmacol. 45(3):627–631. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, van Raaij JA, Joe CT, Ebner T, Burchell B. 1993. Multiple udp-glucuronyltransferases for the glucuronidation of thyroid hormone with preference for 3,3’,5’-triiodothyronine (reverse t3). FEBS Lett. 315(1):65–68. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Farrell S. 2004. Aic model selection using akaike weights. Psychon Bull Rev. 11(1):192–196. [DOI] [PubMed] [Google Scholar]
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Lougee RR, Richard AM, Laws SC, Stoker TE. 2019. High-throughput screening and chemotype-enrichment analysis of toxcast phase ii chemicals evaluated for human sodium-iodide symporter (nis) inhibition. Environ Int. 126:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang Y, Dong J, Wang Y, Min H, Song B, Shan Z, Teng W, Xi Q, Chen J. 2015. Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ Toxicol. 30(11):1264–1274. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PE, van Leeuwen SP, Hamers T. 2009. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol Sci. 109(2):206–216. [DOI] [PubMed] [Google Scholar]
- Wheeler SM, McLelland VC, Sheard E, McAndrews MP, Rovet JF. 2015. Hippocampal functioning and verbal associative memory in adolescents with congenital hypothyroidism. Front Endocrinol (Lausanne). 6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR. 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 20(6):784–794. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, McAndrews MP, Rovet JF. 2014. Accuracy of episodic autobiographical memory in children with early thyroid hormone deficiency using a staged event. Dev Cogn Neurosci. 9c:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SF, Hu AL, Xie L, Liu JJ, Wu Q, Liu J. 2019. Age-associated changes of cytochrome p450 and related phase-2 gene/proteins in livers of rats. PeerJ. 7:e7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Tukey RH. 2016. Triclosan: A widespread environmental toxicant with many biological effects. Annu Rev Pharmacol Toxicol. 56:251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yang M, Zeng L, Liu C. 2018. P38/trhr-dependent regulation of tpo in thyroid cells contributes to the hypothyroidism of triclosan-treated rats. Cell Physiol Biochem. 45(4):1303–1315. [DOI] [PubMed] [Google Scholar]
- Zoeller R, Rovet J. 2004. Timing of thyroid hormone action in the developing brain - clinical observations and experimental findings. J Neuroendocrinol. 16(10):809–818. [DOI] [PubMed] [Google Scholar]
- Zoeller R, Tan SW, Tyl RW. 2007. General background on the hypothalamic-pituitary-thyroid (hpt) axis. Crit Rev Toxicol. 37(1–2):11–53. [DOI] [PubMed] [Google Scholar]
- Zorrilla LM, Gibson EK, Jeffay SC, Crofton KM, Setzer WR, Cooper RL, Stoker TE. 2009. The effects of triclosan on puberty and thyroid hormones in male wistar rats. Toxicol Sci. 107(1):56–64. [DOI] [PubMed] [Google Scholar]
- Zucchi R, Rutigliano G, Saponaro F. 2019. Novel thyroid hormones. Endocrine. 66(1):95–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.