Abstract
Chronic lymphocytic leukemia (CLL) is the most common leukemia affecting the western adult population. While CLL is known to be a risk factor for morbidity and mortality from coronavirus disease 2019 (COVID-19), COVID-19 has not been shown to be a risk factor for the development of CLL. We report a case of a 55-year-old man who presented with COVID-19 pneumonia and developed overt CLL during hospitalization. Four other cases were culled from the literature. We discuss mechanistic possibilities for the unmasking of CLL in susceptible individuals with COVID-19.
Keywords: chronic lymphocytic leukemia (cll), severe acute respiratory syndrome coronavirus -2 (sars-cov-2), hemato-oncology, pneumonia, covid-19 pneumonia, covid-19
Introduction
Patients with hematological malignancies are particularly at risk when infected with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), with case fatality rates approaching 34% due to inadequate immune responses [1]. Chronic lymphocytic leukemia (CLL) is the most common leukemia affecting the western adult population [2], and there are several reports elaborating on the disease course in CLL patients. While CLL is known to be a risk factor for morbidity and mortality from coronavirus disease 2019 (COVID-19), COVID-19 has not been shown to be a risk factor for the development of CLL. We report a case of a 55-year-old man who presented with COVID-19 pneumonia and developed overt CLL during hospitalization. Four other cases were culled from the literature. The data raise the question of whether COVID-19 infection can unmask CLL in susceptible individuals.
Case presentation
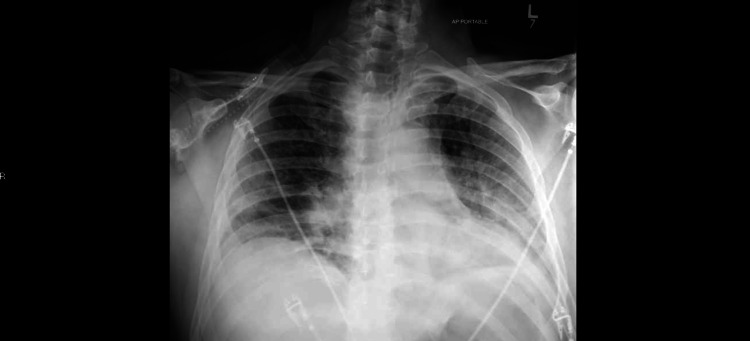
A 55-year-old man with a past medical history of hypertension and hypothyroidism presented to the hospital after 8 days of shortness of breath, dry cough, and fever. His COVID-19 PCR test came back positive. He was tachypneic and hypoxic on arrival, requiring oxygen support via a venturi mask. His temperature was 100.1°F, blood pressure 124/79 mmHg, and pulse 74 beats per minute. Initial laboratory workup included neutrophilic leukocytosis of 12k with normal lymphocyte, hemoglobin, and platelet counts. The metabolic panel revealed hyponatremia with a serum sodium of 131 mmol/L, and mild hypochloremia. Inflammatory markers, including d-dimer, fibrinogen, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and ferritin were all elevated, while pro-calcitonin was within normal limits. Chest X-ray demonstrated bilateral airspace disease (Figure 1).
Figure 1. Chest X-Ray on the day of admission.
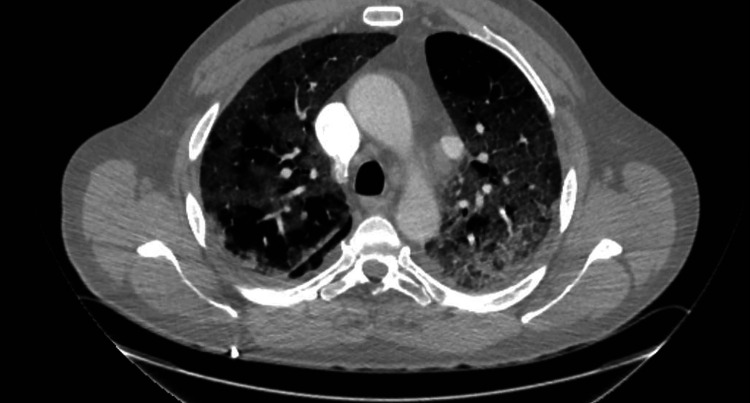
COVID-19 was treated initially with dexamethasone and remdesivir, with the addition of antibiotics for possible bacterial pneumonia. The patient received a dose of tocilizumab on day 3 of hospitalization. He suffered a progressive decline, with worsening oxygenation. A computed tomographic (CT) scan of the chest on day 7 of admission showed acute pulmonary emboli and extensive bilateral parenchymal disease (Figure 2). He was anticoagulated, and steroid dosing was increased due to concern for post-infectious organizing pneumonia. His illness progressed and included bilateral pneumothoraxes and noninvasive ventilation followed by intubation. He succumbed to his illness on day 29 of hospitalization.
Figure 2. CT scan of the chest on day 7 of admission .
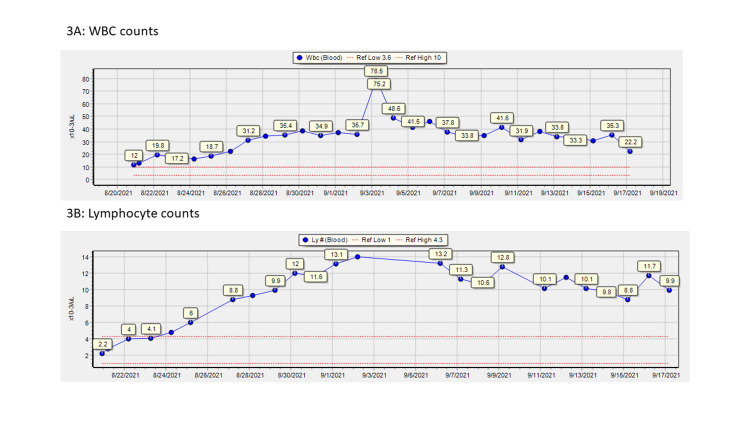
Over the course of his stay, he had developed progressive lymphocytosis (peaked at 14 × 103/µL) and neutrophilia (peaked at 32 × 103/µL), with white blood cell (WBC) count peaking at 78.5 × 103/µL on day 15 of admission (Figure 3). A peripheral smear done early during hospitalization (day 9) had shown leukocytosis with neutrophilia and lymphocytosis without any myelocytes or blasts. A repeat smear on day 15 of admission showed smudge cells concerning for CLL. Flow cytometry of peripheral blood obtained on day 16 was diagnostic for CLL; it showed malignant cells expressing CD45, CD19, CD5, CD20, CD22, CD23, and kappa light chain. Malignant cells were negative for expression of CD10, FMC7, CD79B, and CD38.
Figure 3. Leukocyte counts during admission.
WBC: white blood cells
Discussion
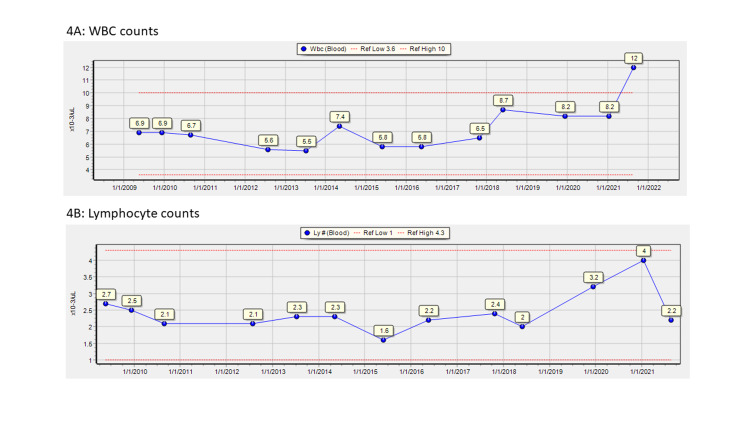
The data are consistent with the concept that COVID-19 infection caused or unmasked CLL. Ten years of pre-admission data, shown in Figure 4, had shown no evidence of lymphocytosis, and admission data showed no evidence of CLL. Subsequent data during this patient’s hospital stay differed from those on admission and were diagnostic of CLL.
Figure 4. Leukocyte data 2010–admission .
WBC: white blood cells
The concept of infection as a precipitant for CLL is not new. In two large population studies, Landgren et al. demonstrated that prior cases of pneumonia and chronic inflammatory conditions were significant risk factors for subsequent CLL. The two most significant risk factors for CLL in their populations were pneumonia in the preceding year (OR: 5.9, 95% CI: 4.5-7.7) and multiple cases of pneumonia (p<0.001 for the trend) [3,4]. Similarly, there have been studies reporting increased use of antimicrobials in the preceding 6-10 years among future CLL patients [5].
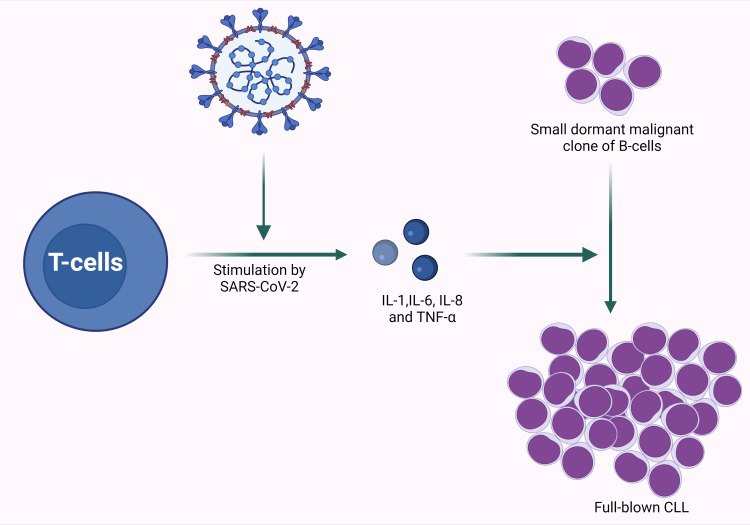
With the advent of immunotherapy, it has become evident that many malignancies are associated with and even infiltrated by immune cells that have the capacity to recognize and attack the malignant population but that have been “silenced;” immunotherapy potentiates this innate and already-present capacity [6]. If a silenced immune response can be activated, it is likely that the opposite can occur, that an effective immune response can be inactivated, allowing “escape” of a malignancy whose genetic substrate had been dormant. This mechanistic understanding is supported by the cited literature and by this case. Given the rapidity with which CLL emerged in this case, it is far more likely that the acute inflammatory response to COVID-19 infection broached a previously effective immune control than that the patient developed and expressed the required mutation over the course of two weeks (Figure 5).
Figure 5. Graphic representation of SARS-CoV-2 stimulating cytokine production, possibly leading to unmasking of CLL.
CLL: chronic lymphocytic leukemia; TNF: tumor necrosis factor; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2 Created with BioRender.com
A Medline search was performed with the goal of finding other case reports of CLL newly diagnosed during acute COVID-19 infection. The keywords “Leukemia, Lymphocytic, Chronic, B-Cell”[Mesh] OR “Leukemia, Prolymphocytic, T-Cell”[Mesh] OR “CLL” AND “COVID-19” led to the identification of four case reports [7-10]. The available data, this case included, are presented in Table 1. Documentation was not thorough enough to rule out the possibility that these additional cases may simply reflect rare instances of the serendipitous diagnosis of CLL during COVID-19 infection; there are a few reported cases in which patients with established CLL developed “Covid-induced lymphocytosis” [11]. Some of the data, however, are consistent with the mechanistic understanding proposed for this case. The median age for diagnosis of CLL is 72, and two of the cases, like the case presented herein, were unusually young at 49 and 55 years of age, consistent with (though far from diagnostic of) the concept of early unmasking due to an infectious trigger [7,9]. The case presented by Largeaud et al.’s study was of an 83-year-old gentleman, but the authors provided evidence of a normal WBC count both one year before and at the time of admission, lymphocytosis with studies diagnostic of CLL on day 11 of admission, and subsequent resolution of lymphocytosis 5 months after discharge although the CLL clone was still detectable [10]. The case is consistent with the hypothesis of immune escape during infection and some improvement in immunosurveillance after the acute infection. Further epidemiological studies are needed to determine causality, if any, between COVID-19 infection and the occurrence of CLL.
Table 1. Reported cases of discovery of CLL during COVID-19 admission.
CLL: chronic lymphocytic leukemia; COVID-19: coronavirus disease 2019
| Author | Age of index patient (years) | Gender of index patient | Comorbidities | Reported day of hospitalization with maximum lymphocytes | Lymphocyte count (Ly #) | Any lymphadenopathies reported | Positivity on flow cytometry OR Immunophenotype results | Death or Follow-up |
| This Case | 55 | Male | Hypertension and hypothyroidism | Day 15 | 14 × 103/µL | Mediastinal lymphadenopathy | Positive for CD45, CD19, CD5, CD20, CD22 (dim), CD23, and kappa light chain | In-hospital mortality 29 days after admission |
| Largeaud et al. (Letter to the editor) [10] | 83 | Male | Radiotherapy-treated lung cancer | ~Day 30 | ~40 × 103/L | Retroperitoneal and mediastinal lymphadenopathy | CD5 positive | At 5 months, resolution of lymphocytosis, but CLL-type-clone detectable |
| Ali et al. [7] | 49 | Male | None | Day 1 | 26.7 × 103/µL | None documented on exam | Positive for CD19, CD5, CD23, CD20 (dim), CD43, CD200 with a dim expression of kappa light chain restriction and FMC7 | At 1 month, plan for observation for early stage CLL |
| Jerez and Ernst [9] | 55 | Male | None | Day 1 | 7.8 × 103/µL | No comment | Positive for CD23, CD200, with dim expression of CD20 and CD43 | No comment |
| Charra et al. [8] | 76 | Male | Surgically treated colon cancer | Day 7 | 154 × 103/µL | Multiple adenopathies at presentation | Immunophenotyping showed B-cells expressing one light chain Immunoglobulin | Death after 10 days of admission |
Conclusions
We posit that the present case report is of interest not because of its rarity but because of the possible mechanistic implications. The case underscores deficiencies in our knowledge with respect to the development or revelation of malignancy during an acute inflammatory process. COVID-19 stimulates an intense immune response, with elevated levels of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, and tumor necrosis factor (TNF)-α. We believe that the cited literature and this case are consistent with a mechanistic understanding that a clone with malignant potential that had previously been controlled by effective immunosurveillance can be unmasked by immunologic modulations caused by infection/inflammation. In the presented case, that trigger was hypothesized to be acute COVID-19 infection.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Vijenthira A, Gong IY, Fox TA, et al. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Hallek M, Cheson BD, Catovsky D, et al. Blood. 2018;131:2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 3.Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Landgren O, Rapkin JS, Caporaso NE, Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Blood. 2007;109:2198–2201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acquired immune-related and inflammatory conditions and subsequent chronic lymphocytic leukaemia. Landgren O, Gridley G, Check D, Caporaso NE, Morris Brown L. Br J Haematol. 2007;139:791–798. doi: 10.1111/j.1365-2141.2007.06859.x. [DOI] [PubMed] [Google Scholar]
- 5.Common community acquired infections and subsequent risk of chronic lymphocytic leukaemia. Anderson LA, Landgren O, Engels EA. Br J Haematol. 2009;147:444–449. doi: 10.1111/j.1365-2141.2009.07849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.T cell dysfunction in cancer. Thommen DS, Schumacher TN. Cancer Cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chronic lymphocytic leukemia concomitant with COVID 19: a case report. Ali E, Badawi M, Abdelmahmuod E, Kohla S, Yassin MA. Am J Case Rep. 2020;21:0. doi: 10.12659/AJCR.926062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19 and fortuitous discovery of chronic lymphocytic leukemia: biological findings and therapeutic challenges. Charra B, Ellouadghiri A, Magramane A, et al. Pan Afr Med J. 2020;36:1–5. doi: 10.11604/pamj.2020.36.286.24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.High percentage of smudge cells in a patient with COVID19: rediscovering their utility. Jerez J, Ernst DM. eJHaem. 2020;1:374–375. doi: 10.1002/jha2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Major rise of a chronic lymphoid leukemia clone during the course of COVID-19. Largeaud L, Ribes A, Dubois-Galopin F, et al. Int J Lab Hematol. 2021;43:0–3. doi: 10.1111/ijlh.13383. [DOI] [PubMed] [Google Scholar]
- 11.Covid-19 infection in therapy-naive patients with B-cell chronic lymphocytic leukemia. Paneesha S, Pratt G, Parry H, Moss P. Leuk Res. 2020;93:106366. doi: 10.1016/j.leukres.2020.106366. [DOI] [PMC free article] [PubMed] [Google Scholar]