Abstract
Observations from early clinical pharmacology studies of amprenavir, an inhibitor of human immunodeficiency virus type 1 (HIV-1) protease that is highly bound to human plasma proteins (∼90%), showed the single-dose pharmacokinetics of amprenavir to be variable between and within individuals. A cross-study analysis of various demographic, laboratory, and clinical covariates was therefore performed. Differences in amprenavir pharmacokinetics could be due to variable concentrations in α1-acid glycoprotein (AAG), the predominant plasma protein to which amprenavir binds. Therefore, AAG was considered an important factor to study since the literature suggested that AAG levels vary by race, age, and weight and following trauma or infection, including HIV disease. Pooled data from three single-dose studies analyzed by stepwise linear regression indicated that AAG concentrations significantly correlated with age and race and that only AAG concentrations were a significant predictor of amprenavir apparent total clearance (CL/F). A significant inverse linear relationship was found between AAG and amprenavir CL/F. Compared to white subjects, black subjects had significantly lower AAG concentrations and therefore significantly higher amprenavir CL/F. Although AAG has a significant influence on the variability of total drug pharmacokinetics, unbound, or free, drug concentrations are not affected by AAG concentrations. Incorrect conclusions could be drawn on the pharmacokinetics of highly protein-bound drugs if AAG concentration is not included in the analysis.
Amprenavir is a potent inhibitor of the human immunodeficiency virus type 1 (HIV-1) protease enzyme, with an inhibition constant (Ki) of 0.6 nM (5). Like almost all of the commercially available HIV protease inhibitors, amprenavir exhibits a high degree of binding to plasma proteins (∼90%); indinavir is the notable exception, with approximately 60% binding to human plasma proteins (prescribing information for Crixivan, Merck & Co.). In vitro studies have shown that the plasma proteins to which amprenavir primarily binds are α1-acid glycoprotein (AAG) and albumin, with fractional binding of 89 and 42%, respectively (10). Although serum albumin concentrations in humans are relatively stable (3.5 to 4.5 g/dl), AAG concentrations fluctuate widely (15 to 250 mg/dl), depending on such factors as HIV serostatus, age, and weight (2, 6, 11). In addition, AAG concentrations have been suggested to vary by race or ethnicity (7).
For several reasons, we chose to investigate whether variations in AAG concentration could explain initial conflicting observations of the effect of race on amprenavir pharmacokinetics. During clinical development, findings from six single-dose and three multiple-dose studies were inconsistent with respect to a race effect on amprenavir pharmacokinetics. Since the calculated pharmacokinetic parameters obtained in these studies were based on the total concentration of amprenavir in plasma (both protein-bound and unbound drug), we suspected that the differences in pharmacokinetic parameters between blacks and whites identified in some studies might be attributable to racial and infection status differences in AAG concentration.
AAG is one of several plasma proteins whose synthesis and concentration increase during the host's acute-phase response to infections, trauma, inflammatory processes, and some malignant diseases. The acute-phase response was so named because the characteristic changes in various plasma proteins were typically seen within hours or days following the onset of infection or injury; however, some acute-phase changes are also indicative of chronic disease (6), including HIV and AIDS (11; S. D. Goodwin, C. J. Renehan, R. T. Schooley, and J. A. Pieper, Abstr. XI Int. Conf. AIDS, abstr. Mo.B.1327, 1996; K. Stellrecht, G. L. Drusano, D. S. Stein, and J. A. Bilello, Abstr. 3rd Annu. Nat. Conf. Hum. Retrovir. Opportunistic Infect., abstr. 170, 1996). It is well documented that plasma protein binding can adversely influence the in vitro activity of anti-infectives and antiretrovirals by decreasing the amount of free drug available for interaction with the drug target (2, 3, 4, 9, 15). However, in vivo data of the effect of protein binding on the pharmacokinetics and activity of these agents have been less well documented.
This paper presents a cross-study analysis of data from three single-dose studies in HIV-positive and -negative subjects (Glaxo Wellcome Protocols PROA1004, PROA1010, and PROA1011). Two of these studies (PROA1010 and PROA1011) were designed to examine the effect of race on amprenavir clearance in healthy volunteers. They were chosen because the two studies yielded different results with regard to amprenavir pharmacokinetics and race and because one of the studies' entry criterion stipulated that the minority study population, either blacks or whites, must constitute at least one-third of the total number of study subjects. The third study (PROA1004) was designed to examine the effect of race on amprenavir clearance in HIV-infected volunteers and was chosen because there was a significant effect of race on amprenavir clearance and because AAG measurements were made for all subjects in each of the three dosing periods. The numbers of black and white subjects were well balanced in all three studies.
In addition to race, various demographic, laboratory, and clinical covariates were analyzed for their potential effect on the pharmacokinetics of amprenavir. Preliminary results of this cross-study analysis have been reported previously (B. M. Sadler, C. Gillotin, Y. Lou, and D. S. Stein, Abstr. 6th Conf. Retrovir. Opportunistic Infect., abstr. 375, 1999).
MATERIALS AND METHODS
A summary of each of the three single-dose studies of amprenavir whose data were included in the cross-study analysis is provided in Table 1.
TABLE 1.
Overview of three studies whose data were included in the cross-study analysis of amprenavir pharmacokinetics and demographic, laboratory, and clinical covariates
| Studya | Major entry criteria | No. of subjects enrolled (blacks/whites/other) | Amprenavir treatmentsb |
|---|---|---|---|
| PROA1004 | HIV-positive males or females, at least 18 years of age | 18c (7/10/1) | (i) 600 mg as 150-mg hard gelatin capsules, after 8-h fast (ii) 600 mg as 150-mg soft gelatin capsules, after 8-h fast (iii) 600 mg as 150-mg soft gelatin capsules, after 8-h fast and consumption of standardized (high-calorie, high-fat) breakfast |
| PROA1010 | HIV-negative healthy male volunteers; aged 18–45 years; 55–95 kg; body mass index of 20–28 kg/m2; ethnic minority population had to constitute at least 1/3 of total study population | 39 (14/25/0) | (i) 1,200 mg as 150-mg soft gelatin capsules, after 8-h fast (ii)1,200 mg as 150-mg soft gelatin capsules, after 8-h fast (iii)1,200 mg as 150-mg soft gelatin capsules, after 8-h fast and consumption of standardized (high-calorie, high-fat) breakfast |
| PROA1011 | HIV-negative healthy male volunteers; aged 18–45 years; 55–95 kg; body mass index of 20–28 kg/m2; ethnic minority population had to constitute at least 1/3 of total study population | 29 (12/17/0) | (i) 600 mg as 150-mg soft gelatin capsules, after 8-h fast (ii) 600 mg as 50-mg soft gelatin capsules, after 8-h fast (iii) 600 mg as 15-mg/ml oral solution, after 8-h fast |
All studies were designed to be single-center, open-label, randomized, balanced, three-period, single-dose, crossover studies.
Each study had three treatment periods, with each dosing period separated by at least 4 days.
The study group was composed of 15 males and 3 females. Of the three female subjects in the study, one reported her ethnic origin as “black,” one as “white,” and one as “other.”
Study population.
The primary difference in entry criteria for the three studies was HIV serostatus. Study PROA1004 was the only one of the three studies that enrolled HIV-positive subjects (males and females, ages of 18 to 55 years). The inclusion and exclusion criteria for this study have been previously reported (13). Studies PROA1010 and PROA1011 enrolled healthy, HIV-negative males (ages of 18 to 45 years, body weight of 55 to 95 kg, body mass index of 20 to 28 kg/m2). In both of the healthy-volunteer studies, a primary entry criterion stipulated that the minority population, either blacks or whites, must constitute at least one-third of the total number of study subjects.
Study design.
The three studies were single-center, open-label, randomized, balanced, three-period, single-dose, crossover pharmacokinetic and safety studies. At least 4 days separated the doses in each study. Follow-up evaluations were to be completed between 7 and 10 days after the last dose of study drug. The treatments for each study are listed in Table 1.
In all studies, subjects were not permitted to take concomitant medications (either prescription or over-the-counter) or alcohol-containing food and beverages within 48 h before each dosing and 24 h after dosing. However, the use of medications to manage adverse events was allowed, and the use of antiretroviral drugs and/or chemoprophylaxis for opportunistic infections was permitted up until 24 h prior to dosing. The consumption of methyl-xanthine-containing foods and beverages was prohibited on the day of dosing.
In both PROA1010 and PROA1011, the consumption of grapefruit and grapefruit juice was not allowed and water was not permitted 2 h predose and 2 h postdose. In PROA1004, water was not permitted for 4 h predose and 4 h postdose. All subjects took their study medication with either 200 ml (PROA1004) or 240 ml (PROA1010 and PROA1011) of water.
All study protocols were approved by an independent Ethics Committee or the Institutional Review Board affiliated with each study center. All subjects provided written informed consent prior to study participation.
Blood collection.
Blood samples were collected to determine both amprenavir and AAG concentrations. AAG concentrations were determined from a single predose sample taken prior to the subjects' dose administration in the same period in all three studies, and for PROA1004, additional samples were taken in each of the other two treatment periods.
Pharmacokinetic evaluation.
For each study, noncompartmental models (WinNonlin version 1.5; Scientific Consulting Inc., Cary, N.C.) were used to calculate pharmacokinetic parameters for amprenavir following single-dose administration based on samples taken at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 16 (PROA1010 and PRO1011 only), and 24 h. The observed peak concentration of amprenavir in serum (Cmax) and the time to reach peak concentration were obtained by inspection of the individual serum drug concentration-time data. Individual estimates of the apparent terminal elimination rate constant (λz) for amprenavir were obtained by log-linear regression of the terminal portions of the serum drug concentration-time curves. Half-life was then calculated as ln(2)/λz. The area under the concentration-time curve from 0 h to the time of the last quantifiable sample (tlast) (AUC0→t) was calculated for each volunteer by using the linear trapezoidal rule. AUC0→t was used to extrapolate from tlast to infinity (AUC0→∞) by adding Clast/λz. The apparent total clearance from serum (CL/F) was calculated as dose/AUC0→∞. The apparent volume of distribution during the elimination phase was calculated as (CL/F)/λz. For this analysis, CL/F of amprenavir was chosen as the representative pharmacokinetic parameter, since CL/F is dose corrected and the three studies used different doses of amprenavir.
Assay for amprenavir.
Concentrations of amprenavir in plasma were quantified using validated reversed-phase high-performance liquid chromatographic methods as previously described (13).
Assay for AAG.
AAG assays were performed using a commercially available assay by Quest Diagnostics, Inc., San Juan Capistrano, Calif. Concentrations of AAG in serum were quantified using a fixed-time nephelometric method. The limits of quantification were 20 to 660 ng/dl, with an interassay variability of <6%.
Statistical analyses.
Descriptive statistics were performed by comparing study and race to AAG concentration, ln(CL/F), age, and weight. CL/F was loge transformed prior to all analyses. Differences in AAG concentrations between blacks and whites across all studies were compared using Student's t test (SAS PROC TTEST, version 6.12). Stepwise linear regression (SAS PROC REG, version 6.12) was used to determine the linear combination of variables (age, bilirubin concentration, dosing period, race, height, weight, and body mass index) that could be independent predictors of AAG concentration. Separate analyses were performed on data from HIV-positive subjects (PROA1004) and pooled data from HIV-negative subjects (PROA1010 and PROA1011). In the analysis of the pooled data from HIV-negative subjects, a period effect was not considered since AAG data were only available for one dosing period.
Stepwise linear regression (SAS PROC REG, version 6.12) was also used to determine the linear combination of variables (AAG concentration, age, bilirubin concentration, dosing period, race, height, weight, body mass index, treatment, food, and dose) that could be independent predictors of ln(CL/F). Separate analyses were performed on data from HIV-positive subjects (PROA1004) and pooled data from HIV-negative subjects (PROA1010 and PROA1011). In the analysis of data from HIV-positive subjects, a dose effect was not considered since all subjects received the same 600-mg dose. Analysis of a period effect from the pooled data of HIV-negative subjects was not performed since, as noted above, AAG data were available from only one period.
RESULTS
Subject accountability and demographics.
Only subjects with amprenavir concentration-time profile data and corresponding AAG concentration data were included in this cross-study analysis. Of the 86 subjects enrolled in the three studies, 83 were included in the analysis: 18 subjects were HIV positive (PROA1004) and 65 subjects were HIV negative (PROA1010, n = 38; PROA1011, n = 27). Of the HIV-positive subjects, 15 were male and 3 were female. All HIV-negative subjects were male, per the studies' inclusion and exclusion criteria. Of the 83 subjects included in the analysis, 33 reported their ethnicity as “black,” 49 as “white,” and 1 as “other.” This last subject was pooled with the white subjects in the analysis.
Relationship between race and AAG concentration.
Pooled data from all three studies were used to analyze the relationship between race and AAG concentration. The mean concentration of AAG in subjects whose race was black (77.2 ± 13.8 mg/dl) was significantly lower than that of those whose race was white (90 ± 20.2 mg/dl) (P ≤ 0.0001, Student's t test).
Relationship between AAG and ln(CL/F).
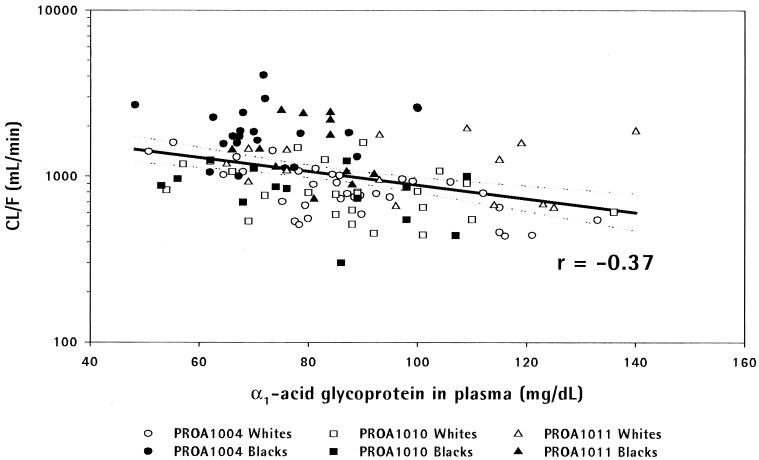
Using pooled data from all three studies, a significant inverse linear relationship was found between AAG concentration and ln(CL/F) (P ≤ 0.0001, linear regression) (Fig. 1).
FIG. 1.
Scatter plot of amprenavir CL/F versus AAG concentration in studies PROA1004, PROA1010, and PROA1011.
Factors that affect AAG.
In the two separate stepwise regression analyses of data from HIV-positive subjects (PROA1004) and HIV-negative subjects (PROA1010 and PROA1011 pooled data), AAG concentration was used as the dependent variable, and the independent variables investigated were age, bilirubin concentration, dosing period, race, height, weight, and body mass index. Table 2 lists those variables found to be significant independent predictors of AAG concentration at least at the α = 0.1 level. Race was found to be a significant predictor of AAG concentration in both HIV-positive and -negative subjects at a P value of < 0.05.
TABLE 2.
Independent predictors of AAG concentration, as determined by stepwise linear regression
| Variablea | Coefficient | Partial r2 | P value |
|---|---|---|---|
| HIV-positive subjects | |||
| Bilirubin concn | −16.66 | 0.175 | 0.0005 |
| Race | −17.29 | 0.216 | 0.0001 |
| HIV-negative subjects | |||
| Weight | 0.85 | 0.137 | 0.0015 |
| Race | −10.74 | 0.072 | 0.019 |
| Age | 0.51 | 0.039 | 0.08 |
Data for HIV-positive subjects came from study PROA1004. Data for HIV-negative subjects are pooled data from studies PROA1010 and PROA1011. Bilirubin concentration was measured in milligrams per deciliter, weight was measured in kilograms, and age was measured in years. For race, “black” was assigned a value of 1 and “white” and “other” were assigned a value of 0.
Factors that affect ln(CL/F).
In the two separate stepwise regression analyses of data from HIV-positive subjects (PROA1004) and HIV-negative subjects (PROA1010 and PROA1011 pooled data), ln(CL/F) was used as the dependent variable, and the independent variables investigated were AAG concentration, age, bilirubin concentration, dosing period, ethnicity, treatment, taking study drug with food or while fasting, dose, and body mass index. Table 3 lists those variables found to be significant independent predictors of CL/F at least at the α = 0.1 level. AAG was found to be a significant predictor in both groups, with a significant interaction between race and AAG noted for the HIV-positive subjects.
TABLE 3.
Independent predictors of ln(CL/F) as determined by stepwise linear regression
| Variablea | Coefficient | Partial r2 | P value |
|---|---|---|---|
| HIV-positive subjects | |||
| AAG concn | −0.013 | 0.124 | 0.0001 |
| Race | −0.369 | 0.004 | 0.438 |
| Food | 0.194 | 0.029 | 0.033 |
| AAG∗raceb | 0.014 | 0.03 | 0.03 |
| HIV-negative subjects | |||
| AAG concn | −0.007 | 0.077 | 0.008 |
| Dose | −0.001 | 0.362 | 0.001 |
| Food | 0.309 | 0.054 | 0.026 |
Data for HIV-positive subjects came from study PROA1004. Data for HIV-negative subjects are pooled data from studies PROA1010 and PROA1011. AAG concentration was measured in milligrams per deciliter. For food consumption, “fed” was assigned a value of 1 and “fasted” was assigned a value of 0. Dose was measured in milligrams. For race, “black” was assigned a value of 1 and “white” and “other” were assigned a value of 0.
AAG∗race is the term for the interaction between AAG concentration and race.
DISCUSSION
Univariate analysis of the pooled data from all three studies revealed a significant inverse relationship between AAG concentration and amprenavir CL/F, such that lower AAG concentrations were associated with a higher CL/F. This is consistent with the analyses of the pooled data which showed that plasma AAG concentrations were significantly lower in black subjects than in white subjects (Student's t test and analysis of variance). Thus, the statistically significant findings of this cross-study analysis indicate that the observed differences in pharmacokinetic parameters between blacks and whites (in studies PROA1004 and PROA1011) are due to the racial differences in AAG concentrations.
The implications of our study findings require an examination of the effect of AAG concentration on the pharmacokinetics of both protein-bound and unbound, or free, drug. Changes in AAG concentration result in an inverse change in the percentage of unbound drug with respect to total drug, not the concentration of unbound drug (i.e., lower percentage of free-drug concentration as total drug increases with higher AAG). Because there are no changes in intrinsic clearance, only apparent total clearance, and because the fractions of bound and free amprenavir are in dynamic equilibrium (see reference 12), no change occurs in the absolute concentration of free drug. Therefore, the apparent difference in total drug clearance, which could lead to the wrong conclusion about differences in amprenavir pharmacokinetics between blacks and whites and between HIV-infected and uninfected volunteers, can be explained by correcting for differences in the concentration of AAG, the principle binding protein of amprenavir. No dose adjustments are therefore indicated for black subjects, and a significant source of variability in total drug concentrations can be accounted for by including AAG concentrations in the analysis.
The findings of this cross-study analysis are consistent with the observations from a multiple-dose study of amprenavir in HIV-positive subjects, which found an inverse relationship between AAG concentrations and CL/F (14). In that study, after 3 weeks of amprenavir monotherapy, AAG concentrations were decreased while CL/F increased (relative to measurements obtained on the first day of dosing). The percent change and the absolute difference in AAG concentrations between single dose on day 1 and steady state at week 3 were significantly associated with the ratio of AUC0→∞/AUCss in a stepwise linear regression model. Although amprenavir CL/Fss was dose dependent in the analysis without AAG data, no dose dependence was observed when AAG concentration was considered in the analysis. The higher doses of amprenavir, which produce the greatest antiviral activity, resulted in the largest decrease in AAG concentration, which led to the greatest changes in total drug concentration.
In the analysis of factors that could potentially affect AAG concentration, race and weight were found to be significant predictors of plasma AAG concentration and age had a borderline association with AAG in HIV-negative subjects, an observation consistent with previously reported pharmacokinetic studies with HIV-negative subjects (1, 7, 8). The analysis also found that in HIV-positive subjects, race and bilirubin concentration were significant predictors of AAG concentration. The inverse relationship between bilirubin and AAG concentrations may be reflective of an underlying subclinical hepatic insufficiency or infection in this immunocompromised population.
In HIV-negative subjects, the only variables found to have an effect on amprenavir CL/F were dose, concomitant food consumption, and AAG concentration. When AAG concentration is taken into account, weight, height, body mass index, race, age, and bilirubin concentration were found not to be predictors of CL/F. In HIV-positive subjects, AAG concentration and concomitant food consumption were significant predictors of CL/F. In addition, there was a significant interaction between AAG concentration and race noted.
The results of the present analyses indicate that for HIV protease inhibitors, as with all drugs highly bound to plasma proteins, AAG concentration should be taken into consideration when evaluating potential pharmacokinetic differences. Any change in total drug clearance should be evaluated in light of a change in AAG concentration. However, of the currently marketed protease inhibitors, only indinavir has been studied in this regard; no racial differences in pharmacokinetic parameters have been found for indinavir (prescribing information for Crixivan, Merck & Co.). The analyses of amprenavir pharmacokinetics reported here indicate that AAG significantly influenced the pharmacokinetics of total amprenavir concentrations and, if AAG were not accounted for, erroneous conclusions regarding amprenavir's pharmacokinetics, including apparent racial differences, could have been made.
ACKNOWLEDGMENTS
We thank Cindy Rawls for bioanalytical support, David Morris for bioanalytical contract support, and the volunteers and clinical investigators who participated in the three studies.
REFERENCES
- 1.Abernethy D, Greenblatt D, Divoll M, Harmatz J, Slader R. Alterations in drug distribution and clearance due to obesity. J Pharmacol Exp Ther. 1981;217:681–685. [PubMed] [Google Scholar]
- 2.Bilello J A, Bilello P A, Prichard M, Robins T, Drusano G L. Reduction of the in vitro activity of A77003, an inhibitor of human immunodeficiency virus protease, by human serum α1 acid glycoprotein. J Infect Dis. 1995;171:546–551. doi: 10.1093/infdis/171.3.546. [DOI] [PubMed] [Google Scholar]
- 3.Dudley M N, Blaser J, Gilbert D, Zinner S H. Significance of “extravascular” protein binding for antimicrobial pharmacodynamics in an in vitro capillary model of infection. Antimicrob Agents Chemother. 1990;34:98–101. doi: 10.1128/aac.34.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gooding A E, Hsieh A, Woolley J L. Proceedings of the International Society for the Study of Xenobiotics 7th North American ISSX Meeting. Vol. 10. Bethesda, Md: International Society for the Study of Xenobiotics; 1996. Plasma protein binding and erythrocyte partitioning studies in rats, dogs, and humans with the HIV-1 protease inhibitor, 141W94 (VX-478) p. 347. [Google Scholar]
- 5.Kim E E, Baker C T, Dwyer M D, Mureko M A, Rao B G, Tung R D, Navia M A. Crystal structure of HIV-1 protease in complex with VX-478, a potent and orally bioavailable inhibitor of the enzyme. J Am Chem Soc. 1995;117:1181–1182. [Google Scholar]
- 6.Kremer J M H, Wilting J, Janssen L H M. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev. 1988;40:1–47. [PubMed] [Google Scholar]
- 7.Johnson J. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci. 1997;86:1328–1333. doi: 10.1021/js9702168. [DOI] [PubMed] [Google Scholar]
- 8.Lalonde R, Tenero D, Burlew B, Herring V, Bottorf M. Effects of age on the protein binding and disposition of propranolol stereoisomers. Clin Pharmacol Ther. 1990;47:447–455. doi: 10.1038/clpt.1990.56. [DOI] [PubMed] [Google Scholar]
- 9.Lazdins J K, Mestan J, Goutte G, Walker M R, Bold G, Capraro H G, Klimkait T. In vitro effect of α1-acid glycoprotein on the anti-human immunodeficiency virus (HIV) activity of the protease inhibitor CGP 61755: a comparative study with other relevant HIV protease inhibitors. J Infect Dis. 1997;175:1063–1070. doi: 10.1086/520352. [DOI] [PubMed] [Google Scholar]
- 10.Livingston D J, Pazhanisamy S, Porter D J T, Partaledis J A, Tung R D, Painter G R. Weak binding of VX-478 to human plasma proteins and implications for anti-human immunodeficiency virus therapy. J Infect Dis. 1995;172:1238–1245. doi: 10.1093/infdis/172.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.O/ie S, Jacobson M A, Abrams D I. α1-Acid glycoprotein levels in AIDS patients before and after short-term treatment with zidovudine (ZDV) J Acquir Immune Defic Syndr Hum Retrovirol. 1993;6:531–533. [PubMed] [Google Scholar]
- 12.Rolan P E. Plasma protein binding displacement interactions—why are they still regarded as clinically important? Br J Clin Pharmacol. 1994;37:125–128. doi: 10.1111/j.1365-2125.1994.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadler B M, Hanson C D, Chittick G E, Symonds W T, Roskell N S. Safety and pharmacokinetics of amprenavir (141W94), an human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob Agents Chemother. 1999;43:1686–1692. doi: 10.1128/aac.43.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadler B M, Gillotin C, Lou Y, Stein D S. A pharmacokinetic and pharmacodynamic study of the human immunodeficiency virus protease inhibitor amprenavir after multiple oral dosing. Antimicrob Agents Chemother. 2001;45:30–37. doi: 10.1128/AAC.45.1.30-37.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X Q, Schooley R T, Gerber J G. The effect of increasing alpha1-acid glycoprotein concentration on the antiviral efficacy of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:1833–1837. doi: 10.1086/315123. [DOI] [PubMed] [Google Scholar]