Abstract
Immune dysregulation has been highlighted as a key player in the pathogenesis of myelodysplastic syndromes (MDS), but little is known about cytokine profiles in patients with unexplained cytopenia with or without mutations in MDS-associated genes (clonal cytopenias of undetermined significance [CCUS] and idiopathic cytopenias of undetermined significance [ICUS], respectively), which often precede MDS. Here, we study the cytokine profiles in 111 patients with ICUS (N = 41), CCUS (N = 30), lower-risk MDS (LR-MDS; N = 22) and higher-risk MDS (HR-MDS; N = 18), and in healthy elderly controls (N = 21). Twenty cytokines were examined in blood plasma at time of diagnosis using Luminex assays and enzyme linked immunosorbent assays. The cytokine levels were compared between patient groups, and in patients versus controls. Associations between cytokines and MDS-associated mutations were evaluated. An aberrant cytokine profile was observed in all patient groups relative to healthy elderly controls. Patients had significantly higher levels of IL-6 (P< 0 .001), tumor necrosis factor α (P < 0.001), IL-10 (P < 0.001), and C-X-C motif chemokine 10 (P < 0.001) and lower levels of transforming growth factor beta 1 (P < 0.001), CCL5/regulated on activation normal T-cell expressed and secreted (P < 0.001), and S100A4 (P < 0.001) compared with healthy controls. Survival was significantly shorter in CCUS and MDS patients with a high systemic inflammatory cytokine load (median overall survival [OS] 21 months) compared with those with low-moderate systemic inflammatory cytokine load (median OS 64 months; P < 0.0001). These data suggest that patients with ICUS and CCUS have cytokine levels as abnormal as in LR-MDS. Indeed, high cytokine levels are present before MDS is diagnosed and cytokine levels are elevated irrespective of the presence or size of the myeloid clones. Cytokines may have a prognostic impact at a very early premalignant stage of myeloid disorders.
INTRODUCTION
Myelodysplastic syndromes (MDS) encompass a heterogeneous group of related clonal hematopoietic stem cell malignancies characterized by ineffective hematopoiesis, dysplastic cell morphology, and abnormal myeloid differentiation that clinically manifest as peripheral blood cytopenias.1 The revised International Prognostic Scoring System (IPSS-R) has been introduced to provide prognostic risk assessment of progression to acute myeloid leukemia (AML) and overall survival (OS) for this heterogenetic disease.2 The IPSS-R includes clinical characteristics like anemia, which has long been considered one of the most important independent prognostic factors for OS in patients with MDS.3
Recently, next-generation sequencing (NGS) panels have made it possible to distinct patients who do not fulfill the MDS diagnostic criteria. Patients with persistent unexplained blood cytopenias (>6 months) and MDS-associated somatic mutations are now classified as having clonal cytopenias of undetermined significance (CCUS) and, if no MDS-associated somatic mutations are present, idiopathic cytopenias of undetermined significance (ICUS).4–6 Long-term follow-up studies have shown that patients with CCUS have a high risk of developing MDS within 1–10 years, while patients with ICUS (without known genetic aberrations) rarely develop myeloid malignancies, but still may suffer from severe cytopenias.7,8
Immune dysregulation is directly affecting hematopoiesis and altered innate and adaptive immunity, with pro- and anti-inflammatory cytokine secretion, is a hallmark of MDS.9–12 The immune profiles of lower-risk MDS (LR-MDS) and higher-risk MDS (HR-MDS) are substantially different. LR-MDS is characterized by a persistent inflammatory environment in the bone marrow with activated nucleotide binding oligomerization domain-like receptor protein 3 inflammasomes and expansion of pro-inflammatory T-cells like Th17.13,14 On the contrary, HR-MDS and AML are immunosuppressive states dominated by aberrant cellular immune responses and expansion of regulatory T-cells and myeloid-derived suppressor cells.15,16
Two recent mouse studies demonstrated that increased pro-inflammatory cytokine production is critical for the expansion of myeloid leukemic blasts with TET2 and DNMT3A mutations. Interestingly, both studies showed promising results to reverse blast counts by inhibiting IL-6 through antibiotic treatment and inhibiting tumor necrosis factor α (TNF-α) with etanercept, respectively.17,18
The potential role of systemic inflammatory cytokine signaling in early human myeloid cancer pathogenesis is currently largely unknown. Therefore, we set out to investigate the cytokine levels in peripheral blood plasma of patients with ICUS, CCUS, LR-MDS, and HR-MDS as well as healthy elderly controls, and correlated these data to mutation status, clinical presentation patterns and outcome.
METHODS
Patients and healthy donors
A total of 111 patients (65 males, 46 females) with a median age of 70 (range: 39–93 years old) were included from 2014 to 2017 at their first visit to Department of Hematology, Rigshospitalet, University of Copenhagen, Denmark. Twenty-one healthy elderly blood donors (10 males, 11 females) with a median age of 63 (range: 60–65 years old) were recruited among donors at Rigshospitalet’s Blood Bank in October 2018 and served as the control group. Blood donors are carefully screened by a health questionnaire when they are first recruited and then a follow-up is performed before each donation. Blood donors did not suffer from cardiovascular disease, serious organ disease, acute or chronic infectious diseases, autoimmune disease, serious allergies to drugs or food, bleeding disorders, diabetes that need medical treatment, or a history of malignant diseases. Additionally, they are not allowed to have an intake of medicine that may affect blood components or as treatment for acute or chronic serious disease. The study was approved by the Danish Regional Science Ethics Committee (H-D-2009-003) and the Data Protection Agency (03026/30-1255). The patients were included according to diagnosis: ICUS (N = 41), CCUS (N = 30), LR-MDS (N = 22), or HR-MDS (N = 18). All patients with ICUS and CCUS had persistent cytopenias for >6 months, and other common causes of cytopenias such as vitamin deficiency were ruled out. Peripheral blood cytopenias were defined as platelets <150 × 109/L, neutrophils < 1.8 × 109/L, and hemoglobin <12.0 g/dL for women, or <13.0 g/dL for men. MDS was defined according to the WHO 2008 diagnostic criteria and risk stratified according to the IPSS-R and HR-MDS was categorized as IPSS-R >4.0.19 All patients with MDS included in the study had not received any treatment at the time of inclusion. In addition, bone marrow morphology assessment and cytogenetic analysis using G-band karyotyping were performed on samples from all patients at their first visit to the clinic. Clinical characteristics of MDS patients are summarized in Suppl. Table S1. Using Danish nationwide registers, we obtained clinical data for all patients included in the study. Co-morbidities present in >5% of the patients at time of diagnosis were included in the dataset. Follow-up data were collected from patient files. Median follow-up from the time of plasma sample collection was 50 months (range: 51–92). During this period, 59 (53%) patients died and 24 (22%) patients had disease progression to more aggressive myeloid malignancy (AML or chronic myelomonocytic leukemia). Treatment for hematological conditions during follow-up was received by 48 of all 111 patients. All relevant clinical characteristics are summarized in Table 1.
Table 1.
Clinical Characteristics of Four Groups of Patients (N = 111) and Healthy Elderly Controls (N = 21)
| Controls | ICUS | CCUS | LR-MDS | HR-MDS | Total Patients | P Value | |
|---|---|---|---|---|---|---|---|
| (N = 21) | (N = 41) | (N = 30) | (N = 22) | (N = 18) | (N = 111) | ||
| Sex, n (%) | 0.597 | ||||||
| Male | 10 (47.6) | 25 (61.0) | 19 (63.3) | 10 (45.5) | 11 (61.1) | ||
| Female | 11 (52.4) | 16 (39.0) | 11 (36.7) | 12 (54.5) | 7 (38.9) | ||
| Age, years | 63.1 (1.79) | 66.5 (11.7) | 70.1 (10.9) | 74.1 (10.5) | 73.4 (9.46) | 0.001 | |
| Anemia grade, n (%) | <0.001 | ||||||
| None | 15 (36.6) | 9 (30.0) | 2 (9.1) | 0 (0) | 47 (35.6) | ||
| Mild | 21 (51.2) | 10 (33.3) | 9 (40.9) | 3 (16.7) | 43 (32.6) | ||
| Moderate | 5 (12.2) | 8 (26.7) | 10 (45.5) | 11 (61.1) | 34 (25.8) | ||
| Severe | 0 (0) | 3 (10.0) | 1 (4.5) | 4 (22.2) | 8 (6.1) | ||
| Anemia | 0% | 65.90% | 70% | 90.90% | 100% | 64.40% | 0.008 |
| Neutropenia | 0% | 2.40% | 6.67% | 4.55% | 5.56% | 10.60% | <0.001 |
| Thrombocytopenia | 0% | 75.60% | 70% | 36.40% | 72.20% | 55.30% | 0.012 |
| No. of cytopenias | <0.001 | ||||||
| Unipenia | 58.50% | 53.30% | 68.20% | 5.60% | 50.50% | ||
| Bipenia | 41.50% | 46.70% | 31.80% | 61.10% | 44.10% | ||
| Pancytopenia | 0% | 0% | 0% | 33.30% | 5.40% | ||
| Hgb, g/dL, mean (IQR) | 12.1 (10.8–13.1) | 11 (9.35–12.6) | 9.97 (8.74–10.9) | 8.88 (8.25–9.98) | 10.9 (9.26–12.4) | <0.001 | |
| Thrombocytes, 109/L, mean (IQR) | 121 (83–144) | 122 (73–168) | 223 (113–324) | 125 (80–156) | 142 (77–176) | <0.001 | |
| Leukocytes, 109/L, mean (IQR) | 5.18 (3.8–6.2) | 5.24 (3.15–5.68) | 5.05 (3.42–6.88) | 3.67 (1.6–4.78) | 4.92 (3.1–5.9) | 0.227 | |
| Neutrophils, 109/L, mean (IQR) | 3.3 (1.9–3.7) | 2.92 (1.35–3.38) | 2.59 (1.5–3.2) | 1.52 (0.43–1.9) | 2.77 (1.5–3.55) | 0.046 | |
| MCV, mean (IQR) | 90.8 (86–95.2) | 92 (86–98.8) | 99.6 (88.8–111) | 98.4 (90.2–106) | 94.1 (86–101) | 0.001 | |
| LDH, U/I, mean (IQR) | 198 (164–229) | 229 (176–246) | 205 (172–224) | 229 (181–235) | 213 (172–236) | 0.207 | |
| Tobacco use, n (%) | 16 (39.0) | 10 (33.3) | 7 (31.8) | 4 (22.2) | 37 (33.3) | 0.814 | |
| Comorbidity (DM, IHD, or AID) | 21 (51.2%) | 16 (53.3%) | 6 (27.3%) | 5 (27.8%) | 48 (43.2%) | 0.149 | |
| Treatment during follow-up | <0.001 | ||||||
| None | 31 (75.6%) | 15 (50.0%) | 5 (22.7%) | 3 (16.7%) | 54 (48.6%) | ||
| ESA/G-CSF | 5 (12.2%) | 8 (26.7%) | 9 (40.9%) | 3 (16.7%) | 25 (22.5%) | ||
| Hypomethylating agents | 1 (2.4%) | 1 (3.3%) | 1 (4.5%) | 6 (33.3%) | 9 (8.1%) | ||
| Chemotherapy | 2 (4.9%) | 2 (6.7%) | 4 (18.2%) | 1 (5.6%) | 9 (8.1%) | ||
| BMT | 0 (0%) | 2 (6.7%) | 2 (9.1%) | 1 (5.6%) | 5 (4.5%) | ||
| Missing | 2 (4.9%) | 2 (6.7%) | 4 (18.2%) | 1 (5.6%) | 9 (8.1%) |
Anemia grade: mild: hemoglobin 10.0 g/dL to lower limit of normal. Moderate: hemoglobin 8.0–10.0 g/dL. Severe: hemoglobin 6.5–7.9 g/dL. Anemia: hemoglobin <12.0 g/dL for women or <13.0 g/dL for men; neutropenia: neutrophils <1.8 × 109/L; thrombocytopenia: thrombocytes <150 × 109/L.
AID = autoinflammatory disease; BMT = bone marrow transplant; CCUS = clonal cytopenias of undetermined significance; DM = diabetes mellitus; ESA = erythropoiesis stimulating agent; G-CSF = granulocyte colony-stimulating factor; HR-MDS = higher-risk myelodysplastic syndromes; ICUS = idiopathic cytopenias of undetermined significance; IHD = ischemic heart disease; IQR = interquartile range; LDH = lactate dihydrogen; LR-MDS = lower-risk myelodysplastic syndromes; MCV = mean corpuscular volume.
Cytokine measurement
Blood samples were centrifuged at 4°C (1300 g for 10 min) within 4 hours of collection and aliquots were stored at −80°C. Blood plasma concentrations of interleukin 1α (IL-1α), IL-1β, IL-1-ra, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL17A, interferon γ (IFN-γ), TNF-α, C-X-C motif chemokine 10 (CXCL10), “regulated on activation normal T-cell expressed and secreted” (RANTES)/CCL5, calcium-binding protein S100A9, vascular endothelial growth factor A, granulocyte macrophage colony-stimulating factor, and thrombopoietin were determined in duplicate using Luminex analyses of commercial assays (R&D Systems, Minneapolis, MN). Active transforming growth factor beta 1 (TGF-β1) was measured using enzyme linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) and calcium-binding protein S100A4 was measured using sandwich ELISA as previously reported.20 Additionally, high-sensitivity C-reactive protein was analyzed in all plasma samples retrospectively. Cytokines and other immune mediators possible to analyze with the applied immunoassay were selected a priori to provide a broad picture of the immune response (ie, Th1, Th2, Th17, Tregs, and acute inflammatory proteins). Patients and controls were run in duplicate and analyzed on the same plates to avoid the influence of intra- and inter assay variation. Coefficient of variations are presented in Suppl. Table S2.
Mutational profiles
NGS was carried out on DNA extracted from bone marrow aspirate collected in the workup to transplantation and stored in local biobanks (for patients in whom material was available). Bone marrow mononuclear cells were separated by density gradient centrifugation. DNA extraction was performed according to standard protocols for human tissues. Gene mutation analysis was performed on a total of 102 patient samples. DNA sequencing methods have changed during the course of the study; therefore, two different NGS assays were used in our cohort. For 87 samples, targeted DNA sequencing of 20 genes known to be involved in myeloid malignancies was performed using a custom-designed multiplex Ion Ampliseq panel (Ampliseq designer, Thermo Fischer Scientific, Waltham, MA). Initial DNA quantification was carried out using Qubit broad range assay. Library construction was performed by means of Ion Ampliseq technology and quantification of the final library was performed by qPCR with TaqMan Ion library quantification kit. Template preparation was automatically carried out on the Ion Chef Instrument using Hi-Q technology and reagents. Five samples, with unique barcodes, were simultaneously loaded on a 318 Ion Chip. Subsequent sequencing was carried out on the Ion PGM System using Hi-Q technology. All of the aforementioned steps were carried out according to manufacturer’s instructions and reagents and equipment manufactured by Thermo Fischer Scientific (Waltham, MA). Alignment and variant calling were performed on the Torrent Suite server (Thermo Fischer Scientific). For easier interpretation of variant detection, variant call format files were analyzed using Ion Reporter 5.0 software (Thermo Fischer Scientific), which provides direct access to full annotation information, functional properties, and external database information. Common single nucleotide polymorphisms (SNPs) referred to as >1% minor allele frequency were excluded from further analysis. We only report mutations that were predicted to result in a change in amino acid sequence and that were not reported as a SNP. Two mutations in ASXL1 and one in TET2 are commonly reported as both point mutations and SNPs, these were reported as mutations. The lower limit of allele detection on the Ion Torrent platform is usually set to 5% mutant allele reads. For the purpose of this study, low-level variations between 2% and 5% were included in a special category, as these variations may be of interest in this specific study population. Minimum coverage for variant detection was set to 500 X coverage. Both sequencing and data analysis were carried out at the Department of Hematology, Rigshospitalet as reported previously.6 Variants with a variant allele frequency (VAF) <5% were not considered for further analyses. DNA from 15 additional patients were subjected to HaloPlex (Agilent Technologies, Santa Clara, CA) target enrichment for mutational screening using panels of 42 mutated genes in myeloid malignancies on the Illumina HiSeq 2000 platform at the SciLife Laboratory, Karolinska Institutet, Stockholm, Sweden, as reported previously.21 Variants with a VAF <3% were excluded from further analyses. Data on mutational status were included in the final analysis of the association between cytokine profiles and mutations if they were present in at least 5% of the patients.
Statistical analysis
All analyses of cytokines were completed after applying a log2-transformation due to a left screwed distribution to reduce the impact of outliers and to obtain a normal distribution of residual in analysis of variances (ANOVAs). Missing values for cytokines, that is, concentrations below the limit of detection (LOD), were replaced by a value corresponding to half the LOD.22 Two of the cytokines (IFN-γ, IL-1β) were undetectable in more than 90% of the samples and were excluded from the analyses. The level of each cytokine was compared among the patient groups and the healthy control group using one-way ANOVA and results were corrected for multiple testing using the Bonferroni method. To compare demographics and clinical variables between groups, we used a Mann–Whitney U test for continuous variables in two groups comparison, Kruskal–Wallis test for continuous variables in multiple groups comparison, and Fischer’s exact test for dichotomous variables. Subanalyses were used to stratify patients based on mutational status (patients with CCUS and MDS). Cytokine–cytokine correlations for 16 cytokines were performed using Spearman’s correlation analysis. OS was measured from the first day of referral to the clinic to death from any cause. Survival curves were made according to the Kaplan–Meier method. Log-rank tests were used to determine if individual cytokines (categorized as normal/abnormal) were independently associated with OS. Cox proportional hazard model was used for multivariable analysis. Comorbidities included in the multivariate analysis were diabetes mellitus type 1 and 2 (DM), ischemic heart disease (IHD), and autoinflammatory diseases (AID) due to their highly possible role in inflammation. Cytokine levels were categorized as normal or high/low according to the upper/lower 95% limit of the cytokine levels observed in the healthy elderly controls. Unsupervised hierarchical clustering analysis was completed on log-transformed, normalized cytokine data using the R package pheatmap. A P value of <0.05 was considered statistically significant. Statistical analyses were conducted using the statistical software R 3.4.3.
Results
Abnormal inflammatory cytokine profile in patients with ICUS, CCUS, LR-MDS, and HR-MDS compared with healthy elderly controls
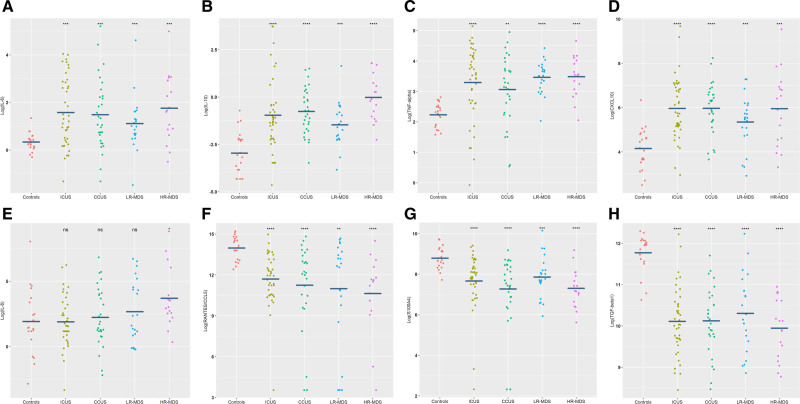
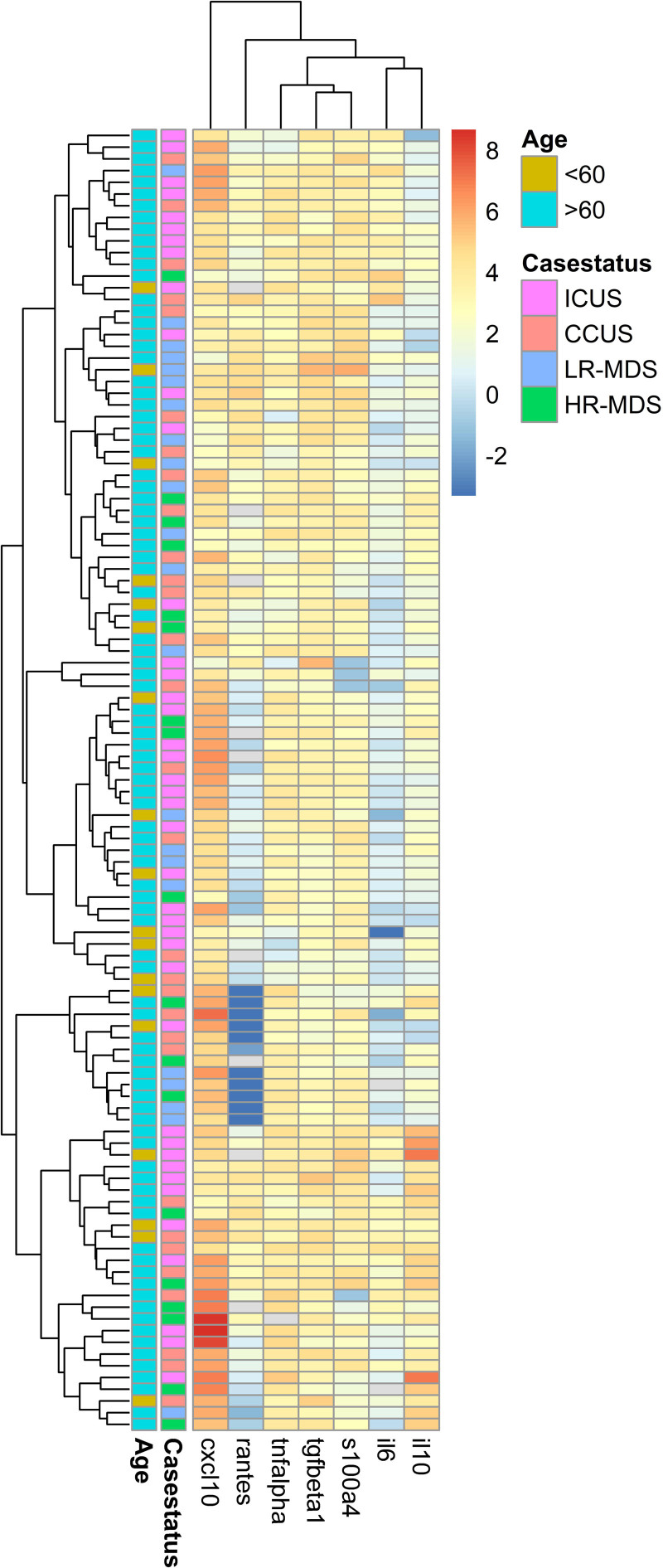
The levels of 20 cytokines were determined for each of 132 peripheral blood plasma samples. The levels of seven of the 20 cytokines were significantly different between patients and controls. Patients had higher levels of the proinflammatory cytokines IL-6 (P < 0.001), TNF-α (P < 0.001), and CXCL10 (P < 0.001) as well as IL-10 (P < 0.001), while lower levels of active TGF-β1 (P < 0.001), RANTES (P < 0.001), and S100A4 (P < 0.001) were observed compared with controls (Figure 1A–H). The results retained statistical significance after Bonferroni correction. The cytokine levels in ICUS and CCUS were comparable to those seen in LR-MDS. Only HR-MDS differed from the other disease groups with significantly higher IL-10 and IL-8 levels compared with patients with LR-MDS, CCUS, and ICUS (P = 0.01, P = 0.007). The difference in IL-10 and IL-8 levels were not statistically significant after Bonferroni correction. Concentrations of peripheral blood plasma cytokines in each group are shown in Table 2. Unsupervised hierarchical clustering of patients reveals a wide variation between patient cytokine profiles. No clusters of cytokines were related to specific disease groups (ICUS, CCUS, LR-MDS, or HR-MDS) or age groups (over/under 60 years old; Figure 2).
Figure 1.
Blood plasma levels of IL-6 (A), IL-10 (B), TNF-α (C), CXCL10 (D), IL-8 (E), RANTES/CCL5 (F), S100A4 (G), and transforming growth factor beta 1 (TGF -β1) (H) in four groups of patients (N = 111) compared with healthy elderly controls (N = 21). All cytokine levels are shown after log2-transformation. One-way ANOVA test is used for comparison between the two groups for each cytokine; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ANOVA = analysis of variance; CCUS = clonal cytopenias of undetermined significance; CXCL10 = C-X-C motif chemokine 10; HR-MDS = higher-risk myelodysplastic syndromes; ICUS = idiopathic cytopenias of undetermined significance; IL = interleukin; LR-MDS = lower-risk myelodysplastic syndromes; RANTES = regulated on activation normal T-cell expressed and secreted; TNF-α = tumor necrosis factor α.
Table 2.
Concentrations of 16 Inflammatory Cytokines in Peripheral Blood Plasma of Four Groups of Patients (N = 111) and Healthy Elderly Controls (N = 21)
| Controls | ICUS | CCUS | LR-MDS | HR-MDS | P Value | |
|---|---|---|---|---|---|---|
| (N = 21) | (N = 41) | (N = 30) | (N = 22) | (N = 18) | ||
| IL-1RA (pg/mL) | 700 [142, 5680] | 636 [160, 3450] | 641 [160, 6170] | 542 [244, 7720] | 796 [170, 4610] | 0.88 |
| IL-1α (pg/mL) | 7.69 [1.26, 94.5] | 6.34 [0.84, 63.7] | 4.10 [0.70, 110] | 4.64 [0.90, 136] | 7.75 [1.41, 40.6] | 0.55 |
| IL-6 (pg/mL) | 1.24 [0.81, 2.53] | 2.30 [0.10, 16.5] | 2.31 [0.30, 36.9] | 2.01 [0.36, 24.4] | 3.16 [0.710, 31.8] | <0.001 |
| IL-7 (pg/mL) | 2.47 [0.05, 301] | 1.16 [0.03, 39.0] | 0.905 [0.05, 56.6] | 2.07 [0.05, 43.0] | 1.39 [0.05, 14.7] | 0.12 |
| IL-8 (pg/mL) | 2.75 [0.140, 263] | 2.96 [0.100, 75.4] | 2.98 [0.220, 114] | 4.11 [0.860, 106] | 9.78 [1.27, 455] | 0.08 |
| IL-10 (pg/mL) | 0.120 [0.050, 0.610] | 0.410 [0.040, 13.3] | 0.570 [0.090, 2.84] | 0.350 [0.070, 3.11] | 0.950 [0.210, 3.46] | <0.001 |
| IL-12p70 (pg/mL) | 148 [2.05, 3280] | 107 [1.11, 1660] | 13.9 [2.00, 3220] | 116 [1.11, 2200] | 71.4 [2.00, 815] | 0.69 |
| TNF-α (pg/mL) | 5.00 [2.99, 7.01] | 11.7 [0.950, 35.3] | 9.25 [1.45, 30.9] | 11.5 [4.08, 21.5] | 11.7 [4.15, 25.3] | <0.001 |
| CXCL10 (pg/mL) | 18.0 [5.65, 80.6] | 53.3 [7.72, 818] | 67.1 [12.6, 303] | 46.2 [7.55, 155] | 72.3 [9.87, 744] | <0.001 |
| CCL5/RANTES (pg/mL) | 17200 [5440, 37800] | 3940 [11.7, 31900] | 4560 [11.7, 29000] | 6390 [11.7, 26200] | 2910 [11.7, 23100] | <0.001 |
| S100A4 (ng/mL) | 415 [210, 845] | 234 [0.00, 679] | 180 [0.00, 579] | 226 [61.0, 1130] | 151 [49.0, 580] | <0.001 |
| S100A9 (pg/mL) | 133 [13.4, 2020] | 135 [42.5, 6500] | 96.6 [7.92, 6500] | 93.4 [13.4, 315] | 76.0 [13.4, 1140] | 0.09 |
| TGF-β1(pg/mL) | 3980 [1590, 5020] | 1050 [349, 4770] | 1130 [354, 3330] | 1190 [465, 4810] | 1030 [437, 1980] | <0.001 |
| VEGF (pg/mL) | 26.9 [11.8, 134] | 21.6 [10.2, 140] | 19.7 [2.93, 188] | 25.7 [8.11, 189] | 27.7 [10.1, 114] | 0.57 |
| GM-CSF (pg/mL) | 6.11 [1.10, 531] | 6.11 [0.0400, 141] | 5.58 [0.0400, 158] | 6.11 [0.0400, 208] | 7.58 [0.520, 97.9] | 0.29 |
| TPO (pg/mL) | 1280 [33.6, 81800] | 846 [27.3, 70000] | 674 [55.6, 81800] | 1230 [134, 81800] | 912 [33.4, 81800] | 0.41 |
P values are shown after Bonferroni correction. IL-1β, IL-4, IL-17A, and IFN-γ are not included in the table as more than 20% of blood plasma levels of these mediators are below detection rate. Concentrations are shown as median [min, max]. Bold = P-values < 0.001.
CXCL10 = C-X-C motif chemokine 10; GM-CSF = granulocyte macrophage colony-stimulating factor; ICUS = idiopathic cytopenias of undetermined significance; IL = interleukin; CCUS = clonal cytopenias of undetermined significance; LR-MDS = lower-risk myelodysplastic syndromes; HR-MDS = higher-risk myelodysplastic syndromes; RANTES = regulated on activation normal T-cell expressed and secreted; TGF-β1 = transforming growth factor beta 1; TNF-α = tumor necrosis factor α; TPO = thrombopoietin; VEGF = vascular endothelial growth factor.
Figure 2.
Clustering of cytokine profiles (IL-6, IL-10, CXCL10, TNF-α, S100A4, RANTES, and TGF-β1) in blood plasma of 111 patients with ICUS, CCUS, LR-MDS, and HR-MDS. Age = patients <60 and >60 years old. All cytokine levels are shown after log2-transformation and normalization. CCUS = clonal cytopenias of undetermined significance; CXCL10 = C-X-C motif chemokine 10; HR-MDS = higher-risk myelodysplastic syndromes; ICUS = idiopathic cytopenias of undetermined significance; IL = interleukin; LR-MDS = lower-risk myelodysplastic syndromes; RANTES = regulated on activation normal T-cell expressed and secreted; TNF-α = tumor necrosis factor α.
Cytokine patterns show robustness
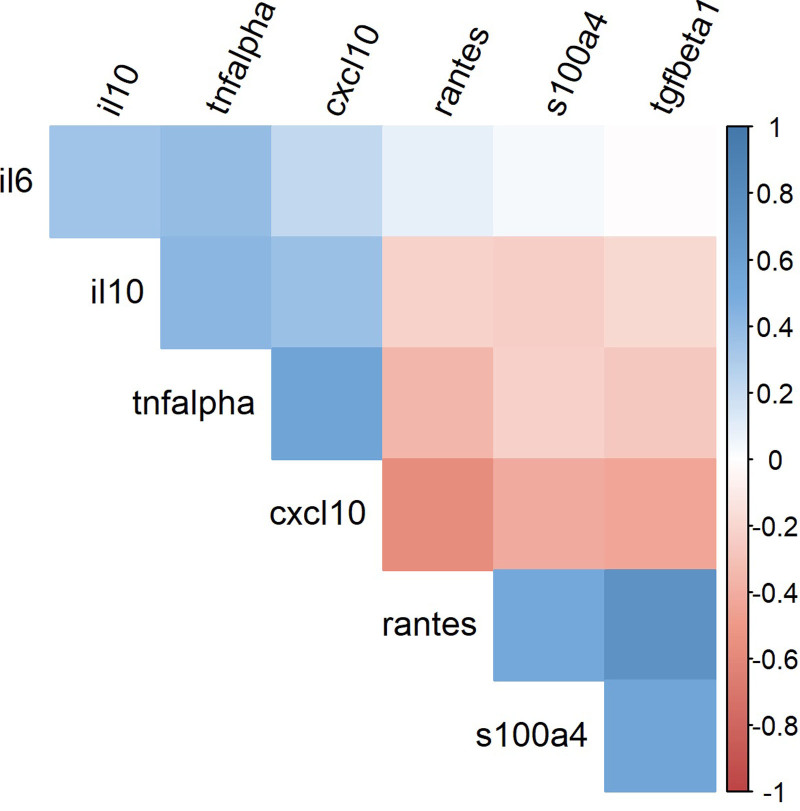
Significant positive correlations between abnormal cytokine levels were seen; direct correlations were observed between the increased cytokines IL-6, TNF-α, IL-10, and CXCL10, and between the decreased cytokines TGF-β1, RANTES, and S100A4 across all groups. Concordantly, TNF-α, IL-10, and CXCL10 levels were negatively correlated with TGF-β1, RANTES, and S100A4. Thus, the cytokine pattern is robust and comparable on an individual level in all four patient groups. Cytokine correlations are shown in Figure 3.
Figure 3.
Spearman’s correlation coefficient displaying cytokine–cytokine correlations in seven cytokines measured in 111 patients with ICUS, CCUS, LR-MDS, and HR-MDS and 21 controls. Positive Spearman’s correlations are shown in blue and negative Spearman’s correlations are shown in red. CCUS = clonal cytopenias of undetermined significance; HR-MDS = higher-risk myelodysplastic syndromes; ICUS = idiopathic cytopenias of undetermined significance; LR-MDS = lower-risk myelodysplastic syndromes.
Anemia is associated with high levels of proinflammatory cytokines
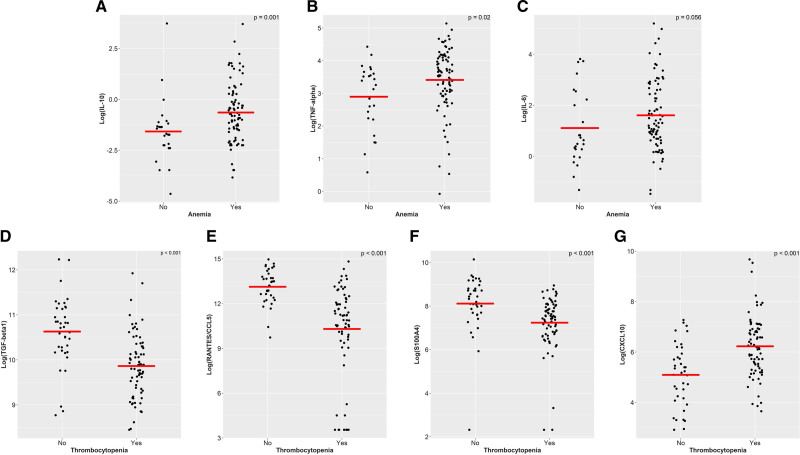
Anemia was associated with increased IL-10, TNF-α, and IL-6 levels compared to patients without anemia (P = 0.001, P = 0.02, P = 0.06, respectively; Figure 4). Of these, only IL-10 remained significant after Bonferroni correction. In multivariate analysis (including age, sex, thrombocytopenia, comorbidities, and inflammatory load) anemia was not associated with any of the seven significant cytokines (Suppl. Table S3).
Figure 4.
Blood plasma levels of IL-10 (A), TNF-α (B), IL-6 (C), transforming growth factor beta 1 (TGF-β1) (D), RANTES/CCL5 (E), S100A4 (F), and CXCL10 (G) in patients with ICUS, CCUS, and MDS (N = 111) with and without blood cytopenias. Cytokine levels are shown after log2-transformation. Anemia was defined as hemoglobin <12.0 g/dL for women or <13.0 g/dL for men. Thrombocytopenia was defined as platelets < 150 × 109/L. Mann–Whitney U test was used to compare plasma levels of cytokines in patients with and without blood cytopenia. CCUS = clonal cytopenias of undetermined significance; CXCL10 = C-X-C motif chemokine 10; HR-MDS = higher-risk myelodysplastic syndromes; ICUS = idiopathic cytopenias of undetermined significance; IL = interleukin; MDS = lower-risk myelodysplastic syndromes; RANTES = regulated on activation normal T-cell expressed and secreted; TNF-α = tumor necrosis factor α.
Thrombocytopenia is independently associated with low levels of TGF-β1, RANTES, and S100A4 levels and high levels of CXCL10 in multivariate analysis
Levels of TGF-β1, RANTES, and S100A4 were significantly lower in patients with thrombocytopenia compared with patients without thrombocytopenia (all P < 0.001) and levels of CXCL10 were significantly higher in this group of patients (P < 0.001). These results were statistical significance after Bonferroni correction (Figure 4) and an independent association was seen in multivariate analysis including age, sex, thrombocytopenia, comorbidities, and inflammatory load (all P < 0.001; Suppl. Table S3).
Under normal physiological conditions, thrombocytes secrete high levels of TGF-β1, but notably, patients with normal thrombocyte counts (>150 × 109/L) did also have lower TGF-β1 levels compared to elderly controls (median 1686 versus 3983 pg/mL; P < 0.001). No significant correlations were found between neutropenia and cytokine levels.
Subgroups of patients with specific MDS-associated mutations
We next stratified patients according to mutational status (Suppl. Figure S1) and observed that the limited group of cases with RUNX1 mutations (N = 8) had lower TGF-β1 levels compared with cases with wtRUNX1 (P = 0.003; Suppl. Figure S2). Interestingly, the size of the VAF of RUNX1 did not correlate with the level of TGF-β1. No other associations were seen between MDS-associated mutations and cytokine levels.
An imbalanced cytokine profile is equally present in patients over and under 60 years
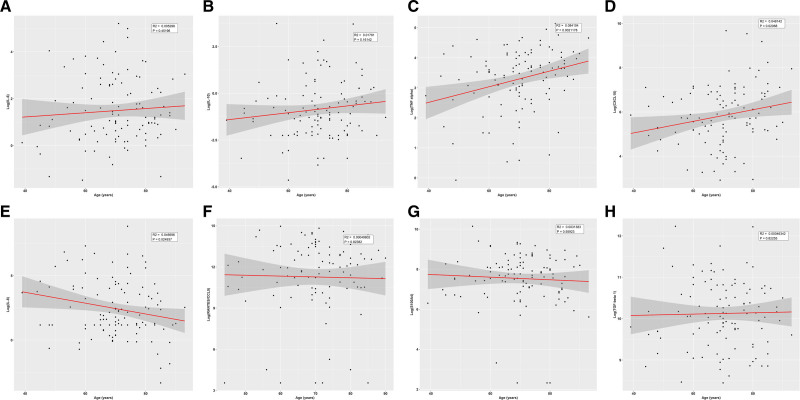
The cytokine levels were not significantly different when patient groups over and under 60 years old were compared. To analyze cytokine levels of younger patients, the group of patients under 60 years (N = 18, mean age = 51 years old, age range: 39–59 years old) were compared with healthy controls (N = 21, mean age = 63 years old, age range: 61–66 years old). This analysis revealed that patients under 60 years had higher levels of TNF-α, IL-10, and CXCL10 and lower levels of transforming growth factor beta 1 (TGF-β1), RANTES, and S100A4 compared with controls (Suppl. Table S4). IL-6 showed a trend toward higher levels in patients compared with controls (P = 0.08). Additionally, age was analyzed as a continuous variable and positive associations to age were seen with TNF-α (R2 = 0.08, P = 0.002) and CXCL10 (R2 = 0.05, P = 0.02; Figure 5). In multivariate analysis, TNF-α and CXCL10 remained statistically significant associated with age (Suppl. Table S3). Finally, age was adjusted for in the multivariate survival analysis of CCUS and MDS patients.
Figure 5.
Relationship between patient’s age (years) and blood plasma levels of IL-6 (A), IL-10 (B), TNF-α (C), CXCL10 (D), IL-8 (E), RANTES/CCL5 (F), S100A4 (G), and transforming growth factor beta 1 (TGF-β1) (H). All cytokine levels are shown after log2-transformation. Linear regression was used to show the correlation between patient’s age and cytokine levels. CXCL10 = C-X-C motif chemokine 10; IL = interleukin; RANTES = regulated on activation normal T-cell expressed and secreted; TNF-α = tumor necrosis factor α.
A high systemic inflammatory cytokine load predicts short OS
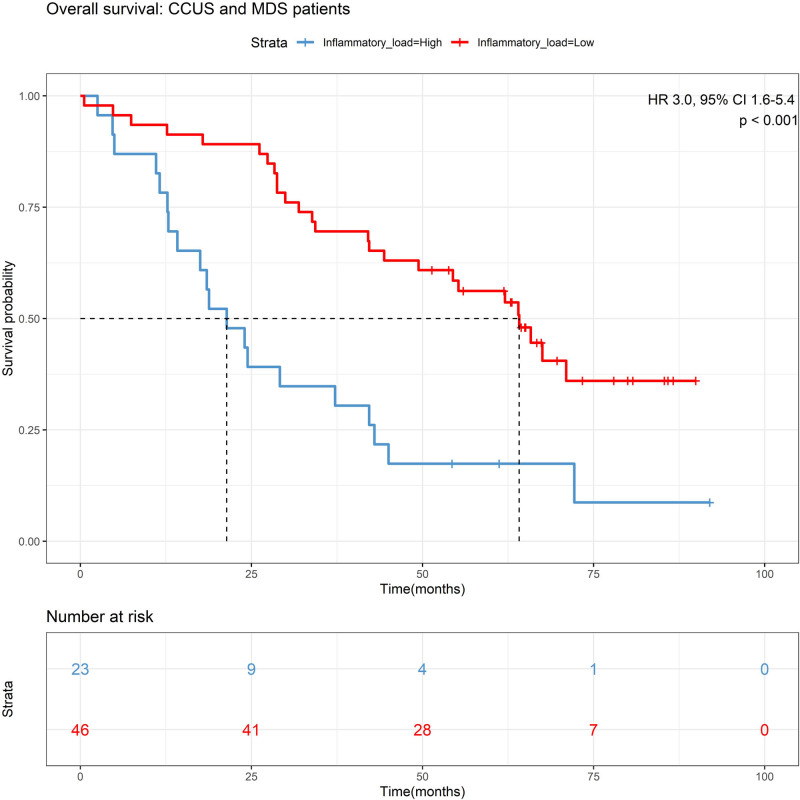
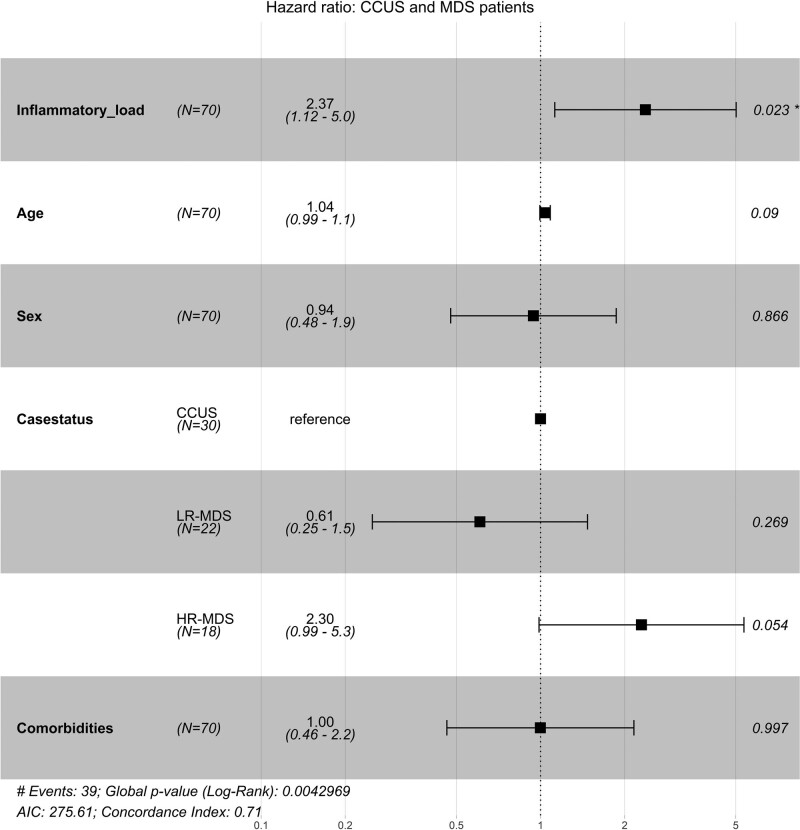
Among the seven cytokines that were aberrantly expressed in all patient groups, increased IL-6 (hazard ratio [HR] 2.6, 95% confidence interval [CI], 1.3-5.1; P = 0.006; Suppl. Figure S3) and increased IL-10 (HR 2.1, 95% CI, 1.1-3.9; P = 0.02; Suppl. Figure S4) were associated with shorter survival in an univariate analysis of patients with CCUS and MDS, but associations did not maintain in multivariate analysis. To identify subjects with the most pronounced systemic chronic inflammatory load we combined patients with high cytokine levels (including IL-6, TNF-α, IL-10, and CXCL10) and classified this group as having a high systemic inflammatory cytokine load. Patients with a high cytokine load had more inflammatory co-morbidities (DM, IHD, and AID) compared with patients with a lower cytokine load (54% vs 37%; P = 0.05; Suppl. Table S5). A high inflammatory cytokine load was observed in 23 of 70 patients with CCUS and MDS. Of 23 patients, 21 died during follow-up. Survival analysis showed that patients with CCUS and MDS with a high systemic inflammatory cytokine load had significantly shorter OS compared with patients with a less inflammatory cytokine load (median OS of 21 vs 64 months; P < 0.001; Figure 6). In multivariate analysis adjusting for age, sex, and disease classification, the high inflammatory cytokine load maintained significantly associated with short OS (HR 2.5, 95% CI, 1.3-4.7; P = 0.006). A high inflammatory load maintained significant influence on survival when adjusting for clinical parameters including anemia (HR 2.1, 95% CI, 1.1-4.1; P = 0.03), thrombocytopenia (HR 2.6, 95% CI, 1.4-5.0; P = 0.004), and co-morbidities (DM, IHD, and AID; HR 2.4, 95% CI, 1.1-5.0; P = 0.02; Figure 7). CCUS patients with a high inflammatory load showed an inferior survival compared with patients with a less inflammatory cytokine load (median OS of 20 vs 66 months; P = 0.001; Suppl. Figure S5). In multivariate analysis adjusting for age and anemia, the high inflammatory cytokine load maintained significantly associated with short OS (HR 3.7, 95% CI, 1.2-11; P = 0.02; Suppl. Figure S6). In addition, survival was in accordance with diagnosis, with longest survival among patients with ICUS and shortest survival among patients with HR-MDS (P < 0.001; Suppl. Figure S7).
Figure 6.
Kaplan–Meier survival curve for overall survival stratified by inflammatory cytokine load in 69 patients with CCUS, LR-MDS, and HR-MDS. Inflammatory cytokine load: low: 0–3 of 4 cytokines increased compared with upper 95% limit of healthy elderly controls. High: 4 of 4 cytokines increased compared with upper 95% limit of healthy elderly controls. Overall survival was measured from the first day of referral to the clinic to death from any cause. Survival curves were made according to the Kaplan–Meier method. CCUS = clonal cytopenias of undetermined significance; CI = confidence interval; HR = hazard ratio; LR-MDS = lower-risk myelodysplastic syndromes; HR-MDS = higher-risk myelodysplastic syndromes.
Figure 7.
Cox model of inflammatory cytokine load in 70 patients with CCUS, LR-MDS, and HR-MDS. Adjusted for age, sex, case status, and comorbidities. Inflammatory cytokine load: low: 0–3 of 4 cytokines increased compared with upper 95% limit of healthy elderly controls. High: 4 of 4 cytokines increased compared with upper 95% limit of healthy elderly controls; case status: CCUS; LR-MDS; HR-MDS; co-morbidities included diabetes mellitus, ischemic heart disease, and autoinflammatory diseases. Events: numbers of death patients during follow-up. *P < 0.05. Log-rank tests were used to determine if individual cytokines (categorized as normal/abnormal) were independently associated with OS. Cox proportional hazard model was used for multivariable analysis. AIC = Akaike’s Information Criterion; CCUS = clonal cytopenias of undetermined significance; HR-MDS = higher-risk myelodysplastic syndromes; LR-MDS = lower-risk myelodysplastic syndromes; OS = overall survival.
DISCUSSION
Although new definitions and guidelines have moved into clinical practice for patients with unexplained persistent cytopenias with or without MDS-associated mutations, little is known about disease pathology and risk stratification in these conditions. Inflammatory cytokine signaling and abnormal cellular immune responses have been well characterized in LR- and HR-MDS and several trials are currently testing immunotherapy in various groups of patients with MDS.23
To the best of our knowledge, this is the first study to investigate the immune profiles of patients with ICUS and CCUS. We measured peripheral blood plasma levels of 20 inflammatory cytokines in patients with ICUS, CCUS, LR-MDS, and HR-MDS and in a control group of healthy elderly blood donors. We clearly demonstrate that all four groups of patients had an increased systemic inflammatory cytokine load (TNF-α, IL-6, CXCL10, IL-10) and reduced concentrations of TGF-β1, RANTES, and S100A4 when compared with controls. Cytokine levels were as aberrant in patients with ICUS/CCUS as in patients with LR-MDS. In addition, patients with HR-MDS had trends toward higher levels of IL-10 and IL-8 and lower levels of RANTES compared with patients with LR-MDS, but numbers of patients were limited.
Our findings support those reported by other studies of patients with MDS, that is that IL-6, TNF-α, IL-10, CXCL10, and RANTES were abnormal in patients with MDS compared with healthy controls.24,25 Previous comparisons of cytokine levels in LR-MDS and HR-MDS have been performed by Kordasti et al15 and Kittang et al.24 In line with our data significant differences in blood plasma levels of IL-10 and RANTES in LR-MDS and HR-MDS were reported in these studies. In addition to the previous studies, we showed that the aberrant levels of IL-10 and RANTES in patients with ICUS and CCUS were comparable to those seen in LR-MDS. Although the studies are incongruent with regards to the difference in RANTES concentrations of healthy donors, their overall results suggest that RANTES levels decrease dramatically from lower risk disease to HR-MDS. In a study of solid tumors, survival was strongly correlated to tumor-infiltrating lymphocytes, whose abundance was associated with RANTES and CXCL9 coexpression.26 We speculate that RANTES may also be of importance to the antitumor response in myeloid malignancies. Pardanani et al27 showed a correlation of increasing IL-8 levels and increasing IPSS risk category in patients with MDS. IL-8 has previously been involved in leukemogenesis as a proangiogenetic mediator.28 This hypothesis is further supported by our data, which revealed no increase in IL-8 levels among patients with ICUS, CCUS, and LR-MDS, but a trend toward higher levels in patients with HR-MDS.
Blood plasma levels of S100A4 have hitherto not been investigated in patients with MDS, nor ICUS or CCUS. S100A4 levels were significantly decreased in all four groups of patients in our study compared with healthy elderly controls. Furthermore, S100A4 showed direct correlation with TGF-β1 and RANTES and negative correlation with CXCL10 (all P values <0.001) in all patient groups. A study of mice with solid cancer revealed that RANTES acts as a critical regulator of S100A4, which might explain why S100A4 is downregulated in this study.29 TGF-β1 has previously been reported to be present in low blood plasma concentrations in patients with AML compared with healthy elderly controls,30 and low TGF-β expression and dysregulation of the TGF-β signaling pathway have been associated with enhanced leukemogenesis.31 Here we showed that even in early myeloid disease stages, such as ICUS and CCUS, TGF-β levels were low. In addition, mutations in the RUNX1 gene have been shown to lead to compromised SMAD signaling, which reduce TGF-β1 signaling.32 This is in line with our results; patients with RUNX1 mutations had lower TGF-β1 levels in blood plasma compared with patients with wtRUNX1, though limited numbers of cases with RUNX1 were available in this study cohort. Notably, patients with wtRUNX1 maintained significantly lower concentrations of TGF-β1 in blood plasma compared with healthy elderly controls.
The mechanisms underlying suppressed erythropoiesis during inflammation are complex and not fully understood. Unexplained anemia might be related to high proinflammatory cytokine levels, especially TNF-α and IL-6.33 Previously, IL-6 has been shown to indirectly decrease erythrocyte production by inducing the synthesis of the iron regulatory hormone hepcidin which leads to functional iron deficiency.34 In this study, IL-6 levels were increased in blood plasma of patients with anemia compared with patients without anemia across all four patient categories (1.7 vs 4.8 pg/mL); however, IL-6 was not independently associated anemia in multivariate analysis.
To our knowledge, cytokines have not been linked to survival in patients with CCUS before. Previous studies of patients with MDS have shown poor OS in patients with high levels of IL-6, CXCL10, and IL-7,30 and in studies of AML, short survival was seen in patients with high levels of IL-6 and low levels of IL-10.27 In the current study, we demonstrated that a high systemic inflammatory cytokine load (IL-6, TNF-α, IL-10, and CXCL10) was an adverse prognostic factor for survival in patients with CCUS, LR-MDS, and HR-MDS (median survival 21 vs median survival 64 months; P < 0.001). In multivariate analysis, a high systemic inflammatory cytokine load was significantly associated with poor survival, even when adjusted for age, sex, disease classification, and comorbidities (HR 2.4, 95% CI, 1.1-5.0; P = 0.02).
In a study by Meisel et al,18 it was shown that bacterial translocation and increased IL-6 production, resulting from dysfunction of the small-intestinal barrier, were critical for leukemic transformation in mice with TET2 mutations. Similarly, SanMiguel et al17 found that mice with DNMT3A mutations have an expansion of clones when increasing the TNF-α levels of the bone marrow, and Hormaechea-Agulla et al35 demonstrated that IFN-γ signaling induced during chronic infection can drive DNMT3A-loss-of-function clonal hematopoiesis in mice. Interestingly, the studies demonstrated reversibility in clone size, when the proinflammatory environment was repressed. These findings support that inhibitors of proinflammatory cytokines may be tested as new therapies to prevent progression in early disease stages as ICUS and CCUS.
The detailed information obtained from the patient files and the Danish national registers enabled us to assess whether cytokines levels associate with clinical characteristics, survival, and MDS-associated mutational profiles. However, this study lacks longitudinal sampling of cytokines to be able to determine the importance of inflammatory cytokines in disease progression. In this study, there could potentially be a confounding effect of age in the comparison of cytokines between healthy elderly controls and patients, hence patients were younger than controls on average. TNF-α and CXCL10 were associated with age in multivariate analysis including all patients. However, the skewed cytokine profile remained significantly different, when analyzing cytokines according to age in controls and the group of younger patients, supporting that the alterations observed in cytokine levels are associated to disease rather than to age difference. Moreover, we cannot exclude that our patient groups were too small to detect some clinical associations to the cytokines measured. In particular, we had low statistical power to detect associations between cytokine levels and MDS-associated mutations. Thus, the correlation of cytokines, somatic mutations, and disease progression warrants investigation in larger cohorts of patients with unexplained cytopenias and MDS.
CONCLUSION
In summary, our data reveal that the inflammatory cytokine signatures of patients with ICUS and CCUS are similar to formerly described signatures of patients with LR-MDS and, in part, HR-MDS. These findings suggest that inflammatory cytokines might be involved in the early pathogenesis of unexplained cytopenias, irrespective of the presence of myeloid clones. It is tempting to speculate whether abnormal cytokine signaling might play a role in disease progression by driving clonal expansion in CCUS; Indeed, our data indicate a significant pathogenetic contribution of the inflammatory cytokines, potentially by contributing to the genomic instability that induce MDS-associated mutations. Taken together, our findings support the idea that inflammatory cytokines may be useful in risk stratification and as potential therapeutic targets in early myeloid disease.36,37
ACKNOWLEDGMENTS
This work was supported by a grant from Rigshospitalet’s Research Foundation and Fru Ingeborg Anna Albinus Larsen’s Mindelegat. KG is funded by a center grant from The Danish Cancer Society (Danish Research Center for Precision Medicine in Blood Cancer; grant 223-A13071-18-S68), from the Novo Nordisk Foundation (Novo Nordisk Foundation Center for Stem Cell Biology, DanStem; grant NNF17CC0027852) and from Greater Copenhagen Health Science Partners (Clinical Academic Group in Blood Cancers).
AUTHOR CONTRIBUTIONS
ABN, JWH, KG, HB, and MS designed the study. ABN, HB, and MS performed the laboratory work. ABN, JWH, and ADØ provided clinical data. HB was responsible for the recruitment of the healthy controls. JWH performed the mutational analysis. All authors helped analyze and interpret the data; ABN, KG, and JWH wrote the manuscript, and all authors revised and approved the final version of the manuscript.
DISCLOSURES
The authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This study was supported by a grant from Rigshospitalet’s Research Foundation and Fru Ingeborg Anna Albinus Larsen’s Mindelegat. KG is funded by a center grant from The Danish Cancer Society (Danish Research Center for Precision Medicine in Blood Cancer; grant 223-A13071-18-S68), from the Novo Nordisk Foundation (Novo Nordisk Foundation Center for Stem Cell Biology, DanStem; grant NNF17CC0027852) and from Greater Copenhagen Health Science Partners (Clinical Academic Group in Blood Cancers).
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malcovati L, Della Porta MG, Strupp C, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica. 2011;96:1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwok B, Hall JM, Witte JS, et al. MDS-associated somatic mutations and clonal hematopoiesis are common in idiopathic cytopenias of undetermined significance. Blood. 2015;126:2355–23w61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cargo CA, Rowbotham N, Evans PA, et al. Targeted sequencing identifies patients with preclinical MDS at high risk of disease progression. Blood. 2015;126:2362–2365. [DOI] [PubMed] [Google Scholar]
- 6.Hansen JW, Westman MK, Sjö LD, et al. Mutations in idiopathic cytopenia of undetermined significance assist diagnostics and correlate to dysplastic changes. Am J Hematol. 2016;91:1234–1238. [DOI] [PubMed] [Google Scholar]
- 7.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen MM, Andersen MA, Grønbæk K, Hansen JW. Long-term clinical outcomes of patients with hematologically unexplained cytopenia after routine assessment: a single center study. Eur J Haematol. 2018;101:595–603. [DOI] [PubMed] [Google Scholar]
- 9.Gañán-Gómez I, Wei Y, Starczynowski DT, et al. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia. 2015;29:1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glenthøj A, Ørskov A, Hansen J, Hadrup S, O’Connell C, Grønbæk K. Immune mechanisms in myelodysplastic syndrome. Int J Mol Sci. 2016;17:944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Yang Y, Gao S, et al. Immune dysregulation in myelodysplastic syndrome: clinical features, pathogenesis and therapeutic strategies. Crit Rev Oncol Hematol. 2018;122:123–132. [DOI] [PubMed] [Google Scholar]
- 12.Méndez-Ferrer S, Bonnet D, Steensma DP, et al. Bone marrow niches in haematological malignancies. Nat Rev Cancer. 2020;20:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basiorka AA, McGraw KL, Eksioglu EA, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128:2960–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordasti SY, Afzali B, Lim Z, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145:64–72. [DOI] [PubMed] [Google Scholar]
- 16.Kittang AO, Kordasti S, Sand KE, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology. 2016;5:e1062208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SanMiguel JM, Loberg M, Heuer S, Stearns T, Young K, Trowbridge J. Cell-extrinsic stressors from the aging Bone Marrow (BM) microenvironment promote Dnmt3a-Mutant clonal hematopoiesis. Blood. 2019;134:5. [Google Scholar]
- 18.Meisel M, Hinterleitner R, Pacis A, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. [DOI] [PubMed] [Google Scholar]
- 20.Klingelhöfer J, Senolt L, Baslund B, et al. Up-regulation of metastasis-promoting S100A4 (Mts-1) in rheumatoid arthritis: putative involvement in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2007;56:779–789. [DOI] [PubMed] [Google Scholar]
- 21.Malcovati L, Karimi M, Papaemmanuil E, et al. SF3B1 mutation identifies a distinct subset of myelodysplastic syndrome with ring sideroblasts. Blood. 2015;126:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitcomb BW, Schisterman EF. Assays with lower detection limits: implications for epidemiological investigations. Paediatr Perinat Epidemiol. 2008;22:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter S, Shoaie S, Kordasti S, Platzbecker U. Integrating the “Immunome” in the stratification of myelodysplastic syndromes and future clinical trial design. J Clin Oncol. 2020;38:1723–1735. [DOI] [PubMed] [Google Scholar]
- 24.Kittang AO, Sand K, Brenner AK, Rye KP, Bruserud Ø. The systemic profile of soluble immune mediators in patients with myelodysplastic syndromes. Int J Mol Sci. 2016;17:E1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng X, Scheinberg P, Wu CO, et al. Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica. 2011;96:602–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dangaj D, Bruand M, Grimm AJ, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. 2019;35:885–900.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardanani A, Finke C, Lasho TL, et al. IPSS-independent prognostic value of plasma CXCL10, IL-7 and IL-6 levels in myelodysplastic syndromes. Leukemia. 2012;26:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryningen A, Wergeland L, Glenjen N, et al. In vitro crosstalk between fibroblasts and native human acute myelogenous leukemia (AML) blasts via local cytokine networks results in increased proliferation and decreased apoptosis of AML cells as well as increased levels of proangiogenic Interleukin 8. Leuk Res. 2005;29:185–196. [DOI] [PubMed] [Google Scholar]
- 29.Forst B, Hansen MT, Klingelhöfer J, et al. Metastasis-inducing S100A4 and RANTES cooperate in promoting tumor progression in mice. PLoS One. 2010;5:e10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61:885–891. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Chen P, Huang HF, et al. Reduction of transforming growth factor-β1 expression in leukemia and its possible role in leukemia development. Leuk Lymphoma. 2012;53:145–151. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43–47. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T. Anemia of inflammation. N Engl J Med. 2019;381:1148–1157. [DOI] [PubMed] [Google Scholar]
- 34.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hormaechea-Agulla D, Matatall KA, Le DT, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell. 2021;28:1428–1442.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azrakhsh NA, Mensah-Glanowska P, Sand K, et al. Targeting immune signaling pathways in clonal hematopoiesis. Curr Med Chem. 2019;26:5262–5277. [DOI] [PubMed] [Google Scholar]
- 37.Steensma DP. The clinical challenge of Idiopathic Cytopenias of Undetermined Significance (ICUS) and Clonal Cytopenias of Undetermined Significance (CCUS). Curr Hematol Malig Rep. 2019;14:536–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.