Abstract
Objective
Phosphate is a fundamental element involved in a number of physiological pathways. A previous study showed abnormal laboratory findings and a higher mortality in hypophosphatemic patients than in normophosphatemic patients with pneumonia. Sporadic cases of pneumonia due to Legionella spp., Streptococcus pneumoniae, and viruses have been reported; however, the significance of hypophosphatemia in patients with pneumonia has not been adequately studied. We determined whether or not hypophosphatemia in patients with community-acquired pneumonia (CAP) was associated with specific pathogens, patient factors, disease severity, and mortality.
Method
We retrospectively analyzed 600 patients with CAP who were admitted to our hospital between January 1, 2010, and December 31, 2019.
Results
Hypophosphatemia was found in 72 (12.0%) of the 600 patients. The most frequent causative microbial agents of CAP in patients with hypophosphatemia were S. pneumoniae, Legionella spp., and influenza virus, whereas in severely ill patients with hypophosphatemia, influenza virus was the most common. Legionella spp., diabetes mellitus, and severe pneumonia were the independent factors for hypophosphatemia in the multivariable analysis. An impaired performance status, severe status on admission, interstitial pneumonia, bacteremia, and guideline-discordant therapy were the independent factors associated with mortality in the multivariable analysis. Hypophosphatemia was not significantly associated with mortality but showed a trend towards higher mortality in the multivariable analysis.
Conclusion
Hypophosphatemia was not associated with the prognosis in patients with CAP. However, the significance of hypophosphatemia for clinicians lies in the laboratory findings that predict abnormal glucose metabolism, Legionella infection, and severe disease.
Keywords: pneumonia, hypophosphatemia, Legionella, severity, outcome
Introduction
Phosphate is a fundamental element involved in numerous physiological pathways, such as skeletal development and mineralization, membrane composition, nucleotide structure, cellular signaling, energy storage and transfer, and maintenance of acid-base equilibrium (1). Because of its importance, phosphate homeostasis is closely regulated and maintained by a dynamic balance between urinary phosphate losses and net phosphate absorption from the gastrointestinal tract, with equal amounts deposited and reabsorbed from bone (2,3). There are three primary mechanisms underlying hypophosphatemia: increased renal excretion, decreased intestinal absorption, and the movement of phosphate from extracellular to intracellular compartments.
Although hypophosphatemia is infrequent in the general population, it is encountered in general hospitalized patients (range, 2.2-3.1%), patients admitted to intensive-care units (28.8-34%), and those with infections, regardless of sepsis (65-80%) (2). Even though pneumonia accounts for a major proportion of hospitalized patients with infection and hypophosphatemia (2), the significance of hypophosphatemia in patients with pneumonia has not been sufficiently investigated.
Some sporadic cases of pneumonia due to Legionella spp. (4), Streptococcus pneumoniae (5), and viruses (6,7), which are major pathogens of severe pneumonia (8), have been reported; however, whether hypophosphatemia reflects a severe condition of these patients or predicts infection by specific pathogens remains unclear.
We therefore examined whether or not hypophosphatemia in patients with pneumonia is associated with specific causative organisms, patient factors, disease severity, and mortality.
Materials and Methods
We conducted a retrospective study of all patients hospitalized with community-acquired pneumonia (CAP) over a 10-year period from January 2009 through December 2018 at our institution in Saitama, Japan. Pneumonia was diagnosed based on symptoms suggestive of lower respiratory tract infections and the development of infiltrations on chest X-ray. The excluded patients comprised those with hospital-acquired pneumonia (9), those showing immunosuppression [acquired immunodeficiency syndrome (AIDS) or receiving chemotherapy for malignancy], and those with tuberculosis, non-resected lung cancer, or a confirmed alternative diagnosis lasting until the end of the follow-up period.
The diagnosis of causative microorganisms was based on the results of semi-quantitative cultures of respiratory samples or blood, paired sera, urinary antigen tests for S. pneumoniae and Legionella pneumophila, and nasopharyngeal swabs for influenza virus, as reported previously (8). The causative microorganisms for which paired-sera were used in the diagnosis were M. pneumoniae, Legionella spp., Chlamydophila pneumoniae, C. psittaci, adenovirus, respiratory syncytial virus, influenza virus, and human parainfluenza viruses 1, 2, 3, and 4. In addition, viral infection was diagnosed when real-time polymerase chain reaction (PCR) was positive.
Respiratory specimens obtained from bronchoalveolar lavage fluid, pharyngeal swab, or sputum were transported on dry ice, stored at -70 °C, and used for the detection of respiratory pathogens on a Rotor-Gene Q instrument (Qiagen, Hilden, Germany) with multiplex reverse-transcription PCR (RT-PCR) using an FTD Resp 21 Kit (Fast Track Diagnostics, Silema, Malta). The kit detects the following respiratory pathogens: influenza A and B viruses; coronaviruses NL63, 229E, OC43, and HKU1; human parainfluenza viruses 1, 2, 3, and 4; human metapneumovirus A/B; rhinovirus; respiratory syncytial virus A/B; adenovirus; enterovirus; human parechovirus; bocavirus; and Mycoplasma pneumoniae. An EZ1 Virus Mini Kit v2.0 was used for nucleic acid extraction (Qiagen). A threshold cycle value of <33 was considered to be a positive result on RT-PCR, as indicated in the manufacturer's instructions.
Disease severity on admission was based on the criteria of the American Thoracic Society/Infectious Disease Society of America (IDSA/ATS) guidelines (9). Severe disease was diagnosed when at least one major criterion or three minor criteria of the guidelines were present. Hypophosphatemia was defined as a serum phosphorus level <2.0 mg/dL on admission. Diabetes mellitus was defined if the patient was previously diagnosed as having the condition and was currently being treated with insulin or oral hypoglycemic agents or had a high serum glucose concentration or high HbA1c levels during hospitalization, according to the Japanese Diagnostic Criteria of Diabetes Mellitus (10).
Rhabdomyolysis was defined as a creatine kinase level of >1,000 IU/L (11). The performance status (PS) was recorded on admission based on anamnesis from the patients and the patients' families and classified based on the criteria suggested by the Eastern Cooperative Oncology Group (ECOG) (12). The definition of healthcare contact was based on the healthcare-associated pneumonia criteria suggested by the ATS/IDSA (13). Patients' comorbidities and laboratory data were searched in their medical records through manual searching and the use of the hospital information system.
The treatment prescribed during the first 24 hours of hospitalization was considered the initial treatment. The initial antibiotic regimen was defined as being concordant when the antibiotics chosen by the attending physician were in accordance with the recommendations of the 2019 IDSA/ATS guidelines (9), regardless of any additionally administered antibiotics.
The following variables were assessed as possible risk factors for the severity on admission and in-hospital mortality of pneumonia: age, male sex, smoking habit, presence of comorbid illnesses, history of healthcare contact, the history of prior antibiotics administered by a local physician, the presence of rhabdomyolysis and hypophosphatemia, and causative pathogens. Concordance of the initial antibiotic therapy with the ATS/IDSA guidelines (9) was also assessed as a possible risk factor for in-hospital mortality.
The study protocol was approved by the Ethical Committee of the Saitama Cardiovascular and Respiratory Center. This same committee approved the verbal consent procedure. We used an opt-out method for patient inclusion and disclosed this to the hospitalized patients. No patients refused to participate.
Statistical analyses
The results are presented as numbers and percentages or mean±standard deviation unless otherwise indicated. Comparisons of patients with and without hypophosphatemia were performed using Fisher's exact test for categorical variables and Student's t-test for continuous variables. Risk factors for hypophosphatemia and in-hospital mortality were evaluated by univariable and multivariable logistic regression analyses. The results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs).
We carefully selected factors considered in the multivariable logistic model by adopting five strategies. First, we forced clinically meaningful factors evaluated in each multivariable logistic model based on expert knowledge, despite the statistical results of factors in the univariable logistic model. Second, factors consisting of more than two categories, namely age and performance status, were simplified by reducing the number of categories to increase precision of the model estimates. Third, factors that were missing in many of the patients or those suspected of causing collinearity were not eliminated. No imputation was made for missing data. Fourth, the number of events and the Hosmer-Lemeshow goodness of fit (14) were considered to select the number of factors included in the multivariable logistic model. Fifth, Firth's bias correction was used to compensate for the small number of events (15).
A p value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed with the SAS software program, version 9.4 (SAS Institute, Cary, USA).
Results
Patient characteristics
We included 600 patients in the present study. The baseline characteristics of the study patients are shown in Table 1. Regarding disease severity on admission, 75 (12.5%) of the patients were classified as having severe pneumonia, and 22 (3.7%) died during their hospital stay. Hypophosphatemia was found in 72 (12.0%) of the 600 patients and was present in 19 (26.4%) of the severely ill patients and in 5 (6.9%) of the patients who died. Three patients had severe hypophosphatemia (<1.0 mg/dL), of whom 1 was severely ill and died, although the other 2 did not. The frequency of non-pulmonary disease and the proportion of severe cases were significantly higher in the patients with hypophosphatemia than in those without it. Interventions for hypophosphatemia were not performed in any of the patients. Among the 72 patients with hypophosphatemia, serum phosphorus levels were not measured in 38 patients after treatment. In the other 34 patients, the serum phosphorus levels improved to >2.0 mg/dL during their subsequent treatment course.
Table 1.
Patient Characteristics.
| Characteristic | Total | Hypophosphatemia | Non-hypophosphatemia | p value | ||||
|---|---|---|---|---|---|---|---|---|
| (n=600) | (n=72) | (n=528) | ||||||
| Female sex | 175 (29.2%) | 17 (23.6%) | 158 (29.9%) | 0.333 | ||||
| Age (years) | 67.9±15.2 | 69.7±14.1 | 67.6±15.3 | 0.265 | ||||
| <65 | 184 (30.7%) | 18 (25.0%) | 166 (31.4%) | 0.485 | ||||
| 65-74 | 200 (33.3%) | 23 (31.9%) | 177 (33.5%) | |||||
| 75-89 | 204 (34.0%) | 29 (40.3%) | 175 (33.1%) | |||||
| 90- | 12 (2.0%) | 2 (2.8%) | 10 (1.9%) | |||||
| Pathogen identified | 304 (50.7%) | 39 (54.2%) | 265 (50.2%) | 0.533 | ||||
| Comorbidity | ||||||||
| Pulmonary disease | 321 (53.5%) | 38 (52.8%) | 283 (53.6%) | 0.901 | ||||
| Non-pulmonary disease | 312 (52.0%) | 47 (65.3%) | 265 (50.2%) | 0.017 | ||||
| Smoking history | 391 (65.2%) | 50 (69.4%) | 341 (64.6%) | 0.510 | ||||
| CAP/HCAP | ||||||||
| CAP without health care contacts | 401 (66.8%) | 52 (72.2%) | 349 (66.1%) | 0.224 | ||||
| CAP with health care contacts | 191 (31.8%) | 18 (25.0%) | 173 (32.8%) | |||||
| Performance status | ||||||||
| 0 | 352 (58.7%) | 45 (62.5%) | 307 (58.1%) | 0.637 | ||||
| 1-2 | 218 (36.3%) | 23 (31.9%) | 195 (36.9%) | |||||
| 3-4 | 28 (4.7%) | 4 (5.6%) | 24 (4.5%) | |||||
| Disease severity on admission | ||||||||
| Severe | 75 (12.5%) | 19 (26.4%) | 56 (10.6%) | <0.001 | ||||
| In-hospital mortality | 22 (3.7%) | 5 (6.9%) | 17 (3.2%) | 0.168 |
CAP: community-acquired pneumonia, HCAP: healthcare-associated pneumonia
Microbiological etiology of pneumonia in patients with and without hypophosphatemia
The diagnostic methods used and the results obtained are listed in Table 2. The use of RT-PCR was begun from 2016 and not performed in cases before that. Furthermore, it was not used in every case from 2016 onward.
Table 2.
Diagnostic Methods and Patient Results (n=600).
| Method | No. of episodes studied | No. of positive diagnostic studies(%) | ||
|---|---|---|---|---|
| Paired sera | 514 | 115 (22.4%) | ||
| Rapid influenza diagnostic test | 591 | 32 (5.4%) | ||
| RT-PCR | 52 | 27 (51.9%) | ||
| Urinary antigen | ||||
| Streptococcus pneumoniae | 588 | 104 (17.7%) | ||
| Legionella sp. | 588 | 16 (2.7%) | ||
| Culture | ||||
| Sputum | 534 | 101 (18.9%) | ||
| Transbronchial aspirate | 30 | 8 (26.7%) | ||
| Protected specimen brush | 3 | 1 (33.3%) | ||
| Bronchial washing | 12 | 4 (33.3%) | ||
| Bronchoalveolar lavage fluid | 39 | 11 (28.2%) | ||
| Blood | 493 | 16 (3.2%) | ||
| Pleural fluid | 13 | 1 (7.7%) |
RT-PCR: reverse-transcription polymerase chain reaction
Pathogens were identified in 39 (54.2%) of the hypophosphatemia patients and 265 (50.2%) of the non-hypophosphatemia patients (Table 1). Polymicrobial infections were identified in 9 (12.5%) of the hypophosphatemia patients and 44 (8.3%) of the non-hypophosphatemia patients (Table 3). The 4 most frequently isolated pathogens in the hypophosphatemia patients were S. pneumoniae (19.4%), Legionella spp. and influenza virus (11.1% each), and a virus other than influenza virus (6.9%). In contrast, the 5 most frequently isolated pathogens in the non-hypophosphatemia patients were S. pneumoniae (18.6%), influenza virus (11.7%), M. pneumoniae (6.3%), C. pneumoniae (5.3%), and Legionella spp. (3.6%) (Table 3). Legionella spp. were identified as etiologies significantly more frequently in the hypophosphatemia patients than in the non-hypophosphatemia patients (11.1% vs. 3.6%, p=0.010). The 3 most frequently isolated pathogens in the hypophosphatemia patients with severe pneumoniae were influenza virus (26.3%), S. pneumoniae (21.0%), and Legionella spp. (15.8%).
Table 3.
Etiology of Pneumonia in the Hypophosphatemia and Non-hypophosphatemia Patients.
| Total | Hypophosphatemia | Non-hypophosphatemia | p value | |||||
|---|---|---|---|---|---|---|---|---|
| (n=600) | (n=72) | (n=528) | ||||||
| Streptococcus pneumoniae | 112 (18.7%) | 14 (19.4%) | 98 (18.6%) | 0.872 | ||||
| Influenza virus | 70 (11.7%) | 8 (11.1%) | 62 (11.7%) | 1.000 | ||||
| Mycoplasma pneumoniae | 36 (6.0%) | 3 (4.2%) | 33 (6.3%) | 0.606 | ||||
| Legionella spp. | 27 (4.5%) | 8 (11.1%) | 19 (3.6%) | 0.010 | ||||
| Pseudomonas aeruginosa | 13 (2.2%) | 2 (2.8%) | 11 (2.1%) | 0.662 | ||||
| Haemophilus influenzae | 13 (2.2%) | 2 (2.8%) | 11 (2.1%) | 0.662 | ||||
| GNEB | 14 (2.3%) | 3 (4.2%) | 11 (2.1%) | 0.230 | ||||
| Chlamydophila pneumoniae | 30 (5.0%) | 2 (2.8%) | 28 (5.3%) | 0.563 | ||||
| Chlamydophila psittaci | 2 (0.3%) | 0 (0.0%) | 2 (0.4%) | 1.000 | ||||
| Moraxella catarrhalis | 6 (1.0%) | 0 (0.0%) | 6 (1.1%) | 1.000 | ||||
| Streptococcus spp. a | 4 (0.7%) | 0 (0.0%) | 4 (0.8%) | 1.000 | ||||
| MSSA | 6 (1.0%) | 1 (1.4%) | 5 (0.9%) | 0.537 | ||||
| Acinetobacter | 1 (0.2%) | 0 (0.0%) | 1 (0.2%) | 1.000 | ||||
| Other bacteria b | 6 (1.0%) | 1 (1.4%) | 5 (1.7%) | 0.537 | ||||
| Virus (other than Influenza virus) | 20 (3.3%) | 5 (6.9%) | 15 (2.8%) | 0.079 | ||||
| Polymicrobial infection c | 53 (8.8%) | 9 (12.5%) | 44 (8.3%) | 0.266 | ||||
| Unknown | 294 (49.0%) | 33 (45.8%) | 261 (49.4%) | 0.616 |
This number includes not only monomicrobial but also polymicrobial infection.
aStreptococcus sp. means other than S. pneumoniae. b "Other bacteria" includes Staphylococcus haemolyticus (n=1) in the hypophosphatemia patients, and Haemophilus parainfluenzae (n=2), Citrobacter freundii, Achromobacter xylosoxidans, Rothia mucilaginosa (n=1 each) in the non-hypophosphatemia patients. c "Polymicrobial infection" includes S. pneumoniae+ influenza virus, C. pneumoniae+Legionella spp., C. pneumoniae+influenza virus, GNEB+influenza virus, human parainfluenza virus 3+respiratory syncytial virus, S. pneumoniae+influenza virus+Staphylococcus haemolyticus, S. pneumoniae+adenovirus+coronaviruses 229E, Legionella spp. +rhinovirus+coronavirus 229E, Legionella spp. +influenza virus+rhinovirus+ human parainfluenza virus 2+parechovirus (n=1 each) in the hypophosphatemia patients, and S. pneumoniae +influenza virus (n=15), S. pneumoniae+C. pneumoniae (n=5), S. pneumoniae+MSSA, S. pneumoniae+GNEB, S. pneumoniae+rhinovirus, M. pneumoniae+P. aeruginosa, M. pneumoniae+S. agalactiae, M. pneumoniae+influenza virus, C. pneumoniae+influenza virus, C. pneumoniae+Rothia mucilaginosa, Legionella spp. +H. influenzae, Legionella spp. +M. catarrhalis, Legionella spp. +influenza virus, MSSA+GNEB, MSSA+influenza virus, C. psittaci+human parainfluenza virus 1, rhinovirus+parechovirus, S. pneumoniae+C. pneumoniae+influenza virus, S. pneumoniae+C. pneumoniae+M. catarrhalis, C. pneumoniae+P. aeruginosa+GNEB, MSSA+GNEB+influenza virus, influenza virus+rhinovirus+coronavirus 229E, rhinovirus+ parechovirus+human parainfluenza virus 2, respiratory syncytial virus+human parainfluenza virus 1+parechovirus, respiratory syncytial virus+human parainfluenza virus 1+coronavirus 229E, rhinovirus+human parainfluenza virus 2+coronavirus NL63+human metapneumovirus (n=1 each) in the non-hypophosphatemia patients. GNEB: Gram-negative enteric bacilli, MSSA: methicillin-susceptible Staphylococcus aureus
Factors contributing to hypophosphatemia
The results of univariable and multivariable logistic regression analyses for hypophosphatemia are provided in Table 4. Univariable analyses showed that Legionella spp. (OR, 3.44; 95% CI, 1.46 to 8.14; p=0.005), chronic non-pulmonary diseases (OR, 1.85; 95% CI, 1.11 to 3.08; p=0.019), diabetes mellitus (OR, 2.72; 95% CI, 1.53 to 4.82; p<0.001), arrhythmia (OR, 2.21; 95% CI, 1.02 to 4.80; p=0.044), rhabdomyolysis (OR, 6.51; 95% CI, 2.48 to 17.08; p<0.001), C-reactive protein (CRP) (OR, 1.06; 95% CI, 1.03 to 1.08; p<0.001), and disease severity on admission (OR, 3.05; 95% CI, 1.69 to 5.50; p<0.001) were independent factors for hypophosphatemia. Next, multivariable analyses showed that Legionella spp. (OR, 2.89; 95% CI, 1.19 to 6.99; p=0.019), diabetes mellitus (OR, 2.53; 95% CI, 1.41 to 4.56; p=0.002), and severe pneumonia (OR, 2.86; 95% CI, 1.57 to 5.22; p=0.001) were independent factors for hypophosphatemia.
Table 4.
Logistic Regression Analysis of the Risk of Hypophosphatemia in the Study Patients.
| n | Hypophosphatemia (%) | Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||||
| Sex | Male | 425 | 55 (12.9%) | 1.36 (0.77, 2.40) | 0.294 | ||
| Age (years) | 90- | 12 | 2 (16.7%) | 2.14 (0.47, 9.74) | 0.324 | ||
| 75-89 | 204 | 29 (14.2%) | 1.51 (0.81, 2.81) | 0.191 | |||
| 65-74 | 200 | 23 (11.5%) | 1.19 (0.62, 2.27) | 0.595 | |||
| <65 | 184 | 18 (9.8%) | 1 | ||||
| Vaccination history | |||||||
| 23-valent pneumococcal polysaccharide | Unknown | 15 | 4 (26.7%) | 3.10 (0.80, 12.10) | 0.103 | ||
| vaccination within 5 years | No | 519 | 61 (11.8%) | 1.06 (0.47, 2.39) | 0.880 | ||
| Influenza vaccination within one year | Unknown | 220 | 23 (10.5%) | 0.70 (0.36, 1.34) | 0.282 | ||
| No | 254 | 31 (12.2%) | 0.83 (0.44, 1.54) | 0.547 | |||
| Prior antibiotic treatment | No | 363 | 47 (12.9%) | 1.23 (0.73, 2.05) | 0.434 | ||
| Identified pathogen | Yes | 304 | 39 (12.8%) | 1.17 (0.72, 1.91) | 0.531 | ||
| Unidentified pathogen | Yes | 294 | 33 (11.2%) | 0.87 (0.53, 1.42) | 0.571 | ||
| Streptococcus pneumoniae | Yes | 112 | 14 (12.5%) | 1.08 (0.58, 2.01) | 0.800 | ||
| Legionella spp. | Yes | 27 | 8 (29.6%) | 3.44 (1.46, 8.14) | 0.005 | 2.89 (1.19, 6.99) | 0.019 |
| Influenza virus | Yes | 70 | 8 (11.4%) | 0.98 (0.46, 2.12) | 0.966 | ||
| Virus (other than influenza virus) | Yes | 20 | 5 (25.0%) | 2.70 (0.96, 7.55) | 0.059 | ||
| Polymicrobial infection | Yes | 53 | 9 (17.0%) | 1.63 (0.77, 3.46) | 0.205 | ||
| Comorbidities | |||||||
| Chronic pulmonary diseases | Yes | 321 | 38 (11.8%) | 0.97 (0.59, 1.58) | 0.891 | ||
| COPD | Yes | 158 | 14 (8.9%) | 0.66 (0.36, 1.21) | 0.180 | ||
| Asthma | Yes | 56 | 10 (17.9%) | 1.74 (0.84, 3.60) | 0.133 | ||
| Bronchiectasis | Yes | 32 | 4 (12.5%) | 1.15 (0.41, 3.27) | 0.788 | ||
| Pulmonary NTM | Yes | 37 | 6 (16.2%) | 1.54 (0.63, 3.77) | 0.341 | ||
| Old tuberculosis | Yes | 21 | 3 (14.3%) | 1.39 (0.42, 4.60) | 0.590 | ||
| Chronic pulmonary aspergillosis | Yes | 9 | 0 (0.0%) | 0.38 (0.02, 7.63) | 0.525 | ||
| Interstitial pneumonia | Yes | 57 | 5 (8.8%) | 0.74 (0.29, 1.86) | 0.521 | ||
| Lung cancer surgery | Yes | 16 | 3 (18.8%) | 1.92 (0.56, 6.62) | 0.301 | ||
| Pneumoconiosis | Yes | 2 | 0 (0.0%) | 1.45 (0.03, 60.37) | 0.845 | ||
| Chronic empyema | Yes | 2 | 0 (0.0%) | 1.45 (0.03, 60.37) | 0.845 | ||
| Chronic non-pulmonary diseases | Yes | 312 | 47 (15.1%) | 1.85 (1.11, 3.08) | 0.019 | ||
| Hypertension | Yes | 93 | 13 (14.0%) | 1.26 (0.67, 2.40) | 0.473 | ||
| Congestive heart failure | Yes | 24 | 5 (20.8%) | 2.13 (0.78, 5.78) | 0.138 | ||
| Ischemic heart diseases | Yes | 24 | 5 (20.8%) | 2.13 (0.78, 5.78) | 0.138 | ||
| Diabetes mellitus | Yes | 86 | 20 (23.3%) | 2.72 (1.53, 4.82) | <0.001 | 2.53 (1.41, 4.56) | 0.002 |
| Valvular disease | Yes | 9 | 1 (11.1%) | 1.29 (0.20, 8.14) | 0.790 | ||
| Arrhythmia | Yes | 42 | 9 (21.4%) | 2.21 (1.02, 4.80) | 0.044 | ||
| Cardiomyopathy | Yes | 5 | 0 (0.0%) | 0.66 (0.03, 15.80) | 0.795 | ||
| Neurological disorders | Yes | 29 | 4 (13.8%) | 1.30 (0.45, 3.71) | 0.627 | ||
| Post surgery of upper digestive system | Yes | 12 | 3 (25.0%) | 2.75 (0.75, 10.11) | 0.127 | ||
| Chronic liver diseases | Yes | 12 | 3 (25.0%) | 2.75 (0.75, 10.11) | 0.127 | ||
| Connective tissue diseases | Yes | 34 | 2 (5.9%) | 0.54 (0.14, 2.05) | 0.366 | ||
| Primary immunodeficiency disease | Yes | 1 | 0 (0.0%) | 2.42 (0.03, 225.72) | 0.703 | ||
| Immunosuppression due to systemic | Yes | 41 | 4 (9.8%) | 0.86 (0.31, 2.39) | 0.774 | ||
| corticosteroids or immunosuppressants | |||||||
| Psychiatric disorders | Yes | 13 | 3 (23.1%) | 2.49 (0.69, 8.94) | 0.163 | ||
| Malignancy | Yes | 16 | 2 (12.5%) | 1.26 (0.31, 5.13) | 0.749 | ||
| Alcoholism | Yes | 3 | 0 (0.0%) | 1.04 (0.03, 31.97) | 0.983 | ||
| CKD | Yes | 7 | 1 (14.3%) | 1.69 (0.25, 11.43) | 0.593 | ||
| Smoking history | Yes | 391 | 50 (12.8%) | 1.23 (0.73, 2.09) | 0.439 | ||
| Long-term oxygen therapy | Yes | 39 | 2 (5.1%) | 0.46 (0.12, 1.74) | 0.255 | ||
| CAP/HCAP | HCAP | 191 | 18 (9.4%) | 0.71 (0.40, 1.24) | 0.232 | ||
| Performance status | Unknown | 0 | 0 (-%) | ||||
| 3-4 | 28 | 4 (14.3%) | 1.24 (0.43, 3.62) | 0.692 | |||
| 1-2 | 218 | 23 (10.6%) | 0.81 (0.48, 1.38) | 0.442 | |||
| 0 | 352 | 45 (12.8%) | 1 | ||||
| Rhabdomyolysis | Yes | 18 | 8 (44.4%) | 6.51 (2.48, 17.08) | <0.001 | ||
| Blood urea nitrogen ≥20 mg/dL | Yes | 169 | 23 (13.6%) | 1.24 (0.73, 2.10) | 0.425 | ||
| Procalcitonin | 161 | 1.04 (1.00, 1.08) | 0.080 | ||||
| C-reactive protein | 600 | 1.06 (1.03, 1.08) | <0.001 | ||||
| Bacteremia | Yes | 16 | 2 (12.5%) | 1.26 (0.31, 5.13) | 0.749 | ||
| Disease severity on admission | Severe | 75 | 19 (25.3%) | 3.05 (1.69, 5.50) | <0.001 | 2.86 (1.57, 5.22) | 0.001 |
| Concordance with CAP guideline-recommended treatment regimen | Discordant | 57 | 4 (7.0%) | 0.58 (0.21, 1.59) | 0.293 | ||
| Concordant | 543 | 68 (12.5%) | 1 | ||||
| Number of antibiotics | Monotherapy | 108 | 7 (6.5%) | 0.48 (0.22, 1.06) | 0.070 | ||
| ≥2 drugs | 492 | 65 (13.2%) | 1 | ||||
Values represent p value for category against the reference. CAP: community-acquired pneumonia, CI: confidence interval, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, HCAP: healthcare-associated pneumonia, NTM: nontuberculous mycobacteriosis, OR: odds ratio
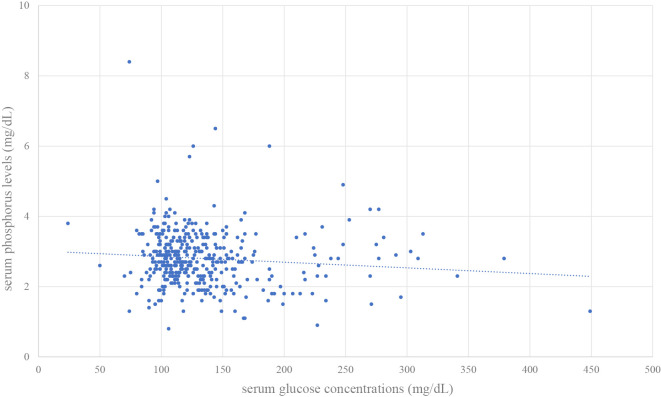
In the 398 cases in which serum glucose and phosphorus were measured simultaneously, we created a scatter plot of blood glucose and phosphorus levels (Figure). The Pearson's product rate correlation coefficient was -0.098.
Figure.
Scatter plot of serum glucose and phosphorus levels in the 398 cases in which the levels were measured simultaneously. The Pearson’s product rate correlation coefficient was -0.098.
The subspecies of Legionella in the cases of pneumonia with hypophosphatemia were L. pneumophila serogroup 1 in two cases, L. pneumophila serogroup 2 in one case, L. longbeachae in no cases, and unknown in five cases. The subspecies in the cases without hypophosphatemia were L. pneumophila serogroup 1 in 5 cases, L. pneumophila serogroup 2 in 0 cases, L. longbeachae in 1 case, and unknown in 13 cases.
Risk factors for mortality
The results of the univariable and multivariable logistic regression analyses for morality are given in Table 5. Univariable analyses showed that advanced age (≥65 years old) (OR, 3.77; 95% CI, 1.00 to 14.24; p=0.050), interstitial pneumonia (OR, 3.15; 95% CI, 1.15 to 8.62; p=0.025), hypertension (OR, 2.76; 95% CI, 1.12 to 6.81; p=0.028), long-term oxygen therapy (OR, 3.72; 95% CI, 1.24 to 11.14; p=0.019), PS (ECOG 3-4) (OR, 7.40; 95% CI, 2.57 to 21.32; p<0.001), rhabdomyolysis (OR, 6.53; 95% CI, 1.82 to 23.34; p=0.004), blood urea nitrogen (≥20 mg/dL) (OR, 4.65; 95% CI, 1.95 to 11.07; p<0.001), procalcitonin (OR, 1.06; 95% CI, 1.01 to 1.12; p=0.016), CRP (OR, 1.06; 95% CI, 1.02 to 1.09; p=0.002), bacteremia (OR, 21.33; 95% CI, 6.95 to 65.44; p<0.001), disease severity on admission (OR, 11.74; 95% CI, 4.90 to 28.13; p<0.001), and concordance with guideline-recommended treatment (i.e., discordant therapy) (OR, 3.15; 95% CI, 1.15 to 8.62; p=0.025) were independent factors. Next, multivariable analyses revealed that interstitial pneumonia (OR, 7.06; 95% CI, 2.12 to 23.46; p=0.001), PS (ECOG 3-4) (OR, 11.48; 95% CI, 3.32 to 39.72; p<0.001), bacteremia (OR, 11.80; 95% CI, 2.77 to 50.18; p=0.001), disease severity on admission (OR, 7.11; 95% CI, 2.53 to 20.01; p<0.001), and discordant therapy (OR, 4.46; 95% CI, 1.38 to 14.36; p=0.012) were independent factors associated with mortality.
Table 5.
Logistic Regression Analysis of the Risk of In-hospital Mortality in the Study Patients.
| n | Non-survivors (%) | Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | ||||
| Sex | Male | 425 | 15 (3.5%) | 0.85 (0.35, 2.07) | 0.718 | ||
| Age (years) | 65- | 416 | 20 (4.8%) | 3.77 (1.00, 14.24) | 0.050 | 1.53 (0.37, 6.38) | 0.558 |
| <65 | 184 | 2 (1.1%) | 1 | 1 | |||
| Vaccination history | |||||||
| 23-valent pneumococcal polysaccharide | Unknown | 15 | 1 (6.7%) | 1.88 (0.24, 14.54) | 0.546 | ||
| vaccination within 5 years | No | 519 | 18 (3.5%) | 0.67 (0.21, 2.18) | 0.505 | ||
| Influenza vaccination within one year | Unknown | 220 | 5 (2.3%) | 0.90 (0.23, 3.52) | 0.880 | ||
| No | 254 | 14 (5.5%) | 2.13 (0.65, 7.00) | 0.214 | |||
| Prior antibiotic treatment | No | 363 | 17 (4.7%) | 2.10 (0.79, 5.56) | 0.136 | ||
| Identified pathogen | Yes | 304 | 12 (3.9%) | 1.17 (0.50, 2.70) | 0.719 | ||
| Unidentified pathogen | Yes | 294 | 9 (3.1%) | 0.72 (0.31, 1.69) | 0.454 | ||
| Streptococcus pneumoniae | Yes | 112 | 5 (4.5%) | 1.38 (0.52, 3.69) | 0.522 | ||
| Legionella spp. | Yes | 27 | 0 (0.0%) | 0.45 (0.03, 7.93) | 0.582 | ||
| Influenza virus | Yes | 70 | 5 (7.1%) | 2.46 (0.91, 6.68) | 0.076 | ||
| Virus (other than influenza virus) | Yes | 20 | 0 (0.0%) | 0.60 (0.03, 11.07) | 0.735 | ||
| Polymicrobial infection | Yes | 53 | 3 (5.7%) | 1.88 (0.58, 6.13) | 0.297 | ||
| Comorbidities | |||||||
| Chronic pulmonary diseases | Yes | 321 | 15 (4.7%) | 1.84 (0.76, 4.47) | 0.180 | ||
| COPD | Yes | 158 | 7 (4.4%) | 1.37 (0.56, 3.34) | 0.494 | ||
| Asthma | Yes | 56 | 2 (3.6%) | 1.17 (0.30, 4.54) | 0.817 | ||
| Bronchiectasis | Yes | 32 | 1 (3.1%) | 1.21 (0.22, 6.76) | 0.826 | ||
| Pulmonary NTM | Yes | 37 | 3 (8.1%) | 2.83 (0.85, 9.43) | 0.090 | ||
| Old tuberculosis | Yes | 21 | 1 (4.8%) | 1.90 (0.33, 10.94) | 0.473 | ||
| Chronic pulmonary aspergillosis | Yes | 9 | 1 (11.1%) | 4.68 (0.72, 30.68) | 0.107 | ||
| Interstitial pneumonia | Yes | 57 | 5 (8.8%) | 3.15 (1.15, 8.62) | 0.025 | 7.06 (2.12, 23.46) | 0.001 |
| Lung cancer surgery | Yes | 16 | 1 (6.3%) | 2.54 (0.43, 15.05) | 0.305 | ||
| Pneumoconiosis | Yes | 2 | 0 (0.0%) | 5.13 (0.12, 216.23) | 0.392 | ||
| Chronic empyema | Yes | 2 | 0 (0.0%) | 5.13 (0.12, 216.23) | 0.392 | ||
| Chronic non-pulmonary diseases | Yes | 312 | 15 (4.8%) | 1.96 (0.80, 4.75) | 0.139 | ||
| Hypertension | Yes | 93 | 7 (7.5%) | 2.76 (1.12, 6.81) | 0.028 | ||
| Congestive heart failure | Yes | 24 | 2 (8.3%) | 3.02 (0.74, 12.29) | 0.123 | ||
| Ischemic heart diseases | Yes | 24 | 2 (8.3%) | 3.02 (0.74, 12.29) | 0.123 | ||
| Diabetes mellitus | Yes | 86 | 2 (2.3%) | 0.71 (0.19, 2.73) | 0.622 | ||
| Valvular disease | Yes | 9 | 0 (0.0%) | 1.33 (0.06, 27.49) | 0.852 | ||
| Arrhythmia | Yes | 42 | 1 (2.4%) | 0.90 (0.16, 4.97) | 0.907 | ||
| Cardiomyopathy | Yes | 5 | 0 (0.0%) | 2.32 (0.09, 56.78) | 0.606 | ||
| Neurological disorders | Yes | 29 | 2 (6.9%) | 2.44 (0.61, 9.80) | 0.207 | ||
| Post surgery of upper digestive system | Yes | 12 | 1 (8.3%) | 3.44 (0.56, 21.31) | 0.184 | ||
| Chronic liver diseases | Yes | 12 | 0 (0.0%) | 1.01 (0.05, 19.67) | 0.996 | ||
| Connective tissue diseases | Yes | 34 | 1 (2.9%) | 1.14 (0.20, 6.31) | 0.884 | ||
| Primary immunodeficiency disease | Yes | 1 | 0 (0.0%) | 8.43 (0.09, 807.94) | 0.360 | ||
| Immunosuppression due to systemic | Yes | 41 | 3 (7.3%) | 2.52 (0.76, 8.33) | 0.130 | ||
| corticosteroids or immunosuppressants | |||||||
| Psychiatric disorders | Yes | 13 | 1 (7.7%) | 3.16 (0.52, 19.31) | 0.212 | ||
| Malignancy | Yes | 16 | 0 (0.0%) | 0.76 (0.04, 14.20) | 0.852 | ||
| Alcoholism | Yes | 3 | 0 (0.0%) | 3.65 (0.12, 114.85) | 0.462 | ||
| CKD | Yes | 7 | 0 (0.0%) | 1.70 (0.08, 37.17) | 0.738 | ||
| Smoking history | Yes | 391 | 11 (2.8%) | 0.52 (0.23, 1.20) | 0.127 | ||
| Long-term oxygen therapy | Yes | 39 | 4 (10.3%) | 3.72 (1.24, 11.14) | 0.019 | ||
| Healthcare contacts | Yes | 191 | 11 (5.8%) | 2.16 (0.94, 5.00) | 0.071 | ||
| Performance status | Unknown | 0 | 0 (-%) | ||||
| 3-4 | 28 | 5 (17.9%) | 7.40 (2.57, 21.32) | <0.001 | 11.48 (3.32, 39.72) | <0.001 | |
| 0-2 | 570 | 17 (3.0%) | 1 | 1 | |||
| Rhabdomyolysis | Yes | 18 | 3 (16.7%) | 6.53 (1.82, 23.34) | 0.004 | 3.54 (0.70, 17.91) | 0.126 |
| Blood urea nitrogen (≥20 mg/dL) | Yes | 169 | 14 (8.3%) | 4.65 (1.95, 11.07) | <0.001 | ||
| Hypophosphatemia | 72 | 5 (6.9%) | 2.38 (0.88, 6.45) | 0.088 | 2.28 (0.72, 7.27) | 0.163 | |
| Procalcitonin | 161 | 1.06 (1.01, 1.12) | 0.016 | ||||
| C-reactive protein | Yes | 600 | 1.06 (1.02, 1.09) | 0.002 | |||
| Bacteremia | Yes | 16 | 6 (37.5%) | 21.33 (6.95, 65.44) | <0.001 | 11.80 (2.77, 50.18) | 0.001 |
| Disease severity on admission | Severe | 75 | 13 (17.3%) | 11.74 (4.90, 28.13) | <0.001 | 7.11 (2.53, 20.01) | <0.001 |
| Concordance with CAP guideline-recommended treatment regimen | Discordant | 57 | 5 (8.8%) | 3.15 (1.15, 8.62) | 0.025 | 4.46 (1.38, 14.36) | 0.012 |
| Concordant | 543 | 17 (3.1%) | 1 | 1 | |||
| Number of antibiotics | Monotherapy | 108 | 6 (5.6%) | 1.83 (0.72, 4.67) | 0.205 | ||
| ≥2 drugs | 492 | 16 (3.3%) | 1 | ||||
Values represent p value for category against the reference. CAP: community-acquired pneumonia, CI: confidence interval, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, HCAP: healthcare-associated pneumonia, NTM: nontuberculous mycobacteriosis, OR: odds ratio
Discussion
The most frequently isolated pathogen in the hypophosphatemia patients was S. pneumoniae, followed by Legionella spp. and influenza virus. In severe cases, however, influenza virus was the most common. Legionella spp., diabetes mellitus, and severe pneumonia were independent factors for hypophosphatemia in the multivariable analysis. Impaired PS, disease severity, interstitial pneumonia, bacteremia, and concordance with guideline-recommended treatment (i.e. discordant therapy) were independent factors associated with mortality in the multivariable analysis. Hypophosphatemia was not significantly associated with mortality but showed a trend towards an increased mortality.
Several different pathogens have been reported in patients with hypophosphatemia associated with pneumonia (4-7). In the present study, Legionella spp. was the only pathogen associated with hypophosphatemia, independent of the other factors in the patients with pneumonia. The main mechanism of hypophosphatemia in Legionella pneumonia is speculated to be increased renal phosphate excretion (4), and the following three sub-mechanisms have been suggested (16): rhabdomyolysis- and myoglobinuria-induced tubular injury, direct Legionella infection in the renal tubules, and a complication of acute tubulointerstitial nephritis. Legionella spp. are pathogens that account for 1-8% of pneumonias and often causes severe pneumonia (17-19). Therefore, Legionella pneumonia needs to be diagnosed and treated promptly with appropriate antibiotics. A urinary antigen test is used for the rapid diagnosis of Legionella, but it is important to predict Legionella pneumonia because the sensitivity of this test is not sufficient. Multiple scoring systems have been proposed to predict Legionella spp. as pathogens of pneumonia (20-23). Among them, however, only one scoring system includes hypophosphatemia (22). Our study suggested that hypophosphatemia may be a laboratory finding that contributes to the prediction of Legionella pneumonia. It would be desirable to construct a highly accurate scoring system that includes hypophosphatemia. The relationship between Legionella subspecies and hypophosphatemia has not been reported before to our knowledge. Statistical analysis was not possible in this study because of the small number of cases, so further studies with a larger number of cases will be required in the future.
In the present study, diabetes mellitus was significantly associated with hypophosphatemia. Elevated serum glucose concentrations depolarize the transmembrane electrochemical Na+ gradient of the brush border membrane for inorganic phosphate entry into the tubular cells and decrease intracellular phosphate levels, leading to hyperphosphaturia (24,25). A previous study showed that hypophosphatemic patients with CAP manifested higher levels of serum glucose (26), suggesting phosphorus metabolism in diabetic patients. However, stress hyperglycemia, defined as a transient increase in the blood glucose concentration during acute physiological illness, is observed in patients with CAP (27,28). Hypophosphatemia may reflect the effects of hyperglycemia associated with pneumoniae itself as well as underlying diabetes mellitus. A previous study showed hyperglycemia to be associated with a poor outcome from CAP (29). Furthermore, another study reported that undiagnosed diabetes mellitus was prevalent among patients with CAP (27) and was associated with a poor 180-day survival rate. These findings suggest the importance of the prompt recognition of abnormal glucose metabolism in CAP when hypophosphatemia is observed in a patient with CAP. However, the Pearson's correlation coefficient was -0.098 in the scatter plot of plasma phosphorus and blood glucose levels in this study, suggesting that the plasma phosphorus level may be an independently important electrolyte because plasma phosphorus does not necessarily correlate with the blood glucose level measured at the same time.
Redistribution across the cell membrane is reported to be the most common cause of hypophosphatemia in severe cases (30). High levels of catecholamines in serum, stimulation of carbohydrate metabolism by the actions of blood glucose and insulin (31), and respiratory alkalosis (32) cause an intracellular shift of phosphorus in such patients. Hypophosphatemia appears early in severe cases and has been reported to be associated with mortality (33-35). A previous study showed a higher mortality in hypophosphatemic patients with pneumonia than in those with normophosphatemia (26). In the present study, hypophosphatemia was more common in severe cases and was not significantly associated with in-hospital mortality. In a previous retrospective study, severe hypophosphatemia (<1.0 mg/dL) was reported to be an independent predictor of mortality in sepsis (35). However, there were only three cases of severe hypophosphatemia in the present study. The degree of hypophosphatemia in patients with pneumonia may be related to the severity and prognosis of the disease, but the number of cases of severe hypophosphatemia was small, making it difficult to evaluate. Because the numbers of severely ill patients and deaths were limited in the present study, it will be necessary to increase the number of cases and study this further in the future. However, as hypophosphatemia was not significantly associated with the prognosis in the univariate analysis, it may be more important as a marker of metabolic disorders, severity, and specific pathogen infections than as a factor affecting the prognosis itself.
In the present study, 8 (44.4%) of the 18 patients with rhabdomyolysis showed hypophosphatemia, and rhabdomyolysis was associated with hypophosphatemia in a univariable analysis. Hypophosphatemia can cause rhabdomyolysis (36) by mechanisms such as impaired mitochondrial respiration and oxidative phosphorylation in skeletal muscle (37,38). The limited number of patients with rhabdomyolysis may be a reason that neither hypophosphatemia nor rhabdomyolysis showed a significant association with mortality in our multivariable analysis.
Several limitations associated with the present study warrant mention. First, because this was a retrospective, observational study, the level of confidence is reduced, and a complete diagnostic workup to determine etiology was not possible in every patient. Second, this was a single-center study, and the results may not be applicable in other settings. Finally, our study was limited to patients in whom serum phosphorus was measured, so there may have been some bias in the patient selection.
Conclusion
The significance for clinicians of hypophosphatemia in patients with pneumonia lies in the laboratory findings that predict abnormal glucose metabolism, Legionella infection, and severe disease. However, hypophosphatemia was not associated with the prognosis in our patients with pneumonia. Patient characteristics (PS, comorbidities), laboratory findings (bacteremia), disease severity on admission, and antimicrobial treatment after admission contributed to their prognosis.
The authors state that they have no Conflict of Interest (COI).
Financial Support
Saitama Cardiovascular and Respiratory Center Funding, E-16, E-17, E-18, E-19.
References
- 1.Florenzano P, Cipriani C, Roszko KL, et al. Approach to patients with hypophosphatemia. Lancet Diabetes Endocrinol 8: 163-174, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Gaasbeek A, Meinders AE. Hypophosphatemia: an update on its etiology and treatment. Am J Med 118: 1094-1101, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Amanzadeh J, Reilly RF Jr. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol 2: 136-148, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe S, Kono K, Fujii H, Nakai K, Goto S, Nishi S. Two cases of hypophosphatemia with increased renal phosphate excretion in Legionella pneumonia. Case Rep Nephrol Dial 6: 40-45, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soh JXJ, Goh RKH, Zheng S. Invasive pneumococcal disease associated with Fanconi-like syndrome. Eur J Case Rep Intern Med 6: 001230, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunha BA. Adenovirus pneumonia mimicking Legionnaire's disease with acute pancreatitis. Conn Med 80: 347-348, 2016. [PubMed] [Google Scholar]
- 7.Cunha BA, Irshad N, Connolly JJ. Adult human metapneumonovirus (hMPV) pneumonia mimicking Legionnaire's disease. Heart Lung 45: 270-272, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiguro T, Takayanagi N, Yamaguchi S, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med 52: 317-324, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 200: e45-e67, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seino Y, Nanjo K, Tajima N, et al. ; Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 19: 212-228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takayanagi N, Tokunaga D, Kubota M, et al. Community-acquired pneumonia with rhabdomyolysis. Nihon Kokyuki Gakkai Zasshi 43: 731-735, 2005. [PubMed] [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655, 1982. [PubMed] [Google Scholar]
- 13. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171: 388-416, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. John Wiley & Sons, Hoboken, NJ, 2013. [Google Scholar]
- 15.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 21: 2409-2419, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Koda R, Itoh R, Tsuchida M, et al. Legionella pneumonia complicated with acquired Fanconi syndrome. Intern Med 57: 2975-2980, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med 333: 1618-1624, 1995. [DOI] [PubMed] [Google Scholar]
- 18.File TM. Community-acquired pneumonia. Lancet 362: 1991-2001, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373: 415-427, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha BA. Hypophosphatemia: diagnostic significance in Legionnaire's disease. Am J Med 119: e5-e6, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Sabé N, Rosón B, Carratalà J, Dorca J, Manresa F, Gudiol F. Clinical diagnosis of Legionella pneumonia revisited: evaluation of the community-based pneumonia incidence study group scoring system. Clin Infect Dis 37: 483-489, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Cunha BA. Severe Legionella pneumonia: rapid presumptive clinical diagnosis with Winthrop-University Hospital's weighted point score system (modified). Heart Lung 37: 311-320, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiumefreddo R, Zaborsky R, Haeuptle J, et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med 9: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ditzel J, Brøchner-Mortensen J, Kawahara R. Dysfunction of tubular phosphate reabsorption related to glomerular filtration and blood glucose control in diabetic children. Diabetologia 23: 406-410, 1982. [DOI] [PubMed] [Google Scholar]
- 25.Ditzel J, Lervang HH. Disturbance of inorganic phosphate metabolism in diabetes mellitus: its impact on the development of diabetic late complications. Curr Diabetes Rev 6: 323-333, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Sankaran RT, Mattana J, Pollack S, et al. Laboratory abnormalities in patients with bacterial pneumonia. Chest 111: 595-600, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Jensen AV, Fauholt-Jensen D, Egelund GB, et al. Undiagnosed diabetes mellitus in community-acquired pneumonia: a prospective cohort study. Clin Infect Dis 65: 2091-2098, 2017. [DOI] [PubMed] [Google Scholar]
- 28.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 87: 978-982, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ 344: 3397, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE, Schultzt MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care 147: 1-8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunelli SM, Goldfarb S. Hypophosphatemia: clinical consequences and management. J Am Soc Nephrol 18: 1999-2003, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Brautbar N, Leubovici H, Massry SG. On the mechanism of hypophosphatemia during acute hyperventilation: evidence for an increased muscle glycolysis. Miner Electrolyte Metab 9: 45-50, 1983. [PubMed] [Google Scholar]
- 33.Brotfain E, Schwartz A, Boniel A, et al. Clinical outcome of critically ill patients with thrombocytopenia and hypophosphatemia in the early stage of sepsis. Anaesthesiol Intensive Ther 48: 294-299, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki S, Egi M, Schneider AG, et al. Hypophosphatemia in critically ill patients. J Crit Care 28: 536-543, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Shor R, Halabe A, Rishver S, et al. Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci 36: 67-72, 2006. [PubMed] [Google Scholar]
- 36.Amanzadeh J, Reilly RF Jr. Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol 2: 136-148, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Knochel JP, Barcenas C, Cotton JR, Fuller TJ, Haller R, Carter NW. Hypophosphatemia and rhabdomyolysis. J Clin Invest 62: 1240-1246, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brautbar N, Carpenter C, Baczynski R, Kohan R, Massry SG. Impaired energy metabolism in skeletal muscle during phosphate depletion. Kidney Int 24: 53-57, 1983. [DOI] [PubMed] [Google Scholar]