Abstract
Objective
For risk stratification of sudden cardiac death in patients with structural heart disease, more precise predictors in addition to left ventricular ejection fraction (LVEF) are clinically needed. The present study assessed the utility of galectin-3 as an independent indicator for the prognosis of heart failure patients with implantable cardioverter-defibrillators (ICD).
Methods
The study population consisted of 91 consecutive patients who underwent a routine ICD checkup in our ICD outpatient clinic. Circulating galectin-3 was assessed using a commercially available enzyme-linked immunosorbent assay kit. The enrolled patients were prospectively followed. The primary endpoint was defined as the occurrence of appropriate ICD therapy (AIT), and the secondary endpoint was defined as the occurrence of unplanned overnight hospitalization due to decompensated heart failure (dHF).
Results
During a mean follow-up of 472±107 days, AIT occurred in 18 patients (20%). Unplanned hospitalizations due to dHF were noted in 12 patients (13%). A receiver-operative characteristics analysis demonstrated a sensitivity of 83% and specificity of 68% for AIT occurrences with a galectin-3 cut-off value of 13.1 ng/mL (area under the curve =0.82). A Kaplan-Meier analysis demonstrated that patients with galectin-3 >13.1 ng/mL had significantly higher incidences of AIT as compared to those with lower galectin-3 (log-rank, p<0.001). This significance was also observed in both subgroup analyses with ischemic and non-ischemic etiology. Cox regression demonstrated that higher galectin-3 was an independent predictor of AIT and dHF, even after adjusting for previous arrhythmic events.
Conclusion
The circulating galectin-3 level can be used as a clinical indicator of subsequent occurrence of ventricular arrhythmic events and decompensated heart failure, regardless of a history of ventricular arrhythmias.
Keywords: ventricular tachycardia, heart failure, galectin-3, implantable cardioverter-defibrillator, ischemic cardiomyopathy, non-ischemic cardiomyopathy
Introduction
Implantable cardioverter-defibrillators (ICDs) have been standard therapy to prevent sudden cardiac death in patients with structural heart disease, particularly heart failure (HF) patients, for at least a quarter of a century. At present, the left ventricular ejection fraction (LVEF) is still the most important indicator for the implantation of ICDs (1). Due to possible long-term complications caused by ICDs, more reliable and efficient risk-predictors are clinically needed. Various circulating markers and risk stratifiers based on imaging modalities have been reported and proposed, although conclusive recommendations, e.g. to exclude the necessity of ICD implantation, have yet to be obtained in clinical practice (2-4).
Given the above, a circulating serum marker to predict ventricular arrhythmic risk that can be easily tested may enable us to initiate intensified therapies before a clinical event occurs.
Galectin-3 (Gal-3) has been reported to be a possible prognostic marker of HF and cardiac arrhythmia (5-7). The recent guidelines for HF therapy give Gal-3 measurement a Class IIb indication, reflecting the disputable usefulness according to reports (8-10). As a risk predictor of ventricular arrhythmias, Gal-3 has been reported to be useful in patients with primary prophylactic ICDs; however, its utility in both patients with primary and secondary prophylactic ICDs remains to be clarified (4).
We hypothesize that circulating Gal-3 predicts ventricular arrhythmic events independent of the primary or secondary prophylaxis ICD indication in patients with HF. Thus far, the usability of Gal-3 independent of prior ventricular arrhythmic events has not yet been assessed. Therefore, in the present study, we evaluated the utility of Gal-3 as a ventricular arrhythmic marker rather than as a marker for HF decompensation.
Materials and Methods
Study population
We consecutively enrolled patients who presented to our device clinics for routine follow-up between September 2016 and March 2017 and who had already undergone ICD implantation for primary prevention according to the current guidelines at least six months prior to the index presentation (1,11). Blood samples were obtained only once at enrollment, and all ICDs have already been implanted in all patients for at least six months at the time of phlebotomy.
Participants were eligible for inclusion if they had received optimal medical therapy for HF for at least three months. Patients were excluded if they had undergone ICD implantation within the prior six months. Patients who had worsening HF or myocardial infarction within the prior six months or who had undergone catheter ablation for ventricular arrhythmias within the prior six months were also excluded.
The study protocol was approved by the Medical Ethics Commission of the University Hospital Düsseldorf. All participants gave their written informed consent in accordance with the Declaration of Helsinki.
Regarding our calculation of the necessary number of patients in the present study, for the patients with elevated Gal-3 concentrations, we estimated the ventricular arrhythmic incidence (primary endpoint) as 40%, and for the remaining patients with a reduced Gal-3 concentration, we estimated the incidence as 10%. With a 10% drop-out rate, 90 participants were calculated to be necessary (statistical power of 0.9).
Blood samples and laboratory assays
Blood samples were collected from a peripheral vein and immediately centrifuged and stored in serum-containing tubes at -80 °C until being assayed. The serum Gal-3 level was measured using commercially available enzyme-linked immunosorbent assay kits (eBioscience, Europe/International, Vienna, Austria), according to the manufacturer's instructions at the University Hospital Düsseldorf. Serum samples underwent a maximum of two freeze-thaw cycles before analyses.
Device programming and interrogation
All devices were programmed as per protocol according to the institutional standard and the current consensus statement at the time (12). All participants were followed up routinely in accordance with the institutional standard. At each visit, the participants were clinically assessed, and the devices were evaluated.
Adjudication of events and therapy
All incidences of ICD therapies were assessed based upon intracardiac electrograms. To adjudicate the arrhythmias, two electrophysiologists categorized the underlying cause/rhythm present at the time of intervention. If the two electrophysiologists did not reach an agreement, an additional electrophysiologist participated in the arrhythmia assessment.
Appropriate ICD therapy (AIT) was defined as anti-tachykardia pacing (ATP) or shock rendered for ventricular tachykardia (VT) or ventricular fibrillation (VF). Inappropriate ICD therapy was defined as ATP or shock rendered when VT or VF was not present.
Clinical endpoints
Prospective patient follow-up started after enrollment. Patients were followed-up routinely in our outpatient clinic. Follow-up data, including clinical parameters, as well as device parameters and the occurrence of ICD therapies were collected in a prospective database [Düsseldorf University Device Registry (ClinicalTrials.gov, NCT03360227)].
The primary endpoint of this study was the occurrence of sustained (>30 seconds) VT/VF or AIT during follow-up. The time from enrollment to the occurrence of any sustained VT/VF (>30 seconds) or AIT was recorded. Rhythm documentation of VT/VF was obtained from the ICD storage or an electrocardiogram.
The secondary endpoint was defined as the occurrence of unplanned hospitalization due to worsening HF. Unplanned hospitalization due to worsening HF was defined as an unplanned overnight hospital admission due to worsening of subjective/objective signs of congestive HF and the necessity of adjunctive therapies including an increase of oral diuretics, intravenous administration of diuretics, vasodilator and inotropic agents. The signs of congestive HF included pulmonary rales, worsening dyspnea, peripheral edema, increased N-terminal pro-brain natriuretic peptide (NT-proBNP) above baseline or radiological evidence of pulmonary congestion.
Statistical analyses
Continuous data are shown as the mean ± standard deviation (SD) for normally distributed data. Non-normally distributed data are shown as the median values (lower-upper quartile). Categorical data are shown as numbers and percentages. The chi-square test, Kruskal-Wallis test, Student's t-test or Fisher's exact test was performed when appropriate. To dichotomize our cohort with regard to Gal-3, we performed a receiver operating characteristic (ROC) analysis of the primary endpoint and determined the cut-off value of Gal-3 using Youden's J-statistic. Time to events was analyzed according to the Kaplan-Meier method and were compared using the log-rank test. To evaluate the association of clinical baseline variables with the primary or secondary endpoints, a Cox regression analysis was performed. After verifying that the proportional hazard assumption was satisfied, a multivariable Cox regression analysis was conducted that incorporated all variables with a p value <0.1 in the univariable analysis. For global test statistics, we used a significance level of 5%. Analyses were performed using the JMP software program, Version 11 (SAS Institute, Cary, USA).
Results
Patient population and distribution of Gal-3
During the enrollment period, we found 111 eligible patients. After 11 patients ultimately declined participation, we were left with 100 patients recruited to the present study. A total of 91 consecutive patients were able to be analyzed after 9 dropped out due to hemolysis. The baseline patient characteristics are shown in Table 1. The mean age of the study population was 67.4±12.5 years old, and 78% were men. Of the 91 patients, 55 (60%) had ischemic cardiomyopathy, and the mean LVEF was 35.8%±10.2%. In 19 (21%) patients, at least 1 previous episode of appropriate ICD intervention was noted. Forty-four patients (48%) had a single-chamber ICD, 21 (23%) had a dual-chamber ICD, 24 (26%) had a cardiac resynchronization therapy-defibrillator (CRT-D), and 2 (2%) had a subcutaneous ICD.
Table 1.
Baseline Patient Characteristics.
| n=91 | ||
|---|---|---|
| Age | 67.4±12.5 | |
| Male | 71 (78%) | |
| Hypertension | 62 (68%) | |
| Diabetes mellitus | 29 (32%) | |
| Ischemic etiology | 55 (60%) | |
| Atrial fibrillation | 43 (47%) | |
| LVEF (%) | 35.8±10.2 | |
| NYHA classification I/II/III/IV | 40/38/13/0 | |
| Estimated glomerular filtration rate (mL/min/1.73m2) | 66.2±19.6 | |
| Previous history of appropriate ICD therapy | 19 (21%) | |
| ACEi or ARB | 81 (89%) | |
| β-blocker | 83 (91%) | |
| Amiodarone | 12 (13%) | |
| Galectin-3 (ng/mL) | 12.4±3.8 | |
| NT-proBNP (pg/mL) | 984.5 (359-2,272) (n=86) |
ACEi: angiotensin-converting-enzyme inhibitor, ARB: angiotensin II receptor blocker, ICD: implantable cardioverter-defibrillator, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association
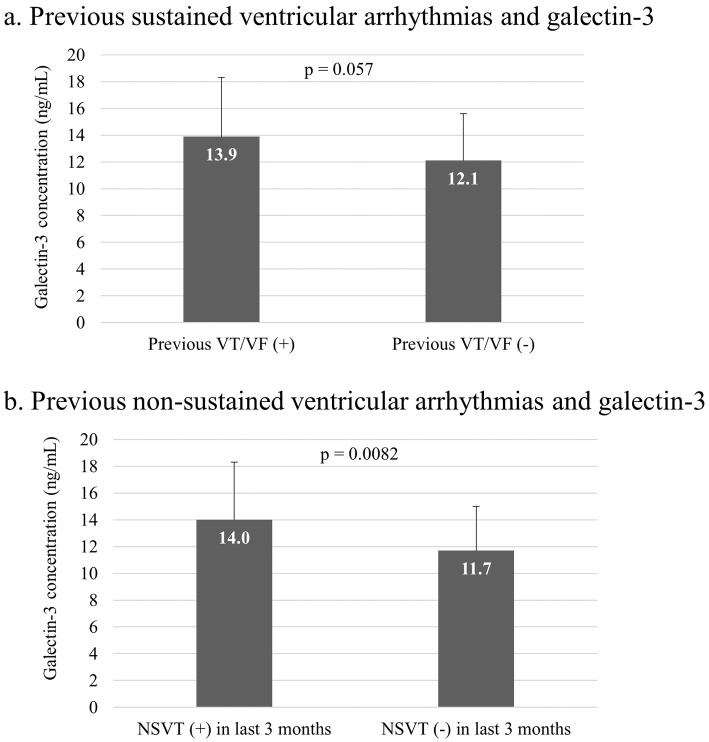
The mean value of Gal-3 was 12.4±3.8 ng/mL. In Figs. 1a and b, the Gal-3 values in patients with and without a history of AIT and in patients with and without documentation of non-sustained VT (NSVT) within the last three months according to the storage of the implanted devices are shown. Gal-3 tended to be higher in the patients with a history of AIT than in those without a history (13.9±4.4 vs. 12.1±3.5 ng/mL, p=0.057). Gal-3 levels in patients with documented NSVT within 3 months were significantly higher than in those without such documentation (14.0±4.3 vs. 11.7±3.3 ng/mL, p=0.0082).
Figure 1.
Ventricular arrhythmias and galectin-3. a: Circulating galectin-3 (Gal-3) levels in patients with precedent sustained ventricular tachykardia (VT), ventricular fibrillation and appropriate ICD intervention tended to be higher than in patients without such a history (13.9±4.4 vs. 12.1±3.5 ng/mL, p=0.057). b: The documentation of non-sustained VT (NSVT) within 3 months prior to enrollment was associated with higher circulating Gal-3 levels (14.0±4.3 vs. 11.7±3.3 ng/mL, p=0.0082).
Primary endpoint
During the follow-up period of 472±107 days, AIT was observed in 18 patients (20%). Of these 18 patients, 17 (94%) experienced ATP, and 6 (33%) experienced shock therapy. Five patients (29%) experienced both ATP and shock therapy. One patient experienced only shock therapy due to VF, and the remaining 12 only received ATP therapy. The patients' characteristics according to AIT occurrence are shown in Table 2. A history of AIT before enrollment was significantly more frequent in those who experienced AIT during follow-up than in those without such experience [8/18 (44%) vs. 11/73 (15%), p=0.006]. The patients who experienced subsequent AIT had a significantly higher Gal-3 concentration at enrollment in the present study than those without subsequent AIT. The other characteristics did not differ significantly between the patients with and without AIT during follow-up.
Table 2.
Patients’ Characteristics according to Appropriate ICD Therapy.
| AIT (+) (n=18) |
AIT (-) (n=73) |
p value | ||||
|---|---|---|---|---|---|---|
| Age | 63.1±12.7 | 68.5±12.2 | 0.10 | |||
| Male | 17 (94%) | 54 (74%) | 0.06 | |||
| Hypertension | 11 (61%) | 51 (70%) | 0.48 | |||
| Diabetes mellitus | 5 (28%) | 24 (33%) | 0.68 | |||
| Ischemic etiology | 13 (72%) | 42 (58%) | 0.25 | |||
| Atrial fibrillation | 7 (39%) | 37 (51%) | 0.37 | |||
| LVEF (%) | 32.0±7.6 | 36.8±10.5 | 0.07 | |||
| NYHA classification I/II/III/IV | 10/4/4/0 | 30/34/9/0 | n.a. | |||
| Estimated glomerular filtration rate (mL/min/1.73m2) | 67.1±16.4 | 66.0±20.4 | 0.84 | |||
| Previous history of appropriate ICD therapy | 8 (44%) | 11 (15%) | 0.006 | |||
| ACEi or ARB | 17 (94%) | 64 (88%) | 0.41 | |||
| β-blocker | 16 (89%) | 67 (92%) | 0.70 | |||
| Amiodarone | 2 (11%) | 10 (14%) | 0.77 | |||
| Galectin-3 (ng/mL) | 15.7±3.4 | 11.6±3.4 | <0.0001 | |||
| NT-proBNP (pg/mL) | 1,014 (467-3,449) (n=18) | 984 (351-2,241) (n=68) | 0.52 |
ACEi: angiotensin-converting-enzyme inhibitor, ARB: angiotensin II receptor blocker, ICD: implantable cardioverter-defibrillator, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association
The ROC analysis on the incidence of subsequent AIT and the value of Gal-3 revealed that a cut-off Gal-3 value of 13.13 ng/mL predicted the incidence of subsequent AIT with 68% specificity and 83% sensitivity during follow-up (Fig. 2a, C-statistic =0.82).
Figure 2.
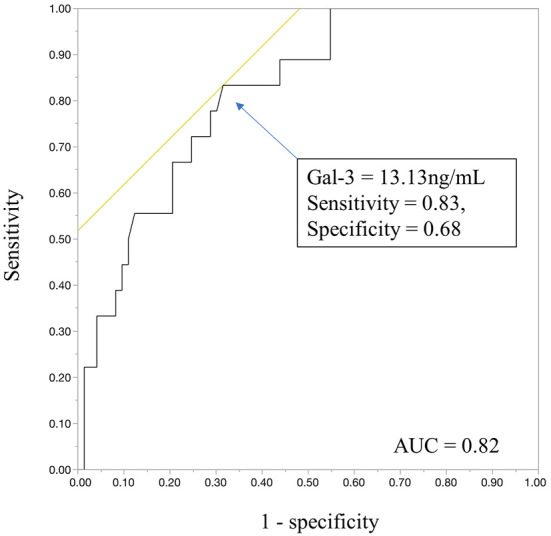
A receiver operating characteristic analysis of galectin-3 (Gal-3) and subsequent appropriate ICD therapy. A receiver operating characteristic analysis revealed that a cut-off galectin-3 value of 13.13 ng/mL had a sensitivity of 0.83 and specificity of 0.68 for subsequent appropriate ICD interventions [area under curve (AUC)=0.82].
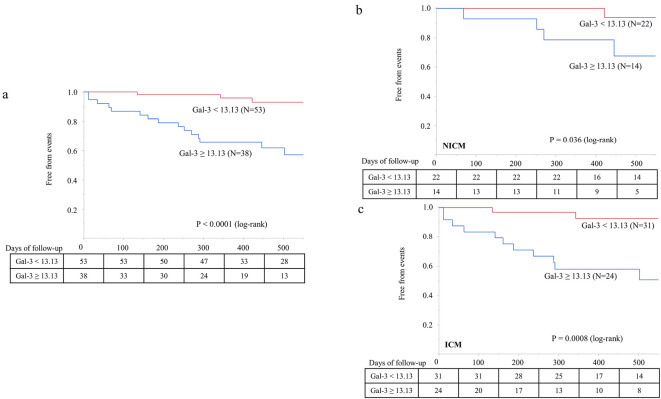
The patients were divided into 2 groups based on the cut-off Gal-3 value of 13.13 ng/mL. There were no statistically significant differences in patients' characteristics except for NT-proBNP, which tended to be higher in the patients with increased Gal-3 levels than in those with lower levels (Table 3). In addition, the patients with increased Gal-3 levels had a higher incidence of AIT than those with lower Gal-3 levels (Fig. 3a, log-rank, p<0.0001). This statistical significance remained even when dividing these patients into ischemic versus non-ischemic etiologies (Fig. 3b, c).
Table 3.
Basic Patient Characteristics according to Galectin-3.
| Gal-3 ≥13.13 (n=38) |
Gal-3 <13.13 (n=53) |
p value | ||||
|---|---|---|---|---|---|---|
| Age | 67.3±12.7 | 67.5±12.4 | 0.93 | |||
| Male | 30 (79%) | 41 (77%) | 0.86 | |||
| Hypertension | 28 (74%) | 34 (64%) | 0.34 | |||
| Diabetes mellitus | 9 (24%) | 20 (38%) | 0.16 | |||
| Ischemic etiology | 24 (63%) | 31 (58%) | 0.65 | |||
| Atrial fibrillation | 20 (53%) | 24 (45%) | 0.49 | |||
| LVEF (%) | 34.5±9.9 | 36.8±10.4 | 0.30 | |||
| NYHA classification I/II/III/IV | 23/10/5/0 | 17/28/8/0 | n.a. | |||
| Estimated glomerular filtration rate (mL/min/1.73m2) | 61.1±19.6 | 69.9±18.9 | 0.033 | |||
| Previous history of appropriate ICD therapy | 11 (29%) | 8 (15%) | 0.11 | |||
| ACEi or ARB | 35 (92%) | 46 (87%) | 0.42 | |||
| β-blocker | 35 (92%) | 48 (91%) | 0.80 | |||
| Amiodarone | 6 (16%) | 6 (11%) | 0.53 | |||
| NT-proBNP (pg/mL) | 1,198 (471-3,447) (n=37) | 930 (320-2,137) (n=49) | 0.21 |
ACEi: angiotensin-converting-enzyme inhibitor, ARB: angiotensin II receptor blocker, ICD: implantable cardioverter-defibrillator, LVEF: left ventricular ejection fraction, NYHA: New York Heart Association
Figure 3.
Galectin-3 concentration and appropriate ICD therapy. a: A Kaplan-Meier analysis demonstrated that a Gal-3 concentration ≥13.13 ng/mL predicted higher AIT incidence. b and c: A Gal-3 concentration ≥13.13 ng/mL predicted higher AIT incidence in both NICM and ICM patients. AIT: appropriate ICD therapy, ICM: ischemic cardiomyopathy, NICM: non-ischemic cardiomyopathy
Secondary endpoint
During the follow-up period, unplanned hospitalizations due to worsening HF were noted in 12 patients (13%). The patients with unplanned hospitalizations due to worsening HF had a significantly higher Gal-3 concentration at the enrollment in the present study than those without elevated concentrations (15.2±2.6 vs. 12.0±3.8 ng/mL, p=0.0064). An ROC analysis on unplanned hospitalizations due to worsening HF revealed that a cut-off Gal-3 value of 12.41 ng/mL predicted the incidence of worsening HF with 60% specificity and 92% sensitivity during follow-up (Fig. 4, C-statistic =0.78).
Figure 4.
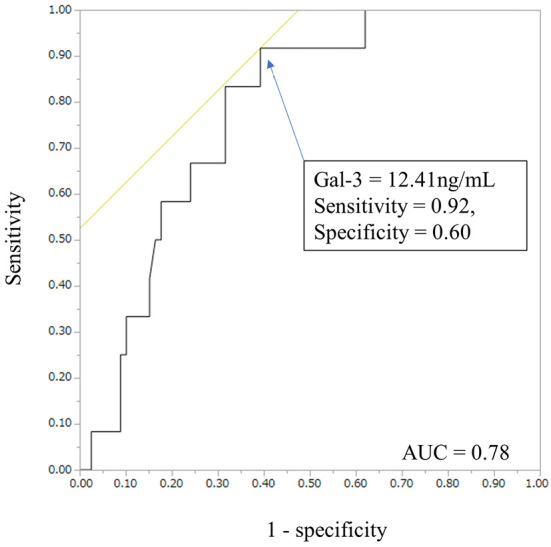
A receiver operating characteristic analysis of galectin-3 and unplanned HF hospitalization. ROC analysis revealed that a cut-off Gal-3 value of 12.41 ng/mL had a sensitivity of 0.92 and specificity of 60% for unplanned HF hospitalization (AUC=0.78). HF: heart failure
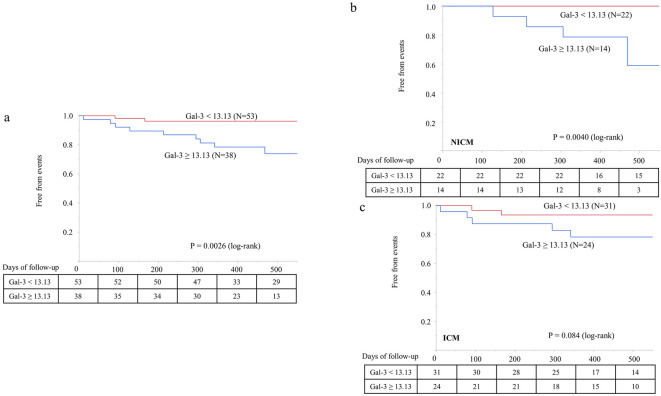
The patients with increased Gal-3 (cut-off of 13.13 ng/mL based on the ROC analysis of the primary endpoint) had a higher incidence of hospitalizations due to worsening HF than those with lower Gal-3 levels [10/38 (26%) vs. 2/53 (4%), log-rank, p=0.0026, Fig. 5a].
Figure 5.
Galectin-3 concentration and unplanned HF hospitalization. a: A Kaplan-Meier analysis demonstrated that Gal-3 ≥13.13 ng/mL predicted a higher incidence of HF hospitalization. b and c: Gal-3 concentration ≥13.13 ng/mL predicted HF hospitalization in NICM patients, but not in ICM patients. HF: heart failure, ICM: ischemic cardiomyopathy, NICM: non-ischemic cardiomyopathy
In the subgroup of NICM patients (n=36), increased Gal-3 levels predicted unplanned hospitalizations during follow-up (log-rank, p=0.0040, Fig. 5b). In ICM patients (n=55), increased Gal-3 levels were not associated with an increased incidence of unplanned hospitalizations (log-rank, p=0.084, Fig. 5c).
Predictors of outcome
Regarding the primary endpoint, in univariable analyses, an increased Gal-3 level, history of VT/VF and precedent NSVT were significant predictors of subsequent AIT during follow-up (Table 4). In the multivariable Cox regression analysis, increased Gal-3 levels remained a significant predictor of subsequent AIT (hazard ratio: 6.95, 95% confidence interval: 1.97-24.6, p=0.0026).
Table 4.
Predictive Capability of Indices for Appropriate ICD Therapy.
| Indices | Univariable Cox regression | Multivariable Cox regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p value | Hazard Ratio | 95% Confidence Interval | p value | |||||||
| Galectin-3 ≥13.13 | 8.40 | 2.43-29.1 | 0.0008 | 6.95 | 1.97-24.6 | 0.0026 | ||||||
| Previous Hx of VT/VF | 3.71 | 1.46-9.43 | 0.0058 | 2.40 | 0.83-7.00 | 0.11 | ||||||
| NSVT in last 3 months | 3.18 | 1.25-8.08 | 0.015 | 1.65 | 0.56-4.85 | 0.36 | ||||||
| Age (1y) | 0.97 | 0.93-1.00 | 0.080 | |||||||||
| LVEF (1%) | 0.96 | 0.90-1.01 | 0.12 | |||||||||
| Male | 4.86 | 0.65-36.6 | 0.12 | |||||||||
| Atrial fibrillation | 0.50 | 0.19-1.35 | 0.17 | |||||||||
| Ischemic etiology | 1.98 | 0.71-5.57 | 0.19 | |||||||||
| NT-proBNP (100 pg/mL) | 1.01 | 0.99-1.02 | 0.43 | |||||||||
| eGFR (1 mL/min/1.73 m2) | 1.00 | 0.98-1.03 | 0.81 | |||||||||
eGFR: estimated glomerular filtration rate, Hx: history, LVEF: left ventricular ejection fraction, NSVT: non-sustained ventricular tachycardia
In the univariable analysis, indexes predictive of unplanned hospitalizations were a history of VT/VF, increased Gal-3 levels and elevated NT-proBNP levels (Table 5). In a multivariable analysis, a history of VT/VF and increased Gal-3 levels remained significant predictors of unplanned hospitalizations.
Table 5.
Predictive Capability of Indices for HF Hospitalization.
| Indices | Univariable Cox regression | Multivariable Cox regression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p value | Hazard Ratio | 95% Confidence Interval | p value | |||||||
| Previous Hx of VT/VF | 4.70 | 1.50-14.8 | 0.0080 | 4.19 | 1.27-13.8 | 0.019 | ||||||
| Galectin-3 ≥13.13 | 7.33 | 1.61-33.5 | 0.010 | 4.79 | 1.00-22.9 | 0.049 | ||||||
| NT-proBNP (100 pg/mL) | 1.02 | 1.00-1.04 | 0.048 | 1.02 | 1.00-1.03 | 0.078 | ||||||
| NSVT in last 3 months | 2.48 | 0.79-7.78 | 0.12 | |||||||||
| Age (1y) | 0.99 | 0.95-1.04 | 0.78 | |||||||||
| LVEF (1%) | 0.94 | 0.87-1.01 | 0.11 | |||||||||
| Male | 1.26 | 0.27-5.77 | 0.77 | |||||||||
| Atrial fibrillation | 0.76 | 0.24-2.40 | 0.64 | |||||||||
| Ischemic etiology | 1.36 | 0.41-4.52 | 0.62 | |||||||||
| eGFR (1 mL/min/1.73 m2) | 0.99 | 0.97-1.02 | 0.62 | |||||||||
eGFR: estimated glomerular filtration rate, Hx: history, LVEF: left ventricular ejection fraction, NSVT: non-sustained ventricular tachycardia
Discussion
This study showed that, in patients with ICDs, circulating Gal-3 can be used as an independent risk marker for 1) ventricular arrhythmic events and 2) unplanned hospitalizations due to dHF. The predictive capability of circulating Gal-3 for these types of events was independent of prior VT/VF events. This is the first focused cohort study to elucidate the predictive impact of circulating Gal-3 levels, independent of the ventricular arrhythmic history. Previous reports have included only primary prevention ICD patients (4), and prior VT/VF events were not assessed as a predictor in the multivariable analyses (13,14). We enrolled patients with a primary prophylactic ICD indication at the time of ICD implantation, but some of the patients had experienced appropriate ICD interventions due to VT/VF before study enrollment.
Gal-3 has been given a Class IIb classification as an additive risk stratification marker according to the ACC/AHA 2017 HF guidelines (8). In previous reports, however, the results varied concerning its utility (5-7,9,10). Zile et al. reported that Gal-3 had no additive value as a prognostic marker if adjusted for BNP, NT-proBNP, or high sensitive Troponin T (hsTnT) and eight profibrotic biomarkers (9). Felker et al. also reported that the predictive value of Gal-3 did not persist after adjusting for NT-proBNP (10). In the present study, the prognostic predictivity of Gal-3 was maintained in the multivariable analysis. We included patients with a history of sustained ventricular arrhythmias and appropriate ICD interventions (approximately 20% of all enrolled patients), and all patients had ICDs. This is distinct from the patient populations in previous reports. In contrast, the interaction between Gal-3 and NT-proBNP, which were noted in the Cox regression analysis for HF hospitalization (Table 5), corresponded to the findings in previous reports. The correlation between Gal-3 and NT-proBNP tended to be statistically significant (p=0.055); however, the correlation coefficient was 0.21 (Fig. 6).
Figure 6.
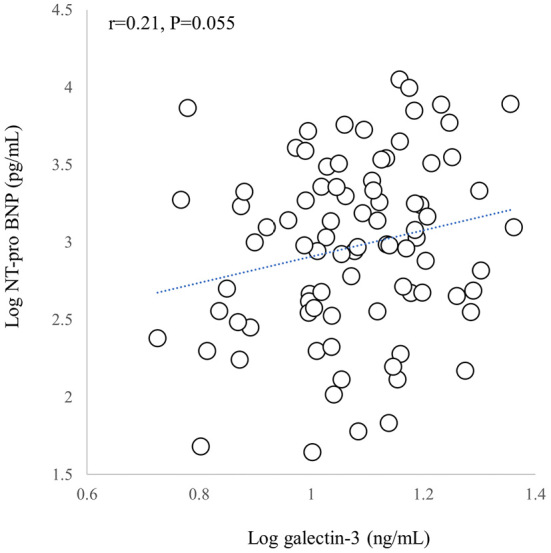
Relationship between galectin-3 (Gal-3) and NT-proBNP. The X-Y plot of logarithmic Gal-3 and logarithmic NT-proBNP is shown. While there was a trend toward a statistically significant association between Gal-3 and NT-proBNP (p=0.055), the correlation coefficient was 0.21. See text for details.
The severity of HF symptoms in the present study also differed from that in previous reports. We enrolled patients in the ambulant setting and excluded those with a history of hospital admission due to worsening HF. In the present study, 44% (41/91) of patients were in the NYHA Class I category, and only 14.3% (13/91) were in the NYHA Class III/IV category. These differences in the enrolled patient population may explain the lower median Gal-3 value in our cohort (12.0 ng/mL) than that reported in previous studies.
We previously reported that the presence of NSVT is an independent prognostic indicator in patients with primary prophylactic ICDs (15). In the present study, in the patients with previous episodes of appropriate ICD interventions (VT/VF) the presence of NSVT was a prognostic predictor in the univariable analysis. After adjusting for Gal-3, NSVT did not maintain its statistical significance; however, in addition to its possible prognostic value in the univariable analysis, it is noteworthy that circulating Gal-3 levels were significantly higher in patients with recorded NSVT within three months prior to their enrollment than in those without such a history. Our results suggest the utility of Gal-3 as a marker of the arrhythmogenic property, which may contribute to the exacerbation of HF, rather than as a direct prognostic marker of HF. Zile et al. reported that Gal-3 was not affected and remained unchanged by the implementation of optimal HF therapy, including sacubitril/valsartan, which also corresponds to our hypothesis (9). This hypothesis should be assessed in future research and may explain why the results of previous reports differed from those of the present study (possibly due to the different arrhythmogenic backgrounds of the enrolled patients).
Gal-3 has been known to be involved in the pathological fibrotic mechanism, and collagen synthesis in the extracellular matrix by fibroblasts is increased by Gal-3. Clinical and basic research studies have reported that Gal-3 levels reflect the progression of ventricular myocardial fibrosis (16-18). Ventricular myocardial fibrosis is associated with the development of ventricular dysfunction and the risk of sudden cardiac death (SCD) and ventricular arrhythmias (19,20). In the present study, elevated circulating Gal-3 levels predicted subsequent ventricular arrhythmias independently of a history of sustained ventricular arrhythmias or appropriate ICD intervention. This predictability was significant in both patients with ICM and those with NICM. Based on these data and our results, we hypothesize that elevated Gal-3 levels may aggravate myocardial fibrosis via the activation of fibroblasts, which precipitates the occurrence of ventricular arrhythmias, including NSVT. This hypothesis may lead to the development of upstream therapy to impede ventricular remodeling, although further research is needed to confirm this.
Several limitations associated with the present study warrant mention. This is a single-center prospective, observational cohort study. The number of patients enrolled was small. Therefore, the utility of Gal-3 should be evaluated further in larger, multi-center studies. Due to the institutional characteristics, referral bias should be taken into account. Furthermore, blood sampling for Gal-3 was performed once at enrollment, so serial changes in Gal-3 and its relationship to ventricular arrhythmias and HF should be further investigated.
Conclusion
Elevated circulating galectin-3 levels independently predicted the subsequent occurrence of ventricular arrhythmic events and decompensated HF. Our data indicate the possible utility of circulating galectin-3 as an arrhythmogenic marker, independent of a history of arrhythmia, both in ischemic and non-ischemic cardiomyopathies.
Ethics approval and consent to participate: This study was conducted in the context of the Düsseldorf University Device Registry (NCT03360227) and approved by the local institutional review board. All patients gave their written, informed consent.
The authors state that they have no Conflict of Interest (COI).
Hisaki Makimoto and Patrick Müller contributed equally to this work.
References
- 1. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 36: 2793-2867, 2015.26320108 [Google Scholar]
- 2.Gulati A, Japp AG, Raza S, et al. Absence of myocardial fibrosis predicts favorable long-term survival in new-onset heart failure. Circ Cardiovasc Imaging 11: e007722, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 309: 896-908, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Francia P, Adduci C, Semprini L, et al. Osteopontin and galectin-3 predict the risk of ventricular tachycardia and fibrillation in heart failure patients with implantable defibrillators. J Cardiovasc Electrophysiol 25: 609-616, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Ueland T, Aukrust P, Broch K, et al. Galectin-3 in heart failure: high levels are associated with all-cause mortality. Int J Cardiol 150: 361-364, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Lok DJ, Van Der Meer P, de la Porte PW, et al. Prognostic value of galectin-3, a novel marker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol 99: 323-328, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang WH, Shrestha K, Shao Z, et al. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol 108: 385-390, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 70: 776-803, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Zile MR, O'Meara E, Claggett B, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol 73: 795-806, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Felker GM, Fiuzat M, Shaw LK, et al. Galectin-3 in ambulatory patients with heart failure: results from the HF-ACTION study. Circ Heart Fail 5: 72-78, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 138: e272-e391, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Wilkoff BL, Fauchier L, Stiles MK, et al. ; Document Reviewers. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter-defibrillator programming and testing. Europace 18: 159-183, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Gullestad L, Ueland T, Kjekshus J, et al. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA). Am Heart J 164: 878-883, 2012. [DOI] [PubMed] [Google Scholar]
- 14.de Boer RA, Lok DJ, Jaarsma T, et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med 43: 60-68, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makimoto H, Zielke S, Clasen L, et al. Clinical significance of precedent asymptomatic non-sustained ventricular tachycardias on subsequent ICD interventions and heart failure hospitalization in primary prevention ICD patients. Eur J Med Res 25: 5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma UC, Pokharel S, van Brakel TJ, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 110: 3121-3128, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Vergaro G, Del Franco A, Giannoni A, et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int J Cardiol 184: 96-100, 2015. [DOI] [PubMed] [Google Scholar]
- 18.de Boer RA, Voors AA, Muntendam P, van Gilst WH, van Veldhuisen DJ. Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 11: 811-817, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Chery G, Kamp N, Kosinski AS, et al. Prognostic value of myocardial fibrosis on cardiac magnetic resonance imaging in patients with ischemic cardiomyopathy: systematic review. Am Heart J 229: 52-60, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zegard A, Okafor O, de Bono J, et al. Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 77: 29-41, 2021. [DOI] [PubMed] [Google Scholar]