Abstract
Duloxetine is widely used for pain control and depressive syndromes. One of its potential side effects is syndrome of inappropriate secretion of antidiuretic hormone (SIADH). Duloxetine-induced SIADH causes hyponatremia, which leads to a variety of symptoms and has previously been reported in the elderly. In the present case, we experienced a case of the rapid onset of SIADH in a super-elderly woman receiving low-dose duloxetine. Elderly patients tend to have lower duloxetine doses and an earlier onset than non-elderly patients. When hyponatremia occurs after duloxetine administration, duloxetine-induced SIADH should be considered, especially in high-risk elderly patients, regardless of the duloxetine dose or duration of treatment.
Keywords: duloxetine, syndrome of inappropriate secretion of antidiuretic hormone (SIADH), hyponatremia, elderly
Introduction
Duloxetine is widely used for pain control and depressive syndromes around the world. One of its reported side effects is syndrome of inappropriate secretion of antidiuretic hormone (SIADH), which can cause hyponatremia and lead to indeterminate complaints in the elderly (1).
While duloxetine-induced SIADH has been reported most frequently in elderly patients (2), its distinctive characteristics in elderly, non-elderly, and super-elderly populations remain unclear. As the aging population is increasing worldwide, especially in developed countries, it is important to clarify how duloxetine-induced SIADH presents in each age group.
We herein report on our experience with a case of duloxetine-induced SIADH in a 92-year-old woman who visited our hospital with indeterminate complaints.
Case Report
A 92-year-old woman who had a history of osteoarthritis of the lumbar spine, chronic heart failure, and insomnia presented with headache and fatigue. She had frequently reported various indeterminate complaints, including low back pain and heart palpitations. She had been taking loxoprofen 60 mg, verapamil 40 mg, lansoprazole 15 mg, furosemide 20 mg, magnesium oxide 330 mg, and zolpidem 10 mg for several years. She had not drunk alcohol or smoked for the past 10 years. She was 150.0 cm tall, weighed 32.2 kg, and her body mass index (BMI) was 14.3 kg/m2. On August X-5, 2019, she complained of low back pain, and her primary care physician prescribed 20 mg duloxetine for the pain. On August X-1, she developed a headache. She was referred to our hospital on August X for a close examination.
At the time of admission to our hospital, she was conscious, her blood pressure was 146/72 mmHg, pulse rate was 76 beats per minute, respiratory rate was 12 breaths per minute, SpO2 was 95% (room air), and body temperature was 36.5 °C. She complained of headache but showed no nausea or vomiting. She also complained of general fatigue and low back pain. Her oral cavity and axillae were not dry, and she had no abnormal heart or breathing sounds. There were no abnormalities in the abdomen and no edema in the face or extremities. No obvious neurological abnormalities were found.
On admission, a laboratory blood examination revealed the following findings: serum sodium concentration, 112 mEq/L; urine sodium concentration, 60.2 mEq/L; serum osmotic pressure, 238 mOsm/kg; urine osmotic pressure, 360 mOsm/kg; blood urea nitrogen, 19.0 mg/dL; creatinine, 1.08 mg/dL; free triiodothyronine, 2.5 pg/mL; free thyroxine, 1.29 ng/dL; thyroid-stimulating hormone, 1.33 μIU/mL; early morning free cortisol level, 21.7 μg/dL; and adrenocorticotropic hormone, 44.6 pg/mL. Her electrocardiogram was normal. Computed tomography showed no obvious mass lesions in the head or chest. Given that hyponatremia occurred after duloxetine was started, we suspected that hyponatremia induced by SIADH was responsible for these clinical symptoms.
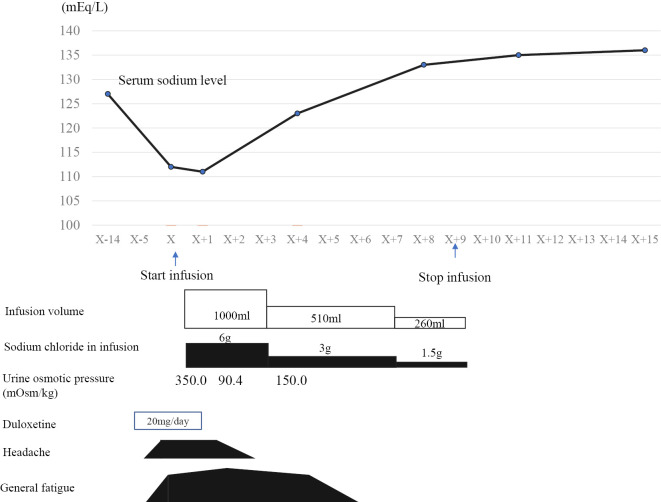
Duloxetine was discontinued on day X+1. Extracellular fluid infusion was started (infusion volume and sodium chloride amount in infusion: 1,000 mL/day and 6 g, respectively). The patient's headache disappeared on day X+2. On day X+4, the serum sodium concentration was 123.0 mEq/L and rising; by day X+8, it was 133.0 mEq/L and continuing to improve. A laboratory analysis on the day of admission revealed an antidiuretic hormone (ADH) level of 0.8 pg/mL, which was above the measurement sensitivity. There were no abnormalities in the renal, thyroid, or adrenal functions, nor were there any obvious mass lesions on whole-body CT. Based on these diagnostic criteria, we diagnosed the patient with SIADH. Infusion was discontinued from day X+9. On day X+11, the serum sodium concentration was 135.0 mEq/L; it did not subsequently decrease. The urine osmotic pressures at days X+2 and X+4 were 90.4 mOsm/kg and 150.0 mOsm/kg, respectively. Considering the patient's medical history, laboratory examinations, and clinical course, the diagnosis of duloxetine-induced SIADH was confirmed. The clinical course, including serum sodium levels and treatment, is shown in Figure.
Figure.
Clinical course.
Discussion
To our knowledge, this is the oldest reported patient with duloxetine-induced SIADH. The previous literature on duloxetine-induced SIADH, which we assessed via Pub Med and the Central Journal of Medicine, consisted of 20 cases, including the present case (Table 1). A previous review identified risk factors for duloxetine-induced SIADH as old age, female sex, use of diuretics, a history of hyponatremia, a body weight <60 kg, psychiatric disorders, and chronic obstructive pulmonary disease (3,4). Duloxetine increases ADH secretion in the posterior pituitary gland by inhibiting the serotonin reuptake and increasing the dopamine secretion (5). As duloxetine is metabolized by CYP1A2 and CYP2D6, the concomitant use of competing drugs is thought to enhance its effect and increase the risk of SIADH (5).
Table 1.
Clinical Characteristics of Duloxetine-induced SIADH.
| Age/ Sex |
Duloxetine dosage (mg) | Initial symptoms | Number of medications (n) | Concomitant medications | Medical history | Risk factors | Time to disease onset (days) | Serum sodium level at onset (mEq/L) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 92/F | 20 | Headache, fatigue | 6 | Loxoprofen, verapamil, lansoprazole, furosemide, magnesium oxide, zolpidem | Chronic heart failure, osteoarthritis of the lumbar spine | Elderly, female sex, low body weight, history of hyponatremia | 5 | 112 | Present case (2021) |
| 86/F | 20 | Low back pain, nausea, disorientation | 8 | Trichlormethiazide, tocopherol, oxybutynin, diphenidol, benidipine, domperidone, thiazolam, lorazepam | HL, dizziness, aphasia | Elderly, female sex, mental illness | 6 | 116 | 6 |
| 85/F | 60 | Disorientation | 0 | None | Major depression | Elderly, female sex, mental illness | 6 | 110 | 7 |
| 85/F | 20 | Headache, anorexia | Unknown | Oral hypoglycemic agents (details unknown) | DM, diabetic neuropathy | Elderly, female sex, low body weight | 7 | 118 | 8 |
| 79/F | 50 | Headache, anorexia | 5 | Benidipine, valsartan, quetiapine, ramelteon, clonazepam | HT, HL, and old tuberculosis | Elderly, female sex, mental illness | 10 | 118 | 9 |
| 78/F | 40 | Disorientation | 0 | None | None | Elderly, female sex, mental illness | 5 | 119 | 10 |
| 78/M | 60 | Headache, nausea | 3 | Titropium, amlodipine, gabapentin | HL, COPD, herpetic neuralgia | Elderly, low body weight, COPD | 1 | 125 | 11 |
| 77/F | Unknown (20 or 30) | Headache, nausea | 6 | Warfarin, zolpidem, telmisartan | Deep vein thrombosis, HT, HL, restlessness | Elderly, female sex | 1 | 119 | 1 |
| 76/F | 30 | Fatigue, nausea | 4 | Aspirin, pantoprazole, polyethylene glycol, quinapril | Neuromuscular pain, DM, HL | Elderly, female sex | 1 | 124 | 12 |
| 76/F | 30 | Muscle weakness, nausea, vomiting, disorientation | 2 | Metoprolol, metformin | DM | Female sex | 3 | 113 | 13 |
| 75/M | 30 | Lethargy, nausea, headache | 1 | Pregabalin | Neuropathic pain | Elderly | 3 | 118 | 14 |
| 74/F | 20 | Headache, nausea | 2 | Telmisartan, benidipine | HL | Elderly, female sex | 4 | 110 | 2 |
| 74/F | 60 | Nausea, vomiting, headache | 6 | Angiotensin II receptor antagonists, aspirin, NSAIDs, pregabalin, tramadol, acetaminophen | Sciatica | Female sex | 6 | 112 | 3 |
| 68/M | 30 | Dizziness, gait disturbance | 3 | Bupropion, lorazepam, agomelatine | General anxiety disorder, DM, anterior gland hypertrophy | Mental illness | 28 | 127 | 15 |
| 68/F | 60 | Fatigue, nausea | 4 | Gabapentin, warfarin, zolpidem, oxycodone | Sciatica, lumbar radiculopathy | Female sex | 2 | 121 | 4 |
| 66/F | 20 | Fatigue, lethargy | 0 | None | Functional gastrointestinal disorder | Elderly, female sex, mental illness | 3 | 115 | 16 |
| 66/F | 60 | Lethargy, muscle weakness, nausea | More than 1 | Olmesartan, etc. | Epilepsy, heart disease, HT, HL, depression | Female sex, mental illness | 90 | 129 | 5 |
| 58/M | 30 | Tingling sensation, pain in extremities, anxiety, depressed mood, insomnia | 0 | None | None | Low body weight | 5 | 122 | 17 |
| 50/F | 60 | Seizure, disturbance of consciousness, polydipsia, polyuria | 1 | Ziprasidone | Major depression | Female sex, mental illness | 10 | 117 | 18 |
| 48/F | 60 | Seizure | 0 | None | None | Female sex, mental illness | 2 | 103 | 19 |
DM: Diabetes mellitus, HT: Hypertension, HL: hyperlipidemia, COPD: chronic obstructive pulmonary disease, NSAIDS: non-steroidal anti-inflammatory drugs
We investigated the characteristics, including the sex, initial symptoms, time to the disease onset (days), duloxetine dose at the onset (mean or median), serum sodium concentration, and number of concomitant medications in previous duloxetine-induced SIADH patients as well as in the present case (1-19). The overall mean patient age was 72.7 years old, and the proportion of men was 25% (4/20). We divided the patients into 2 groups based on the onset age (20): 1) a non-elderly group comprising all patients <75 years old and 2) an elderly group comprising all patients ≥75 years old. The proportion of men, mean time to the onset, dose of duloxetine at the onset, serum sodium concentration, and number of concomitant medications were markedly different between the non-elderly group (22.2%, 18.3 days, 44.4 mg/day, 119.1 mEq/L, and 2.0, respectively) and the elderly group (11.1%, 4.3 days, 36 mg/day, 116.8 mEq/L, and 3.5, respectively). In addition, the initial symptoms differed between the non-elderly and elderly groups: in the non-elderly group, nausea was the most common initial symptom, reported in 44.4% of patients (4/9); in the elderly group, headache and nausea were the most common initial symptoms, reported in 54.5% of patients (6/11). These results suggest that duloxetine-induced SIADH develops more rapidly in the elderly and at lower doses of duloxetine than in younger patients. In addition, elderly patients tended to have lower serum sodium levels at the time of the SIADH diagnosis and more frequently experienced headache and nausea than younger patients (Table 2).
Table 2.
Differences in Clinical Characteristics between Non-elderly and Elderly Patients with Duloxetine-induced SIADH.
| Non-elderly (less than 75 years, n=9) |
Elderly (75 years and older, n=11) |
|||
|---|---|---|---|---|
| Male sex, n (%) | 2/9 (22.2) | 2/11 (11.1) | ||
| Major initial symptoms | Nausea | Headache, nausea | ||
| Time to disease onset, days (mean, median) | 18.3 days, 5 days | 4.3 days, 5 days | ||
| Serum sodium level at onset, mEq/L, mean (range) | 119.1 (103-129) | 116.8 (110-125) | ||
| Average duloxetine dosage (range) | 44.4 mg (20-60) | 36 mg (20-60) | ||
| Median duloxetine dosage (range) | 45 mg (20-60) | 40 mg (20-60) | ||
| Average number of medications, n | 2 | 3.5 |
We next performed a similar analysis with all reported patients divided into a “<85 years old group” (n=17) and a “≥85 years old group” (n=3). The proportion of men, mean time to the onset, mean dose of duloxetine at the onset, serum sodium concentration at the onset, and number of concomitant medications differed markedly between the <85 years old group (24%, 10.7 days, 41 mg/day, 118.2 mEq/L, and 2.7, respectively) and the ≥85 years old group (0%, 5.7 days, 33 mg/day, 112.6 mEq/L, and 3.7, respectively). In the <85 years old group, the most common initial symptom was nausea (52.9%); in the ≥85 years old group, 2 out of 3 patients (66.7%) experienced disturbance of consciousness as the initial symptom. The small number of reported duloxetine-induced SIADH cases in the ≥85 years old group (n=3) prevented us from performing a statistical analysis or drawing firm conclusions about their characteristics. Further research regarding the clinical differences in duloxetine-induced SIADH between the super-elderly and non-super-elderly is warranted.
The mechanism underlying the increased frequency of duloxetine-induced SIADH in the elderly is unclear. Previous reports have suggested that the concomitant use of competing drugs that interact with duloxetine may increase the blood concentration of duloxetine and predispose patients to SIADH (1). Elderly patients tend to take a larger number of medications than younger ones (19), meaning that drug interactions are more likely to occur; this may be related to the observation that lower doses of duloxetine can cause duloxetine-induced SIADH in elderly patients. In addition, the frequency of hyponatremia increases with age, as the ability to retain sodium decreases with age (9,12,21). It also remains unclear why SIADH is more likely to develop in elderly women at smaller doses of duloxetine and after shorter durations of treatment than in other patients. In general, the body weight is lower in women than in men. A low body weight, delayed drug metabolism due to an impaired hepatic and renal function, multiple drug use, and an impaired CYP function due to aging might increase blood duloxetine concentrations and make duloxetine-induced SIADH more likely to occur (13).
In the present case, water restriction could not be performed due to the patient's high psychological anxiety. As the patient did not consent to an MRI scan, we could not thoroughly evaluate the intracranial lesions. We were also unable to measure the uric acid, renin, or aldosterone levels at admission. Some cases of SIADH due to pain have been reported in the perioperative period of surgical procedures (22); the patient's low back pain may thus have contributed to her SIADH. As all of her symptoms, including hyponatremia, disappeared after the discontinuation of duloxetine, we are able to confirm the diagnosis of duloxetine-induced SIADH.
Most cases of duloxetine-induced SIADH have been reported within the last 10 years, which may indicate that the rate of duloxetine-induced SIADH is increasing or may merely suggest that it is being recognized more often. Additional case reports are needed to clarify the differences in clinical symptoms and susceptibility to duloxetine-induced SIADH that are associated with aging.
In conclusion, medical professionals should keep duloxetine-induced SIADH in mind as a differential diagnosis, especially in elderly women, who are at high risk of developing SIADH, regardless of the duloxetine dose or duration of duloxetine treatment.
Author's disclosure of potential Conflicts of Interest (COI).
Shinya Furukawa: Honoraria, Sanofi, Eli Lilly, Novo Nordisk and Ono Phamacy.
References
- 1.Yoshida K, Aburakawa Y, Suzuki Y, Kuroda K, Kimura T. Acute hyponatremia resulting from duloxetine-induced syndrome of inappropriate antidiuretic hormone secretion. Intern Med 58: 1939-1942, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takayama A, Nagamine T, Matsumoto Y, Nakamura M. Duloxetine and angiotensin II receptor blocker combination potentially induce severe hyponatremia in an elderly woman. Intern Med 58: 1791-1794, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anwari JS, Hazazi AA. Another cause of headache after epidural injection. Neurosciences (Riyadh) 20: 167-169, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safdieh JE, Rudominer R. A case of hyponatremia induced by duloxetine. J Clin Psychopharmacol 26: 675-676, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Stovall R, Brahm NC, Crosby KM. Recurrent episodes of serotonin-reuptake inhibitor-mediated hyponatremia in an elderly patient. Consult Pharm 24: 765-768, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Mori M, Koide T, Imanishi Y, Matsui Y, Matsuda T. Duloxetine- induced hyponatremia in an elderly patient treated with thiazide diuretics. Indian J Pharmacol 46: 657-659, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müssig K, Mörike K, Häring HU. Severe and symptomatic hyponatremia following duloxetine treatment. J Psychopharmacol 23: 338-339, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Kamei S, Kaneto H, Tanabe A, et al. Rapid onset of syndrome of inappropriate antidiuretic hormone secretion induced by duloxetine in an elderly type 2 diabetic patient with painful diabetic neuropathy. J Diabetes Investig 6: 343-345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura M, Satoh Y, Hiraoka A, Fujita Y, Nagamine T. Duloxetine-induced hyponatremia in the elderly. CNPT 3: 33-36, 2012. [Google Scholar]
- 10.Sakamoto S, Morigaki Y, Iga J, Omori T. A case of recurrent depressive patient with SIADH induced by three different class of antidepressants. Clin Psychiatry 55: 803-806, 2013(in Japanese, Abstract in English). [Google Scholar]
- 11.Wang D, Lai J, Lu S, Huang M, Hu S, Xu Y. Rapid-onset hyponatremia and delirium following duloxetine treatment for postherpetic neuralgia. Medicine (Baltimore) 97: e13178, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amoako AO, Brown C, Riley T. Syndrome of inappropriate antidiuretic hormone secretion: a story of duloxetine-induced hyponatremia. BMJ Case Rep 24: bcr2014208037, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beydilli I, Akguc L, Korkmaz I, Gencoglan S, Yılmaz F, Uysal Z. Low dose duloxetine induced hyponatremia in an elderly patient: a case report. Bull Clin Psychopharmacol 22: 283-285, 2012. [Google Scholar]
- 14.Demirci S, Demirci K, Altuntaş A, Söylemez A, Kutluhan S. Rapid-onset hyponatremia induced by duloxetine in an elderly patient. New Yeni Symposium 53: 20-22, 2015. [Google Scholar]
- 15.Sun CF, Chen YL, Li YH, Kumaraswamy M, Lo YC, Chen YT. Duloxetine-induced hyponatremia in an elderly male patient with treatment-refractory major depressive disorder. Case Rep Psychiatry 2019: 4109150, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yabe M, Kusabe Y, Shindo M, Ishikawa S, Ozaki S, Nomoto Y. Duloxetine-induced syndrome of inappropriate secretion of antidiuretic hormone. Niigata Shimin Hosp Med J 40: 28-34, 2019(in Japanese). [Google Scholar]
- 17.Choi JS, Lee HW, Lee JY, Jung HY. Rapid-onset hyponatremia induced by duloxetine in a middle-aged male with depression and somatic symptoms. Psychiatry Investig 9: 83-84, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li RM, Wang C, Liu ZW, Zhao B. A case of severe hyponatremia induced by duloxetine and ziprasidone. Chin Med J (Engl) 125: 3750-3751, 2012. [PubMed] [Google Scholar]
- 19.Maramattom BV. Duloxetine-induced syndrome of inappropriate antidiuretic hormone secretion and seizures. Neurology 66: 773-774, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi Y, Rakugi H, Arai H, et al.; the Joint Committee of Japan Gerontological Society (JGLS); Japan Geriatrics Society (JGS) on the Definition and Classification of Elderly. Redefining the elderly as aged 75 years and older: proposal from the Joint Committee of Japan Gerontological Society and the Japan Geriatrics Society. Geriatr Gerontol Int 17: 1045-1047, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg G, Hattis D, Russ A, Sonawane B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environ Health Perspect 113: 1243-1249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fieldman NR, Forsling ML, Le Quesne LP. The effect of vasopressin on solute and water excretion during and after surgical operations. Ann Surg 201: 383-390, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]