Abstract
Background.
Posttransplant mineral and bone diseases are causes of fractures, and their association with cardiovascular events is being studied.
Methods.
We analyzed the evolution of biochemical, histological, and imaging parameters pre– and 1 y post–renal transplantation in 69 patients and correlated mineral and bone findings with coronary calcifications. At inclusion and after 12 mo, clinical data and echocardiographic findings were recorded, and laboratory evaluations, radiography of the pelvis and hands, and bone biopsy were performed. Noncontrast cardiac computed tomography was performed during the second evaluation.
Results.
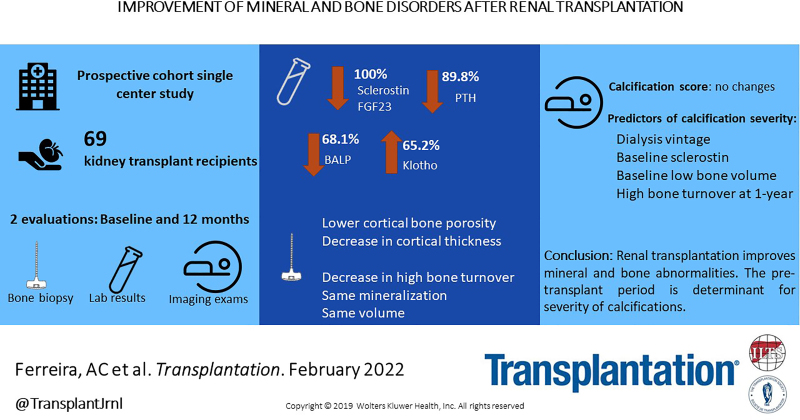
Serum levels of fibroblast growth factor 23 and sclerostin decreased in all patients, parathyroid hormone levels decreased in 89.8% of patients, bone alkaline phosphatase levels decreased in 68.1% of patients, and alpha-Klotho levels increased in 65.2% of patients. More than half of the patients presented with renal osteodystrophy at both biopsies, but histological findings improved: a significant transition from high to normal or low turnover and no significant differences in volume, mineralization defect, or cortical porosity at the 2 evaluations. Alpha-Klotho, sclerostin, and bone alkaline phosphatase shifts affect bone changes. Neither echocardiographic findings nor vascular calcification scores differed between the 2 points. Both the pretransplant period (dialysis vintage, sclerostin, and low bone volume at baseline) and the maintenance of abnormalities in the posttransplant period (high turnover posttransplant) were the most reliable predictors of the severity of the coronary calcification percentile.
Conclusions.
Renal transplantation improved bone and mineral abnormalities. The pretransplant period determines the severity of calcification.
INTRODUCTION
With improved long-term outcomes in kidney transplantation,1,2 cardiovascular (CV) disease and fractures have emerged as events that reduce both the quality of life and survival of renal transplant patients.3,4 Posttransplant mineral and bone disease is considered to be one of the major causes of these outcomes. The evolution of bone disease after transplantation is not well defined, and studies are contradictory,5-13 which may mirror the fact that many studies rely on only 1 bone biopsy. In this context, early biopsies in the posttransplant period can only reflect abnormalities in the pretransplant period.5 Three recent double bone biopsy studies enrolling 36,14 31,15 and 27 patients16 showed that the remodeling pathology observed after renal transplantation was predominantly low-turnover disease; 2 of those studies showed no major changes in bone volume,17 although in the Brazilian study by Marques et al,15 the presence of low bone volume changed from 15% to 26%, and in all 3 studies, the presence of mineralization defects increased after transplantation. It should be noted that in the study by Marques et al,15 trabecular microarchitecture worsened, characterized by decreases in trabecular number and spacing. Although posttransplant mineral and bone disease improvement has a positive influence on the calcification progression rate,4 to the best of our knowledge, no studies have explored the link between fibroblast growth factor 23 (FGF23) and sclerostin levels or metabolic bone disease and their role in vascular calcification in renal transplant recipients.
The aim of this study was to determine the prevalence, phenotype, and evolution of bone disease before and 1 y after renal transplantation and to correlate bone-associated biomarkers (FGF23, alpha-Klotho, sclerostin, parathyroid hormone [PTH], and bone alkaline phosphatase [BALP]) with bone histomorphometric parameters and CV disease.
MATERIALS AND METHODS
This was a prospective observational cohort study approved by the local ethics committees of the institutions and performed under the Strengthening the Reporting of Observational Studies in Epidemiology guidelines18 of patients aged 18 to 66 y admitted to de novo renal transplant from November 2015 to February 2018 (ClinicalTrials.gov; ID: NCT02751099). The exclusion criteria were admission for double (liver-kidney and pancreas-kidney) transplantation, age outside the determined range, and major cognitive impairment. Written consent was obtained from all the participants. The patients were monitored for 12 mo.
This study was designed to perform evaluations at 2 time points: before engraftment (T0) and at the end of the first year (T1). At inclusion, demographic and medical past history were collected, transplant and donor data registered, as well as the evaluation of the last echocardiography. At both time points, routine laboratory analysis was performed using standard methods, and serum and plasma samples were stored at –80 °C for further analysis of BALP, FGF23, alpha-Klotho, and sclerostin. Intact PTH level was measured by immunochemiluminescence using a second-generation assay (Immulite 2000; Siemens Medical Solutions Diagnostics, Los Angeles, CA). Vitamin D (25(OH)D) levels were measured using a radioimmunoassay provided by immunodiagnostic systems (Boldon, United Kingdom). BALP was measured using an enzyme immunoassay with a monoclonal anti-BALP antibody (MIcroVue BAP). FGF23 levels were measured using a second-generation ELISA kit that detects epitopes within the carboxyl-terminal (C-Term) portion of FGF23 (Immunotopics, San Clement, CA). Alpha-Klotho was determined using a human soluble α-klotho assay kit, consisting of a solid-phase sandwich ELISA using 2 highly specific antibodies (Immuno-Biological Laboratories America, MN). Sclerostin levels were measured using a high-sensitivity enzyme immunoassay kit, which is a 96-well immune-capture ELISA (TECOmedical).
Horizontal manual puncture transiliac bone biopsies were obtained from the anterior iliac crest using a 7G trocar (Osteobell T) under general anesthesia (first biopsy) or with local anesthesia (second biopsy). In the case of the second bone biopsy, tetracycline hydrochloride, 500 mg, 12/12 h, 3 d, was given 1 mo and 1 wk before the biopsy. Biopsy specimens (4.5 mm × 1.0 – 1.5 cm) were fixed in 70% alcohol and dehydrated in 96% and 99.9% alcohol. The fragments were cleared with xylene and embedded in methyl methacrylate. Undecalcified 5-μm sections were stained with modified Masson-Goldner trichrome, toluidine blue, von Kossa, acid phosphatase, alkaline phosphatase, Perls, and solochrome azurine for static histomorphometric evaluation. Unstained 10-μm sections were prepared for fluorescent dynamic analysis in the second biopsies. Each sample was composed of 2 cortices and a cylinder of trabecular bone. One observer evaluated all biopsies: for cortical bone, cortical thickness and porosity, and for trabecular bone, volume (normal if bone/tissue volume ≥16%), bone remodeling (normal if osteoblast surface/bone surface [BS] 0.2%–3.5% and osteoclast surface/BS 0.1%–7.25%, plus bone formation rate/BS 18–38 μm3/μm2/y19,20 in the biopsies with dynamic evaluation), and efficacy of mineralization (abnormal if osteoid thickness ≥12.5 μm plus no active osteoblasts in mineralization front or mineralization lag time >100 d in the biopsies with dynamic evaluation).21,22 Based on our findings (median values and interquartile range), we considered an abnormal cortical porosity >10%. This decision was made because of the missing reference values in the literature. Mixed lesions were identified if both high remodeling and abnormal mineralization were present. Renal osteodystrophy (ROD) is defined as abnormal turnover or mineralization. Whenever static and dynamic evaluations provided different information, we reviewed the bone biopsy. Bone histomorphometry was analyzed using a semiautomatic technique with Osteomeasure software (Osteometrics, Atlanta, GA).
Echocardiography performed in M mode and 2 dimensions, to access both valve calcifications and left ventricular mass index (LVMI), calculated using the Devereux formula, indexed to body surface area, was performed at the time of the second bone biopsy and was compared with the one performed pretransplant. Female patients were considered to have left ventricular hypertrophy if LVMI was >95 g/m2, whereas in male patients, LVMI was >115 g/m2. Radiography of the pelvis and hands was performed to classify vascular calcifications using the Adragão score23 at baseline and after 1 y. At the end of the study, patients who underwent a second bone biopsy underwent noncontrast cardiac computed tomography (CT) in a low-radiation exposure technique to quantify the coronary artery calcification score using the Agatston method,24 with the exception of 3 patients who undertook prior angioplasty. This examination was performed only once, 1 y after the transplant.
Immunosuppression
Patients received induction immunosuppression (basiliximab or thymoglobulin, depending on the immunologic risk) and intravenous 500 mg of methylprednisolone intraoperatively and daily for 2 d, followed by maintenance of 20 mg of oral prednisolone (tapered throughout the year), mycophenolate mofetil (2 g/d with dose adjustments and reduction throughout the year), and tacrolimus (adjusted for levels of 8–12 ng/mL for 3 mo and 5–8 ng/mL thereafter). In 5 patients, low doses of everolimus were added to low doses of tacrolimus to minimize calcineurin inhibitor toxicity. Globally, patients are treated with steroid-based immunosuppression.
Statistical Analysis
The outcome variables of interest were bone histomorphometric parameters (turnover, mineralization, and volume) and extraosseous calcifications (Agatston percentiles score). The predictor variables were laboratory measurements of PTH, BALP, alpha-Klotho, FGF23, and sclerostin. Continuous variables are presented as medians (with interquartile ranges), and categorical variables are expressed as frequencies.
Evolution from baseline (bone biopsies, biochemical parameters, vascular calcification scores, or echocardiographic findings) was assessed using the Wilcoxon matched-pair signed-rank test or the McNemar test. Associations between demographic or laboratory data and bone biopsy data were obtained using the Mann-Whitney test, Fisher exact test, or Spearman correlation test. The different degrees of severity of coronary calcifications were evaluated using ordered logistic regression, and multivariate ordered logistic regression was performed to detect possible risk factors for coronary artery calcification. In this analysis, the outcome variable was the 3 levels of severity of coronary calcification percentiles, and we included plausible predictor variables that had a P value of ≤0.1 in the univariate model. The final model evaluated the relationship between high bone turnover and severity of coronary calcifications, adjusted for potential confounders that could theoretically interact with both turnover and calcifications (previous time on dialysis, estimated glomerular filtration rate by epidemiology collaboration equation, sclerostin baseline values, BALP 1 y after transplant, and bone volume at baseline).
All tests were performed using STATA version 13 software package, and statistical significance was set at P < 0.05.
RESULTS
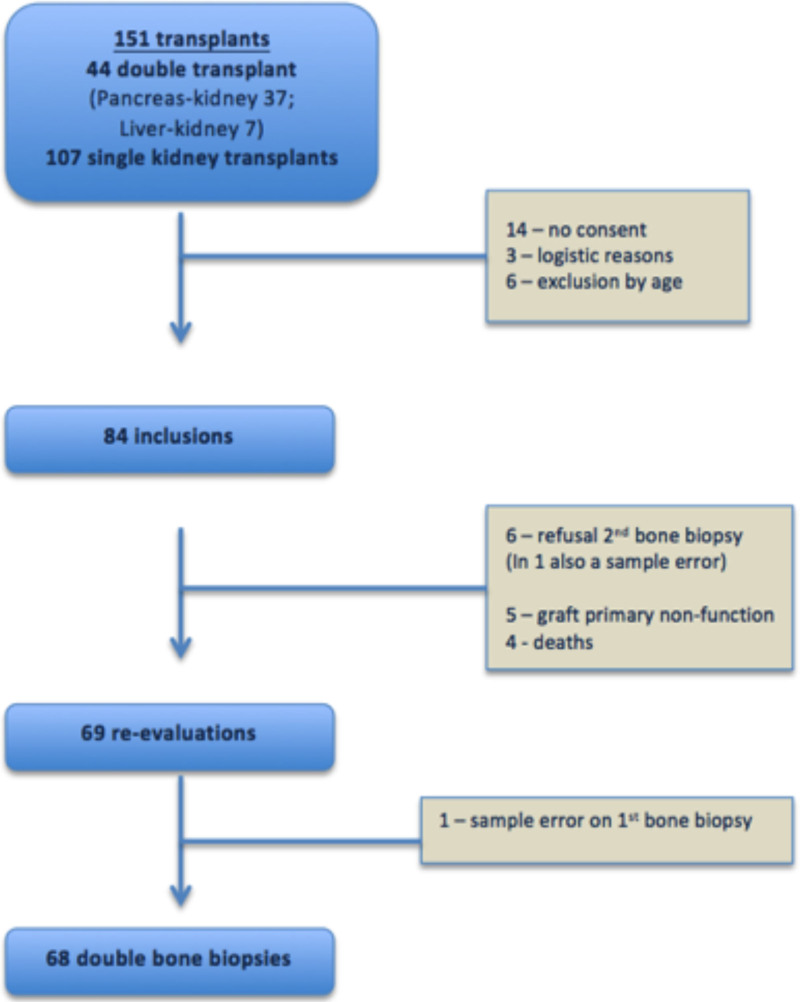
During the recruitment phase, 151 patients underwent renal transplantation at our center: 84 were recruited, and 69 underwent a second evaluation, as shown in Figure 1. These patients were middle-aged, mostly Caucasian, and male, with a median dialysis vintage of almost 5 y. Six patients underwent parathyroidectomy before transplantation, presenting lower levels of PTH both at baseline and 1 y after the transplant but no other differences regarding other bone-related hormones or minerals or histomorphometric bone parameters, specifically bone formation rate/BS. None of the patients were prescribed antiosteoporotic drugs during the posttransplant period. Of the 16 patients who were on cinacalcet, 7 retained the drug after transplantation (Supplementary Data, SDC, http://links.lww.com/TP/C378). The demographic data are presented in Table 1.
FIGURE 1.
Flowchart of the study.
TABLE 1.
Demographics and past history of the population
| Demographic characterization (N = 69) | |
|---|---|
| Age (y), median (IQR) | 53.0 (41.0–62.0) |
| Gender (M:F), n (%) | 48 (69.6):21 (30.4) |
| Caucasian race, n (%) | 53 (76.8) |
| BMI at transplant, kg/m2, median (IQR) | 24.5 (22.7–27.8) |
| PD (previous or current):HD, n (%) | 9 (13.0):65 (94.2) |
| Dialysis vintage (mo), median (IQR) | 55.0 (42.0–84.0) |
| Diabetes/PTD, n (%) | 9 (13.0)/35 (50.7) |
| Hyperparathyroidism at transplant, n (%)a | 50 (72.5) |
| Parathyroidectomy prior transplantation, n (%) | 6 (8.7) |
| Cause of renal disease, n (%) | |
| Unknown | 13 (18.8) |
| Hypertensive nephrosclerosis | 11 (15.9) |
| ADPKD | 11 (15.9) |
| Diabetic nephropathy (type 1 and 2) | 6 (8.9) |
| Alport disease | 2 (2.9) |
| Glomerulonephritis | |
| Chronic glomerulonephritis | 5 (7.2) |
| IgA nephropathy/mensangial proliferation | 6 (8.8)/1 (1.4) |
| HIVAN | 1 (1.4) |
| FSGS | 1 (1.4) |
| Membranous nephropathy | 2 (2.9) |
| Lupus nephritis | 1 (1.4) |
| Vasculitis | |
| Pauci-immune/Goodpasture | 2 (2.9)/1 (1.4) |
| Lithiasis | 3 (4.4) |
| CAKUT | 3 (4.4) |
| Living kidney donor, % | 10.1 |
| Preemptive transplantation, % | 0 |
| GFR by CKD-EPI 1 y after transplant | 53.0 (37.3–69.0) mL/min/1.73 m2 |
| Bone-related medication, n (%) | 61 (88.4) |
| Phosphate binders | 28 (40.6) |
| Cholecalciferol | 21 (30.4) |
| Vitamin D analogs/calcitriol | 44 (69.0) |
| Calcimimetics | 22 (31.9) |
| Cumulative steroid dose (mg) | 3580.0 (3257.5–4072.5) |
aWe assume a hyperparathyroidism diagnosis if patients were receiving vitamin D analogs or calcimimetics at the time of transplantation.
ADPKD, autosomal polycystic kidney disease; BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CKD-EPI, chronic kidney disease–epidemiology collaboration equation; FSGS, focal segmental glomerulosclerosis; GFR, glomerular filtration rate; HD, hemodialysis; HIVAN, HIV-associated nephropathy; IgA, immunoglobulin A; IQR, interquartile range; M:F, male:female; PD, peritoneal dialysis; PTD, posttransplant diabetes.
Metabolic Evaluation
Table 2 shows the differences between the pre- and 1-y follow-up after renal transplantation in 69 patients. In all patients, both sclerostin and FGF23 serum levels had decreased (sclerostin from 1.9 [1.3–2.7] ng/mL to 0.7 [0.5–1.0] ng/mL; FGF23 from 1806.5 [613.7–6281.6] pg/mL to 135.2 [101.1–168.6] pg/mL) with a median percentage reduction of 62.0% and 91.1%, respectively. PTH, BALP, and alpha-Klotho expressed some behavioral variability: 89.8% of patients had decreased PTH levels (from 475.0 [301.0–748.7] pg/mL to 135 [90.1–232.7] pg/mL), 68.1% had decreased BALP levels (from 33.8 [26.7–44.7] U/L to 23.0 [17.2–35.2] U/L), and 65.2% of patients had increased alpha-Klotho levels (from 571.0 [363.5–846.0] pg/mL to 945.2 [485.0–2044.2] pg/mL). Additional data regarding the metabolic evaluation are provided in the Supplementary Data (SDC, http://links.lww.com/TP/C378).
TABLE 2.
Laboratory evaluation at baseline and 1 y after transplantation
| Variable | Median (IQR) | P | |
|---|---|---|---|
| Baseline (N = 69) | 12 mo (N = 69) | ||
| Hemoglobin (g/dL) | 11.5 (10.9–12.6) | 12.9 (12.2–14.3) | <0.0001 |
| Glucose (mg/dL) | 88.0 (79.0–102.0) | 92.0 (81.0–103.0) | 0.248 |
| Urea (mg/dL) | 104.0 (66.0–138.0) | 60.0 (44.0–78.0) | <0.001 |
| Creatinine (mg/dL) | 8.2 (5.7–10.6) | 1.4 (1.1–1.8) | <0.001 |
| Uric acid (mg/dL) | 5.1 (3.5–7.0) | 6.4 (5.6–7.1) | <0.001 |
| Alkaline phosphatase (U/L) | 83.0 (61.0–103.0) | 78.0 (57.0–119.0) | 0.859 |
| Albumin (g/dL) | 4.2 (4.0–4.5) | 4.3 (4.1–4.5) | 0.509 |
| Calcium (mg/dL) | 9.3 (8.7–9.6) | 9.8 (9.3–10.4) | <0.0001 |
| Phosphorus (mg/dL) | 4.2 (3.3–5.1) | 3.1 (2.8–3.5) | <0.0001 |
| Magnesium (mg/dL) | 2.3 (2.1–2.5) | 1.7 (1.6–1.8) | <0.001 |
| Vitamin D (ng/mL) | 20.2 (15.0–30.4) | 22.5 (14.3–29.0) | 0.881 |
| iPTH (pg/mL) | 475.0 (301.0–748.7) | 135.0 (90.1–232.7) | <0.001 |
| BALP (U/L) | 33.8 (26.7–44.7) | 23.0 (17.2–35.2) | 0.001 |
| FGF23 (RU/mL) | 1806.5 (613.7–6281.6) | 135.2 (101.1–168.5) | <0.001 |
| Klotho (pg/mL) | 571.0 (363.5–846.0) | 945.2 (485.0–2044.2) | <0.001 |
| Sclerostin (ng/mL) | 1.9 (1.3–2.7) | 0.7 (0.5–1.0) | <0.001 |
Statistical analysis was performed with the Wilcoxon matched-pair signed-rank test. Bold values when P < 0.05.
Normal range for PTH: 14.8 to 83.1 pg/mL; for vitamin D: 4.8 to 52.8 ng/mL; for FGF23: ≤180 RU/mL; Klotho normal value: 845 ± 330 pg/mL.
BALP is dependent on sex and age; the normal range in premenopausal women is 11.6 to 29.6 U/L, in postmenopausal women is 14.2 to 42.7 U/L, and in men is 15 to 41.3 U/L.
Sclerostin is dependent on sex and age; the normal values in premenopausal women are 0.45 ± 0.15 ng/mL, in postmenopausal women are 0.51 ± 0.14 ng/mL, and in men are 0.59 ± 0.13 ng/mL.
BALP, bone alkaline phosphatase; FGF23, fibroblast growth factor 23; iPTH, intact parathyroid hormone; IQR, interquartile range.
Histologic Evaluation
In the second bone biopsy, it was possible to analyze the dynamic parameters of the trabecular bone, as shown in Table 3. Overall, the histological findings improved, as patients with high bone turnover, low bone volume, abnormal mineralization, or bone porosity >10% decreased.
TABLE 3.
Static and dynamic parameters of the bone biopsies
| Histomorphometric bone parameters | Baseline (N = 68) | 12 mo (N = 69) | P |
|---|---|---|---|
| Cortical bone | |||
| Porosity (%) | 7.4 (4.9–10.6) | 5.9 (3.8–9.8) | 0.094 |
| Thickness (μm) | 737.9 (552.7– 973.9) | 629.2 (403.5–849.2) | 0.006 |
| Porosity >10%, n (%) | 23 (33.3) | 18 (26.1) | 0.297 |
| Trabecular bone | |||
| Bone volume/tissue volume (%) | 18.8 (14.3–24.3) | 19.3 (15.8–24.8) | 0.339 |
| Osteoid surface/bone volume (%) | 3.2 (1.7–4.9) | 4.2 (2–5.8) | 0.660 |
| Osteoid thickness (μm) | 7.8 (6.7–10.3) | 9.1 (6.8–12.6) | 0.005 |
| Osteoid volume/bone volume (%) | 3.2 (1.7–4.9) | 4.2 (2.0–5.8) | 0.261 |
| Mineralized bone volume/tissue volume (%) | 18.3 (13.7–23.0) | 18.4 (14.7–23.6) | 0.389 |
| Osteoblast surface/bone surface (%) | 2.3 (0.7–5.5) | 1.9 (1.1–3.2) | 0.030 |
| Osteoclast surface/bone surface (%) | 1.3 (0.2–2.5) | 0.4 (0–0.9) | <0.001 |
| Adjusted mineral apposition rate (μm/d) | – | 0.3 (0.1–0.4) | – |
| Bone formation rate (μm3/μm2/d) | – | 21.4 (4.7–32.2) | – |
| Mineralization lag time (d) | – | 40.3 (25.5–85.0) | – |
| Low bone volume, n (%) | 22 (32.3) | 18 (26.5) | 0.513 |
| Normal bone turnover, n (%) | 33 (48.5) | 31(44.9) | 0.590 |
| Low-turnover bone disease, n (%) | 15 (22.1) | 31 (44.9) | 0.001 |
| Adynamic bone disease | 7 (10.3) | 10 (14.5) | 0.405 |
| Osteomalacia | 1 (1.5) | 3 (4.3) | 0.157 |
| High-turnover bone disease, n (%) | 20 (29.4) | 7 (10.1) | <0.001 |
| Mixed renal osteodystrophy | 2 (2.9) | 0 (0) | 0.157 |
Statistical analysis was performed using the Wilcoxon matched-pair signed-rank test or the McNemar test. Bold values when P < 0.05.
Cortical Bone
A nonsignificant trend toward lower cortical bone porosity was observed. The cortical thickness decreased significantly. The difference between cortical thicknesses at the 2 evaluations did not correlate with the cumulative steroid dose (P = 0.269).
Remodeling
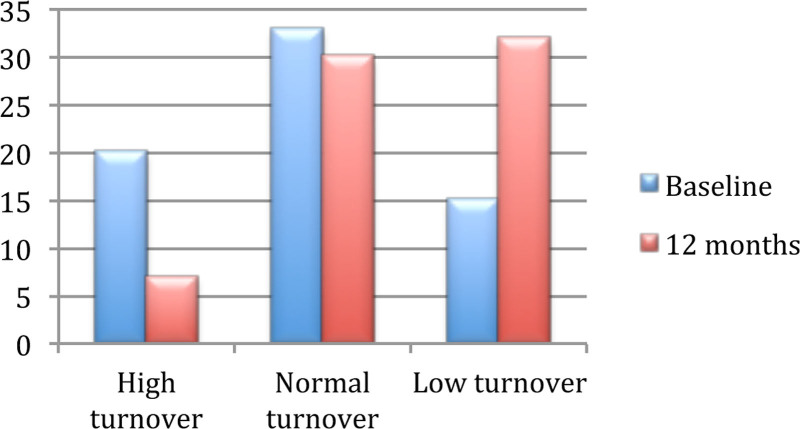
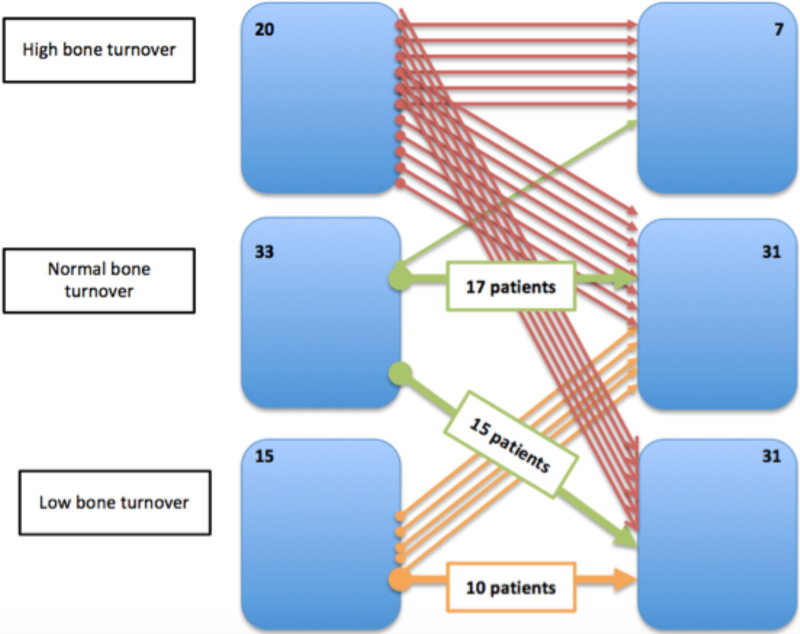
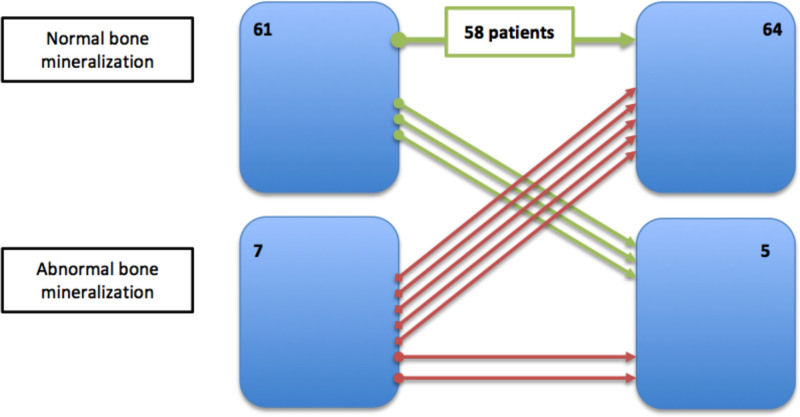
Less than half of the patients had normal remodeling parameters in both biopsies (n = 33 versus n = 31; P = 0.590). We observed a large decrease in the number of patients with high-remodeling disease (20 versus 7 patients; P < 0.001) and a significant increase in patients with low-remodeling disease (15 versus 31 patients; P = 0.002), as demonstrated in Figure 2. Nevertheless, 33 (48.5%) maintained their original turnover, 29 (42.1%) decreased their turnover, and 6 (15.8%) increased their turnover, as shown in Figure 3.
FIGURE 2.
Differences in bone remodeling before and after transplantation (according to the number of patients).
FIGURE 3.
Changes in bone remodeling after transplantation.
Notably, increased bone remodeling occurred mostly in patients with low turnover at baseline (P = 0.001). Patients who had an increase in turnover had lower PTH levels at baseline (179.4 versus 481.1 pg/mL; P = 0.032), had higher calcium levels at baseline (10.3 versus 9.3 mg/dL), had a significant difference in the percentage of change of vitamin D (–95.1% versus 7.5%; P = 0.016), and had lower cumulative prednisolone dose (3150.0 versus 3755.0 mg; P = 0.042); 50% of those patients were under everolimus (versus 3.2% of patients who did not have an increased in bone turnover and were under everolimus; P = 0.004). Patients who had experienced decreases in bone remodeling categories had higher alpha-Klotho levels after 1 y of transplantation (1266.2 [619.0–2335.2] pg/mL versus 687.5 [453.3–1310.2] pg/mL; P = 0.050), greater increases in alpha-Klotho than at baseline (delta-Klotho of 798.4 [155.8–1908.2] pg/mL versus 14.2 [–375.7–967.7] pg/mL; P = 0.036), lower levels of sclerostin 1 y after transplantation (0.5 [0.3–0.9] ng/mL versus 0.8 [0.6–1.0] ng/mL; P = 0.029), and a higher percentage reduction in sclerostin (68.9% versus 59.4%; P = 0.006). These patients did not present with different estimated glomerular filtration rates.
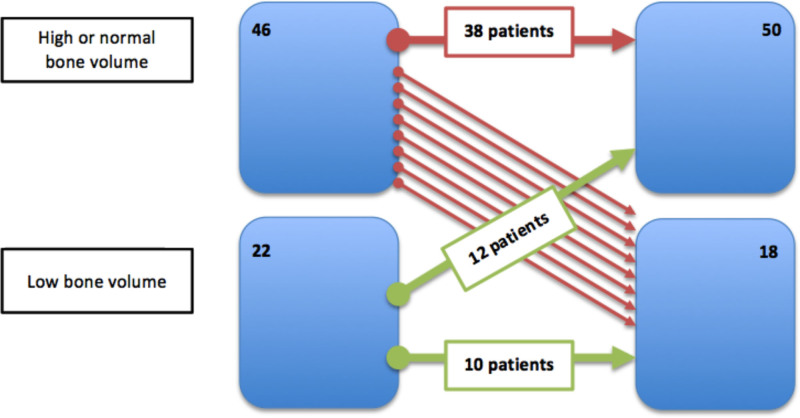
Volume
We found that 12 of 22 patients with low volume at baseline normalized the volume (54.5%), as shown in Figure 4. Only 8 of 46 (17.5%) patients with normal bone volume had decreased bone volume. We notice that patients whose bone volume decreased after transplant were the ones with the highest body mass index at baseline (28.1 [26.4–29.4] kg/m2 versus 24.2 [22.4–27.3] kg/m2; P = 0.007), the highest levels at sclerostin at baseline (2.5 [2.2–4] ng/mL versus 1.7 [1.2–2.7] ng/mL; P = 0.006), and a significant decrease in sclerostin values from baseline (–2.2 [–3.1 to –1.2] versus –1.1 [–1.6 to 0.7]; P = 0.004). No association with cumulative steroid dose was observed.
FIGURE 4.
Changes in volume after transplantation.
Mineralization
Very few patients had abnormal mineralization: there were 7 in the first bone biopsy (1 osteomalacia, 2 mixed ROD, 3 with normal volume and remodeling, and 1 with low volume and normal remodeling), and this number decreased to 5 in the second bone biopsy (3 osteomalacia, 2 with normal volume and remodeling), without statistical significance (P = 0.479). There were no differences in the mixed ROD (P = 0.157) or osteomalacia (P = 0.157) between the 2 points. Of the 7 patients classified as having abnormal mineralization, 5 (71.4%) had normalized mineralization, as shown in Figure 5; however, 3 additional patients (4.9%) moved from the normal group, leaving a total of 5 patients with this condition. The only factor associated with the reduced mineralization was the delta value of BALP: median was 7.3 (4.4–23.5) U/L higher at 12 mo versus –12.4 (–21.0 to 1.4) U/L in normal mineralization cases.
FIGURE 5.
Changes in mineralization after transplantation.
Imaging Exams Evaluation
The major echocardiographic findings did not differ from those at the baseline. Similarly, the vascular calcification score did not change, as shown in Table 4.
TABLE 4.
Differences in imaging results comparing baseline to 1 y of follow-up
| Imaging examinations | Baseline (N = 69) | 12 mo (N = 69) | P |
|---|---|---|---|
| Echocardiographic findings | |||
| Left ventricular mass index (g/m2) | 107.0 (91.5–140.5) | 108.5 (98.0–138.0) | 0.091 |
| Interventricular septal thickness (mm) | 11.0 (10.0–12.0) | 11.0 (9.0–12.0) | 0.492 |
| Left ventricular hypertrophy, n (%) | 29 (42) | 26 (39.4) | 1.000 |
| Valve calcifications, n (%) | 15 (21.7) | 16 (23.5) | 0.781 |
| Left ventricular fractional shortening (%) | 40.0 (35.0–43.0) | 43.0 (37.0–47.0) | 0.015 |
| Vascular calcification score (Adragão score) | |||
| Hands score | 0 (0–2) | 1 (0–2) | 0.589 |
| Pelvis score | 0 (0–1) | 0 (0–1) | 0.873 |
| Total score | 1 (0–2) | 1 (0–2) | 0.196 |
| Low score (0 and 1)/high score (≥2) | 63.8%/36.2% | 56.5%/43.5% | 0.165 |
Statistical analysis was performed using the Wilcoxon matched-pair signed-rank or McNemar test. Bold values when P < 0.05.
A CT scan was performed to quantify coronary artery calcification using the Agatston score. The percentiles of severity were homogeneous in the population: one third of the patients had mild coronary artery calcification (n = 22; 33.3%), one third had moderate coronary artery calcification (n = 24; 36.4%), and less than a third of the population had severe coronary artery calcification (n = 20; 30.3%).
Comparing the 3 levels of severity of the percentiles of coronary calcifications in univariate analysis, we observed that a longer time on dialysis; high sclerostin at baseline; high serum levels of calcium, BALP, and PTH 1 y after transplant; higher osteoid volume/bone volume; and higher cortical porosity 1 y after transplant were associated with calcification severity, as shown in Table 5. In the multivariate analysis, dialysis vintage (P = 0.001), baseline sclerostin levels (P = 0.006), baseline low bone volume (P = 0.016), and high bone turnover at 1 y after transplant (P = 0.040) were the main predictors of coronary calcification percentiles, as shown in Table 6.
TABLE 5.
Predictors for the percentile of Agatston coronary artery calcium score
| Agatston percentile ≤50% (n = 22) | Agatston percentile 51%–90% (n = 24) | Agatston percentile >90% (n = 20) | P | |
|---|---|---|---|---|
| Agatston percentile | 0 (0–0) | 84 (74.0–87.5) | 97.5 (94.0–99.0) | – |
| Age (y) | 42.5 (33.0–50.0) | 59.5 (49.5–63.0) | 56.5 (41.5–60.5) | – |
| Male gender (%) | 63.6 | 66.7 | 78.3 | – |
| Caucasian race (%) | 63.6 | 79.2 | 85.0 | – |
| Valve calcifications | 9.1% | 20.8% | 36.8% | 0.030 |
| Vascular calcifications | 0 (0–1) | 2 (1–2.5) | 1 (0.5–2) | 0.021 |
| PD/only HD | 27.3%/86.4% | 4.2%/95.8% | 10.0%/100% | 0.053 |
| Dialysis vintage (mo) | 51.5 (24.0–64.0) | 48.5 (43.0–70.5) | 79.5 (55.5–100.0) | 0.003 |
| T0 PTH (pg/mL) | 468.2 (308.0–671.3) | 529.9 (290.1–774.4) | 470.8 (273.0–956.2) | 0.249 |
| T0 calcium (mg/mL) | 9.0 (8.7–9.5) | 9.3 (8.6–9.6) | 9.5 (9.0–10.1) | 0.056 |
| T0 phosphorus (mg/dL) | 3.9 (3.2–4.9) | 4.1 (3.3–5.0) | 4.7 (3.5–5.2) | 0.189 |
| T0 magnesium (mg/dL) | 2.2 (2.1–2.4) | 2.1 (2.0–2.4) | 2.3 (2.1–2.7) | 0.199 |
| T0 BALP (U/L) | 32.1 (23.9–43.0) | 34.3 (26.7–43.6) | 36.4 (28.4–49.5) | 0.399 |
| T0 sclerostin (ng/mL) | 1.7 (1.2–2.2) | 2.1 (1.3–2.9) | 2.2 (1.7–2.9) | 0.030 |
| T0 FGF23 (RU/mL) | 1402.7 (463.9–6220.9) | 1575.4 (599.4–3673.6) | 4749.6 (778.6–8699.8) | 0.151 |
| T1 PTH (pg/mL) | 122.3 (84.9–179.3) | 128.0 (88.3–181.0) | 150.8 (113.4–256.4) | 0.023 |
| T1 calcium (mg/mL) | 9.6 (9.2–9.9) | 9.6 (9.3–10) | 10.3 (9.6–10.8) | 0.044 |
| T1 phosphorus (mg/dL) | 3.1 (2.9–3.5) | 3.1 (2.9–3.4) | 2.8 (2.3–3.9) | 0.681 |
| T1 magnesium (mg/dL) | 1.7 (1.6–1.8) | 1.6 (1.4–1.8) | 1.7 (1.6–1.9) | 0.460 |
| T1 BALP (U/L) | 18.2 (13.3–30.8) | 26.4 (19.7–36.4) | 27.6 (18.9–57.3) | 0.008 |
| T1 sclerostin (ng/mL) | 0.6 (0.4–0.8) | 0.7 (0.5–0.9) | 0.9 (0.5–1.2) | 0.156 |
| T1 FGF23 (RU/mL) | 119.3 (88.5–143.2) | 123.6 (96–164.1) | 145.4 (119.9–196.1) | 0.592 |
| T0 BV/TV (%) | 20.2 (14.1–26.0) | 20.2 (16.3–25.1) | 17.6 (13.3–21.5) | 0.138 |
| T1 porosity (%) | 4.4 (3.5–7.5) | 5.3 (3.9–10.6) | 7.3 (5.4–10.9) | 0.017 |
| T1 BV/TV (%) | 20.0 (15.0–24.8) | 19.0 (17.1–23.4) | 19.4 (14.3–26.3) | 0.797 |
| T1 OtV/BV | 4 (1.9–5.2) | 3.9 (1.5–4.9) | 5.3 (3.0–9.1) | 0.021 |
| T1 high turnover | 4.5% | 4.2% | 20.0% | 0.069 |
Statistical analysis performed with ordered logistic regression. Bold values when P < 0.05.
BALP, bone alkaline phosphatase; BV/TV, bone volume/tissue volume; FGF23, fibroblast growth factor 23; HD, hemodialysis; OtV/BV, osteoid volume/bone volume; PD, peritoneal dialysis; PTH, parathyroid hormone; T0, at inclusion; T1, after 12 mo.
TABLE 6.
Ordered logistic regression for independent associations with Agatston percentiles
| Agatston percentiles | |||
|---|---|---|---|
| OR | 95% CI | P | |
| Dialysis vintage (mo) | 1.26a | 1.09-1.45 | 0.001 |
| T0 sclerostin (ng/mL) | 2.61 | 1.38-4.92 | 0.006 |
| T1 BALP (U/L) | 1.38 | 1.04-1.90 | 0.050 |
| GFR (mL/min/1.73 m2) | 0.95a | 0.74-1.21 | 0.699 |
| T0 BV/TV (%) | 0.90 | 0.83-0.97 | 0.016 |
| T1 high bone turnover | 10.4 | 1.18-92.6 | 0.040 |
aFor each 10-unit increase.
BALP, bone alkaline phosphatase; BV/TV, bone volume/tissue volume; CI, confidence interval; GFR, glomerular filtration rate; OR, odds ratio; T0, at inclusion; T1, after 12 mo.
DISCUSSION
Laboratory evaluation 1 y after transplant was as expected, considering the significant improvement in renal function: calcium and alpha-Klotho increased significantly, and phosphorus, magnesium, PTH, BALP, FGF23, and sclerostin decreased significantly. Despite these results, ROD was present in 68.1% of our patients 1 y after transplantation (adding remodeling and mineralization abnormalities); however, overall, we believe that the histological results improved. One important finding was that bone volume did not change pretransplant and posttransplant, and mineralization defects were not different between the 2 time points. Considering bone remodeling, a significant increase in low-turnover disease was observed (15–31 patients). In contrast, high bone turnover decreased, and hyperparathyroid bone disease was present in only 7 patients after transplantation. Neither echocardiographic findings nor vascular calcification scores differed between the 2 points. Nonetheless, 12 mo is a short period to observe major differences in the main echocardiographic findings. Dialysis vintage, sclerostin at baseline, low bone volume, and high bone turnover at 1 y after transplantation were the most reliable predictors of the percentile severity of coronary calcifications in this population.
We should recognize that this is a small, unicentric, and observational study; therefore, associations do not indicate a cause–effect relationship. Likewise, only the second bone biopsies had dynamic evaluations; the first biopsies could not benefit from tetracycline labeling, as patients were recruited on the day they were admitted to the hospital, meaning that turnover could be misclassified in the first bone biopsies.20 Nevertheless, we did not find many discrepancies between quantitative and qualitative assessments of bone turnover in the second biopsies. We performed bone biopsies with a 7G trocar (4.5-mm inner diameter), but these were comparable with full 7.5-mm samples.25 As we stored at –80 °C blood samples for nonroutine analysis (BALP, FGF23, alpha-Klotho, and sclerostin), alpha-Klotho values can be inexact, as lower results can be obtained in stored versus fresh serum samples.26,27 As this was a population listed for kidney transplantation, meaning that these are the healthiest patients among patients with end-stage renal disease (ESRD), these results cannot be generalized to all ESRD patients. Finally, we classified 72.5% of the patients with secondary hyperparathyroidism based on the fact that those were receiving either vitamin D analogs, calcimimetics, or both at the time of transplantation. Despite this fact, the median value of PTH at baseline was 475.0 (301.0–748.7) pg/mL, which is consistent with most of the studies.
Overall, trabecular bone dynamics were similar to those published in recent literature,14-16 although different reference ranges for turnover were applied (Salusky, composite parameters, and Malluche, respectively), as we still lack agreement in the turnover diagnostic cutoffs. Although a similar approach (double bone biopsy) was used in the aforementioned studies, we included an extraosseous calcification analysis, which has not been studied by other authors. In addition, the Belgian and Finnish studies included only Caucasian patients, a high number of males, and a high number of diabetic patients. Although our population characteristics were closer to those of the Brazilian cohort, they only addressed living-donor recipients and excluded those with low PTH levels and low bone turnover diagnosed by a bone biopsy before entry into the study.
We observed a significant reduction in the number of patients with high-turnover bone disease and a significant increase in the number of patients with low-turnover bone disease, with stable numbers of normal turnover. Six of our patients experienced an increase in bone remodeling, but this occurred mostly in patients with low turnover at baseline, which normalized their turnover. These patients presented with lower cumulative steroid doses, highlighting the importance of glucocorticoids in the activity of bone cells (inhibiting osteoblast function and promoting apoptosis).28 Additionally, based on exploratory analysis, we observed that patients receiving everolimus also had increased bone turnover, which is in line with the belief that mammalian target of rapamycin inhibitors are sparing bone immunosuppressive agent.5
The reduction in bone turnover was associated with a greater increase in the levels of alpha-Klotho and higher levels of alpha-Klotho at 1 y after transplantation but not with PTH or BALP levels, as expected. In fact, PTH did not have an influence on trabecular bone dynamics. Although its median levels were above the normal level (135 pg/mL), its optimal range is unknown in renal transplanted patients.5 In addition, changes in BALP were not associated with turnover deviations, but we noted that the development of abnormal mineralization posttransplantation occurred in patients with a greater increase in BALP levels than at baseline. A recent study showed that alkaline phosphatase levels could predict mineralization defects in a pediatric population.29 In our population, BALP correlated well with nonmineralized bone (Supplementary Data, SDC, http://links.lww.com/TP/C378), and as this marker is produced by osteoblasts during bone formation,30 inactivating pyrophosphate (which inhibits mineralization31) and osteopontin (which is a calcification inhibitor32), we can suspect some resistance to BALP actions at the bone after transplantation. In fact, after transplantation, osteoblast cells can become dysfunctional, have lower alkaline phosphatase expression,33 and increase their own apoptosis, which contributes to bone disorders in these patients.9 Although low bone turnover increased significantly, only 3 patients presented with osteomalacia, and only 10 patients presented with low turnover and low volume (adynamic bone disease). Even so, it is important to note that the presence of adynamic bone disease (defined as low turnover and low volume) did not increase with the transplant, which is a good result, as we recently published an association between adynamic bone disease and valve calcifications at baseline.34 More important than having a low bone turnover after transplantation is having or maintaining a high bone turnover after the transplant, as this is associated with the severity of coronary calcifications. The introduction of vitamin D analogs (if calcium levels allow) or calcimimetics to halt bone turnover seems to be protective.35
Contrary to what is reported in the literature, we observed no loss of bone volume, even with a slight increase, as the number of patients with normal or high bone volume increased from 44 to 50 patients. It should be noted that we found no relationship between the cumulative steroid dose and bone volume or loss of bone volume. The decrease in bone volume after the transplantation was associated with the highest levels of sclerostin at baseline, which could be explained by the fact that sclerostin is an inhibitor of bone formation36-38; however, these results should be interpreted with caution, as decreased bone volume occurred in only 8 patients. Low bone volume and high sclerostin levels at baseline were correlated with calcification severity 1 y after the transplant, so it is of utmost importance to identify these patients to implement CV protective measures to safeguard them from an early CV event. We believe that it could be beneficial to perform a multicenter study to analyze the importance of performing bone densitometry some months after transplantation. For instance, it could rule out volume abnormalities in patients with a normal examination39 and recognize patients who could benefit from more invasive studies (such as bone biopsies), especially those with higher calcification scores,40 before starting antiresorptive or osteoformer therapies. These therapies could also be effective in those who do not benefit from the normalization of bone volume after transplantation.
Our transplant patients did not show progression in vascular calcification scores obtained by radiography of the hands and pelvis and did not show progression in valve calcifications. The fact that transplantation can slow the progression of calcifications has been suggested by other studies.4 Because we did not have a control population, we cannot conclude that renal transplantation halts the evolution of calcifications. The Agatston percentiles correlated well with the vascular calcification score (and the presence of valve calcifications). We would expect to see more associations between bone-related hormones at baseline and Agatston percentile severity, as we suppose that coronary calcifications did not change significantly during a 12-mo period. FGF23 levels did not differ according to an increase in calcification percentile severity. Although increased expression of FGF23 in human calcified tissue has been shown,41 Scialla et al42 described that FGF23 does not have a role in inducing arterial calcification. The opposite was found with sclerostin, a soluble Wnt pathway antagonist and a negative regulator of bone metabolism.37 Wnt signaling is involved in vascular calcification, and increased sclerostin expression has been demonstrated during vascular smooth muscle cell calcification in an animal model.38,43 Different studies have reached different conclusions regarding patients with chronic kidney disease. Some studies have demonstrated that high sclerostin levels are associated with better survival in hemodialysis patients,44,45 suggesting a protective role through inhibition of vascular calcifications,44,46 whereas other studies have found an association between high sclerostin levels and CV mortality in dialysis patients,47 justified by the propensity for vascular calcifications via low-turnover bone disease,37 leading investigators to speculate that a U-shaped dose effect could be the cause of these findings.38 A recent study performed in patients with ESRD showed that sclerostin is associated with the degree of vascular calcifications.48 Nevertheless, the role of sclerostin in CV health is very important to clarify, as a sclerostin antibody is being evaluated for osteoporosis treatment in postmenopausal women.49 Patients with higher cortical porosity at 1 y after transplant also presented more severe percentiles of coronary calcification in univariate analysis but not in multivariate analysis, where only trabecular features were possible determinants of its severity (low bone volume and high bone turnover). This highlights the importance of trabecular bone in extraosseous calcifications in comparison with cortical bone, which is less metabolically active and probably has a more robust role in fracture prevention, which is relevant in these patients.50
CONCLUSIONS
Renal transplantation improves bone and mineral abnormalities. Not only the transplantation environment (ie, the presence of high bone turnover) but also the period on dialysis determines the severity of calcifications: dialysis vintage, sclerostin serum values, and bone volume at baseline were found to be predictors of severe calcifications. Preventing CV events through the timely identification of patients who would benefit from antiosteoporotic/bone remodeling control drugs should be considered in a prospective study.
ACKNOWLEDGMENTS
The authors thank CHULC for their assistance in this study. They thank the Portuguese Society of Nephrology and Portuguese Society of Transplantation/Astellas for awarding them a research grant. They would like to thank Sandra Silva for help with administrative work and Dr Isabel Mesquita for help with sample storage and handling. They also thank the surgeons Ana Pena and Sofia Carrelha and all operating room staff for their assistance with the bone biopsies performed in the operating room. They thank the Radiology Department of Hospital Santa Marta from CHULC for performing CT scans. They thank the patients for their consent to participate in the study.
Supplementary Material
Footnotes
This study was supported by a grant from the Portuguese Society of Nephrology and the Portuguese Society of Transplantation/Astellas.
The authors declare no conflicts of interest.
The results presented in this article have not been published previously in whole or part, except in the abstract format.
The data supporting the findings of this study are available upon reasonable request from the corresponding author. The data were not publicly available because of privacy or ethical restrictions.
A.C.F. and A.F. conceptualized and designed the study. A.C.F., M.M., C.S., P.C., I.A., D.N., F.C., R.R., R.S., B.C., and G.C. collected data and performed bone biopsies or laboratory evaluations. A.C.F., M.M., R.S., B.C., G.C., F.N., and A.F. analyzed the data. A.C.F. drafted the article. M.M., C.S., P.C., I.A., D.N., F.C., R.R., R.S., B.C., G.C., F.N., and A.F. revised the article. All authors approved the final version of the article and agreed with all aspects of the work.
Supplemental Visual Abstract; http://links.lww.com/TP/C379.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Malluche HH, Monier-Faugere MC, Herberth J. Bone disease after renal transplantation. Nat Rev Nephrol. 2010;6:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wesseling-Perry K, Bacchetta J. CKD-MBD after kidney transplantation. Pediatr Nephrol. 2011;26:2143–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeherunvong W, Wolf M. Tertiary excess of fibroblast growth factor 23 and hypophosphatemia following kidney transplantation. Pediatr Transplant. 2011;15:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianciolo G, Capelli I, Angelini ML, et al. Importance of vascular calcification in kidney transplant recipients. Am J Nephrol. 2014;39:418–426. [DOI] [PubMed] [Google Scholar]
- 5.Evenepoel P. Recovery versus persistence of disordered mineral metabolism in kidney transplant recipients. Semin Nephrol. 2013;33:191–203. [DOI] [PubMed] [Google Scholar]
- 6.Martins L, Queirós J, Ferreira A, et al. Renal osteodystrophy: histologic evaluation after renal transplantation. Transplant Proc. 2000;32:2599–2601. [DOI] [PubMed] [Google Scholar]
- 7.Carlini RG, Rojas E, Weisinger JR, et al. Bone disease in patients with long-term renal transplantation and normal renal function. Am J Kidney Dis. 2000;36:160–166. [DOI] [PubMed] [Google Scholar]
- 8.Montalban C, de Francisco AL, Marinoso ML, et al. Bone disease in long-term adult kidney transplant patients with normal renal function. Kidney Int Suppl. 2003;85:S129–S132. [DOI] [PubMed] [Google Scholar]
- 9.Rojas E, Carlini RG, Clesca P, et al. The pathogenesis of osteodystrophy after renal transplantation as detected by early alterations in bone remodeling. Kidney Int. 2003;63:1915–1923. [DOI] [PubMed] [Google Scholar]
- 10.Cruz EA, Lugon JR, Jorgetti V, et al. Histologic evolution of bone disease 6 months after successful kidney transplantation. Am J Kidney Dis. 2004;44:747–756. [PubMed] [Google Scholar]
- 11.Abdallah KA, Jorgetti V, Pereira RC, et al. Improvement of adynamic bone disease after renal transplantation. Braz J Med Biol Res. 2006;39:31–41. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann G, Ott U, Stein G, et al. Renal osteodystrophy after successful renal transplantation: a histomorphometric analysis in 57 patients. Transplant Proc. 2007;39:3153–3158. [DOI] [PubMed] [Google Scholar]
- 13.Borchhardt K, Sulzbacher I, Benesch T, et al. Low-turnover bone disease in hypercalcemic hyperparathyroidism after kidney transplantation. Am J Transplant. 2007;7:2515–2521. [DOI] [PubMed] [Google Scholar]
- 14.Evenepoel P, Behets GJ, Viaene L, et al. Bone histomorphometry in de novo renal transplant recipients indicates a further decline in bone resorption 1 year posttransplantation. Kidney Int. 2017;91:469–476. [DOI] [PubMed] [Google Scholar]
- 15.Marques IDB, Araújo MJCLN, Graciolli FG, et al. A randomized trial of zoledronic acid to prevent bone loss in the first year after kidney transplantation. J Am Soc Nephrol. 2019;30:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keronen S, Martola L, Finne P, et al. Changes in bone histomorphometry after kidney transplantation. Clin J Am Soc Nephrol. 2019;14:894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshayeb HM, Josephson MA, Sprague SM. CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis. 2013;61:310–325. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 19.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salam S, Gallagher O, Hughes D, et al. The role of static bone histomorphometry in diagnosing renal osteodystrophy. Bone. 2021;142:115689. [DOI] [PubMed] [Google Scholar]
- 21.Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhan A, Qiu S, Rao SD. Bone histomorphometry in the evaluation of osteomalacia. Bone Rep. 2018;8:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adragao T, Pires A, Lucas C, et al. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1480–1488. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 25.Novel-Catin E, Pelletier S, Fouque D, et al. Quantitative histomorphometric analysis of halved iliac crest bone biopsies yield comparable ROD diagnosis as full 7.5 mm wide samples. Bone. 2020;138:115460. [DOI] [PubMed] [Google Scholar]
- 26.Barker SL, Pastor J, Carranza D, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neyra JA, Moe OW, Pastor J, et al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J. 2020;13:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canalis E, Mazziotti G, Giustina A, et al. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. [DOI] [PubMed] [Google Scholar]
- 29.Soeiro EMD, Castro L, Menezes R, et al. Association of parathormone and alkaline phosphatase with bone turnover and mineralization in children with CKD on dialysis: effect of age, gender, and race. Pediatr Nephrol. 2020;35:1297–1305. [DOI] [PubMed] [Google Scholar]
- 30.Bover J, Ureña-Torres P, Cozzolino M, et al. The non-invasive diagnosis of bone disorders in CKD. Calcif Tissue Int. 2021;108:512–527. [DOI] [PubMed] [Google Scholar]
- 31.Vervloet MG, Brandenburg VM; CKD-MBD working group of ERA-EDTA. Circulating markers of bone turnover. J Nephrol. 2017;30:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nizet A, Cavalier E, Stenvinkel P, et al. Bone alkaline phosphatase: an important biomarker in chronic kidney disease—mineral and bone disorder. Clin Chim Acta. 2020;501:198–206. [DOI] [PubMed] [Google Scholar]
- 33.Neves CL, dos Reis LM, Batista DG, et al. Persistence of bone and mineral disorders 2 years after successful kidney transplantation. Transplantation. 2013;96:290–296. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira AC, Cotovio P, Aires I, et al. The role of bone volume, FGF23 and sclerostin in calcifications and mortality; a cohort study in CKD stage 5 patients. Calcif Tissue Int. 2022;110:215–224. [DOI] [PubMed] [Google Scholar]
- 35.Torregrosa JV, Ferreira AC, Cucchiari D, et al. Bone mineral disease after kidney transplantation. Calcif Tissue Int. 2021;108:551–560. [DOI] [PubMed] [Google Scholar]
- 36.Vervloet MG, Massy ZA, Brandenburg VM, et al. ; CKD-MBD Working Group of ERA-EDTA. Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol. 2014;2:427–436. [DOI] [PubMed] [Google Scholar]
- 37.Moysés RM, Schiavi SC. Sclerostin, osteocytes, and chronic kidney disease—mineral bone disorder. Semin Dial. 2015;28:578–586. [DOI] [PubMed] [Google Scholar]
- 38.Jean G, Chazot C. Sclerostin in CKD-MBD: one more paradoxical bone protein? Nephrol Dial Transplant. 2013;28:2932–2935. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira AC, Mendes M, Silva C, et al. Bone densitometry versus bone histomorphometry in renal transplanted patients: a cross-sectional study. Transpl Int. 2021;34:1065–1073. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira AC, Cohen-Solal M, D’Haese PC, et al. ; European Renal Osteodystrophy (EUROD), an initiative of the CKD-MBD working group of the ERA-EDTA. The role of bone biopsy in the management of CKD-MBD. Calcif Tissue Int. 2021;108:528–538. [DOI] [PubMed] [Google Scholar]
- 41.Donate-Correa J, Martín-Núñez E, Hernández-Carballo C, et al. Fibroblast growth factor 23 expression in human calcified vascular tissues. Aging (Albany NY). 2019;11:7899–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scialla JJ, Lau WL, Reilly MP, et al. ; Chronic Renal Insufficiency Cohort Study Investigators. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu D, Mackenzie NC, Millán JL, et al. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6:e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drechsler C, Evenepoel P, Vervloet MG, et al. ; NECOSAD Study Group. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2015;30:288–293. [DOI] [PubMed] [Google Scholar]
- 45.Viaene L, Behets GJ, Claes K, et al. Sclerostin: another bone-related protein related to all-cause mortality in haemodialysis? Nephrol Dial Transplant. 2013;28:3024–3030. [DOI] [PubMed] [Google Scholar]
- 46.Claes KJ, Viaene L, Heye S, et al. Sclerostin: another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013;98:3221–3228. [DOI] [PubMed] [Google Scholar]
- 47.Gonçalves FL, Elias RM, dos Reis LM, et al. Serum sclerostin is an independent predictor of mortality in hemodialysis patients. BMC Nephrol. 2014;15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qureshi AR, Olauson H, Witasp A, et al. Increased circulating sclerostin levels in end-stage renal disease predict biopsy-verified vascular medial calcification and coronary artery calcification. Kidney Int. 2015;88:1356–1364. [DOI] [PubMed] [Google Scholar]
- 49.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. [DOI] [PubMed] [Google Scholar]
- 50.Evenepoel P, Claes K, Meijers B, et al. Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int. 2019;95:1461–1470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.