Abstract
Background:
The use of radiofrequency in aesthetic surgery has evolved significantly since it was first introduced in the early 2000s. Nonexcisional correction of the lower one-third of the face and neck has long been a challenging problem. The purpose of this prospective study was to assess the safety and efficacy of the first handsfree thermoregulated bipolar radiofrequency device for face and neck contouring.
Methods:
This prospective multicenter (New York, Nevada) IRB-approved study evaluated healthy candidates who desired noninvasive correction of their lower face and neck laxity. The primary objective of this study was to evaluate safety and soft tissue remodeling pretreatment and at 1-, 3-, and 6-months post last treatment. Assessment was made using blinded evaluators, 3D photographic analysis (Quantificare, France), and volumetric measurements. Investigator and subject assessments were obtained using a 0-4 point Likert scale.
Results:
A total of 34 patients completed both the cheek and chin applicator treatment series. Average age of patients was 38 (STD 3.4), BMI 27 (STD 2.2), average Baker Face & Neck classification 2.6 (STD 1.1), and average Fitzpatrick type 2.4 (STD 1.2). Mean treatment time was 41 min (STD 3.5) with a temperature of 42°C–43°C. Patient discomfort data were statistically very low based on t-test analysis. Satisfaction metrics measured at 1- and 3-month follow-up demonstrated a significant change in subject skin appearance, subject overall satisfaction, and investigator improvement perception. More patients were satisfied at the 3-month follow-up compared with the 1-month follow-up for all three measures. Volumetric data demonstrated an average change of −3.2 cm3 (STD ±1.2 cm3) per side for the cheek applicator and −4.1 (STD ±2.3) for the submental applicator. Of note there were cases where volume increases were noted that were believed to be related to soft tissue contraction.
Conclusions:
This is the first prospective study to evaluate a handsfree thermoregulated bipolar radiofrequency device for face and neck contouring. This device demonstrates a significant advance in the control and delivery of radiofrequency for aesthetic purposes. With a favorable safety and comfort profile, this device is able to concentrate thermal energy consistently at a depth that allows for fibroseptal network tightening to improve lower third of face and submental soft tissue contraction.
Takeaways
Question: How does radiofrequency produce soft tissue contraction?
Findings: This prospective study demonstrated safety and efficacy of a handsfree noninvasive bipolar radiofrequency device in soft tissue contraction of the lower face and submental area. Consistent with previous studies, acquisition of temperatures (42°C–43°C) for the duration of the treatments produce soft tissue contraction by stimulation of neocollagenesis and elastin reorganization.
Meaning: Noninvasive bipolar radiofrequency can achieve safe and effective soft tissue contraction of the lower one-third of the face and neck.
INTRODUCTION
The multifactorial etiology of facial aging and increasing demand for noninvasive and minimally invasive treatments have led to development of various modalities to address the components of the aging face.1,2 As a result, industry for noninvasive and minimally invasive facial aesthetics continues to grow at an all-time high, with a current estimated market size of 19 billion USD in 2019. This includes treatments such as skin resurfacing (chemical peels, lasers, microneedling), energy-based devices (radiofrequency, ultrasound), suspension methods (threads), and volumizing methods (biologic and nonbiologic fillers).3–13
Our current method of aesthetic facial analysis systematically divides the face into horizontal thirds to identify and treat the predictable changes that occur per facial region. Among the most challenging areas to correct has been the lower face and neck. As opposed to the upper thirds of the face where volume deflation is a primary target for correction, the lower third of the face and neck requires correction of soft tissue laxity and descent (jowls and submental region), which has long been an elusive goal for the noninvasive or minimally invasive technologies.14,15 In fact, even traditional excisional surgery (ie, facelift/necklift) is often challenging due to the tendency for recurrent laxity in these areas postoperatively.16
Numerous energy-based technologies have developed to address aesthetic concerns in the lower third of the face and neck, including laser, cryolypolysis, high-intensity focused ultrasound (HIFU), and radiofrequency (RF).17–24 Over the past 20 years, RF has emerged as a safe and efficacious method to produce soft tissue contraction through thermal energy.3,5,14,15,25–29 Today’s RF devices allow for closely regulated delivery of electrical currents to achieve precise thermal effect in accordance with Ohm’s law (energy = current2 × impedance × time).14,15 As RF is applied to different tissue types (ie, muscle, fat, skin), the inherent resistance or impedance leads to generation of thermal energy. Tissues with higher impedance (ie, dermis, fibroseptal networks, fat) generate more heat than tissues with lower impedance (muscle). Once target temperatures are acquired and maintained, RF has been shown to achieve soft tissue remodeling by two mechanisms: (1) cleavage of hydrogen bonds in the collagen triple helix structure, leading to immediate fibril denaturation and contraction/thickening of collagen, and (2) a wound healing cascade is triggered to induce neocollagenesis, angiogenesis, and elastogenesis, leading to long-term soft tissue contraction.14,15 Clinical and animal studies have both shown that after 10 minutes of exposure to temperatures of 39°C–43°C, the amount of collagen increases from approximately 15% to 20% after a 3-month follow-up period.30 Other studies have similarly shown through electron microscopy that collagen fibrils had a greater diameter after RF treatment.31 In addition, Northern blot analysis has confirmed microinflammatory stimulation of fibroblasts and other substances that enhance dermal structure.31 RF has not only been proved effective for skin tightening, but it has also been shown to trigger apoptosis in adipocytes.32
Today’s aesthetic RF devices have advanced significantly over the first FDA approved RF device for facial wrinkle reduction in 2004 (ThermaCool, Thermage, Inc., Hayward, Calif.).33 The major advance has not only been in the RF energy delivery method itself but in depth, control, and consistency of energy delivery. The balance between temperatures that trigger a nonablative wound healing response to remodel collagen as opposed to ablating collagen is relatively narrow.14,15,29 The depth and degree of energy transferred depends on several factors, including the size and configuration of the treatment device, energy settings, time of treatment, and inherent conductive properties of the tissue.
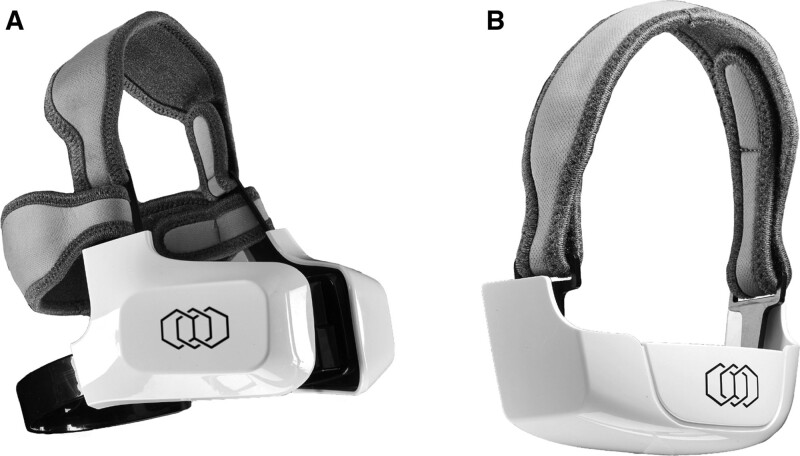
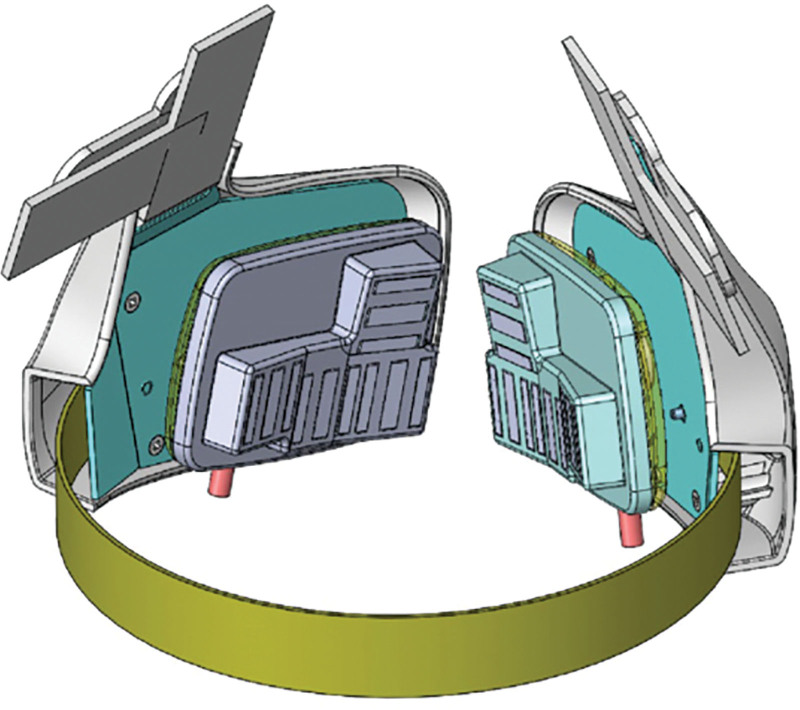
The purpose of this study was to prospectively evaluate the first “hands free” thermoregulated noninvasive bipolar radiofrequency technology (EVOKE; InMode, Lake Forest, Calif.) specifically intended to target lower one-third of the face soft tissue laxity. (Fig. 1) To our knowledge, this is the first device designed to provide soft tissue contraction through noninvasive bipolar radiofrequency in an automated delivery process.
Fig 1.
Evoke Bipolar noninvasive radiofrequency device. A, Cheek applicator. B, Chin applicator.
METHODS
This multicenter prospective study was IRB approved and complied with the Declaration of Helsinki. Subjects were healthy adults between the ages of 36–75 years with visible signs of facial aging, seeking skin tightening treatments. Three centers conducted this study (New York, N.Y. & Reno/Tahoe, Nev.) with the goal of enrolling 10–20 subjects per site. Exclusion criteria included any type of electrical implant (pacemaker, defibrillator), permanent implant in the treatment areas (metal plates, silicone implants, screws), current or history of skin cancer, immune compromise, history of skin disorders (keloids, wound healing conditions), 6 months post any skin treatment (filler, microneedling, laser), or use of isotretinoin.
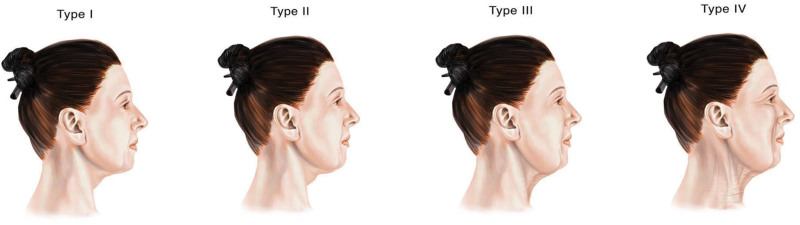
The primary objective of this study was to evaluate soft tissue remodeling pretreatment and at 1-, 3-, and 6-months post last treatment. This was assessed using blinded evaluators (objective plastic surgeons not familiar with the device/study), 3D photographic analysis (Quantificare, France), and volumetric measurements. Investigator and subject assessments were obtained using a 0-4 point Likert scale at the 1-, 3-, and 6-month follow-up visits. Baker Face & Neck classification was also utilized to classify patients (Fig 2). Adverse events and treatment discomfort were closely monitored after each treatment. The treatment discomfort metrics were chin discomfort and cheek discomfort at each of the three treatments. The six subject satisfaction metrics were measured on an 11-point scale, where 0 = most comfortable, and 10 = most uncomfortable. The follow-up visit satisfaction metrics were (1) subject skin appearance evaluation, (2) subject overall satisfaction, and (3) investigator improvement rating. Because follow-up visits occurred twice (1-month follow-up and 3-month follow-up), there were six satisfaction metrics in all. These six metrics were measured along the same scale, where 0 = no change, and 4 = significantly marked improvement.
Fig. 2.
Baker face and neck classification.
RESULTS
This study was composed of 40 patients (n = 40) who completed all treatments, of which 34 participants completed at least one follow-up visit. All subjects sought noninvasive treatment for facial aging and were in satisfactory health. Patient demographics included an average age of 38 (STD 3.4), BMI 27 (STD 2.2), average Baker Face & Neck classification of 2.6 (STD 1.1), and average Fitzpatrick type 2.4 (STD 1.2) All patients were treated with both the cheek and chin applicators. Mean treatment parameters include temperature of 42°C–43°C for a mean duration of 41 min (STD 3.5).
Given the nature of this study, the treatment results were reported both descriptively and inferentially. Starting first with the descriptive results, the vast majority of respondents reported no or very low chin or cheek discomfort as a result of the procedure. In general, the discomfort rate improved at each successive treatment. The discomfort was rated on a 1–10 point Likert scale, with 0 being no discomfort and 10 being maximal discomfort (Table 1).
Table 1.
Chin and Cheek Discomfort Metrics at Each Treatment Interval
| Discomfort | Chin | Cheek | ||||
|---|---|---|---|---|---|---|
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 1 | Treatment 2 | Treatment 3 | |
| 0 | 52.5% | 59.5% | 65.7% | 53.8% | 54.1% | 70.6% |
| 1 | 7.5% | 8.1% | 8.6% | 15.4% | 16.2% | 14.7% |
| 2 | 17.5% | 10.8% | 11.4% | 7.7% | 10.8% | 2.9% |
| 3 | 7.5% | 8.1% | 0.0% | 7.7% | 8.1% | 5.9% |
| 4 | 2.5% | 2.7% | 2.9% | 5.1% | 5.4% | 2.9% |
| 5 | 7.5% | 5.4% | 5.7% | 5.1% | 5.4% | 2.9% |
| 6 | 2.5% | 2.7% | 0.0% | 0.0% | 0.0% | 0.0% |
| 7 | 2.5% | 2.7% | 5.7% | 2.6% | 0.0% | 0.0% |
| 8 | 0.0% | 0.0% | 0.0% | 2.6% | 0.0% | 0.0% |
Next, these values were tested inferentially, utilizing a one-sample t test against a hypothesized mean. Note that given the above distributions are skewed toward zero, the Wilcoxon signed-rank test was conducted in addition. The conclusions were the same, given the means and medians were closely aligned. Thus, only the one-sample t test results will be reported. First, a cutoff/test value of 5 was utilized, as 5 represented the middle value of the scale. For all three time points for chin and cheek measures, the observed means were statistically significantly different. That is, participants evaluated their chin and cheek discomfort well below the middle of the scale (moderate discomfort), indicating low discomfort with the procedures. Decreasing the test value to be 2 (the first quartile cutoff value, a low discomfort level) largely tells the same story, though not all comparisons are statistically different from one another. That is, treatment 1 of the chin discomfort evaluation did not differ from a hypothesized cutoff value of 2 (though it was near the 0.05 P-value threshold, indicating marginal significance); thus, the discomfort caused by this treatment can be considered low. The time 2 and 3 chin discomfort evaluations were below a P-value of 0.05, indicating the discomfort levels were considered very low. The other three cheek discomfort evaluations were nearly identical. The mean difference for the first evaluation was marginally different from the hypothesized value of 2, indicating the discomfort caused by this procedure was considered low. For the second and third treatments, the mean differences were significantly lower than 2, indicating the discomfort in these visits was considered very low. The following table demonstrates the means, t scores, degrees of freedom, and P-values of the size tests utilizing the cutoff values of 2 and 5 (Table 2).
Table 2.
One-Sample t-test Statistics Results
| Measure | t | df | P | Mean | Mean Difference |
|---|---|---|---|---|---|
| Test value = 2 | |||||
| T1 chin discomfort | −1.816 | 39 | 0.08 | 1.44 | −0.563 |
| T2 chin discomfort | −2.273 | 36 | 0.03 | 1.27 | −0.73 |
| T3 chin discomfort | −2.587 | 34 | 0.01 | 1.11 | −0.886 |
| T1 cheek discomfort | −1.919 | 38 | 0.06 | 1.37 | −0.628 |
| T2 cheek discomfort | −3.519 | 36 | 0.001 | 1.11 | −0.892 |
| T3 cheek discomfort | −6.181 | 33 | <0.001 | 0.65 | −1.353 |
| Test value = 5 | |||||
| T1 chin discomfort | −11.504 | 39 | <0.001 | 1.44 | −3.563 |
| T2 chin discomfort | −11.617 | 36 | <0.001 | 1.27 | −3.73 |
| T3 chin discomfort | −11.347 | 34 | <0.001 | 1.11 | −3.886 |
| T1 cheek discomfort | −11.082 | 38 | <0.001 | 1.37 | −3.628 |
| T2 cheek discomfort | −15.355 | 36 | <0.001 | 1.11 | −3.892 |
| T3 cheek discomfort | −19.885 | 33 | <0.001 | 0.65 | −4.353 |
Post-treatment Satisfaction and Evaluation Metrics
As previously mentioned, the satisfaction metrics, measured at 1-month follow-up and 3-month follow-up, were (1) subject skin appearance evaluation, (2) subject overall satisfaction, and (3) investigator improvement rating, all along the same scale. The majority of respondents/investigators identified some to significant change compared with no change; thus, the descriptive statistics are reported (Table 3), accordingly. That is, at 1-month, 3-month, and 6-month follow-up, subject skin appearance, subject overall satisfaction, and investigator improvement perception were in agreement; more people reported some to significant change compared with those who reported no change, by a large plurality. There is also anecdotal evidence to suggest more people were satisfied at the 3-month follow-up compared with the 1-month follow-up for all three measures.
Table 3.
Post-treatment Satisfaction and Evaluation Metrics
| Measure | n | % No Change | % Some to Significant Change |
|---|---|---|---|
| 1MFU sub skin appearance | 33 | 33.3% | 66.7% |
| 3MFU sub skin appearance | 26 | 19.2% | 80.8% |
| 6MFU sub skin appearance | 14 | 21.4% | 78.6% |
| 1MFU sub overall sat | 33 | 24.2% | 75.8% |
| 3MFU sub overall sat | 26 | 15.4% | 84.6% |
| 6MFU sub overall sat | 15 | 20.0% | 80.0% |
| 1MFU invest improve | 31 | 35.5% | 64.5% |
| 3MFU invest improve | 27 | 33.3% | 66.7% |
| 6MFU invest improve | 14 | 35.7% | 64.3% |
One-sample t-tests against hypothesized means were conducted for the following nine comparisons. The one-sample t-test results were consistent with the descriptive statistics results. The hypothesized mean value used in this series of tests was 0, as 0 represented no change. Each of the nine tests was significant, indicating that for each metric, at all three time points, there was an observed improvement compared with the hypothesized baseline (no change). Another one-sample t-test was conducted using the hypothesized mean value of 0.5; all tests were also statistically significant, which indicates that all metrics were better compared with even a slight change. In other words, subjects at all time points and for all measurements reported larger improvements compared with minor improvement. The following table represents the one-sample t-test results for the hypothesized comparison value of 0.5 (Table 4).
Table 4.
One-Sample t-test Statistics Results (Test Value = 0.5)
| Measure | t | df | P | Mean | Mean Difference |
|---|---|---|---|---|---|
| 1MFU skin appearance | 3.04 | 32 | 0.01 | 1.06 | 0.56 |
| 1MFU overall sat | 4.56 | 32 | 0.00 | 1.64 | 1.14 |
| 1MFU invest improvement | 2.72 | 30 | 0.01 | 0.94 | 0.44 |
| 3MFU skin appearance | 4.41 | 25 | 0.00 | 1.39 | 0.88 |
| 3MFU overall sat | 4.64 | 25 | 0.00 | 1.77 | 1.27 |
| 3MFU invest improvement | 2.99 | 26 | 0.01 | 1.07 | 0.57 |
| 6MFU skin appearance | 2.96 | 13 | 0.01 | 1.29 | 0.79 |
| 6MFU overall sat | 2.28 | 14 | 0.04 | 1.07 | 0.57 |
| 6MFU invest improvement | 2.14 | 13 | 0.05 | 1.07 | 0.57 |
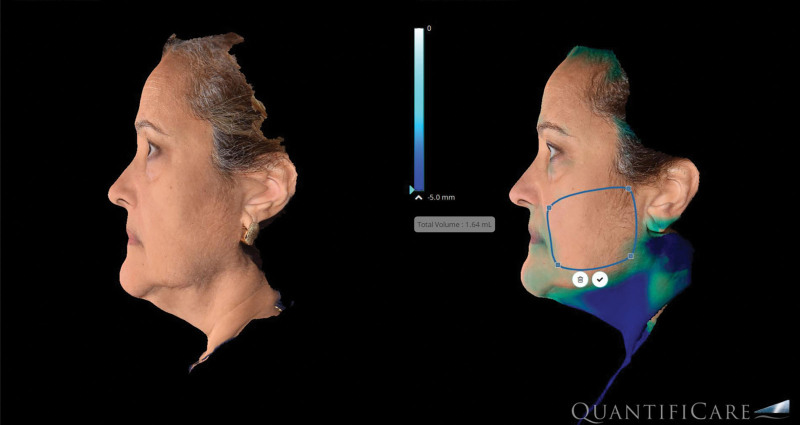
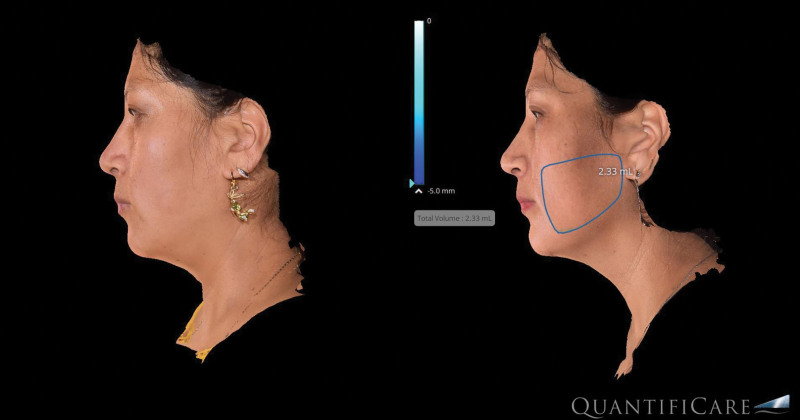
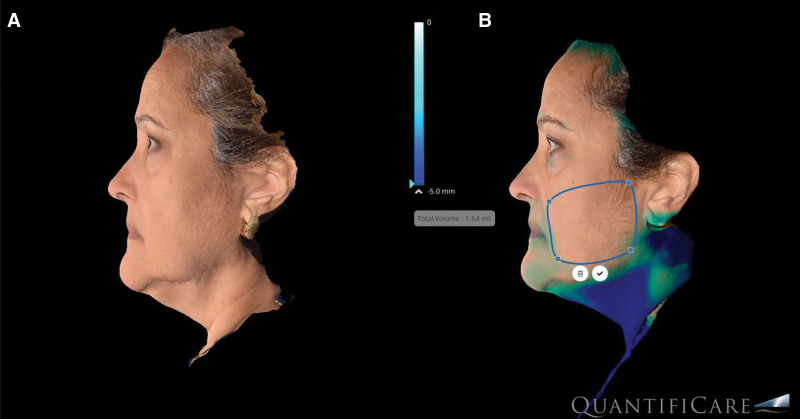
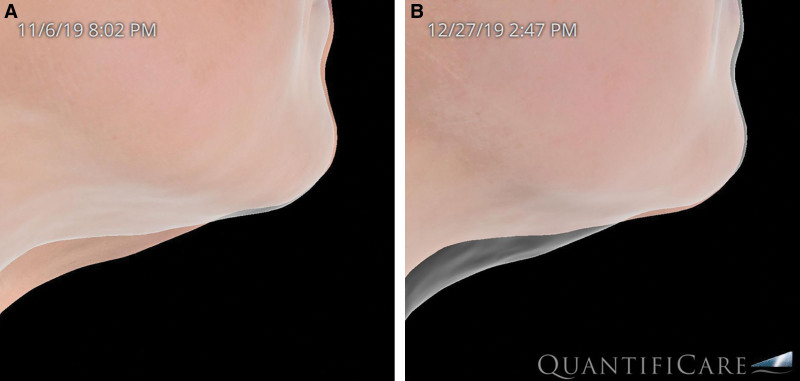
The analysis of volumetric data obtained from our imaging software (Quantificare, France) provided valuable insights to soft tissue changes resulting from this treatment. The research grade camera used digitally generates volumetric changes (Fig. 3). The RF technology used in this study produced two effects seen on the volumetric imaging: (1) soft tissue contraction and (2) adipose remodeling. The average volume changes from the initial treatment to 6-month follow-up were −3.2 cm3 (STD ±1.2) per side for the cheek applicator and −4.1 (STD ±2.3) for the submental applicator. Interestingly, there were cases where volume increases were noted (Fig. 4). Although this could be due to weight gain, analysis of the images indicates that that contraction of the soft tissue envelope caused the imaging software to identify a volume increase rather than soft tissue contraction.
Fig 3.
Volumetric image analysis (Quantificare, France).
Fig. 4.
Increased volumetric assessment due to soft tissue contraction.
In all, there is significant evidence to suggest this noninvasive RF treatment is effective from treatment to treatment (by way of the chin and cheek discomfort measures) and 1-, 3-, and 6-month follow-up (by way of the subject skin appearance, subject overall satisfaction, and investigator improvement rate). The descriptive statistics results largely align with the results of the one-sample t-tests.
DISCUSSION
The noninvasive thermoregulated bipolar radiofrequency device evaluated in this study represents significant development in the delivery and control of RF energy. As opposed to prior RF devices, this is the first device to provide a handsfree treatment modality. This means that the device could be placed on the patient, pre-set to target temperatures, and activated, not requiring manual provider application. The device automatically reaches target temperature within 1–2 minutes and regulates electromagnetic current delivery to achieve the predetermined temperature settings consistently throughout the treatment. This feature eliminates user variability previously seen with other manual RF deliver methods, leading to inconsistent energy delivery and outcomes.3,5,14,25
Another unique feature is the use of deep bipolar RF energy, which reaches 4-mm depth and targets the lower one-third of the face and submental laxity through two separate components (cheek and submental). Each side of the cheek headpiece contains four applicators, and the submental headpiece has three applicators, with each applicator comprising three electrodes (Fig. 1). The bipolar RF travels directionally from the central electrode to outer electrodes to achieve a volumetric bulk heating at approximately a 4-mm depth, which impacts fibroseptal networks between the superficial musculoaponeurotic system and dermal layers. Intervening thermostats were engineered between the electrodes to be in contact with the skin and achieve real-time and responsive temperature monitoring (Fig. 5). As opposed to other RF devices, surface cooling is not necessary due to the tight thermal control, and depth of heat achieved with this device configuration. The purpose of this configuration was to maximally impact fibroseptal network contraction, which has been shown to be more effective than only subdermal heating. The deep heating effect further allows for a wider gradient of volumetric heat to be applied. The electrode size and coverage area also allows for bulk heating of the entire anatomic subunit. Rather than prior devices that claim a “lift” effect, it has become clear that RF does not induce “lift,” but rather soft tissue contraction. Thus, a wider treatment area is required to achieve a clinically impactful effect.
Fig. 5.
Thermostats between electrodes.
Traditionally, noninvasive energy-based aesthetic devices have produced modest results due to the limited amount of energy that can be delivered safely at the dermal/subdermal level without causing ablative injury to the skin surface. Without precise temperature control, predecessor devices either were “underpowered” and caused a few complications with limited to no results, or conversely the devices were “overpowered” and led to unacceptable percentage of thermal injuries. Radiofrequency microneedling devices were a partial solution to this by bypassing the skin level and delivering deeper RF energy; however, most of these devices had energy concentrated between the needle tips, thus not volumetrically heating the soft tissue envelope.
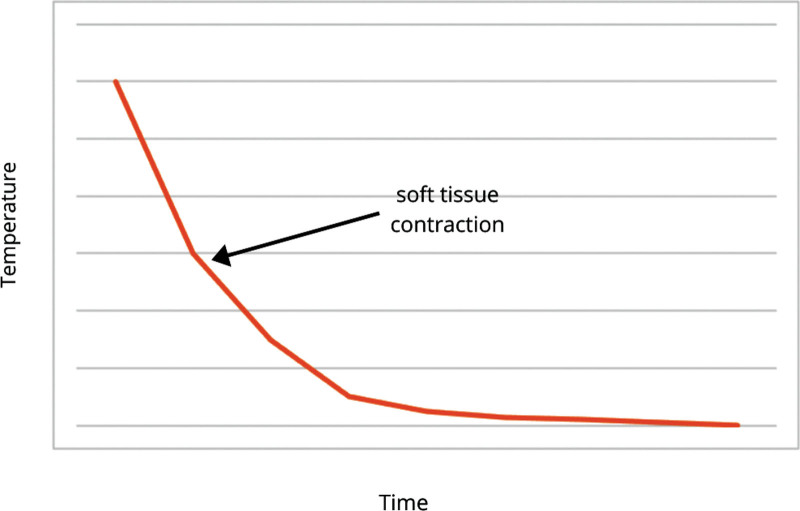
The aesthetic application of radiofrequency energy delivery stimulates neocollagenesis functions through an Arrhenius time versus temperature relationship (Fig. 6). Studies suggest that for millisecond exposures, the shrinkage temperature is above 85°C, whereas for exposures for several seconds, the shrinkage temperature is in the range of 60°C–65°C.31,34 For every 5°C decrease in temperature, a 10× increase in time is required to achieve a comparable collagen contraction.31,34 Although the “ideal time” has been elusive, it has been well established that using lower temperatures (fluence) for a longer period of time is more effective at collagen stimulation, rather than using higher temperatures and shorter time.14,15,29 Further, it has been postulated that lower temperatures may additionally stimulate fibroblasts to form collagen. Seo et al compared facial soft tissue laxity improvements with RF versus surgical facelift using blinded grading of photographs.35 They demonstrated a 49% improvement in skin laxity relative to baseline for the surgical facelift compared with 16% for bipolar RF. Further the mean laxity improvement form a single bipolar RF treatment was 37% of the surgical facelift.35 Peterson et al36 also studied objective measurements of mechanical skin properties and demonstrated a statistically significant improvement (5%–12% decrease in Young’s modulus and 10%–16% decrease in retraction time), as well as 1.42 grade improvement on the Fitzpatrick scale for wrinkles, and 0.66 on the Alexiades scale for skin laxity, increasing to 1.57 and 0.70 improvement at 6 months.36 Patient satisfaction was noted to be “very high” for more than 90% of patients.36 Multiple other studies have corroborated these findings by showing efficacy of nonablative multisource RF as a single modality for face/body contouring.37–39 Similar to the findings of Mulholland and Hruza et al, this technology allows for thermal stimulation from an “inside-outside” method, directing the heat gradient from 4 mm of depth toward the dermis.10 The patient comfort and 1-, 3-, and 6-month follow-up measurements utilized in this study (subject skin appearance, subject overall satisfaction, and investigator improvement rate) all are statistically consistent with this explanation. The 3D volumetric data obtained in this study demonstrate that the soft tissue contraction effect of the RF supercedes volume loss. One consideration that arises with the use of this technology is the possibility that RF will impact adipocyte viability and lead to undesireable volume loss. This is of concern, as modern aesthetics aims to revolumize facial fat compartments that decrease with age. However, our volumetric data demonstrate that some volume loss in the lower one-third of the face is aesthetically desirable, especially when combined with a more significant soft tissue contraction (Figs. 7, 8). (See figure, Supplemental Digital Content 1, which displays (A) before and (B) after results EVOKE (InMode, Lake Forest, Calif.). http://links.lww.com/PRSGO/B967.)
Fig. 6.
Arrhenius time versus temperature relationship.
Fig. 7.
A 52-year-old female patient before (A) and after (B) Evoke treatment.
Fig. 8.
A 54-year-old female patient 7 weeks before (A) and after (B) EVOKE treatment.
There were a number of limitations to this study. Although this study was appropriately powered for statistical analysis, there were only 34 patients who completed the entire study follow-up period. We would have preferred a larger sample size to obtain more information on the efficacy of this treatment on various age brackets to better ascertain expectations for treatment and who is the ideal candidate. The volumetric data obtained by the 3D camera system used did not account for soft tissue laxity, and data may have been confounded by changes in soft tissue laxity that may skew volume readings. It also would have been preferable to exclude patients who had any procedure on their faces/neck in the past as some patients may have had lingering effects (ie, filler) that would have confounded the effect of RF alone. Also, there are more objective scales that are currently being applied to future prospective studies, including the Fitzpatrick wrinkle scale and Alexiades skin laxity scale as well as surface area measurements.
This is the first prospective study to evaluate a handsfree thermoregulated bipolar radiofrequency device for face and neck contouring. This device demonstrates a significant advance in the control and delivery of radiofrequency for aesthetic purposes. With a favorable safety and comfort profile, this device is able to concentrate thermal energy consistently at a depth that allows for fibroseptal network tightening to improve lower third of face and submental soft tissue contraction.
Supplementary Material
Footnotes
Published online 28 March 2022.
Disclosure: Dr. Dayan is a consultant at InMode, Codeveloper at CoreAesthetics.com, and receives book royalties from Thieme. Dr. Chapas, Chia, and Theodorou are consultants at InMode. Dr. Marte has no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Farkas JP, Pessa JE, Hubbard B, et al. The science and theory behind facial aging. Plast Reconstr Surg Glob Open. 2013;1:e8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohrich RJ. The “Science of Aging” supplement. Plast Reconstr Surg. 2021;147:1S–2S. [DOI] [PubMed] [Google Scholar]
- 3.Abraham MT, Mashkevich G. Monopolar radiofrequency skin tightening. Facial Plast Surg Clin North Am. 2007;15:169–177, v. [DOI] [PubMed] [Google Scholar]
- 4.Aslam A, Alster TS. Evolution of laser skin resurfacing: from scanning to fractional technology. Dermatol Surg. 2014;40:1163–1172. [DOI] [PubMed] [Google Scholar]
- 5.Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638–642. [DOI] [PubMed] [Google Scholar]
- 6.Ee HL, Barlow RJ. Lasers, lights and related technologies: a review of recent journal highlights. Clin Exp Dermatol. 2007;32:135–137. [DOI] [PubMed] [Google Scholar]
- 7.Fisher GH, Jacobson LG, Bernstein LJ, et al. Nonablative radiofrequency treatment of facial laxity. Dermatol Surg. 2005;31(9 Pt 2):1237–1241; discussion 1241. [DOI] [PubMed] [Google Scholar]
- 8.Gentile RD. Renuvion/J-plasma for subdermal skin tightening facial contouring and skin rejuvenation of the face and neck. Facial Plast Surg Clin North Am. 2019;27:273–290. [DOI] [PubMed] [Google Scholar]
- 9.Greene RM, Green JB. Skin tightening technologies. Facial Plast Surg. 2014;30:62–67. [DOI] [PubMed] [Google Scholar]
- 10.Hruza G, Taub AF, Collier SL, et al. Skin rejuvenation and wrinkle reduction using a fractional radiofrequency system. J Drugs Dermatol. 2009;8:259–265. [PubMed] [Google Scholar]
- 11.Kornstein AN. Ultherapy shrinks nasal skin after rhinoplasty following failure of conservative measures. Plast Reconstr Surg. 2013;131:664e–666e. [DOI] [PubMed] [Google Scholar]
- 12.Laubach HJ, Tannous Z, Anderson RR, et al. Skin responses to fractional photothermolysis. Lasers Surg Med. 2006;38:142–149. [DOI] [PubMed] [Google Scholar]
- 13.Rohrich RJ, Sanniec K, Afrooz PN. Autologous fat grafting to the chin: a useful adjunct in complete aesthetic facial rejuvenation. Plast Reconstr Surg. 2018;142:921–925. [DOI] [PubMed] [Google Scholar]
- 14.Dayan E, Burns AJ, Rohrich RJ, et al. The use of radiofrequency in aesthetic surgery. Plast Reconstr Surg Glob Open. 2020;8:e2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan E, Rovatti P, Aston S, et al. Multimodal radiofrequency application for lower face and neck laxity. Plast Reconstr Surg Glob Open. 2020;8:e2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skouras GA, Skouras AG, Skoura EA. Revision and secondary facelift: problems frequently encountered. Plast Reconstr Surg Glob Open. 2020;8:e2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hruza GJ, Dover JS. Laser skin resurfacing. Arch Dermatol. 1996;132:451–455. [PubMed] [Google Scholar]
- 18.Kaplan H, Kaplan L. Combination of microneedle radiofrequency (RF), fractional RF skin resurfacing and multi-source non-ablative skin tightening for minimal-downtime, full-face skin rejuvenation. J Cosmet Laser Ther. 2016;18:438–441. [DOI] [PubMed] [Google Scholar]
- 19.Laubach HJ, Makin IR, Barthe PG, et al. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34:727–734. [DOI] [PubMed] [Google Scholar]
- 20.Narurkar VA. Lasers, light sources, and radiofrequency devices for skin rejuvenation. Semin Cutan Med Surg. 2006;25:145–150. [DOI] [PubMed] [Google Scholar]
- 21.Pedroso JD, Gutierrez MM, Volker KW, et al. Thermal Effect of J-Plasma(R) energy in a porcine tissue model: implications for minimally invasive surgery. Surg Technol Int. 2017;30:19–24. [PubMed] [Google Scholar]
- 22.Ruiz-Esparza J, Gomez JB. The medical face lift: a noninvasive, nonsurgical approach to tissue tightening in facial skin using nonablative radiofrequency. Dermatol Surg. 2003;29:325–332; discussion 332. [DOI] [PubMed] [Google Scholar]
- 23.Sadick N, Rothaus KO. Minimally invasive radiofrequency devices. Clin Plast Surg. 2016;43:567–575. [DOI] [PubMed] [Google Scholar]
- 24.Sadick N, Rothaus KO. Aesthetic applications of radiofrequency devices. Clin Plast Surg. 2016;43:557–565. [DOI] [PubMed] [Google Scholar]
- 25.Abraham MT, Vic Ross E. Current concepts in nonablative radiofrequency rejuvenation of the lower face and neck. Facial Plast Surg. 2005;21:65–73. [DOI] [PubMed] [Google Scholar]
- 26.Alexiades-Armenakas M, Dover JS, Arndt KA. Unipolar versus bipolar radiofrequency treatment of rhytides and laxity using a mobile painless delivery method. Lasers Surg Med. 2008;40:446–453. [DOI] [PubMed] [Google Scholar]
- 27.Bassichis BA, Dayan S, Thomas JR. Use of a nonablative radiofrequency device to rejuvenate the upper one-third of the face. Otolaryngol Head Neck Surg. 2004;130:397–406. [DOI] [PubMed] [Google Scholar]
- 28.Burns AJ, Holden SG. Monopolar radiofrequency tissue tightening—how we do it in our practice. Lasers Surg Med. 2006;38:575–579. [DOI] [PubMed] [Google Scholar]
- 29.Dayan E, Chia C, Burns AJ, et al. Adjustable depth fractional radiofrequency combined with bipolar radiofrequency: a minimally invasive combination treatment for skin laxity. Aesthet Surg J. 2019;39(Supplement_3):S112–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fritz K, Salavastru C. Ways of noninvasive facial skin tightening and fat reduction. Facial Plast Surg. 2016;32:276–282. [DOI] [PubMed] [Google Scholar]
- 31.Zelickson BD, Kist D, Bernstein E, et al. Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: a pilot study. Arch Dermatol. 2004;140:204–209. [DOI] [PubMed] [Google Scholar]
- 32.Hantash BM, Ubeid AA, Chang H, et al. Bipolar fractional radiofrequency treatment induces neoelastogenesis and neocollagenesis. Lasers Surg Med. 2009;41:1–9. [DOI] [PubMed] [Google Scholar]
- 33.Hodgkinson DJ. Clinical applications of radiofrequency: nonsurgical skin tightening (thermage). Clin Plast Surg. 2009;36:261–268, viii. [DOI] [PubMed] [Google Scholar]
- 34.Ruiz-Esparza J. Near [corrected] painless, nonablative, immediate skin contraction induced by low-fluence irradiation with new infrared device: a report of 25 patients. Dermatol Surg. 2006;32:601–610. [DOI] [PubMed] [Google Scholar]
- 35.Seo KY, Yoon MS, Kim DH, et al. Skin rejuvenation by microneedle fractional radiofrequency treatment in Asian skin; clinical and histological analysis. Lasers Surg Med. 2012;44:631–636. [DOI] [PubMed] [Google Scholar]
- 36.Peterson JD, Palm MD, Kiripolsky MG, et al. Evaluation of the effect of fractional laser with radiofrequency and fractionated radiofrequency on the improvement of acne scars. Dermatol Surg. 2011;37:1260–1267. [DOI] [PubMed] [Google Scholar]
- 37.Royo de la Torre J, Moreno-Moraga J, Munoz E, et al. Multisource, phase-controlled radiofrequency for treatment of skin laxity: correlation between clinical and in-vivo confocal microscopy results and real-time thermal changes. J Clin Aesthet Dermatol. 2011;4:28–35. [PMC free article] [PubMed] [Google Scholar]
- 38.Harth Y. Painless, safe, and efficacious noninvasive skin tightening, body contouring, and cellulite reduction using multisource 3DEEP radiofrequency. J Cosmet Dermatol. 2015;14:70–75. [DOI] [PubMed] [Google Scholar]
- 39.Elman M, Vider I, Harth Y, et al. Non-invasive therapy of wrinkles and lax skin using a novel multisource phase-controlled radio frequency system. J Cosmet Laser Ther. 2010;12:81–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.