Abstract
In solid organ transplant recipients (SOTRs), 2-dose mRNA vaccination offers suboptimal protection, with similar infection rates and modestly decreased mortality when compared with unvaccinated SOTRs.1,2 Encouragingly, a third vaccine dose improves neutralization against variants of concern for some SOTRs, yet others do not mount an antibody response even following a fourth dose.3-6 It is unclear whether continued immune priming can augment vaccine immunogenicity in this population, and the benefit of a fifth dose (D5) remains unstudied. We describe 18 SOTRs who received a D5 vaccine between July 26, 2021, and December 11, 2021, and were followed through January 14, 2022.
SOTRs were enrolled in an observational study as described.5,7 None reported prior coronavirus disease 2019 (COVID-19) infection. Serial semiquantitative antispike serological testing was performed on the Roche Elecsys anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) S enzyme immunoassay (positive ≥0.8 U/mL, ceiling 2500). This study was approved by the Johns Hopkins Institutional Review Board, and participants provided informed consent.
Eighteen SOTRs received D5, with a median (interquartile range) of 114 (66–155) d after fourth dose. Fifteen of 18 received heterologous vaccine combinations. Ten (56%) had 4 preceding mRNA vaccinations, and 8 (44%) had 3 preceding mRNA vaccinations plus 1 Ad.26.COV2.S dose. D5 included 14 mRNA-1273, 3 BNT162b2, and 1 Ad.26.COV2.S doses. The median age was 64 (53–68) y. The median time since transplant was 6.4 (2.8–16.4) y. There were 8 (44%) kidney, 4 (22%) liver, 3 (17%) lung, 2 (11%) heart, and 1 (6%) liver-kidney recipients. Twelve (67%) reported mycophenolate mofetil (MMF) use, and 17 (94%) reported calcineurin inhibitor use. The median MMF daily dosage was 1000 (1000–1500) mg.
Pre-D5, 2 of 18 (11%) SOTRs were seronegative. The median anti-receptor-binding domain value among those who tested positive on the Roche enzyme immunoassay pre-D5 (16/18) was 456 U/mL, with 10 of 16 (63%) ≥250 U/mL and 5 of 16 (31%) ≥1000 U/mL. Of 12 SOTRs on MMF, 4 of 12 (33%) modified their MMF dose surrounding D5: 2 discontinued MMF (1 temporarily) and 2 reduced their doses (25% and 50%, respectively) before receiving D5.
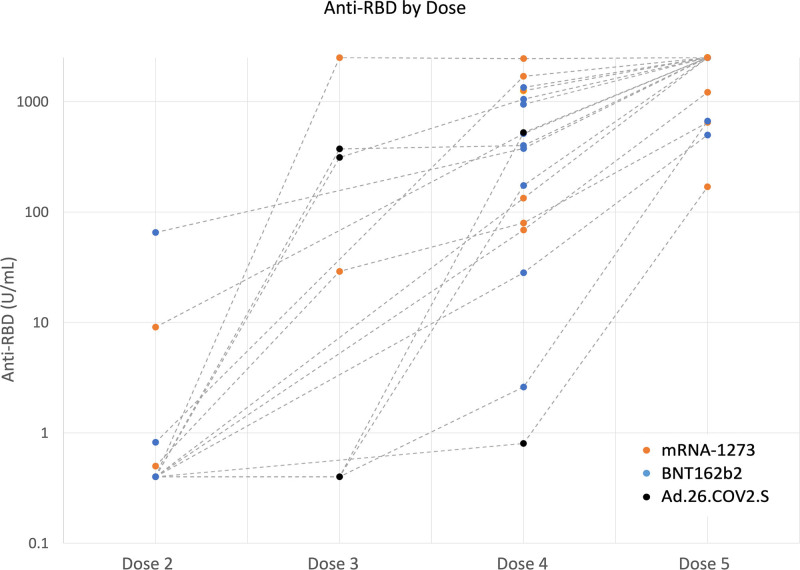
Antibody testing was performed 27 (21–34) d post-D5; 17 of 17 (100%) who tested on the same platform had higher antibody titers, whereas 1 tested seronegative on 2 different platforms before and after D5. The median antireceptor-binding domain value among those who tested positive increased to 2500 U/mL, with 16 of 17 (94%) ≥250 U/mL, 13 of 17 (76%) ≥1000 U/mL, and 12 of 17 (71%) ≥2500 U/mL (Figure 1). The most common symptoms reported were pain at the site of vaccination and fatigue. There were no self-reported episodes of rejection or COVID-19 infection.
FIGURE 1.
Antibody responses after SARS-CoV-2 vaccination among solid organ transplant recipients. RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
In summary, SOTRs with weak vaccine-induced antibody responses continue to have the potential to mount an improved response to additional vaccinations, including to D5. Many participants demonstrated boosting after D5 to levels observed in the general population after a 2-dose mRNA series.8 Reassuringly, D5 side effects were minimal and consistent with previous findings, with no reported rejection7; however, despite 5 vaccines (2 BNT162b2, 2 mRNA-1723, and 1 Ad.26.CoV2.S), 1 SOTR remained seronegative; this participant was also taking the highest MMF dose (2500 mg/d) among all the participants (did not reduce dosage surrounding D5). This emphasizes the need for antibody testing even after booster dosing and consideration of alternative strategies such as passive immunoprophylaxis or immunosuppressive modulation in persistent nonresponders. Limitations include the small sample size, lack of anti-N testing to augment incident COVID-19 ascertainment, and the absence of assays for neutralizing antibody, B-cell memory, and T-cell responses. In conclusion, SOTRs with suboptimal antibody responses to 4 SARS-CoV-2 doses may still benefit from a fifth dose.
ACKNOWLEDGMENTS
The authors thank the participants of the Johns Hopkins COVID-19 Transplant Vaccine Study, without whom this research could not be possible. They also thank the members of the study team, including Brian J. Boyarsky, MD, PhD; Alexa Jefferis, BS; Nicole Fortune Hernandez, BS; Letitia Thomas; Rivka Abedon; Chunyi Xia; Kim Hall; Mary Sears; Alex Alex; and Jonathan Susilo. They also thank Andrew H. Karaba, MD, PhD, and Ms Yolanda Eby for project support and guidance.
Supplementary Material
Footnotes
A.T.A. and M.S.T. contributed equally.
This work was supported by the Ben-Dov family, the Trokhan Patterson family, grant 5T32DK007713 (J.L.A.), the American Society of Transplant Surgeons Fryer Resident Scientist Award (J.M.), K01DK101677 (A.B.M.), and K23DK115908 (J.M.G.-W.) from the National Institute of Diabetes and Digestive and Kidney Diseases; grant K24AI144954 (D.L.S.) from the National Institute of Allergy and Infectious Diseases; and grants U01AI138897 and K23AI157893 (W.A.W.).
D.L.S. received consulting and speaking honoraria from Sanofi, Novartis, CLS Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific, Regeneron, and AstraZeneca. M.L.L. is the Social Media Editor for Transplantation. R.K.A. has grant/research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, and Takeda/Shire. The other authors declare no conflicts of interest.
A.T.A., M.S.T., J.L.A., J.D.K., J.M., T.P.Y.C., R.K.A., A.A.R.T., M.L.L., D.S.W., A.B.M., J.M.G.-W., D.L.S., and W.A.W. participated in conception or design of the work; acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content. A.T.A., M.S.T., J.L.A., D.L.S., and W.A.W. participated in final approval of the version to be published. A.T.A., M.S.T., J.L.A., J.D.K., J.M., T.P.Y.C., R.K.A., A.A.R.T., M.L.L., D.S.W., A.B.M., J.M.G.-W., D.L.S., and W.A.W. agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Visual Abstract; http://links.lww.com/TP/C373.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. [Epub ahead of print. January 4, 2022]. doi:10.1097/TP.0000000000004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman JR, Wigmore SJ. Simple vaccination is not enough for the transplant recipient. Transplantation. [Epub ahead of print. January 4, 2022]. doi:10.1097/TP.0000000000004064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaba AH, Zhu X, Liang T, et al. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;00:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, et al. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients. JAMA Intern Med. 2021;182:e217372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alejo JL, Mitchell J, Chiang TP-Y, et al. Antibody response to a fourth dose of a SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamar N, Abravanel F, Marion O, et al. Assessment of 4 doses of SARS-CoV-2 messenger RNA-based vaccine in recipients of a solid organ transplant. JAMA Netw Open. 2021;4:e2136030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174:1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.