Abstract
The antifungal efficacy, safety, and pharmacokinetics of posaconazole (SCH 56592) (POC) were investigated in treatment and prophylaxis of primary pulmonary aspergillosis due to Aspergillus fumigatus in persistently neutropenic rabbits. Antifungal therapy consisted of POC at 2, 6, and 20 mg/kg of body weight per os; itraconazole (ITC) at 2, 6, and 20 mg/kg per os; or amphotericin B (AMB) at 1 mg/kg intravenously. Rabbits treated with POC showed a significant improvement in survival and significant reductions in pulmonary infarct scores, total lung weights, numbers of pulmonary CFU per gram, numbers of computerized-tomography-monitored pulmonary lesions, and levels of galactomannan antigenemia. AMB and POC had comparable therapeutic efficacies by all parameters. By comparison, animals treated with ITC had no significant changes in outcome variables in comparison to those of untreated controls (UC). Rabbits receiving prophylactic POC at all dosages showed a significant reduction in infarct scores, total lung weights, and organism clearance from lung tissue in comparison to results for UC (P < 0.01). There was dosage-dependent microbiological clearance of A. fumigatus from lung tissue in response to POC. Serum creatinine levels were greater (P < 0.01) in AMB-treated animals than in UC and POC- or ITC-treated rabbits. There was no elevation of serum hepatic transaminase levels in POC- or ITC-treated rabbits. The pharmacokinetics of POC and ITC in plasma demonstrated dose dependency after multiple dosing. The 2-, 6-, and 20-mg/kg dosages of POC maintained plasma drug levels above the MICs for the entire 24-h dosing interval. In summary, POC at ≥6 mg/kg/day per os generated sustained concentrations in plasma of ≥1 μg/ml that were as effective in the treatment and prevention of invasive pulmonary aspergillosis as AMB at 1 mg/kg/day and more effective than cyclodextrin ITC at ≥6 mg/kg/day per os in persistently neutropenic rabbits.
Invasive pulmonary aspergillosis is an important cause of infectious morbidity and mortality in immunocompromised patients, particularly in those with severe and prolonged neutropenia as a consequence of cytotoxic chemotherapy for the treatment of cancer (3, 10, 18, 36). Conventional amphotericin B (AMB), which binds to ergosterol and disrupts membrane integrity, remains the mainstay of therapy for serious fungal infections; however, its clinical utility may be thwarted by dose-limiting nephrotoxicity (2, 15, 42). There clearly is a great need for safer yet effective antifungal compounds against pulmonary aspergillosis, particularly in patients with persistent neutropenia and those undergoing bone marrow transplantation (18, 36, 48).
Posaconazole (SCH 56592) (POC), a new triazole antifungal compound, has a potent and broad spectrum of antifungal activity (24, 37, 43). POC is structurally similar to the broad-spectrum triazole compound itraconazole (ITC) (17). The mechanism of action of the antifungal triazoles is through inhibition of cytochrome P-450-dependent 14α-sterol demethylase, which results in inhibition of ergosterol biosynthesis (6).
In vitro studies have demonstrated the potent antifungal activity of POC against Candida spp. (Candida albicans, Candida dubliniensis, Candida tropicalis, Candida parapsilosis, and Candida krusei), as well as Cryptococcus neoformans (4, 14, 26, 38, 39, 40). Additional studies have demonstrated that POC is more active than ITC and fluconazole against clinical isolates of filamentous fungi such as Aspergillus spp., Fusarium spp., Rhizopus spp., and Pseudallescheria boydii (11, 30, 34). POC is also active against dimorphic fungi such as Blastomyces dermatitidis (45) and Histoplasma capsulatum (8). POC has also been evaluated in animal models of cryptococcal meningitis (20, 38), disseminated aspergillosis (16, 23, 35), disseminated fusariosis (27), coccidioidomycosis (28), histoplasmosis (8), blastomycosis (45), and pheohyphomycosis (1).
Little is known, however, about the activity of POC against primary pulmonary aspergillosis in persistently neutropenic hosts, where this compound may have an important role. We therefore investigated the antifungal efficacy, safety, and pharmacokinetics in plasma of POC in the treatment and prophylaxis of primary pulmonary aspergillosis in persistently neutropenic rabbits. We further investigated the potential correlation between a therapeutic response to POC and galactomannan antigenemia.
MATERIALS AND METHODS
Animals.
Healthy female New Zealand White rabbits weighing 2.6 to 3.7 kg (Hazleton, Deutschland, Pa.) at the time of inoculation were used in all experiments. Rabbits were individually housed and maintained with water and standard rabbit feed ad libitum according to National Institutes of Health guidelines for animal care and in fulfillment of the criteria of the American Association for Accreditation of Laboratory Animal Care (32). A total of 102 rabbits were used for all experiments. Vascular access was established in each rabbit by the surgical placement of a silastic tunneled central venous catheter (50). The silastic catheter permitted nontraumatic venous access for repeated blood sampling for study of biochemical and hematological parameters, study of pharmacokinetics in plasma, study of serum galactomannan, and administration of parenteral agents. Serum samples were drawn, when possible, from all rabbits at the initiation of immunosuppression, during the course of pulmonary aspergillosis, and before death. Rabbits were euthanized by intravenous (i.v.) administration of sodium pentobarbital injection (65 mg [1 ml]/kg of body weight; The Butler Company, Columbus, Ohio) at the end of each experiment, 24 h after administration of the last dose of the drug.
Organism and inoculation.
Aspergillus fumigatus (National Institutes of Health isolate 4215) obtained from a fatal case of pulmonary aspergillosis was used in all experiments. The MIC, determined by proposed NCCLS methods (12, 31), of POC (Schering-Plough Research Institute, Kenilworth, N.J.) was 0.125 μg/ml, that of ITC (Janssen Pharmaceutica NV, Beerse, Belgium) was 0.5 μg/ml, and that of AMB deoxycholate (Bristol-Myers Squibb Company, Princeton, N.J.) was 1.0 μg/ml. The minimum fungicidal concentration (MFC) of POC was 0.125 μg/ml, that of ITC was 0.5 μg/ml, and that of AMB deoxycholate was 1.0 μg/ml.
Pulmonary aspergillosis was established as previously described (13). For each experiment, the A. fumigatus inoculum was prepared from a frozen isolate (stored at −70°C) that was subcultured onto Sabouraud dextrose slants (BBL, Cockeysville, Md.). Those slants were incubated for 24 h at 37°C and then kept at room temperature for 5 days before use. Conidia were harvested under a laminar airflow hood with a solution of 10 ml of 0.025% Tween 20 (Fisher Scientific, Fair Lawn, N.J.) in 0.9% NaCl (Quality Biological, Inc., Gaithersburg, Md.), transferred to a 50-ml conical tube, washed, and counted with a hemacytometer. The concentration was adjusted in order to give each rabbit a predetermined inoculum of 108 conidia of A. fumigatus in a volume of 250 to 350 μl. The concentrations of the inocula were confirmed by serial dilutions cultured on Sabouraud glucose agar (SGA).
Inoculation was performed on day 2 of the experiments under general anesthesia. Each rabbit was anesthetized with 0.8 to 1.0 ml of a 2:1 (vol/vol) mixture of i.v. administered ketamine (100 mg/ml) obtained as Ketaset (Phoenix Scientific, Inc., St. Joseph, Mo.) and xylazine (20 mg/ml; Animal Health, Agriculture Division, Bayer Corp. Shawnee Mission, Kans.) obtained as Rompun. Once satisfactory anesthesia was obtained, a Flagg O straight-blade laryngoscope (Welch Allyn Inc., Skaneateles Falls, N.Y.) was inserted in the oral cavity until the vocal cords were clearly visualized. The A. fumigatus inoculum was then administered intratracheally with a tuberculin syringe attached to a 5 1/4-inc. 16-gauge Teflon catheter (Becton Dickinson Infusion Therapy Systems Inc., Sandy, Utah).
Immunosuppression and maintenance of neutropenia.
To simulate the conditions of persistent neutropenia, therapy with cytarabine (Ara-C) (Cytosar-U; The Upjohn Company, Kalamazoo, Mich.) was initiated i.v. 1 day before the endotracheal inoculation of the animals. Profound and persistent neutropenia (granulocyte count of <100/μl) was achieved by an initial course of 525 mg of Ara-C per m2 for five consecutive days. A maintenance dose of 484 mg of Ara-C per m2 was administered for 4 additional days on days 8, 9, 13, and 14 of the experiment. Concomitant thrombocytopenia ranged from 30,000 to 50,000/μl. Methylprednisolone (Abbott Laboratories, North Chicago, Il.) at 5 mg/kg of body weight was administered on days 1 and 2 of the experiment to inhibit macrophage activity against conidia in order to facilitate establishment of infection.
Ceftazidime (75 mg/kg given i.v. twice daily; Glaxo, Inc., Research Triangle Park, N.C.), gentamicin (5 mg/kg given i.v. every other day; Elkins-Sinn, Inc., Cherry Hill, N.J.), and vancomycin (15 mg/kg given i.v. daily); Abbott Laboratories) were administered from day 4 of immunosuppression until study completion to prevent opportunistic bacterial infections during neutropenia. In order to prevent antibioticassociated diarrhea due to Clostridium spiriforme, all rabbits continuously received 50 mg of vancomycin per liter of drinking water.
Total leukocyte counts and the percentages of granulocytes were monitored twice weekly with a Coulter (Miami, Fla.) counter and by peripheral blood smears and differential counts, respectively.
Antifungal compounds.
POC was provided by Schering-Plough Research Institute as standard powder. Drug stock solution (30 mg/ml) was prepared by dissolving the antifungal powder in a solution of distilled water and Tween 80 (Fisher Scientific, Fair Lawn, N.J.). ITC was purchased as Sporanox (Janssen Pharmaceutica NV) in a 10-mg/ml hydroxypropyl-β-cyclodextrin oral solution. AMB was purchased as Fungizone, an i.v. suspension (Bristol-Myers Squibb Company), and was resuspended for use in sterile water according to the manufacturer's instructions.
Treatment regimens.
Treatment study groups were either untreated controls (UC) or animals treated with POC, ITC, or AMB. POC was administered per os at dosages of 2 mg/kg/day (POC2), 6 mg/kg/day (POC6), and 20 mg/kg/day (POC20). ITC was administered per os at dosages of 2 mg/kg/day (ITC2), 6 mg/kg/day (ITC6), and 20 mg/kg/day (ITC20) as a 10mg/ml oral solution. AMB was administered i.v. at 1 mg/kg of body weight per day (0.1 ml every 10 s). Antifungal therapy was initiated 24 h after endotracheal inoculation. Antifungal therapy was continued throughout the course of the experiments for a maximum of 12 days in surviving rabbits.
Prophylactic regimen.
In order to study the efficacy of POC for prevention of aspergillosis in persistently neutropenic hosts, we compared this azole to ITC and no drug in the persistently neutropenic rabbit model for prophylaxis against pulmonary aspergillosis. The prophylaxis experiments used the same methods as described above with the following exceptions. Rabbits received the same dosages, POC2, −6, or −20 or ITC2, −6, or −20 administered for 4 days before endotracheal inoculation. On the day of inoculation, POC and ITC were administered in the morning and the endotracheal inoculum was administered approximately 4 h later. POC and ITC were then continued for a maximum of 12 more days after inoculation. In order to simulate the low initial tissue burden of A. fumigatus in the setting of antifungal prophylaxis, the administered inoculum was 5 × 107 conidia. All other methods, including outcome variables, were identical for both treatment and prophylaxis experiments.
Outcome variables.
A panel of outcome variables was used to assess antifungal efficacy. These variables consisted of survival, pulmonary infarct score, lung weight, microbiologic clearance (CL) from lung tissue (in log CFU per gram) and from bronchoalveolar lavage (BAL) specimens (log CFU per milliliter), computerized tomography (CT), galactomannan index (GMI), and pathology. Pulmonary infarct score, lung weight, and CT scan are measures of organism-mediated pulmonary injury.
Survival.
The survival time in days postinoculation was recorded for each rabbit in each group. Surviving rabbits were euthanized by sodium pentobarbital anesthesia on the 13th day postinoculation.
Pulmonary lesion scores.
The entire heart-lung block was carefully resected at autopsy. The heart was then dissected away from the lungs, leaving the tracheobronchial tree and lungs intact. The lungs were weighed and inspected by at least two observers who were blinded to the treatment group and recorded hemorrhagic infarct lesions (if any) in each individual lobe. The numbers of positive lobes were added, and the mean value of all positive lobes was calculated for each treatment group. Hemorrhagic infarcts were dark-red consolidated lesions that corresponded histologically to coagulative necrosis and intra-alveolar hemorrhage.
BAL.
BAL was performed on each lung preparation by the instillation and subsequent withdrawal of 10 ml of sterile normal saline two times into the clamped trachea with a sterile 12-ml syringe. The lavage was then centrifuged for 10 min at 1,500 × g. The supernatant was discarded, leaving the pellet, which was then resuspended in 2 ml of sterile normal saline. A 0.1-ml sample of this fluid and 0.1 ml of a dilution (10−1) of this fluid were cultured on 5% SGA plates.
Histopathology.
Pulmonary lesions were excised and fixed in 10% neutral buffered formalin. Paraffin-embedded tissue sections were stained with periodic acid-Schiff and Gomori methenamine silver stains. Tissues were microscopically examined for pulmonary injury and structural changes in Aspergillus hyphae.
Fungal cultures.
Lung tissue from each rabbit was sampled and cultured by a standard excision of tissue from each lobe. Each fragment was weighed individually, placed in a sterile polyethylene bag (Tekmar Corp., Cincinnati, Ohio), and homogenized with sterile saline for 30 s per tissue sample (Stomacher 80; Tekmar) (47). Lung homogenate dilutions (10−1 and 10−2) were prepared in sterile saline. Aliquots (100 μl) from homogenates and homogenate dilutions were plated onto SGA plates and incubated at 37°C for the first 24 h and then at room temperature for another 24 h. The number of CFU of A. fumigatus were counted and recorded for each lobe, and the log CFU per gram was calculated. A finding of one colony of A. fumigatus was considered positive.
CT.
Serial CT of the lungs was performed during all experiments in order to monitor the effects of antifungal therapy on organism-mediated pulmonary injury during the course of infection. Briefly, rabbits were sedated with ketamine and xylazine and then placed prone, head first, on the scanning couch. CT was performed with an ultrafast electron beam CT scanner (model C-100XL; Imatron, Oyster Point, Calif.), as previously described (49). Using the high-resolution, table-incremented, volume acquisition mode, 3-mm-thick ultrafast CT scans were performed every 4 s. A small scan circle and a 9-cm-diameter reconstruction circle with a matrix of 512 by 512 were used, which resulted in a pixel size of less than 1 mm. Scan parameters were 130 kV and 630 mA, and scan duration was 100 ms. In virtually all cases, 30 slices were sufficient to scan the entire thorax of the rabbit. Images were photographed using lung windows with a level of −600 Hounsfield units (HU) and a width of 1,800 HU. The radiologic features of invasive pulmonary aspergillosis observed in this experimental system are similar to those reported for persistently neutropenic patients (7, 21, 25).
Galactomannan assay.
Blood was collected every other day from each rabbit for determination of serum galactomannan concentrations. Serum galactomannan concentrations we determined by the Platelia Aspergillus (Genetic Systems/Sanofi Diagnostic Pasteur, Redmond, Wash.) one-stage immunoenzymatic sandwich microplate assay method. The assay used the rat monoclonal antibody EBA-2, which is directed against Aspergillus galactomannan. The monoclonal antibody is used to sensitize the wells of the microplate and to bind the antigen. Peroxidase-linked monoclonal rat antibody is used as the detector antibody.
Serum samples were heat treated in the presence of EDTA in order to dissociate the immune complexes and to precipitate serum proteins. The treated serum samples and conjugate were added to the wells coated with the monoclonal antibody and then incubated for 90 min at 37°C. A monoclonal antibody-galactomannan-monoclonal antibody–peroxidase complex was formed in the presence of Aspergillus antigen. The strips were washed to remove any unbound material. Next, we added the substrate solution, which reacted with the complexes bound to the well to form a blue color. The enzymatic reaction was stopped by the addition of stopping solution (1.5 N sulfuric acid), which changed the blue color to yellow. The optical absorbance values of specimens and controls were determined with a microplate spectrophotometer equipped with 450- and 620-nm-band-pass filters (Multiscan MMC/340; Titertek, Huntsville, Ala.).
Enzyme immunoassay data were expressed as serum GMIs plotted over time. The GMI for each test serum is equal to the optical density (OD) of the sample divided by the threshold OD in serum. Sera with a GMI less than 1 were considered to be negative. Sera with a GMI greater than 1.5 were considered to be initially positive. Sera with GMIs between 1 and 1.5 were considered to be indeterminate. Serial serum galactomannan levels were plotted over time as a function of antifungal compound and time of initiation of study drug (treatment versus prophylaxis).
Toxicity studies.
A sample of blood was collected from each rabbit every other day, starting from the first day after inoculation and continuing throughout treatment. Plasma samples were stored in Sarsted (Newton, N.C.) tubes at −70°C until all samples were processed simultaneously. Chemical determinations of potassium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, serum urea nitrogen, and total bilirubin concentrations were performed on the penultimate sample drawn from each rabbit.
Pharmacokinetic experiments.
The pharmacokinetics of POC and ITC in plasma were investigated in three infected animals per each dosage group. Time points for minimal plasma sampling were determined on the basis of full plasma concentration profiles from the same species (5, 22, 33) with the aid of the Adapt II computer program (9). Plasma sampling was performed 6 days after inoculation. Blood samples were drawn immediately prior to oral dosing and then at 1, 4, 8, and 24 h postdosing. All samples were immediately centrifuged, and plasma was stored at −80°C until assayed.
Concentrations of POC were determined after solid-phase extraction by liquid chromatography-tandem mass spectrometry at the Schering-Plough Research Institute. The analytical procedure involved dilution of the samples in controlled plasma prior to extraction. The quantifiable range of the assay was 4 to 1,000 ng/ml. Accuracies and intra- and interday variability (precision) were within ±15% and ±20% at the lower limit of quantitation.
Concentrations of ITC were determined after protein precipitation by the reversed-phase high-performance liquid chromatographic method using saperconazole as the internal standard (19). Ten-point standard curves were linear, with R2 values of ≥0.999. Accuracies were within ±12%, and intra- and interday variability (precision) was <6%. The lower limit of quantitation of the assay was 25 ng/ml in plasma.
Pharmacokinetic parameters for POC and ITC were determined using compartmental analysis. Experimental concentration in plasma data were fit to a one-compartment open model with first-order input, oral lag time, and first-order elimination from the central compartment using iterative weighted least-squares regression and the Adapt II computer program. Weighting was by maximum a posteriori probability, and model selection was guided by Akaike's Information Criterion (51). The model fit the data with mean coefficients of determination (r2) ± standard errors of the mean (SEM) of 0.647 ± 0.05 (POC) and 0.720 ± 0.04 (ITC), respectively. Fitted parameters included total CL, volume of distribution (V), and elimination half-life (t1/2).
Statistical analysis.
Comparisons between groups were performed by analysis of variance (ANOVA) with Bonferroni's correction for multiple comparisons or by the Mann-Whitney U test, as appropriate. Kaplan-Meier survival plots were analyzed by the Mantel-Haenzsel chi-square test. All P values were two-sided, and a P value of <0.05 was considered to be statistically significant. Values were expressed as means ± SEM.
RESULTS
Antifungal therapy.
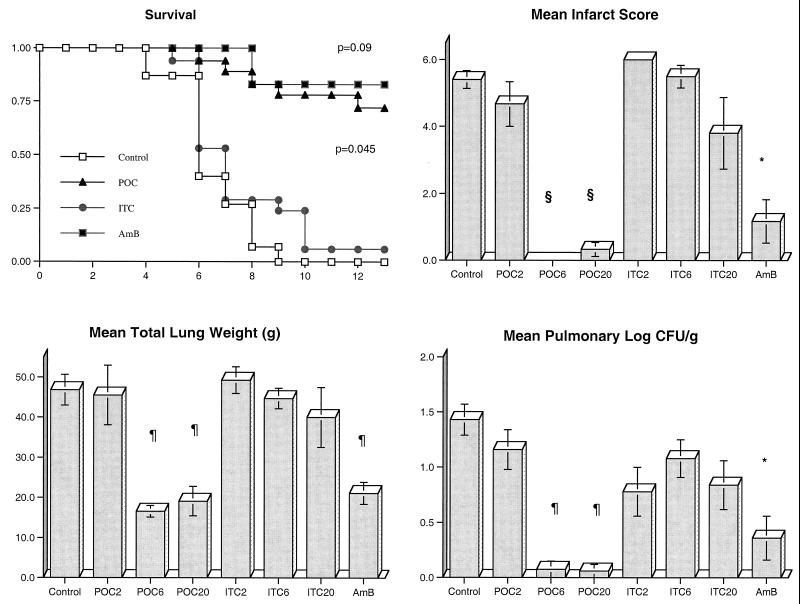
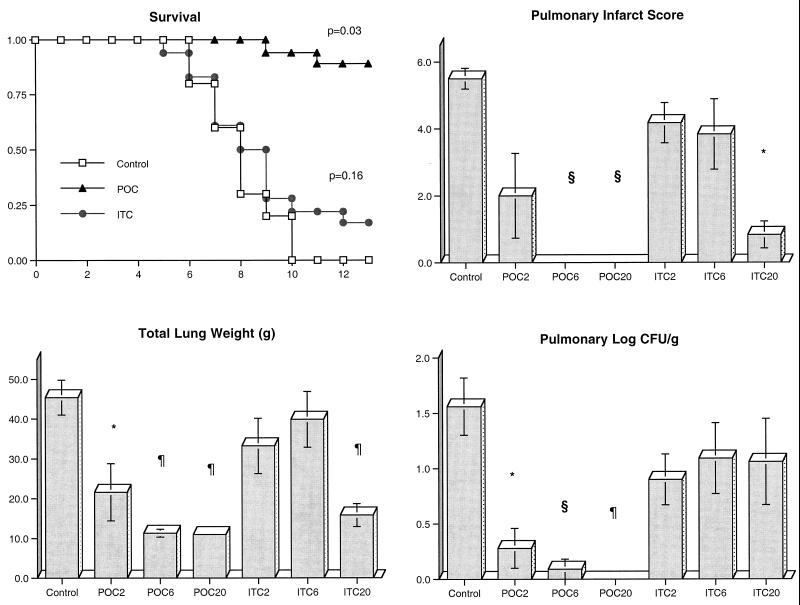
There was a significant improvement in the survival of rabbits treated with POC and AMB compared to that of UC. Through the entire study, 13 of 18 rabbits treated with POC, 5 of 6 rabbits (83%) treated with AMB, 1 of 18 rabbits (5.6%) treated with ITC, and none of the 15 UC survived (Fig. 1). Of the rabbits treated with POC2, only 1 of 6 (16.7%) survived. By comparison, 100% (6 of 6) of the rabbits treated with POC6 and 83% (5 of 6) of the rabbits in the POC20 treatment group survived.
FIG. 1.
Comparative rates of survival of persistently neutropenic rabbits in treatment groups receiving POC, ITC, or AMB versus in the UC group. To gauge survival over 12 days (x axis) dosage groups for each antifungal compound were combined (P value determined by Mantel-Haenzsel chi-square test). The response of persistently neutropenic rabbits with primary pulmonary aspergillosis to antifungal therapy was also measured by pulmonary infarct score, total lung weight, and pulmonary tissue concentration of residual organisms (log CFU/per gram) in UC (n = 15); rabbits treated with POC2 (n = 6), POC6 (n = 6), and POC20 (n = 6); rabbits treated with ITC2 (n = 6), ITC6 (n = 6), and ITC20 (n = 5); and rabbits treated with AMB at 1 mg/kg/day (n = 6). Values are given as means ± SEM (∗, P < 0.05; §, P < 0.01; ¶, P < 0.001) in comparison to values for UC using an ANOVA test with Bonferroni's correction for multiple comparisons.
There also was a significant reduction in organism-mediated tissue injury, as measured by pulmonary infarct score and total lung weight, in rabbits treated with POC and AMB. Rabbits treated with POC6, POC20, and AMB had similarly significant lower mean infarct scores (P < 0.001, P < 0.001, P < 0.05, respectively); however, no differences in infarct scores were noted in UC and rabbits treated with POC2, ITC2, ITC6, and ITC20 (Fig. 1). The mean lung weights in rabbits treated with POC20, POC6, and AMB were significantly reduced in comparison to those of UC (P < 0.001); however, no differences in lung weight were observed between untreated animals and animals treated with POC2, ITC2, ITC6, and ITC20 (Fig. 1).
There was a significant quantitative reduction in the growth of A. fumigatus in lung tissues from rabbits treated with POC and AMB in comparison to lung tissue from UC. Rabbits treated with POC6, POC20 (P < 0.001), and AMB (P < 0.05) showed significant reductions in the number of pulmonary CFU per gram (Fig. 1). In contrast, no difference in levels of A. fumigatus growth was observed between POC2- or ITC-treated rabbits and UC.
There was a higher frequency of BAL cultures negative for A. fumigatus in rabbits treated with POC in all dosage groups than in untreated rabbits (16 of 18 [89%] versus 6 of 15 [40%] rabbits, respectively; P < 0.01). There also was a trend toward more BAL cultures negative for A. fumigatus in ITC-treated rabbits than in UC (13 of 17 [76%] versus 6 of 15 [40%] rabbits, respectively; P = 0.07).
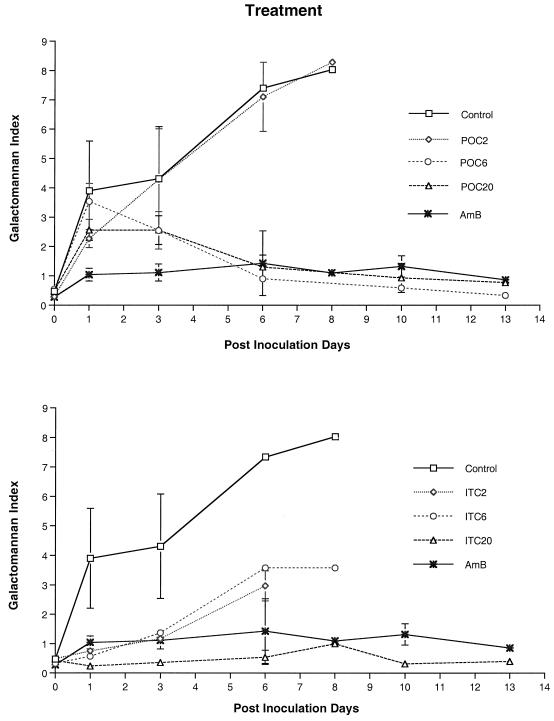
We investigated the expression of galactomannan in the sera of rabbits with pulmonary aspergillosis as a surrogate maker of therapeutic response (Fig. 2 and 3). Expression of galactomannan was studied in rabbits receiving treatment and in rabbits receiving prophylaxis. The following groups were studied: UC and AMB-, POC2-, POC6-, POC20-, ITC2-, ITC6-, and ITC20- treated animals. The GMI was positive (≥ 1.5) in UC 1 day after inoculation. Serial serum samples from UC and POC2-treated rabbits showed progressive galactomannan antigenemia, correlating with progression of invasive pulmonary aspergillosis. By comparison, serum GMI of rabbits treated with POC6 and POC20 demonstrated a significant decline during therapy and correlated with low pulmonary log CFU per gram in those groups (P < 0.001, ANOVA). Serial serum GMI values of rabbits treated with AMB were negative throughout treatment. Serum GMI remained negative after day 6 in POC6- and POC20- treated rabbits. Serum GMI was positive in ITC2 and ITC6 dosage groups, which also correlated with progression of invasive pulmonary aspergillosis and elevated tissue concentrations of A. fumigatus. Rabbits treated with ITC20 demonstrated a negative GMI. The responses of galactomannan antigenemia in rabbits treated with POC6, POC20, and ITC20 were comparable to that of rabbits treated with AMB.
FIG. 2.
Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis in the following treatment groups: UC, AMB-treated controls, and rabbits treated with POC (upper panel) or with ITC (lower panel). The differences between the antigen concentration-time curves of UC versus those of rabbits treated with POC6 and POC20 are significant (P = 0.05, ANOVA). Differences between antigen concentration-time curves of UC versus rabbits treated with ITC2, ITC6, and ITC20 are also significant (P < 0.001, ANOVA).
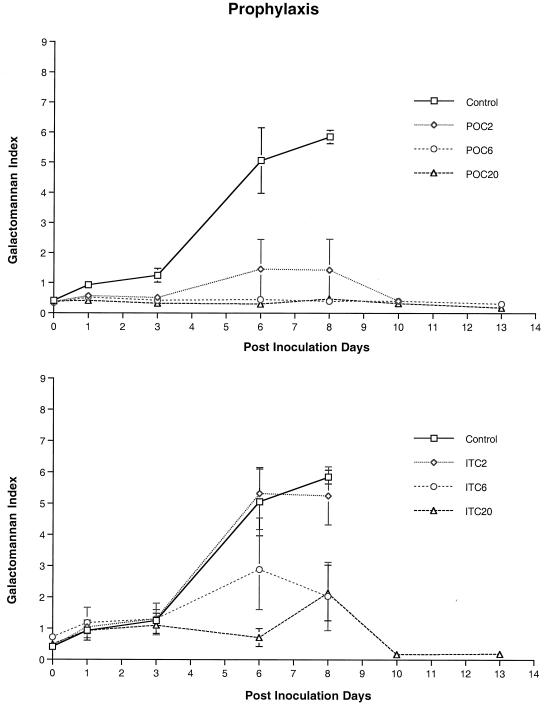
FIG. 3.
Expression of galactomannan antigenemia in persistently neutropenic rabbits with pulmonary aspergillosis in the prophylaxis group. Results for UC and rabbits treated with POC or with ITC are shown. The antigen concentration-time curves of rabbits treated with POC2, POC6, and POC20 are significantly different from those of UC (P < 0.001, ANOVA). The antigen concentration-time curve of ITC20-treated rabbits, but not that of ITC2- or ITC6-treated rabbit is significantly different from that of UC (P < 0.05, ANOVA).
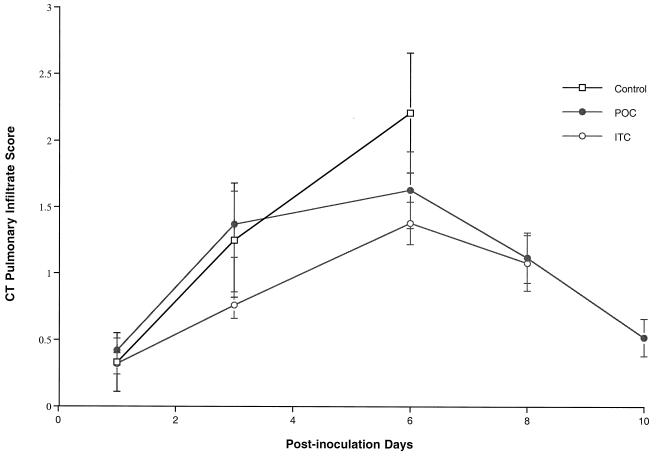
Consistent with the reduction in organism-mediated pulmonary injury, ultrafast CT scans demonstrated resolution of pulmonary infiltrates in rabbits treated with POC (Fig. 4). During the first 6 days of treatment, there was an increase in pulmonary infiltrates. Following day 6 of treatment, there was a substantial reduction of infiltrates in rabbits receiving antifungal therapy. ITC-treated rabbits demonstrated a similar trend of resolution of pulmonary infiltrates during antifungal therapy.
FIG. 4.
Pulmonary infiltrate scores determined by image analysis of serial ultrafast CT scans of UC, POC-treated rabbits, and ITC-treated rabbits. Pulmonary infiltrates increased during the first 6 days in UC and treated rabbits. The pulmonary infiltrate curve ends on day 6 due to mortality in the UC. Pulmonary infiltrates declined following day 6 in both the POC and ITC treatment groups. The ITC curve ends on day 8 due to mortality.
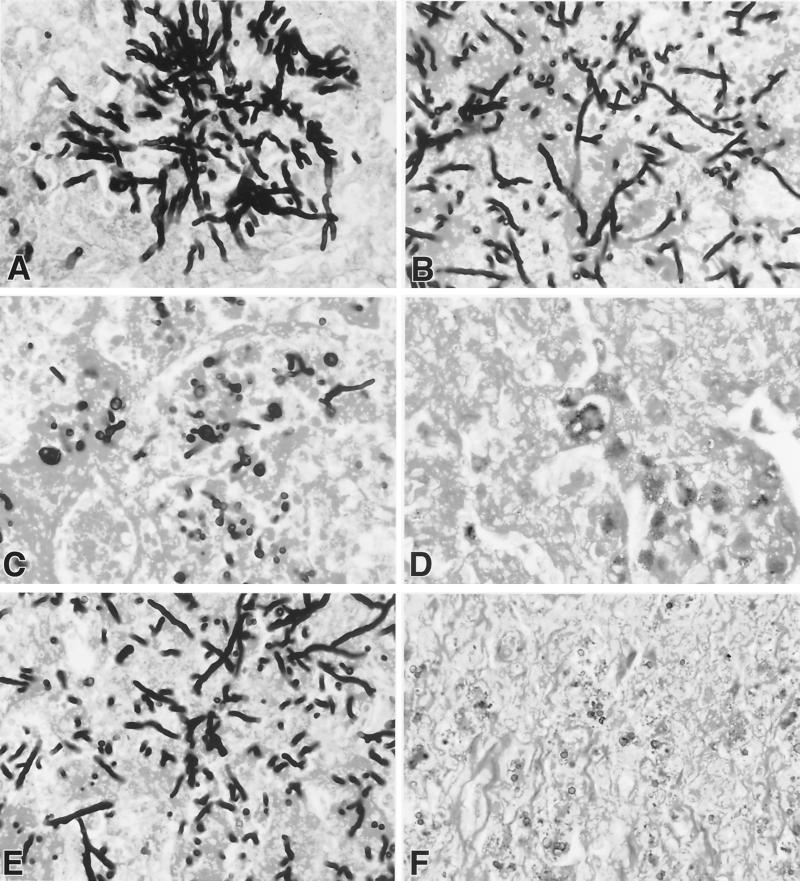
Histopathological features of aspergillosis were studied in the lungs of rabbits in all treatment groups. There was dosage-dependent damage of hyphal structures and clearance of the organism from the lung tissue in animals treated with POC. Each panel in Fig. 5 demonstrates a representative section of hyphal morphology in the POC dosage group. Organisms from UC rabbits demonstrated typical dichotomously branching septate hyphae of A. fumigatus (Fig. 5A). Organisms in Fig. 5B from lung tissue of the rabbit treated with POC2 had shortened hyphae. POC6- and POC20-treated rabbits (Fig. 5C and D) seldom revealed hyphal elements. The pulmonary histopathological features of aspergillosis in POC6- and POC20-treated rabbits were similar to those observed in AMB-treated rabbits. Damaged hyphal elements were observed at all dosages of ITC.
FIG. 5.
Effects of POC, ITC, and AMB on hyphal structures and microbiological CL of A. fumigatus in rabbits with pulmonary aspergillosis. (A to D) Progressive reduction of hyphal elements in a representative section in the lungs from each dosage group. (A) UC; (B) POC2; (C) POC6; (D) POC20; (E) ITC20; (F) AMB at 1 mg/kg.
Antifungal prophylaxis.
Prophylaxis was started 4 days before endotracheal inoculation and continued throughout the experiment. Significantly greater survival was achieved with rabbits treated with POC than with UC and animals treated with ITC (P < 0.03) (Fig. 6). There was also a significant reduction in organism-mediated tissue injury in rabbits treated with POC, as measured by the mean pulmonary infarct score and mean lung weight, in comparison to that in UC. Rabbits treated with POC6 and POC20 had no infarct lesions in the lungs, and animals treated with POC2 and ITC20 showed a significant reduction in infarct score (P < 0.01). Rabbits treated with POC at all dosages and ITC20 had significantly lower mean lung weights than those of UC (P < 0.01). Rabbits receiving prophylactic POC2, POC6, and POC20 had significant organism CL from the lung tissue in comparison to UC (P < 0.01). However, there was no significant CL from lung tissue in rabbits treated with ITC.
FIG. 6.
Comparative rates of survival of persistently neutropenic rabbits receiving antifungal prophylaxis with POC or ITC versus that of UC. To gauge survival over 12 days (x axis) dosage groups for each antifungal compound were combined (P value determined by Mantel-Haenzsel chi-square test). The response of persistently neutropenic rabbits with primary pulmonary aspergillosis to antifungal therapy was measured by pulmonary infarct score, total lung weight, and pulmonary tissue concentration of residual organisms (log CFU per gram) in UC (n = 10); in rabbits treated with POC2 (n = 6), POC6 (n = 6), and POC20 (n = 6); and in rabbits treated with ITC2 (n = 6), ITC6 (n = 6), and ITC20 (n = 6). Values are given as means ± SEM (∗, P < 0.05; §, P < 0.01; ¶, P < 0.001) in comparison to values for UC using an ANOVA test with Bonferroni's correction for multiple comparisons.
There was a higher frequency of negative BAL cultures for A. fumigatus in rabbits treated with POC in all prophylaxis groups than in UC rabbits (17 of 18 [94%] versus 5 of 10 [50%] rabbits, respectively; P = 0.01). However, there was no significant difference in BAL cultures between rabbits receiving prophylactic ITC and UC (13 of 18 [72%] versus 5 of 10 [50%] rabbits, respectively; P = 0.41).
Serum GMI was positive in UC and ITC2- and ITC6-treated rabbits by day 3 after inoculation, which correlated with increased tissue burden of A. fumigatus. By comparison, serum GMI was significantly reduced in POC2, POC6, POC20, and ITC20 dosage groups (P < 0.001, ANOVA). These findings correlated with organism CL from the lungs expressed by log CFU per gram and dose-proportional drug concentrations in serum.
Safety.
Rabbits treated with AMB had significant increases in their mean levels of creatinine and urea nitrogen in serum compared to those of POC- or ITC-treated rabbits or UC. There was no significant elevation of the concentrations of hepatic transaminases in the sera of rabbits treated with POC or ITC. There were no differences in serum potassium and bilirubin concentrations for any of the treatment groups (Table 1).
TABLE 1.
Effects of POC, ITC, and AMB on concentrations of serum creatinine, serum urea nitrogen, serum ALT, and serum LAST in persistently neutropenic rabbits with pulmonary aspergillosisa
| Treatment group (n) | Serum creatinine (mg/dl) | Serum urea nitrogen (mg/dl) | Serum ALT (U/liter) | Serum AST (U/liter) |
|---|---|---|---|---|
| UC (9) | 0.68 ± 0.05 | 21.7 ± 2.3 | 33.2 ± 4.8 | 36.8 ± 10.8 |
| POC (6) | 0.93 ± 0.11 | 21.8 ± 7.1 | 21.7 ± 5.4 | 10.7 ± 2.4 |
| ITC (6) | 0.77 ± 0.06 | 21.8 ± 3.1 | 37.3 ± 5.0 | 22.3 ± 10.8 |
| AMB (6) | 2.97 ± 0.55** | 99.5 ± 14.3* | 7.3 ± 0.8 | 9.3 ± 4.4 |
All values are expressed as means ± SEM. *, P < 0.05 (Kruskal-Wallis nonparametric ANOVA with Dunn's correction for multiple comparisons); **, P < 0.001 (ANOVA with Bonferroni's correction for multiple comparisons).
Pharmacokinetics of POC and ITC in plasma.
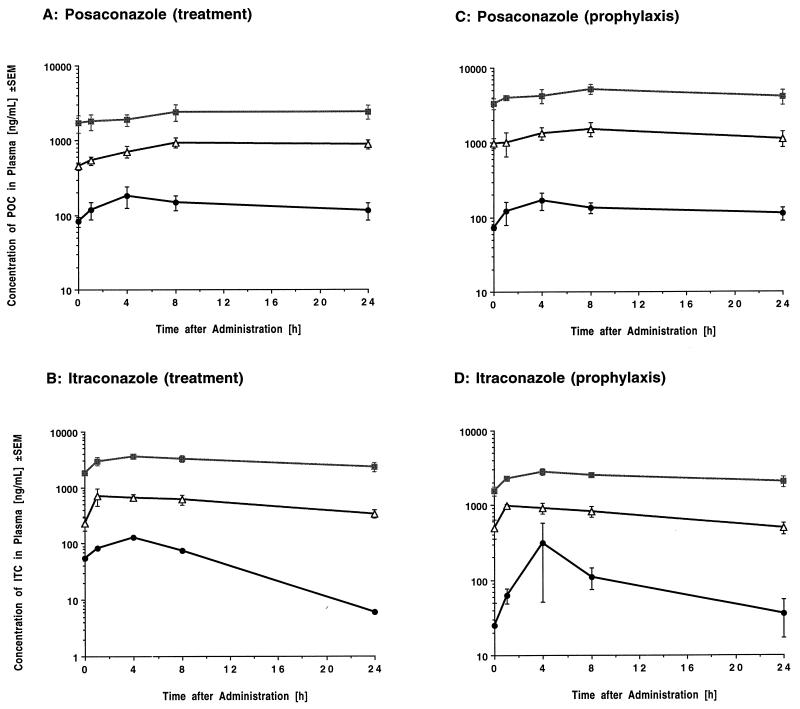
Concentration profiles of POC and ITC in the plasma of infected animals receiving treatment and prophylaxis regimens are depicted in Fig. 7; the corresponding pharmacokinetic parameters are presented in Tables 2 and 3, respectively.
FIG. 7.
Concentration profiles of POC (A) and ITC (B) in plasma after multiple dosing over 6 days (treatment) and concentration profiles of POC (C) and ITC (D) in plasma after multiple dosing over 10 days (prophylaxis). ●, 2 mg/kg/day; ■, 6 mg/kg/day; ▵, 20 mg/kg/day.
TABLE 2.
Pharmacokinetic parameters of POC (SCH 56592) and ITC in plasma after multiple dosing over 6 days (treatment)a
| Drug dose (mg/kg QD) | Cmax (ng/ml) | Tmax (h) | Cmin (ng/ml) | AUC0–24 (ng · h/ml) | V (liter/kg) | CL (liter/kg · h) | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| POC2 | 183 ± 58b | 5.00 ± 1.00c | 82 ± 13b | 3211 ± 657b | 5.64 ± 3.00d | 0.476 ± 0.240d | 9.03 ± 4.50d |
| POC6 | 940 ± 142b | 18.00 ± 3.83c | 454 ± 54b | 20,719 ± 2,104b | 3.45 ± 0.47d | 0.305 ± 0.028d | 7.76 ± 0.35d |
| POC20 | 2,412 ± 602b | 18.37 ± 3.37c | 1,731 ± 461b | 56,153 ± 12,633b | 6.93 ± 0.56d | 0.558 ± 0.055d | 8.74 ± 0.54d |
| ITC2 | 127 ± 0.00e | 4.00 ± 0.00f | 6 ± 0.00g | 2450 ± 0.00g | 15.3 ± 0.00f | 0.829 ± 0.00f | 12.79 ± 0.00g |
| ITC6 | 711 ± 234e | 5.33 ± 1.33f | 229 ± 60g | 12,937 ± 2,379g | 9.79 ± 1.65f | 0.507 ± 0.105f | 13.69 ± 1.67g |
| ITC20 | 3,564 ± 0.195e | 5.33 ± 1.33f | 2,338 ± 0.446g | 71,830 ± 8,152g | 9.17 ± 0.90f | 0.285 ± 0.031f | 22.72 ± 2.82g |
Values are the means ± SEM of results for three rabbits except for the ITC2 cohort (only one rabbit survived until the sampling). QD, once a day; Tmax, time to maximum concentration of drug in serum; Cmin, minimum concentration of drug in serum.
P ≤ 0.005.
P ≤ 0.05.
Not significant by ANOVA. P = 0.058 by ANOVA for AUC per dose versus dose (linearity plot).
P ≤ 0.001.
Not significant by ANOVA. P = 0.1043 (not significant) by t test for AUC per dose versus dose (linearity plot). ANOVA comparing all three cohorts was not feasible due to only one value in the ITC2 cohort.
P ≤ 0.05 for the comparison of the ITC6 and ITC20 dosage level by t test (ANOVA comparing all three cohorts was not feasible due to only one value in the ITC2 cohort).
TABLE 3.
Pharmacokinetic parameters of POC (SCH 56592) and ITC in plasma after multiple dosing over 10 days (prophylaxis)a
| Drug dose (mg/kg QD) | Cmax (ng/ml) | Tmax (h) | Cmin (ng/ml) | AUC0–24 (ng · h/ml) | V (liter/kg) | CL (liter/kg · h) | t1/2 (h) |
|---|---|---|---|---|---|---|---|
| POC2 | 170 ± 44b | 4.0 ± 0.00c | 73 ± 5b | 2990 ± 537b | 5.36 ± 3.10c | 0.719 ± 0.132d | 6.38 ± 4.56c |
| POC6 | 1,528 ± 322b | 4.03 ± 2.03c | 990 ± 145b | 40,466 ± 12,562b | 3.57 ± 1.12c | 0.226 ± 0.051d | 7.86 ± 1.43c |
| POC20 | 5,176 ± 788b | 5.30 ± 2.67c | 3360 ± 563b | 107,500 ± 14,752b | 2.89 ± 0.92c | 0.182 ± 0.025d | 10.75 ± 1.48c |
| ITC2 | 314 ± 263e | 4.33 ± 2.03 | 36 ± 19e | 2017 ± 370e | 15.88 ± 1.30f | 1.075 ± 0.206f | 10.73 ± 1.48g |
| ITC6 | 992 ± 030e | 2.00 ± 1.00 | 491 ± 86e | 18,650 ± 2,660e | 8.51 ± 0.67f | 0.341 ± 0.053f | 18.13 ± 2.96g |
| ITC20 | 2,802 ± 259e | 5.33 ± 1.33 | 2,061 ± 332e | 58,560 ± 3,290e | 11.30 ± 0.84f | 0.348 ± 0.019f | 22.73 ± 2.47g |
Values are the means ± SEM of results with three rabbits each. QD, once a day; Tmax, time to maximum concentration of drug in serum; Cmin, minimum concentration of drug in serum.
P < 0.001.
Not significant by ANOVA. P = 0.0977 by ANOVA for AUC per dose versus dose (linearity plot).
P < 0.005.
P ≤ 0.001.
P ≤ 0.01.
P < 0.05 by ANOVA for comparison among all three cohorts; P < 0.01 by ANOVA for AUC per dose versus dose (linearity plot) among all three cohorts; P = 0.5965 for the comparison of the ITC6 and ITC20 dosage levels by t test.
Over the investigated dosage range, both compounds exhibited dose-dependent pharmacokinetics with a statistically significant decrease in CL and a significant increase in the elimination t1/2 with increasing dosages after multiple dosing over 10 days (Table 3). Consistent with a dose-dependent disposition of both drugs, there was a trend toward increases in the dose-normalized area under the concentration-time curve from 0 to 24 h (AUC0–24) with increasing dosages. Mean peak levels of POC in plasma exceeded the corresponding MIC for the test organism at all dosages and ranged from 170 to 5,176 ng/ml in POC-treated rabbits. Mean peak levels of ITC in plasma exceeded the corresponding MIC at all dosages with the exception of the 2-mg/kg dosage group and ranged from 127 to 3,564 ng/ml (Tables 2 and 3). With the exception of the POC2 dosage group and ITC2 dosage group, mean concentrations in plasma stayed above the MICs for the tested isolate throughout most of the dosing interval. Both compounds exhibited a relatively large V, which is indicative of high protein binding and/or extensive distribution into tissues.
DISCUSSION
POC demonstrated potent dosage-dependent in vivo antifungal activity against A. fumigatus in persistently neutropenic rabbits with primary invasive pulmonary aspergillosis. The antifungal effects of orally administered POC6 were comparable to those of i.v. administered AMB at 1 mg/kg. The antifungal response was observed across all outcome parameters, including microbiologic burden (measured as log CFU per gram of lung tissue, serum galactomannan antigenemia, and histology), as well as organism-mediated pulmonary injury (measured as pulmonary hemorrhage score, lung weight, and CT scan). This antifungal efficacy of POC was superior to that of orally administered cyclodextrin ITC at equal dosages. The antifungal effect of POC was achieved without any detectable hepatotoxicity. ITC was well absorbed and achieved levels in plasma similar to those of POC6 and POC20. Concentrations of POC6 and POC20 in plasma exceeded the MIC for A. fumigatus throughout the 24-h dosing interval.
There was a significant reduction of microbiologic burden as measured by pulmonary log CFU per gram of A. fumigatus. Consistent with this antifungal effect was a parallel reduction of organisms observed histologically in tissue. Additionally, there appeared to be considerable disruption of fungal cell wall morphology of residual organisms, suggesting azole-mediated damage to residual hyphae.
The serum galactomannan levels also paralleled the microbiologic decline in pulmonary tissue burden of A. fumigatus as a surrogate marker for a therapeutic response. The expression of galactomannan in the sera of untreated neutropenic control rabbits with pulmonary aspergillosis demonstrates patterns similar to those observed in neutropenic patients initially diagnosed with pulmonary aspergillosis (29, 41, 44, 46). As declining serum galactomannan levels reflected decreased microbiologic tissue burden of A. fumigatus and a more favorable experimental therapeutic response, serial GMI determinations warrant further study as a surrogate marker of clinical antifungal efficacy.
The histopathological features of rabbits treated with POC demonstrate progressive dose-dependent reduction of hyphal elements in lung tissue. In addition to quantitative reduction, there are marked structural changes in hyphal morphology. For example, hyphal structures in rabbits treated with POC6 reveal marked distortion of fungal elements that include truncated and rounded hyphal fragments, as well as distended chlamydospore-like remnants. These features are highly atypical for A. fumigatus and may lead to misdiagnosis in a clinical setting in patients undergoing lung biopsy while receiving antifungal prophylaxis or therapy. Small sample sizes of biopsies may also miss foci of these distorted hyphal elements.
As the microbiologic tissue burden of A. fumigatus declines, pulmonary injury should also decrease. Indeed, pulmonary injury was significantly reduced at dosages of ≥6 mg/kg/day. The reduction in organism-mediated pulmonary injury, evidenced by reduced total lung weight, mean pulmonary infarct score, and resolution of pulmonary infiltrates on CT scans, correlated with significant improvement in survival in comparison to that of UC. This survival was comparable to that achieved with AMB.
The potent antifungal activity of oral POC against invasive pulmonary aspergillosis in persistently neutropenic rabbits was distinctive. One usually would not consider that an orally administered antifungal compound would attain this level of potent activity in a profoundly and persistently neutropenic host. That POC demonstrates fungicidal properties against A. fumigatus at relatively low MICs of 0.125 μg/ml that were exceeded in all dosage cohorts may explain in part this level of activity.
As an additional treatment control, oral ITC was investigated as the cyclodextrin solution. Cyclodextrin solution was selected in order to optimize oral bioavailability. Despite administration of the oral cyclodextrin solution, levels of ITC in plasma above the MIC were not reliably achieved at the 2-mg/kg dosage. When the ITC6 dosage was administered, concentrations in plasma were above the MIC throughout the dosing interval. However, despite these sustained levels, the ITC6 dosage was less effective than the same dosage of POC.
This disparity in antifungal efficacy between POC and ITC may be explained in part by differences in the potencies of compounds with similar molecular weights. The MICs of POC for A. fumigatus have been reported as being relatively lower than those of ITC (12, 34). As the plasma concentration-time curves and AUCs of POC and cyclodextrin ITC were similar, differences in efficacy between these antifungal triazoles could not be attributed to differences in bioavailability. This level of activity may be understood in terms of sustained levels in plasma being achieved above a relatively low MIC for A. fumigatus. That the POC MIC and MFC were four times lower than those of ITC may contribute to the greater in vivo activity observed in POC-treated rabbits. These lower MICs may be particularly important in tissue sites of aspergillosis, where pulmonary infarcts reduce the access of plasma antifungal compound to the organism. Assuming levels of penetration into such infarcted sites to be similar for both triazoles, the compound with lower MICs and MFCs will likely demonstrate greater in vivo antifungal activity.
The MICs and MFCs demonstrate a consistent fourfold difference in in vitro potencies. Yet, the differences observed in our in vivo experiments suggest more potent activity than is reflected by the MICs and MFCs. This greater disparity between the in vitro and in vivo potencies of POC was also observed by Oakley et al. in a transiently neutropenic murine model of disseminated aspergillosis (35). Among the possible explanations for this difference are tissue penetration, rates of microbicidal activity, and pharmacodynamics. The pharmacodynamics of POC and ITC against A. fumigatus are nonlinear (5; A. H. Groll, D. Mickiene, R. Petraitiene, V. Petraitis, T. Sein, J. Roach, K. Roth, S. C. Piscitelli, and T. J. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., p. 385, abstr. 1675, 2000). Perhaps the differences in antifungal activity between POC and ITC may be greater than a fourfold difference in vivo as the result of the nonlinear in vivo pharmacodynamics. There are no apparent differences in levels of tissue penetration of these two azoles reported at this point. Time-kill assays may prove to be helpful in understanding the differences in rates of kill of A. fumigatus.
Pharmacokinetic features of POC revealed that the maximum concentration of the drug in serum (Cmax) greatly exceeds that of the MIC at dosages of 6 and 20 mg/kg/day. Moreover, the relatively long t1/2 in plasma of 7 to 10 h also maintains existing plasma POC levels well above the MIC for A. fumigatus. Whether peak concentrations in plasma or exposure over time is the more critical pharmacodynamic parameter remains to be further investigated. Nevertheless, features of both properties were observed in these experiments and suggested a favorable pharmacokinetic profile of POC in the treatment of invasive pulmonary aspergillosis. Based upon these findings, as well as the MIC and MFC for the organism, a sustained level of approximately 1,000 ng/ml is considered to be the minimal highly active concentration in plasma.
Thus, POC6 per os generates sustained concentrations in plasma of ≥1 μg/ml that were as effective in treatment and prevention of invasive pulmonary aspergillosis as AMB at 1 mg/kg/dag and more effective than cyclodextrin ITC6 per os in persistently neutropenic rabbits. Given its potent antifungal activity, excellent safety profile, and favorable pharmacokinetic parameters, POC offers the potential for use as a prophylactic agent in the prevention of invasive pulmonary aspergillosis. These in vivo findings provide a foundation for understanding the antifungal activity, safety, and pharmacokinetics of POC in persistently neutropenic hosts and form a foundation for developing clinical trials for the prevention and treatment of invasive pulmonary aspergillosis.
ACKNOWLEDGMENTS
We thank the staff of the Laboratory Animal Science Branch of the National Cancer Institute and the staff of the Surgery Service of the Office of Research Service for their excellent laboratory animal care. We also thank Jeremy Roach and Kenneth Roth for determining levels of POC in plasma, Diana Mickiene for determining levels of ITC in plasma, Joanne Peter for determining MICs, and the staff of the Department of Radiology for CT imaging studies.
REFERENCES
- 1.Al-Abdely H M, Najvar L, Bocanegra R, Fothergill A, Loebenberg D, Rinaldi M G, Graybill J R. SCH 56592, amphotericin B, or itraconazole therapy of experimental murine cerebral phaeohyphomycosis due to Ramichloridium obovoideum (“Ramichloridium mackenziel”) Antimicrob Agents Chemother. 2000;44:1159–1162. doi: 10.1128/aac.44.5.1159-1162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allende M C, Lee J W, Francis P, Garrett K, Dollenberg H, Berenguer J, Lyman C A, Pizzo P A, Walsh T J. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob Agents Chemother. 1994;38:518–522. doi: 10.1128/aac.38.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992;14(Suppl. 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 4.Barchiesi F, Arzeni D, Fothergill A W, Falconi Di Francesco L, Caselli F, Rinaldi M G, Scalise G. In vitro activities of the new antifungal triazole SCH 56592 against common and emerging yeast pathogens. Antimicrob Agents Chemother. 2000;44:226–229. doi: 10.1128/aac.44.1.226-229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer J, Ali N M, Allende M C, Lee J, Garrett K, Battaglia S, Piscitelli S C, Rinaldi M G, Pizzo P A, Walsh T J. Itraconazole for experimental pulmonary aspergillosis: comparison with amphotericin B, interaction with cyclosporin A, and correlation between therapeutic response and itraconazole concentration in plasma. Antimicrob Agents Chemother. 1994;38:1303–1308. doi: 10.1128/aac.38.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossche H V, Koymans L. Cytochromes P450 in fungi. Mycoses. 1997;41(Suppl. 1):32–38. doi: 10.1111/j.1439-0507.1998.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 7.Caillot D, Casasnovas O, Bernard A, Couaillier J F, Durand C, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, Couillault G, Dumas M, Guy H. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147. doi: 10.1200/JCO.1997.15.1.139. [DOI] [PubMed] [Google Scholar]
- 8.Connolly P, Wheat J, Schnizlein-Bick C, Durkin M, Kohler S, Smedema M, Goldberg J, Brizendine E, Loebenberg D. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob Agents Chemother. 1999;43:322–328. doi: 10.1128/aac.43.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Argenio D Z, Schumitzky A. ADAPT II user's guide. University of Southern CaliforniaLos Angeles: Biomedical Simulations Resource; 1990. [Google Scholar]
- 10.De Bock R. Epidemiology of invasive fungal infections in bone marrow transplantation. Bone Marrow Transplant. 1994;14(Suppl. 5):S1–S2. [PubMed] [Google Scholar]
- 11.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis P, Lee J W, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P A, Walsh T J. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 14.Galgiani J N, Lewis M L. In vitro studies of activities of the antifungal triazoles SCH 56592 and itraconazole against Candida albicans, Cryptococcus neoformans, and other pathogenic yeasts. Antimicrob Agent Chemother. 1997;41:180–183. doi: 10.1128/aac.41.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 16.Graybill J R, Bocanegra R, Najvar L K, Luther M F, Loebenberg D. SCH 56592 treatment of murine invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:539–542. doi: 10.1093/jac/42.4.539. [DOI] [PubMed] [Google Scholar]
- 17.Groll A, Piscitelli S, Walsh T J. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343–500. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 18.Groll A H, Kurz M, Schneider W, Witt V, Schmidt H, Schneider M, Schwabe D. Five-year-survey of invasive aspergillosis in a pediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999;42:431–442. doi: 10.1046/j.1439-0507.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 19.Gubbins P O, Gurley B J, Bowman J. Rapid and sensitive high performance liquid chromatographic method for the determination of itraconazole and its hydroxy-metabolite in human serum. J Pharm Biomed Anal. 1998;16:1005–1012. doi: 10.1016/s0731-7085(97)00062-9. [DOI] [PubMed] [Google Scholar]
- 20.Hossain M A, Maesaki S, Mitsutake K, Kakeya H, Sasaki E, Tomono K, Tashiro T, Kohno S. In-vitro and in-vivo activities of SCH56592 against Cryptococcus neoformans. J Antimicrob Chemother. 1999;44:827–829. doi: 10.1093/jac/44.6.827. [DOI] [PubMed] [Google Scholar]
- 21.Hruban R H, Meziane M A, Zerhouni E A, Wheeler P S, Dumler J S, Hutchins G M. Radiologic-pathologic correlation of the CT halo sign in invasive pulmonary aspergillosis. J Comput Assist Tomogr. 1987;11:534–536. doi: 10.1097/00004728-198705000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Likhari P, Kumari P, Lin C, Nomeir A A. High-performance liquid chromatographic analysis of the anti-fungal agent SCH 56592 in dog serum. J Chromatogr B. 2000;738:93–98. doi: 10.1016/s0378-4347(99)00489-2. [DOI] [PubMed] [Google Scholar]
- 23.Kirkpatrick W R, McAtee R K, Fothergill A W, Loebenberg D, Rinaldi M G, Patterson T F. Efficacy of SCH56592 in a rabbit model of invasive aspergillosis. Antimicrob Agents Chemother. 2000;44:780–782. doi: 10.1128/aac.44.3.780-782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koltin Y, Hitchcock C A. The search for the new triazole antifungal agents. Curr Opin Chem Biol. 1997;1:176–182. doi: 10.1016/s1367-5931(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 25.Kuhlman J E, Fishman E K, Siegelman S S. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 26.Law D, Moore C B, Denning D W. Activity of SCH 56592 compared with those of fluconazole and itraconazole against Candida spp. Antimicrob Agents Chemother. 1997;41:2310–2311. doi: 10.1128/aac.41.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozano-Chiu M, Arikan S, Paetznick V L, Anaissie E J, Loebenberg D, Rex J H. Treatment of murine fusariosis with SCH 56592. Antimicrob Agents Chemother. 1999;43:589–591. doi: 10.1128/aac.43.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutz J E, Clemons K V, Aristizabal B H, Stevens D A. Activity of the triazole SCH 56592 against disseminated murine coccidioidomycosis. Antimicrob Agents Chemother. 1997;41:1558–1561. doi: 10.1128/aac.41.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maertens J, Verhaegen J, Demuynck H, Brock P, Verhoef G, Vandenberghe P, Van Eldere J, Verbist L, Boogaerts M. Autopsy-controlled prospective evaluation of serial screening for circulating galactomannan by a sandwich enzyme-linked immunosorbent assay for hematological patients at risk for invasive aspergillosis. J Clin Microbiol. 1999;37:3223–3228. doi: 10.1128/jcm.37.10.3223-3228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marco F, Pfaller M A, Messer S A, Jones R N. In vitro activity of a new triazole antifungal agent, Sch 56592, against clinical isolates of filamentous fungi. Mycopathologia. 1998;141:73–77. doi: 10.1023/a:1006970503053. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 32.National Research Council Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 33.Nomeir A A, Kumari P, Hilbert M J, Gupta S, Loebenberg D, Cacciapuoti A, Hare R, Miller G H, Lin Chin-Chung, Cayen M N. Pharmacokinetics of SCH 56592, a new azole broad-spectrum antifungal agent, in mice, rats, rabbits, dogs, and cynomolgus monkeys. Antimicrob Agents Chemother. 2000;44:727–731. doi: 10.1128/aac.44.3.727-731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakley K L, Moore C B, Denning D W. In vitro activity of SCH-56592 and comparison with activities of amphotericin B and itraconazole against Aspergillus spp. Antimicrob Agents Chemother. 1997;41:1124–1126. doi: 10.1128/aac.41.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1503–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pannuti C, Gingrich R, Pfaller M A, Kao C, Wenzel R P. Nosocomial pneumonia in patients having bone marrow transplant. Attributable mortality and risk factors. Cancer. 1992;69:2653–2662. doi: 10.1002/1097-0142(19920601)69:11<2653::aid-cncr2820691106>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 37.Patterson T F. Role of newer azoles in surgical patients. J Chemother. 1999;11:504–512. doi: 10.1179/joc.1999.11.6.504. [DOI] [PubMed] [Google Scholar]
- 38.Perfect J R, Cox G M, Dodge R K, Schell W A. In vitro and in vivo efficacies of the azole SCH56592 against Cryptococcus neoformans. Antimicrob Agents Chemother. 1996;40:1910–1913. doi: 10.1128/aac.40.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller M A, Messer S A, Hollis R J, Jones R N, Doern G V, Brandt M E, Hajjeh R A. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-20747, Sch 56592, and voriconazole. Antimicrob Agents Chemother. 1998;42:3242–3244. doi: 10.1128/aac.42.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller M A, Messer S, Jones R N. Activity of a new triazole, Sch 56592, compared with those of four other antifungal agents tested against clinical isolates of Candida spp. and Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41:233–235. doi: 10.1128/aac.41.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohrlich P, Sarfati J, Mariani P, Duval M, Carol A, Saint-Martin C, Bingen E, Latge J-P, Vilmer E. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr Infect Dis J. 1996;15:232–237. doi: 10.1097/00006454-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Sarosi G A. Amphotericin B: still the ‘gold standard’ for antifungal therapy. Postgrad Med. 1990;88:151–166. doi: 10.1080/00325481.1990.11716368. [DOI] [PubMed] [Google Scholar]
- 43.Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stynen D, Goris A, Sarfati J, Latge J P. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J Clin Microbiol. 1995;33:497–500. doi: 10.1128/jcm.33.2.497-500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugar A M, Liu X P. In vitro activity and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother. 1996;40:1314–1316. doi: 10.1128/aac.40.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verweij P E, Stynen D, Rijs A J M M, de Pauw B E, Hoogkamp-Korstanje J A A, Meis J F G M. Sandwich enzyme-linked immunosorbent assay compared with pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J Clin Microbiol. 1995;33:1912–1914. doi: 10.1128/jcm.33.7.1912-1914.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh T J, McEntee C, Dixon D M. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Microbiol. 1987;25:931–932. doi: 10.1128/jcm.25.5.931-932.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh T J, Hiemenz J W, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin N Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 49.Walsh T J, Garrett K, Feuerstein E, Girton M, Allende M, Bacher J, Francesconi A, Schaufele R, Pizzo P A. Therapeutic monitoring of experimental invasive pulmonary aspergillosis by ultrafast computerized tomography, a novel, noninvasive method for measuring responses to antifungal therapy. Antimicrob Agents Chemother. 1995;39:1065–1069. doi: 10.1128/aac.39.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–470. [PubMed] [Google Scholar]
- 51.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]