Abstract
A 28-year-old woman experienced gross hematuria after the administration of the second dose of an messenger ribonucleic acid (mRNA) vaccine (BNT162b2). She was diagnosed with Immunogloblin A nephropathy (IgAN) by a renal biopsy two weeks after vaccination, which revealed a mild increase in mesangial cells and a matrix with co-depositions of galactose-deficient IgA1 and C3 in the mesangial region. The gross hematuria and proteinuria gradually improved without any medication, suggesting that immune activation by the mRNA vaccine may not elicit continuous disease progression of IgAN. Thus, further studies investigating the relationship between mRNA vaccines against COVID-19 and the progression of IgAN should be conducted.
Keywords: IgA nephropathy, COVID-19, mRNA vaccine, gross hematuria
Introduction
Since the discovery of severe acute respiratory syndrome coronavirus-2 (SARS-CoV2), the coronavirus disease 2019 (COVID-19) pandemic has affected over 150 million people worldwide (1). Vaccination is one of the most important and effective strategies in ending this issue. Currently, the Centers for Disease Control and Prevention has recommended three types of vaccines for COVID-19: BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and JNJ-78436735 (Johnson & Johnson). Among them, BNT162b2 and messenger ribonucleic acid (mRNA)-1273 are new types of vaccines known as mRNA vaccines. The mRNA vaccine formulated in lipid nanoparticles induces high levels of neutralizing antibodies by generating antigen-specific follicular T cells and germinal center B cells (2) and activated CD4 and CD8 T cells (3). As of July 2021, more than 3 billion shots' worth of COVID-19 vaccines have reportedly been administered worldwide.
Immunogloblin A nephropathy (IgAN) is the most common form of primary glomerulonephritis pathogenically characterized by the deposition of immune complexes (ICs) containing IgA, often with IgG and IgM, and complement C3 in the glomerular mesangial region of the kidney (4). These ICs contain aberrantly glycosylated IgA1, in which the deficiency of galactose in the O-linked glycan of the hinge region (Gd-IgA1) can be detected (5). Patients with IgAN usually present with asymptomatic microscopic hematuria or proteinuria; however, some patients show episodic gross hematuria after infection, especially after upper respiratory infections. Thus, dysregulation of the mucosal immune response is deemed to be involved in IgAN pathogenesis.
The clinical association between mRNA vaccination and glomerular injuries are open for discussion (6,7). We herein report a case of gross hematuria following BNT162b2 mRNA vaccination. A renal biopsy performed 14 days after the onset of gross hematuria demonstrated the deposition of Gd-IgA1 and C3 in the mesangial region, revealing that this was caused by the transient exacerbation of IgAN following an mRNA vaccination. We also found that urinary Gd-IgA1 levels were increased at the time of the renal biopsy. Thus, this case suggests that an enhanced immuno-response due to the mRNA vaccine against COVID-19 may transiently increase the Gd-IgA1 production, leading to gross hematuria.
Case Report
A 28-year-old woman who was a healthcare worker received her first dose of an mRNA-based vaccine, BNT162b2 on March 22, 2021, and her second dose on April 12 (day 0). She developed a headache and fever of 39 ℃ without any upper respiratory syndrome the following day, suggesting that the symptoms were adverse events of the vaccine. Although she reported her fever lowering on day 2, she developed gross hematuria on day 7 and was admitted to Juntendo University Hospital the next day (day 8). At 17 years old, she had been suspected of having IgAN because of a history of gross hematuria and mild proteinuria preceded by an upper respiratory infection. She had not undergone a renal biopsy due to the spontaneous remission of the urinary abnormalities post-tonsillectomy for chronic tonsillitis. However, she was still scheduled to undergo a renal biopsy the next time she presented with abnormal urinary findings.
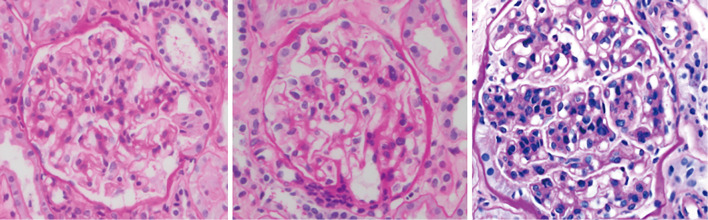
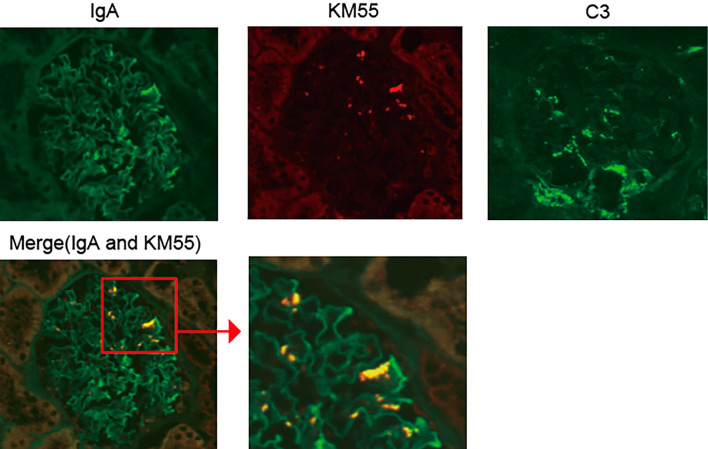
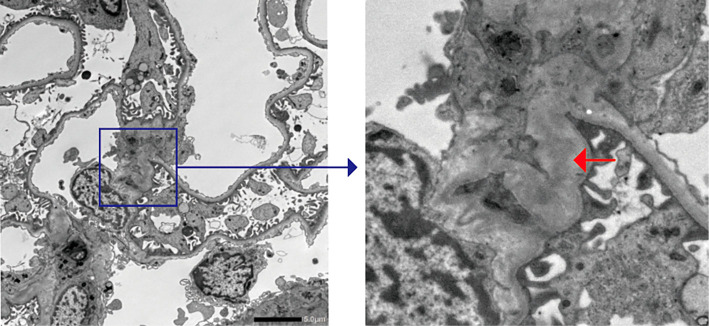
Urinary and laboratory tests on admission revealed hematuria and mild proteinuria without elevated serum creatinine or blood urea nitrogen levels (Table). A renal biopsy was performed on day 14 because the gross hematuria had not improved. On light microscopy, the specimens contained 44 glomeruli; among them, only 6 glomeruli exhibited a mild increase in mesangial cells and matrix, while 1 showed endocapillary proliferation (Fig. 1). No glomeruli with sclerosis or crescents were detected. Tubulointerstitial fibrosis and inflammation were not observed. Collectively, the MEST scores of the present case were M0, S0, E1, T0, and C0. However, immunofluorescence (IF) staining revealed IgA and C3 deposition in the mesangial area (Fig. 2). We also observed colocalization of Gd-IgA1 and IgA in the mesangial region using an anti-Gd-IgA1 monoclonal antibody (KM55) (Fig. 2). Electron microscopy confirmed the electron-dense deposits in the paramesangial region (Fig. 3). Thus, the patient was diagnosed with IgAN. As serum and urinary Gd-IgA1 levels are reportedly increased in patients with IgAN (8,9), we evaluated the levels of these biomarkers over time. Although serum Gd-IgA1 levels were comparable between day 8 (one day after the gross hematuria onset) and day 42 when gross hematuria disappeared, the level of urinary Gd-IgA1 was still the highest on day 8 before it gradually decreased. The hematuria and mild proteinuria gradually improved to within the normal range without any medication (Fig. 4).
Table.
Biochemical and Biomarker Test Results on Admission.
| White blood cell count (×103 cells per μL) | 4.6 | |
| Lymphocyte count (×103 cells per μL) | 0.67 | |
| Hemoglobin (g/dL) | 13.4 | |
| Platelet count (×103 cells per μL) | 247 | |
| Blood urea nitrogen (mg/dL) | 11 | |
| Creatinine (mg/dL) | 0.70 | |
| Total protein (g/dL) | 7.1 | |
| Albumin (g/dL) | 4.2 | |
| IgG (mg/dL) | 1,371 | |
| IgA (mg/dL) | 283 | |
| C3 (U/L) | 85 | |
| Anti-nuclear antibody | <40 | |
| MPO-ANCA | N. D. | |
| PR3-ANCA | N. D. | |
| Anti-GBM antibodies | N. D. | |
| Proteinuria (g/gCr) | 0.13 | |
| Hematuria (RBC/HPF) | >100 |
IgG: immunoglobulin G, IgA: immunoglobulin A, MPO-ANCA: myeloperoxidase anti-neutrophil cytoplasmic antibody, PR3-ANCA: proteinase 3 anti-neutrophil cytoplasmic antibody, anti-GBM antibody: anti-glomerular basement membrane antibody, N. D.: not detected, RBC: red blood cells, HPF: high-power field
Figure 1.
Light microscopic findings of the renal biopsy sample showing the mild increase of mesangial cells and endocapillary proliferation. Periodic acid-Schiff staining was performed.
Figure 2.
Immunofluorescence staining of the renal biopsy samples. IgA, galactose-deficient IgA1 (Gd-IgA1), and C3. The samples were stained with anti-IgA and C3 antibodies (Ab) (FITC) and anti-Gd-IgA1 monoclonal Ab (KM55; PE).
Figure 3.
Electron microscopic findings of renal biopsy samples. The red arrow indicates electron-dense deposits in the paramesangial region.
Figure 4.
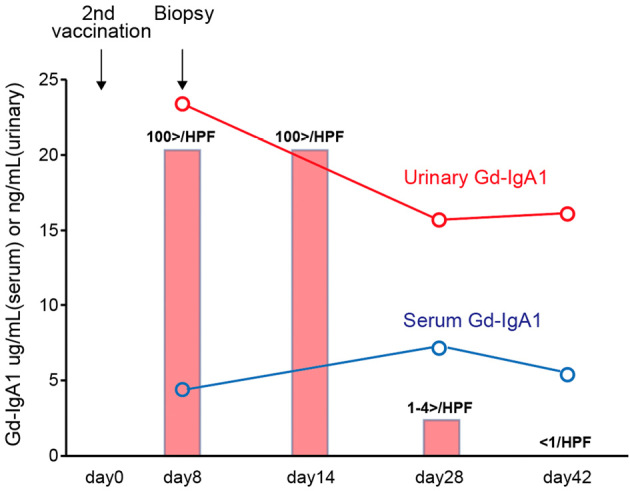
Clinical course of the present case. Serum (blue line) or urinary (red line) Gd-IgA1 levels. These were measured on the indicated days using enzyme-linked immunosorbent assays. The number of urinary red blood cells/high-power field on the indicated days is shown (rectangle).
Discussion
Millions of people worldwide have received various vaccines, including mRNA vaccines. Several reports have recently highlighted the increased risk of glomerular nephritis recurrence following mRNA vaccination (6,7,10). We herein report a case of IgAN with transient disease exacerbation following administration of the SARS-CoV-2 mRNA vaccine BNT162b2.
The mRNA vaccine BNT162b2 is a nucleoside-modified RNA encoding the SARS-CoV-2 full-length spike formulated in a lipid nanoparticle and has demonstrated a high efficacy rate with acceptable adverse effects. However, a pathogenetic association between the mRNA-COVID-19 vaccination and IgAN has been reported (7). Negrea et al. reported that some patients showed gross hematuria after mRNA-COVID-19 vaccination; however, this report was unable to determine whether gross hematuria was caused by flare-up of IgAN itself or the development of other types of glomerular nephritis by the mRNA vaccine (7).
In the present case, we performed a renal biopsy immediately after gross hematuria following a second mRNA-COVID-19 vaccination; the patient was diagnosed with IgAN. Furthermore, there is increasing evidence that IgA deposits in glomeruli in patients with primary IgAN are Gd-IgA1; this procedure involves staining kidney sections using monoclonal antibody against Gd-IgA1 (KM55) (11,12). In this case, Gd-IgA1 colocalized with IgA deposits, indicating that the present patient had gross hematuria due to the transient exacerbation of IgAN following mRNA vaccine BNT162b2.
Although there have been several reports describing conventional vaccine (i.e. influenza vaccine, hepatitis B vaccine and pneumococcal vaccine)-associated kidney diseases, such as acute kidney injury, anti-neutrophil cytoplasmic antibody (ANCA)-related vasculitis, and minimal change disease (13), vaccination-induced flare-up IgAN has been rarely reported. Interestingly, although the patient had received several conventional vaccines (live or live attenuated vaccine), such as those against the hepatitis B virus, mumps, human papillomaviruses, and seasonal influenza, she did not show gross hematuria after these types of vaccination, suggesting that the specific immune response acquired by SARS-CoV2 mRNA vaccines might be involved in the appearance of gross hematuria.
Although the pathogenesis of IgAN has not been clearly elucidated, the overproduction of Gd-IgA1 is thought to be first affected (5,14); the urinary Gd-IgA1 level also increases in patients with IgAN (9). Conversely, Wisnewski et al. recently showed that IgA, not only IgG, against the SARS-CoV-2 spike protein increased after mRNA vaccination (15). Consequently, we hypothesized that mRNA vaccination increases serum and/or urine Gd-IgA1, leading to the transient exacerbation of IgAN. However, we were unable to detect a correlation between the serum Gd-IgA1 level and disease activity; nonetheless, the urine Gd-IgA1 level was highest at the onset of gross hematuria (Fig. 4), suggesting that mRNA vaccination might affect the production of Gd-IgA1 or its delivery to the glomerulus.
As we were unable to measure the serum level of Gd-IgA1 before the appearance of gross hematuria, it is possible that we only measured the serum Gd-IgA1 levels after the peak increase of serum Gd-IgA1. Toll-like receptors (TLRs) are a family of pattern recognition receptors that play a crucial role in sensing microbial infection by the innate immune system. In the pathogenesis of IgAN, we recently reported that TLR9, which recognizes unmethylated CpG motifs abundant in bacterial DNA, is involved in the production of Gd-IgA1 (16,17). In the case of mRNA vaccination against COVID-19, it is possible that the stimulation of other types of TLRs (e.g. TLR7, which recognizes single-stranded RNA) can affect the production of Gd-IgA1 (18) Further studies are necessary to elucidate the mechanism by which mRNA vaccines affect the exacerbation of IgAN.
Although the present patient was diagnosed with IgAN, the hematuria and proteinuria gradually improved and disappeared without any medication. Le et al. evaluated the risk factors for progression to renal failure in 1,155 Chinese adult patients with IgAN and reported that patients with a history of gross hematuria had better renal outcomes than those without such a history (19). Consistent with this report, the renal biopsy in our patient revealed a mild increase in the mesangial cells and matrix; among 44 glomeruli, there was only 1 glomerulus with endocapillary proliferation, and none had crescents. Thus, immune activation by mRNA vaccines against COVID-19 may not induce severe renal damage in patients with IgAN.
In conclusion, this report is the first to evaluate the renal pathology immediately after the appearance of gross hematuria in a patient who developed IgAN following mRNA vaccination against COVID-19. Although we diagnosed the patient with IgAN, the renal biopsy specimen did not have features of severe renal damage; our case therefore suggests that macrohematuria following mRNA vaccination may not worsen the outcome in patients with IgAN.
Informed consent was obtained from the patient in the present case.
The authors state that they have no Conflict of Interest (COI).
Yoshihito Nihei and Monami Kishi contributed equally to this work.
Acknowledgement
The authors would like to thank all of the clinicians involved in our present case.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727-733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med 215: 1571-1588, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med 383: 1920-1931, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med 368: 2402-2414, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Kiryluk K, Novak J, et al. The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795-1803, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebedev L, Sapojnikov M, Wechsler A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 78: 142-145, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int 99: 1487, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasutake J, Suzuki Y, Suzuki H, et al. Novel lectin-independent approach to detect galactose-deficient IgA1 in IgA nephropathy. Nephrol Dial Transplant 30: 1315-1321, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki H, Allegri L, Suzuki Y, et al. Galactose-deficient IgA1 as a candidate urinary polypeptide marker of IgA nephropathy? Dis Markers 2016: 7806438, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masset C, Kervella D, Kandel-Aznar C, Fantou A, Blancho G, Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int 100: 465-466, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karasawa K, Iwabuchi Y, Kyoda M, et al. Primary IgA vasculitis with nephritis in a patient with rheumatoid arthritis diagnosed by anti-galactose-deficient IgA1 immunostaining. Intern Med 58: 2551-2554, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int 93: 700-705, 2018. [DOI] [PubMed] [Google Scholar]
- 13.Patel C, Shah HH. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl 30: 1002-1009, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Lai KN, Tang SC, Schena FP, et al. IgA nephropathy. Nat Rev Dis Primers 2: 16001, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One 16: e0249499, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makita Y, Suzuki H, Kano T, et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int 97: 340-349, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muto M, Manfroi B, Suzuki H, et al. Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J Am Soc Nephrol 28: 1227-1238, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arunachalam PS, Scott MKD, Hagan T, et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596: 410-416, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le W, Liang S, Hu Y, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479-1485, 2011. [DOI] [PubMed] [Google Scholar]