Abstract
Objective
The relationship between the prognosis and magnitude of a decrease in tumor blood flow according to estimated tumor differentiation remains unclear. This study investigated the relationship between reductions in the rate of mean computed tomography (CT) attenuation values and the clinical prognosis.
Methods
We evaluated 63 consecutive patients who received lenvatinib treatment for unresectable hepatocellular carcinoma (HCC). The oncological aggressiveness of the tumors was estimated using classification by dynamic CT enhancement patterns. The utility of changes in mean CT attenuation values of intra-hepatic targets during treatment to estimate the prognosis was investigated by calculating the progression-free survival (PFS) and post-progression survival (PPS). A multivariate analysis was used to identify potential confounders for the survival after progression during lenvatinib therapy.
Results
The rate of decrease in the mean CT attenuation value gradually increased according to the degree of deterioration in estimated tumor differentiation, and the rate of a decrease in attenuation ≥40% showed a tendency to increase (p=0.064). This trend was reflected by a better objective response in oncological aggressiveness heterogeneous enhancement patterns (Type-3 and Type-4) than a homogeneous enhancement pattern (Type-2) (83% vs. 56% of modified Response Evaluation Criteria in Solid Tumors). This resulted in a similar PFS between the groups (p=0.773), whereas the PPS was significantly worse when the rate of decrease in the attenuation value was ≥40% (p=0.012). A multivariate analysis confirmed that a rate of decease in attenuation value ≥40% was a poor prognostic factor for the PPS (hazard ratio, 2.993; 95% confidence interval, 1.196-7.490; p=0.019).
Conclusion
A rate of decrease in attenuation ≥40% may reflect a good response of a highly malignant tumor to lenvatinib. Therefore, this value may have utility as a surrogate marker for estimating the oncological aggressiveness of tumors and their associated prognosis.
Keywords: computed tomography, hepatocellular carcinoma, lenvatinib; malignant potential, poorly differentiated, post-progression survival
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and the third leading cause of cancer (1). The Barcelona Clinic Liver Cancer (BCLC) system is currently the most widely used for staging HCC (2,3). BCLC intermediate-stage disease can be further subclassified based on the Up-to-7 criteria (4) and the liver function by the Child-Pugh system (5). In patients with tumors that meet the Up-to-7 criteria and who have a good liver function, transarterial chemoembolization (TACE) is recommended, whereas in general, patients who fall outside the Up-to-7 criteria are considered a “TACE unsuitable population”.
Recently, Kudo et al. reported the potential efficacy of upfront lenvatinib-TACE sequential therapy in patients with intermediate stage HCC that fell outside the Up-to-7 criteria (6,7). We also reported the utility of lenvatinib-TACE sequential therapy on tumor progression during lenvatinib therapy (8). Lenvatinib is thus considered one of the best molecular-targeted agents for use in combination with TACE.
In contrast, several other treatment algorithms (2,3) are recommend to be used only in the presence or absence of tumor hypervascularity on contrast enhancement studies in order to optimize the selection of treatment for HCC, without evaluating the malignant potential of the target nodules. We previously reported that a “heterogeneous enhancement pattern” in the arterial phase of dynamic computed tomography (CT) accurately predicted the oncological aggressiveness of HCC (e.g., non-simple nodular type and poorly-differentiation) (9). These unique enhancement patterns also correlate with the oncological aggressiveness of HCC (10), and we recently reported the efficacy of lenvatinib for such aggressive types of HCC (11,12). However, these classification systems have potential issues with objectiveness, and in some cases, it may be difficult to classify the enhancement pattern.
The present study therefore estimated the tumor aggressiveness based on an evaluation of the treatment responses and changes observed on dynamic CT. We also evaluated the relationship between the post-progression survival (PPS) and treatment response stratified according to changes in the mean CT attenuation value of the target nodule.
Materials and Methods
Study population
From April 2018 to December 2020, 114 patients received systemic anticancer treatment using lenvatinib for unresectable HCC at the Department of Hepatology, Toranomon Hospital, Tokyo, Japan.
The following inclusion criteria were used in the study: 1) triple-phase dynamic CT performed within 1 month prior to initiation of lenvatinib, 2) tumor with hyperenhancement in the arterial phase on dynamic CT, 3) performance of triple-phase dynamic CT to evaluate the treatment response 2-12 weeks after the initiation of lenvatinib, 4) a Child-Pugh class A liver function at the time of lenvatinib initiation, 5) BCLC stage A to C tumor(s), 6) unresectable HCC with the patient not wanting to undergo local ablation or chemoembolization therapy for various reasons (i.e. tumor size, number and location, extrahepatic metastasis, TACE refractoriness, and various complications), 7) no treatment history of lenvatinib, 8) at least 1 measurable target nodule in the liver, 9) treatment interval of ≥28 days since the previous therapy with a tyrosine kinase inhibitor (TKI; sorafenib or regorafenib), and 10) an observation period of ≥4 weeks. A total of 63 patients met these inclusion criteria.
All procedures in the study were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and complied with the 1975 Declaration of Helsinki. The study was approved by the Institutional Review Board of our hospital (protocol number; 1438-H/B).
The HCC diagnosis
The diagnosis of HCC was based predominantly on the imaging findings using dynamic CT. All patients underwent unenhanced and four-phase dynamic CT using a 64-row multidetector CT (MDCT) scanner (Aquilion 64; Canon Medical Systems, Ohtawara, Japan) or an 80-row MDCT scanner (Aquilion one; Canon Medical Systems) according to a protocol reported elsewhere (12). A liver nodule was diagnosed as HCC when a dynamic study showed hyperattenuation in the arterial phase and washout in the portal or delayed phase.
Imaging findings for HCC and definitions of dynamic CT enhancement patterns
Before treatment, the enhancement pattern of HCC was classified into the following three types according to our CT enhancement classification system (Supplementary material), taking into account their strong relationship to macroscopic classification and histopathological differentiation in non-treated HCC (9): Type-2, a homogeneous enhancement pattern with increased arterial blood flow; Type-3, a heterogeneous enhancement pattern with a septum-like structure; and Type-4, a heterogeneous enhancement pattern with irregularly shaped ring structures. These unique enhancement patterns were established originally in a surgically resected population that confirmed their ability to predict the oncological aggressiveness of HCC. The patterns were validated later using a medical population treated with radiofrequency ablation (RFA) (10).
The enhancement patterns were assessed independently by an expert hepatologist (Y. Kawamura), expert hepatobiliary surgeon (J. Shindoh), and expert radiation oncologist (L. Tominaga) who were blinded to the clinical data. Discrepancies between any two of these examiners were resolved by a consensus review that included input from an additional reviewer (K. Ikeda). All target HCC nodules appeared to be hypervascular in the study, so they were all classified into three enhancement patterns (Type-2 to Type-4). The enhancement pattern that accounted for 70% of the nodules was defined as the predominant pattern, with slightly mixed tumors with a higher malignancy pattern considered less likely to be relevant to the prognosis.
We also calculated the mean CT attenuation value (mean Hounsfield units; mean HU) of the intrahepatic target tumors. A circular or elliptical region of interest (ROI) was drawn on the axial plane to include the largest surface of the target lesion and set to cover the most tumor area without the normal liver parenchyma. The mean HU of each tumor then calculated at a workstation (SYNAPS; Fuji Film Medical, Tokyo, Japan) (13).
Lenvatinib treatment and assessment of adverse events
Lenvatinib (LevimaⓇ; Eisai, Tokyo, Japan) was administered orally to the majority of patients at either 8 mg/day for patients <60 kg or 12 mg/day for patients ≥60 kg. Treatment was discontinued when any unacceptable or serious adverse event (AEs) or significantly clinical progression of the tumor were observed. According to the guidelines for the administration of lenvatinib, the dose should be reduced or the treatment interrupted when a patient develops grade ≥3 severe AEs or any unacceptable grade 2 drug-related AE. AEs were assessed using the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (14). In accordance with the guidelines provided by the manufacturer, if a drug-related AE occurred, dose reduction or temporary interruption was maintained until the symptom was resolved to Grade 1 or 2.
The treatment response evaluation
Treatment response was evaluated in accordance with the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (15). We assessed the best tumor response over 2-12 weeks. The liver was examined by dynamic CT. Tumor assessments were generally performed every four to eight weeks.
The treatment response was assessed independently by an expert hepatologist (Y. Kawamura) and an expert hepatobiliary surgeon (J. Shindoh) who were blinded to the clinical data. Discrepancies between these two examiners were resolved by a consensus review including an additional reviewer (K. Ikeda).
Definition of TACE failure/refractoriness
TACE failure was defined as an insufficient response after at least two consecutive TACE procedures, based on the response evaluation CT or magnetic resonance imaging (MRI) performed after one to three months, even after the chemotherapeutic agent was changed and/or the feeding artery was reanalyzed. In addition, the identification of a greater number of lesions in the liver than that recorded at the previous TACE procedure (other than the nodule being treated) was included in the classification of TACE failure/refractoriness (16).
The assessment of the hepatic functional reserve
The Child-Pugh classification (5) and albumin-bilirubin (ALBI) grade (17) were used to assess the hepatic functional reserve. The modified ALBI (mALBI) grade was based on the ALBI score, calculated from the serum albumin and total bilirubin concentrations using the following formula: [ALBI score = (log10 bilirubin (μmol/L)×0.66)+(albumin (g/L)×-0.085)], and defined by the following cut-off values: ≤-2.60= Grade 1; >-2.60 to -2.27= Grade 2a; >-2.27 to -1.39= Grade 2b; and >-1.39= Grade 3 (18).
Follow-up protocol
Physicians examined the patients every one to two weeks after the initiation of lenvatinib, and biochemical laboratory and urine tests were also performed. After the initiation of lenvatinib, the patients underwent dynamic CT to evaluate their early treatment response during the 2- to 12-week period. Dynamic CT or MRI was performed every one to three months after the first evaluation of the best treatment response.
Statistical analyses
Statistical analyses were performed using the IBM SPSS software program (ver. 27.0; SPSS, USA). Data were expressed as the median and range. The des-γ-carboxy prothrombin (DCP) value and tumor number were analyzed as continuous variables, with the DCP value analyzed in 100-AU/L intervals because of its wide range. Differences in background features between each parameter were analyzed by the chi-squared test, Fisher's exact test, and Kruskal-Wallis test. The Cochran-Armitage trend test was used to investigate the significance of trends in the rate of decrease in the mean CT attenuation value ≥40% after the initiation of lenvatinib, stratified according to the dynamic CT enhancement pattern.
p values <0.05 were considered to indicate statistical significance. The progression-free survival (PFS), PPS, and over-all survival (OS) after the introduction of lenvatinib were estimated by the Kaplan-Meier method, with the values compared using the log-rank test.
To identify the factors associated with the PPS after the initiation of lenvatinib, a multivariate analysis was performed using a Cox proportional hazards model. In this multivariate analysis, the integrated score was excluded in order to detect the true factors. All factors that were at least marginally associated with the PPS (p<0.15) in the univariate analysis were entered into a stepwise Cox regression analysis. Significant variables were selected by the stepwise method. A two-tailed p value <0.05 was considered statistically significant.
Results
Overview
Table 1 summarizes the clinical profile and laboratory data of the 63 HCC patients treated with lenvatinib. The man:woman ratio was 2.71:1. Hepatitis C virus (HCV) antibody was detected in 55.6% of patients. Overall, 58 patients (92%) received an initial dose of lenvatinib according to body weight, while 5 patients (8%) received a reduced starting dose for the following reasons: age >80 years old, platelet count <50×103/μL, and body mass index (BMI) <19 kg/m2. In addition, 4 patients (6%) received an elevated starting dose of lenvatinib according to their body weight, as they were enrolled in a Global Phase II study with fixed dosing (12 mg). Regarding the liver function, 42 (67%) patients had a Child-Pugh score of 5, and 17 patients (27%) had a mALBI grade of 1. Based on the pretreatment image analysis, the median tumor diameter was 31.0 mm, with 29 of 63 patients (46%) presenting with BCLC stage C disease, 13 (45%) of whom had macrovascular invasion and 21 (72%) extrahepatic metastasis. In addition, 6 patients (10%) had a history of treatment with other TKIs, and 43 patients (71%) had TACE failure/refractoriness. The median levels of alfa-fetoprotein (AFP) and des-gamma carboxyprothrombin (DCP) were 87.8 μg/L and 164.0 AU/L, respectively.
Table 1.
Clinical Profiles and Laboratory Data of Patients with HCC Treated with Lenvatinib.*
| Patient characteristics and laboratory data | ||
| Number of patients | 63 | |
| Gender, males: females, n | 46:17 | |
| Age, yr (range)† | 72 (45-91) | |
| Body weight <60 kg:≥60 kg | 33:30 | |
| HCV:HBV:NonB,NonC | 35:5:23 | |
| Platelet count, ×103/µL (range)† | 130 (48-292) | |
| Albumin, g/dL (range)† | 3.7 (2.9-4.5) | |
| Total bilirubin, mg/dL (range)† | 1.0 (0.3-2.8) | |
| Prothrombin activity, % (range)† | 86.3 (64.9-124.8) | |
| AST, IU/L (range)† | 37 (15-351) | |
| AFP, µg/L (range)† | 87.8 (0.8-61,040.7) | |
| DCP, AU/L (range)† | 164.0 (13.0-63,347.0) | |
| Child-Pugh score 5:6, n (%) | 42 (67%):21 (33%) | |
| mALBI score (1:2a:2b:3), n (%) | 17 (27%):24 (38%):22 (35%):0 (0%) | |
| Initial dose of lenvatinib, 4 mg:8 mg:12 mg [n (%)] | 2 (3%):30 (48%):31 (49%) | |
| Reduced starting dose of lenvatinib [n (%)] | 5 (8%) | |
| History of TKI treatment, n (%) | 6 (10%) | |
| Tumor characteristics | ||
| Largest tumor diameter, mm (range)† | 31.0 (11.0-175.0) | |
| Number of tumors, n (range) | 4 (1->200) | |
| Macrovascular invasion, n (%) | 13 (21%) | |
| Extrahepatic metastasis, n (%) | 21 (33%) | |
| BCLC stage A:B:C, n (%) | 11 (17%):23 (37%):29 (46%) | |
| TACE failure/refractoriness, n (%) | 43 (71%) | |
| Pretreatment dynamic CT study enhancement pattern (number and ratio) | ||
| Type -2: -3: -4, n (%) | 16 (25%): 33 (52%): 14 (22%) | |
*AFP: alpha-fetoprotein, BCLC: Barcelona clinic liver cancer, AST: aspartate aminotransferase, DCP: des-γ-carboxyprothrombin, HBV: hepatitis B virus, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, IU: international units, mALBI: modified albumin-bilirubin, NonB, NonC: neither HBV nor HCV infection present, TACE: transarterial chemoembolization, and TKI: tyrosine kinase inhibitor
†Data expressed as median (range).
The composition ratio is rounded off to the first decimal place and therefore the total will not necessarily be 100.
Regarding the pretreatment dynamic CT enhancement patterns, 16 patients (25%) had the Type-2 pattern, 33 (52%) the Type-3 pattern, and 14 (22%) had the Type-4 pattern. Fifteen patients had died at the time of database lock (December 13, 2020). The median duration of lenvatinib administration was 7.6 months, and the median observation period was 12.8 months. The median 2-week relative dose intensities (Type-2, Type-3, and Type-4), defined as the actual dose divided by the standard dose (8 mg/day or 12 mg/day depending on body weight), were 71.4%, 100%, and 100%, respectively.
Rate of decrease in the mean HU after the initiation of lenvatinib
We compared the rate of decrease in the mean CT attenuation value of the target tumors after the initiation of lenvatinib. The therapeutic effect was determined using mRECIST. Mean HU after the initiation of lenvatinib was measured at the time of image evaluation.
In the early treatment response evaluation based on the dynamic CT enhancement pattern determined by mRECIST, the overall response rates (ORRs) of each enhancement pattern (Type-2, Type-3, Type-4) were 56%, 79%, and 93%, respectively. The ORR for the heterogeneous enhancement pattern was significantly higher than that for the homogeneous enhancement pattern (83% vs. 56%, respectively) (p=0.030).
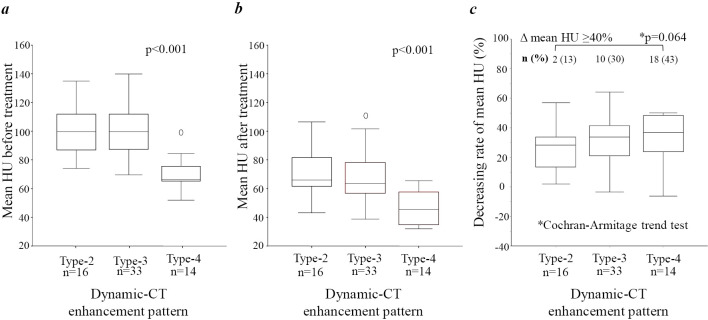
Fig. 1a, b show the mean HU of tumors before and after treatment, respectively, with the data grouped according to the tumor enhancement pattern (Type-2 to Type-4). The heterogeneous enhancement pattern had a significantly lower mean HU than the homogeneous enhancement patterns, both before and after treatment.
Figure 1.
Rate of change in mean Hounsfield units (HU) before and after lenvatinib administration, evaluated according to dynamic CT enhancement patterns and early treatment responses.
Fig. 1c shows the rate of decrease in the mean HU according to the tumor enhancement pattern (Type-2 to Type-4). The rate of decrease in the mean HU value gradually increased according to the degree of deterioration in estimated tumor differentiation. The rate of decrease in mean HU ≥40% also tended to increase with the degree of deterioration in estimated tumor differentiation (p=0.064).
The PPS in lenvatinib-treated patients according to the rate of decrease in the mean HU after the initiation of lenvatinib
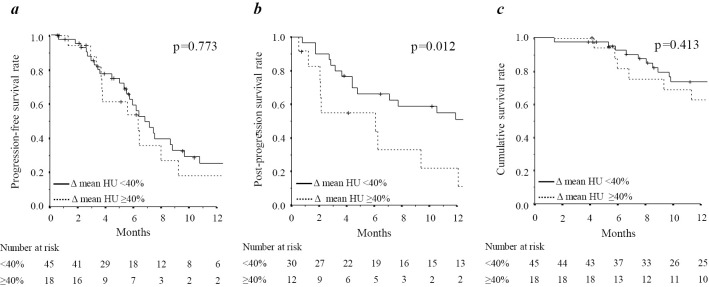
Fig. 2 shows the survival according to the rate of decrease in the mean HU after the initiation of lenvatinib. There was no marked difference in the PFS between the groups (Fig. 2a). The survival after progression was markedly worse when a patient presented with a rate of decrease in mean HU ≥40% after the initiation of lenvatinib (p=0.012) (Fig. 2b).
Figure 2.
Survival outcomes according to the change in mean Hounsfield units (HU) during lenvatinib treatment. (a) Progression-free survival rate, (b) Post-progression survival rate, and (c) overall survival rate.
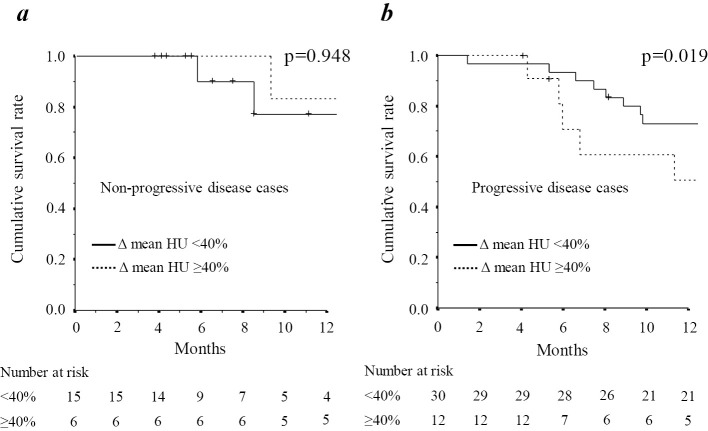
As shown in Fig. 2c, there was no marked difference in the OS between the groups. Patients who presented with non-progressive disease showed no marked difference in the OS between groups (Fig. 3a). However, in patients with progressive disease (PD), there was a significant difference in the OS between the groups (p=0.019) (Fig. 3b).
Figure 3.
The overall survival rate of PD and non-PD subgroups stratified by the change in the mean Hounsfield units (HU) during lenvatinib treatment.
Predictors of the PPS after the introduction of lenvatinib
Table 2 summarizes the results of the multivariate analysis for the PPS during lenvatinib therapy, which included pretreatment variables, the rate of decrease in the mean CT attenuation value evaluated at the time of the best treatment response, and the presence of subsequent treatment after judgement of PD. Of the 18 variables tested, a rate of decrease in mean HU of ≥40% at the time of the evaluation of the best treatment response was significantly associated with a poor PPS [hazard ratio (HR), 2.993; 95% confidence interval (CI), 1.196-7.490; p=0.019]. Subsequent treatment after reaching the PD state was also associated with a better PPS (other subsequent treatment: HR, 0.155; 95% CI, 0.049-0.486; p=0.001 and lenvatinib-TACE sequential therapy: HR, 0.085; 95% CI, 0.025-0.289); p<0.001).
Table 2.
Predictive Factors for Post-progression Survival.
| p* | Coefficients† | SE | Wald χ2 | HR | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean rate of decrease ≥40% in HU | 0.019 | 1.096 | 0.468 | 5.488 | 2.993 | 1.196-7.490 | ||||||
| Subsequent treatment after PD state | ||||||||||||
| No subsequent treatment | ||||||||||||
| Other subsequent treatment | 0.001 | -1.867 | 0.584 | 10.219 | 0.155 | 0.049-0.486 | ||||||
| Lenvatinib-TACE sequential therapy | <0.001 | -2.470 | 0.627 | 15.523 | 0.085 | 0.025-0.289 | ||||||
| DCP +100 AU/L | 0.053 | 0.004 | 0.002 | 3.758 | 1.005 | 1.000-1.009 | ||||||
| Tumor number +1 nodule | 0.060 | 0.007 | 0.004 | 3.543 | 1.007 | 1.000-1.015 |
*Based on the likelihood test adjusted for the other factors in the final model.
†Estimated coefficient for the variable and the associated standard error.
AFP: alpha-fetoprotein, 95% CI: 95% confidence interval, CT: computed tomography, HR: hazard ratio, HU: Hounsfield units, DCP: des-γ-carboxyprothrombin, PD: progressive disease, SE: standard error, TACE: transarterial chemoembolization
Multivariate Cox regression was applied using stepwise backward selection. Of the potential predictors, factors showing a marginal association (p<0.15) with post-progression survival after the introduction of lenvatinib in the univariate analysis were included in the initial model. Factors that showed no or limited statistically significant association (p>0.1) adjusted for the remaining factors in the model were then deleted from the model in a stepwise fashion.
The 18 variables tested were as follows (p values in univariate analysis): age (0.209), gender (0.527), etiology (HCV vs. others) (0.995), serum albumin (0.424), serum total bilirubin (0.715), prothrombin activity (0.839), platelet count (0.477), serum aspartate aminotransferase (0.731), serum alfa-fetoprotein (0.017), plasma des-γ-carboxyprothrombin (0.019), tumor diameter (0.002), tumor number (<0.001), macrovascular invasion (0.016), extrahepatic metastasis (0.003), ROI decreasing rate ≥40% (0.015), TACE failure/refractoriness (0.277), reduced starting dose of lenvatinib (0.456), and subsequent treatment after diagnosis of PD state (other subsequent treatment <0.001 and lenvatinib-TACE sequential therapy <0.001).
Discussion
We previously reported that the Type-3 dynamic CT enhancement pattern was a macroscopic classification that accurately predicted the nodular type of single nodular with extranodular growth (SNEG) and confluent multinodular (CMN) types of HCC and that a Type-4 enhancement pattern accurately predicted the histopathological grade of poorly-differentiated HCC (9). We also confirmed in another study that the heterogeneous enhancement pattern (i.e. Type-3 and Type-4) correlated with a higher response to lenvatinib than the homogeneous pattern (i.e. Type-2) (12).
However, experience and training on reading images is required to maximize the usefulness of these dynamic CT enhancement criteria. In this regard, the current study investigated the utility of estimating the response of tumor aggressiveness and the prognosis. We found that the rate of decrease in mean HU according to the tumor enhancement patterns (Type-2 to Type-4) gradually increased with the deterioration of the estimated tumor differentiation and also that the rate of decrease in mean HU ≥40% tended to increase according to the degree of deterioration in estimated tumor differentiation (p=0.064).
Initially, it was thought that a greater reduction in mean HU suggested a decrease in tumor blood flow and better disease control. In fact, some criteria for tumor control only use a blood flow evaluation. According to the Choi criteria (19) a reduction in tumor diameter of ≥10% or a decrease in the mean HU rate of ≥15% is defined as partial response (PR). In fact, it has been suggested that the Choi criteria are more effective for use in treating gastrointestinal stromal tumors (GISTs) than the RECIST in that they reflect the time to progression and the prognosis. As shown in another research report (20), the evaluation of the rate of decrease in mean HU of HCC was considered useful for determining the therapeutic effect and long-term disease control. Based on the initial response, tumors with a large decrease in mean HU are considered to be well controlled and have a marked reduction in tumor blood flow.
However, against expectations, the results of the current study showed that there was no marked difference in the PFS and that the group with a decreased rate of mean HU ≥40% had a significantly worse PPS than with a decreased rate of mean HU <40%. Unfortunately, tumors that were judged to be very responsive, with a markedly reduced mean HU, may be subsequently defined as having a poor prognosis. Based on these results we consider that the oncological aggressive predisposition becomes clearer after PD. The present findings suggest that even when a marked decrease in the tumor blood flow is observed, this does not reflect tumor necrosis in the majority of cases.
Tumors with high biological malignancy are expected to have a marked decrease in blood flow as a result of the early treatment effect of lenvatinib. This is a unique and strong effect of lenvatinib that other TKIs lack. However, such tumors may be able to survive by shifting their metabolic mechanism from aerobic to anaerobic, even under strong ischemic conditions. Many tumors also show positive findings on 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET)/CT, indicating increased anaerobic metabolism due to enhanced glycolytic pathways. Furthermore, as we reported previously, lenvatinib treatment increases the uptake of 18F-FDG in aggressive oncological tumors, resulting in the survival in many cases (21). Such clinical backgrounds may have contributed to the poor PPS observed in the group with a reduction in the mean HU of ≥40%.
Our analysis showed that a reduction in the mean HU of ≥40% significantly reduced the PPS, although there was no marked difference in the OS. This was because 21 of the 63 (33%) cases were non-PD. Among these cases, there was no marked difference in the OS according to the rate of decrease in mean HU (p=0.948). In contrast, the OS deteriorated in PD cases when the mean HU decreased at a rate ≥40% (p=0.019). These differing clinical backgrounds may account for the non-significant findings we observed for the OS in the case analysis stratified according to the rate of decrease in mean HU.
Finally, a multivariate analysis revealed that a rate of decrease in mean HU ≥40% was a predictive factor for a poor PPS. Subsequent treatment, especially lenvatinib-TACE sequential therapy, was a predictor of a favorable PPS, a finding similar to that described in our previous report (8).
Several limitations associated with the present study warrant mention. First, it was a retrospective, single-center, cohort study that evaluated a small number of patients. Second, the follow-up period of the trial was shorter than that of the global Phase III REFLECT trial (22) (median follow-up period of 12.8 vs. 27.7 months, respectively). It was therefore not possible to perform a high-quality prognostic analysis. Furthermore, the imaging analysis revealed no tumors with unclear margins in this study, but there may be cases where it is difficult to position an ROI. In such cases, it may be wise to consider including imaging procedures along with other modalities. Finally, the diagnosis of HCC was based essentially on the image analysis findings. A large-scale study is required to evaluate the utility of heterogeneous dynamic study enhancement patterns as biomarkers in the treatment of HCC by lenvatinib.
Conclusion
A rate of decrease in attenuation value of ≥40% may indicate a good response of highly malignant tumors to lenvatinib. Therefore, this value may have utility as a surrogate marker for estimating the oncological aggressiveness of tumors and the associated prognosis.
This retrospective, non-intervention study was approved by the Institutional Review Board, Toranomon Hospital (protocol number; 1438-H/B). The study was performed in accordance with the Declaration of Helsinki. Because of the anonymous nature of the data and the opt-out option disclosed on our institution's homepage (https://www.crc-toranomonhosp.jp/wp-content/uploads/2020/01/rinken_1438HB_2.pdf), the requirement for additional informed consent to participate in this study was deemed unnecessary according to the Japanese national regulation “Ethical Guidelines for Medical and Health Research Involving Human Subjects” (https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf).
Author's disclosure of potential Conflicts of Interest (COI).
Yusuke Kawamura: Honoraria, Eisai. Masahiro Kobayashi: Honoraria, Eisai. Junichi Shindoh: Honoraria, Eisai. Hiromitsu Kumada: Honoraria, Eisai.
Financial Support
Okinaka Memorial Institute for Medical Research and Japanese Ministry of Health, Labour and Welfare.
Supplementary Material
The Type-1 pattern represents a homogeneous enhancement pattern with no increase in arterial blood flow, with the entire image appearing uniform during the arterial and portal phases. The Type-2 pattern represents a homogeneous enhancement pattern with increased arterial blood flow, with the entire image appearing uniform during the arterial and portal phases. The Type-3 pattern represents a heterogeneous enhancement pattern with septations, with heterogeneous enhancement and septations in the arterial phase, with the septations resembling a near-uniform tumor tissue periphery in the portal phase. The Type-4 pattern represents a heterogeneous enhancement pattern with irregular ring-like structures. The arterial phase is marked by the presence of irregularly shaped ring areas of enhancement and areas of minimal blood flow relative to the periphery of the tumor tissue, while the portal phase is characterized by areas of reduced blood flow.
Acknowledgement
This work was supported in part by grants from the Ministry of Health, Labour and Welfare in Japan and Japan Agency for Medical Research and Development.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-E386, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Sem Liver Dis 30: 61-74, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 391: 1301-1314, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Mazzaferro V, Llovet JM, Miceli R, et al. ; Mertoticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 10: 35-43, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Brit J Surg 60: 646-649, 1973. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer 8: 299-311, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M, Ueshima K, Chan S, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond Up-to-seven criteria and Child-Pugh A liver function: a proof-of-concept study. Cancers (Basel) 11: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura Y, Kobayashi M, Shindoh J, et al. Lenvatinib-transarterial chemoembolization sequential therapy as an effective treatment at progression during lenvatinib therapy for advanced hepatocellular carcinoma. Liver Cancer 9: 756-770, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura Y, Ikeda K, Hirakawa M, et al. New classification of dynamic computed tomography images predictive of malignant characteristics of hepatocellular carcinoma. Hepatol Res 40: 1006-1014, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura Y, Ikeda K, Seko Y, et al. Heterogeneous type 4 enhancement of hepatocellular carcinoma on dynamic CT is associated with tumor recurrence after radiofrequency ablation. AJR: Am J Roentgenol 197: W665-W773, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura Y, Kobayashi M, Shindoh J, et al. 18F-fluorodeoxyglucose uptake in hepatocellular carcinoma as a useful predictor of an extremely rapid response to lenvatinib. Liver Cancer 9: 84-92, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura Y, Kobayashi M, Shindoh J, et al. Pretreatment heterogeneous enhancement pattern of hepatocellular carcinoma may be a useful new predictor of early response to lenvatinib and overall prognosis. Liver Cancer 9: 275-292, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronot M, Bouattour M, Wassermann J, et al. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist 19: 394-402, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okanoue T, Ebise H, Kai T, et al. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol 53: 129-139, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Sem Liver Dis 30: 52-60, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Kudo M, Matsui O, Izumi N, et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology 87: 22-31, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33: 550-558, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer 6: 325-336, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25: 1753-1759, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko S, Tsuchiya K, Kurosaki M, et al. Three criteria for radiological response on survival in patients with hepatocellular carcinoma treated with lenvatinib. Hepatol Res 50: 137-143, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Yamashige D, Kawamura Y, Kobayashi M, et al. Potential and clinical significance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for evaluating liver cancer response to lenvatinib treatment. Oncology 99: 169-176, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Type-1 pattern represents a homogeneous enhancement pattern with no increase in arterial blood flow, with the entire image appearing uniform during the arterial and portal phases. The Type-2 pattern represents a homogeneous enhancement pattern with increased arterial blood flow, with the entire image appearing uniform during the arterial and portal phases. The Type-3 pattern represents a heterogeneous enhancement pattern with septations, with heterogeneous enhancement and septations in the arterial phase, with the septations resembling a near-uniform tumor tissue periphery in the portal phase. The Type-4 pattern represents a heterogeneous enhancement pattern with irregular ring-like structures. The arterial phase is marked by the presence of irregularly shaped ring areas of enhancement and areas of minimal blood flow relative to the periphery of the tumor tissue, while the portal phase is characterized by areas of reduced blood flow.