Abstract
After BNT162b2 messenger ribonucleic acid (mRNA) coronavirus disease 2019 (COVID-19) vaccination, a 30-year-old man developed bilateral lateral gaze palsy, diplopia, absent tendon reflexes, and ataxic gait. Serum anti-GQ1b and anti-GT1a immunoglobulin G (IgG) antibodies were strongly positive. Based on those findings, he was diagnosed with Miller Fisher syndrome (MFS). Intravenous immunoglobulin therapy was administered, and his symptoms fully recovered within approximately 3 months. To the best of our knowledge, this is the first report to describe the development of MFS after COVID-19 mRNA vaccination.
Keywords: Miller Fisher syndrome, vaccine, COVID-19, SARS-CoV-2, adverse event
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has necessitated large-scale global vaccination campaigns to control the spread of this infection under emergency use authorization. Although the the risks and benefits of vaccination should be considered for the individual, we still do not fully understand the risk of neurologic disorders after COVID-19 vaccine administration. We herein report the case of a patient who developed Miller Fisher syndrome (MFS) after BNT162b2 messenger ribonucleic acid (mRNA) COVID-19 vaccination. To the best of our knowledge, this is the first report to describe the development of MFS after mRNA COVID-19 vaccination.
Case Report
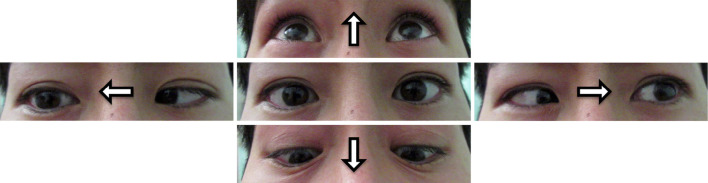
A 30-year-old healthy Japanese man received a second dose of tozinameran (BNT162b2 mRNA COVID-19 vaccine). He reported fever and cough during the first week after the vaccination. On day 7 post-vaccination, he experienced diplopia, dizziness, and difficulty walking. The patient was admitted to our hospital on day 8. A neurological examination revealed multidirectional diplopia, while his eye movement was full and smooth and tandem gait was impossible. A laboratory analysis revealed normal findings. All other clinical findings associated with the cranial, motor, and sensory nerves, as well as reflexes, were normal. Cerebrospinal fluid (CSF) and nerve conduction studies were normal on admission. Respiratory function tests, chest radiography, and contrast-enhanced magnetic resonance imaging of the brain and spine showed normal findings. On day 11, the patient developed bilateral lateral gaze palsy and ataxic gait (Figure). The biceps and patella tendon reflexes were absent, whereas the Achilles reflex was present. Serum anti-GQ1b and anti-GT1a immunoglobulin G (IgG) antibodies were strongly positive. Based on those findings, he was diagnosed with MFS. On day 12, intravenous immunoglobulin (IVIg) therapy (400 mg/kg/day for 5 days) was administered. His symptoms fully recovered by day 105.
Figure.
Limitation of extraocular movements. Bilateral lateral gaze palsy was observed after mRNA COVID-19 vaccination.
Discussion
To date, Guillain-Barré syndrome (GBS), a rare but serious autoimmune neurological disorder affecting the peripheral nervous system (PNS), has been reported in several cases after mRNA COVID-19 vaccination (1-5). In the present case, a typical clinical presentation of MFS, which is a variant form of GBS, with serum anti-GQ1b IgG antibody positivity was observed after mRNA COVID-19 vaccination. MFS is characterised by the clinical triad of ophthalmoplegia, ataxia, and areflexia (6), with a higher incidence in Asian countries than in Western countries (7). It is usually preceded by viral or diarrhoeal illness and is strongly associated with serum anti-GQ1b IgG antibodies (8). The prognosis of MFS is usually good, with a median time to full recovery of 1 month for ataxia and 3 months for ophthalmoplegia (7). The present patient exhibited the clinical triad of MFS accompanied with serum anti-GQ1b IgG antibody positivity. He was treated with IVIg therapy and fully recovered in approximately 3 months.
A score of 5 on the Naranjo adverse drug reaction probability scale (9) suggested an association between the mRNA COVID-19 vaccination and MFS, which was supported by the absence of any other trigger of MFS. Furthermore, the interval between the vaccination and the onset of neurological symptoms was 7 days. Most cases of GBS after mRNA COVID-19 vaccination, as well as that of GBS after influenza vaccination, occurred within 2 weeks of vaccination (10) (Table). In several reports, mRNA COVID-19 vaccination was not associated with GBS (4,11,12). Whereas, adverse events were more commonly reported among the mRNA COVID-19 vaccination group (13). The spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is provided in the mRNA vaccine, binds to sialic acids linked to host cell surface gangliosides (14). This affinity of spike proteins for gangliosides may lead to the development of GBS. Although it remains unclear how mRNA COVID-19 vaccination induced MFS in this case, mRNA COVID-19 vaccination could be associated with MFS.
Table.
Cases with Miller Fisher Syndrome and Guillain-Barré Syndrome after mRNA COVID-19 Vaccination without Infection and Other Vaccination.
| Diagnosis | Country | Age (years)/ Sex |
Vaccine type | Number of dose | Days from inoculation to onset | Neurological symptoms | CSF cells (/μL)/ Protein (mg/dL) |
Anti-ganglioside antibody | NC study | Treatment | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFS | Japan | 30/M | BNT162b2 | Second | 7 | Bilateral lateral gaze palsy, areflexia, and ataxic gait | 1/30.8 | GQ1b GT1a | Normal | IVIg | Recovered | Present case |
| GBS | Qatar | 73/M | BNT162b2 | Second | 16 | Muscle weakness, areflexia | Normal/80 | N.D. | Absent H reflexes | IVIg | Recovered | 2 |
| GBS | US | 82/F | BNT162b2 | First | 14 | Muscle weakness, sensory loss, areflexia | 4/88 | N.D. | N.D. | IVIg | Improved | 1 |
| GBS | US | 86/F | BNT162b2 | First | 1 | Muscle weakness, areflexia | 2/162 | N.D. | N.D. | IVIg | Recovered | 3 |
| GBS | US | 65/M | BNT162b2 | First | 2 | Bilateral facial palsy, muscle weakness, sensory loss, areflexia | 1/107 | N.D. | AIDP | IVIg | Improved | 5 |
| GBS | Mexico | 31/M | BNT162b2 | First | 11 | Muscle weakness, areflexia | Not performed | N.D. | AIDP | IVIg | Improved | 4 |
| GBS | Mexico | 67/F | BNT162b2 | First | 4 | Muscle weakness, areflexia, respiratory failure | 22/30 | N.D. | AMAN | IVIg | Dead | 4 |
MFS: Miller Fisher syndrome, GBS: Guillain-Barré syndrome, N.D.: not detected, AIDP: acute inflammatory demyelinating polyneuropathy, AMAN: acute motor axonal neuropathy, CSF: cerebrospinal fluid
In conclusion, mRNA COVID-19 vaccination may rarely induce MFS.
Written informed consent was obtained by the patient for use and publication of the facial photographs.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Professor Susumu Kusunoki (Department of Neurology, Kinki University School of Medicine, Osaka) for measuring the anti-ganglioside antibodies and all members of the Department of Neurology at Kumamoto University Hospital for helping with the collection of clinical data.
References
- 1.Waheed S, Bayas A, Hindi F, Rizvi Z, Espinosa PS. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus 13: e13426, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Ann Med Surg 67: 102540, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogbebor O, Seth H, Min Z, Bhanot N. Guillain-Barré syndrome following the first dose of SARS-CoV-2 vaccine: a temporal occurrence, not a causal association. IDCases 24: e01143, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.García-Grimshaw M, Michel-Chávez A, Vera-Zertuche JM, et al. Guillain-Barré syndrome is infrequent among recipets of the BNT162b2 mRNA COVID-19 vaccine. Clin Immunol 230: 108818, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplant recipient with favorable treatment response. Liver Transpl 28: 134-137, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher M. An unusual variant of acute idiopathic polyneuritis (syndrome of ophthalmoplagia, ataxia and areflexia). N Engl J Med 255: 57-65, 1956. [DOI] [PubMed] [Google Scholar]
- 7.Mori M, Kuwabara S, Fukutake T, Yuki N, Hattori T. Clinical features and prognosis of Miller Fisher syndrome. Neurology 56: 1104-1106, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chiba A, Kusunoki S, Shimizu T, Kanazawa I. Serum IgG antibody to ganglioside GQ1b is a possible marker of Miller Fisher syndrome. Ann Neurol 31: 677-679, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30: 239-245, 1981. [DOI] [PubMed] [Google Scholar]
- 10.Haber P, DeStefano F, Angulo FJ, et al. Guillain-Barré syndrome following influenza vaccination. JAMA 292: 2478-2481, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 326: 1390-1399, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shasha D, Bareket R, Sikron FH, et al. Real-world safety data for the Pfizer BNT162b2 SARS-CoV-2 vaccine: historical cohort study. Clin Microbiol Infect 28: 130-134, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxylchloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents 55: 105960, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]