Abstract
Human papilloma virus (HPV) infections can cause common warts, which usually resolve spontaneously or become recalcitrant, resistant to multiple treatments. In rare cases, they transform into cutaneous giant horns resulting in the tree-man syndrome (TMS). Defective β-HPVs can cause flat warts in epidermodysplasia verruciformis (EV), a genetic disorder. In typical EV, limited to the skin, the mutated genes are critical for keratinocyte-intrinsic immunity, while atypical, syndromic EV involves genes controlling T-cells. Inborn errors of immunity due to mutations in distinct genes underlying recalcitrant warts and the α-HPV2-driven TMS have been identified, all disrupting T-cell immunity. Collectively, these observations attest to the wide phenotypic spectrum of cutaneous infections caused by different HPV types, at the intersection of the genetic diversity of the viral and human genomes.
Keywords: Cutaneous HPV infections, epidermodysplasia verruciformis, recalcitrant warts, the tree-man syndrome, keratinocyte-intrinsic immunity, T cell immunity
INTRODUCTION
Human papilloma viruses (HPVs), with strict tropism to keratinized and non-keratinized squamous epithelia, are common pathogens; for detailed discussion of HPV virology and pathology, see (Beziat et al., 2021; Bzhalava et al., 2015; McBride, 2021). HPVs are double-stranded DNA viruses with over 400 genotypes identified, grouped into five genera (α-, β-, γ-, μ-, and ν-HPV), on the basis of their nucleotide sequence similarities of the open reading frame encoding the capsid protein L1 (Bzhalava et al., 2015; de Villiers et al., 2004; McBride, 2021). While most cutaneous HPV infections are subclinical and persistent in the absence of immunosuppression, they can cause benign common warts (CW), as well as extensive, unusually severe conditions which can be divided into three distinct clinical categories, (a) recalcitrant common warts (RCW), (b) the tree-man syndrome (TMS), and (c) epidermodysplasia verruciformis (EV) (Figure 1; the clinical pictures are presented with written, informed consent of the patients). While HPV may infect a number of extracutaneous sites, this Perspective will focus on recent progress in understanding the genetic and immunologic underpinnings of cutaneous HPV infections, RCW, TMS and EV, and we suggest updates to wart classification regarding the associated HPV types (Table 1).
Figure 1. Clinical presentation of warts on the backs of hands of patients with cutaneous HPV infections and identification of HPV types by whole transcriptome sequencing.
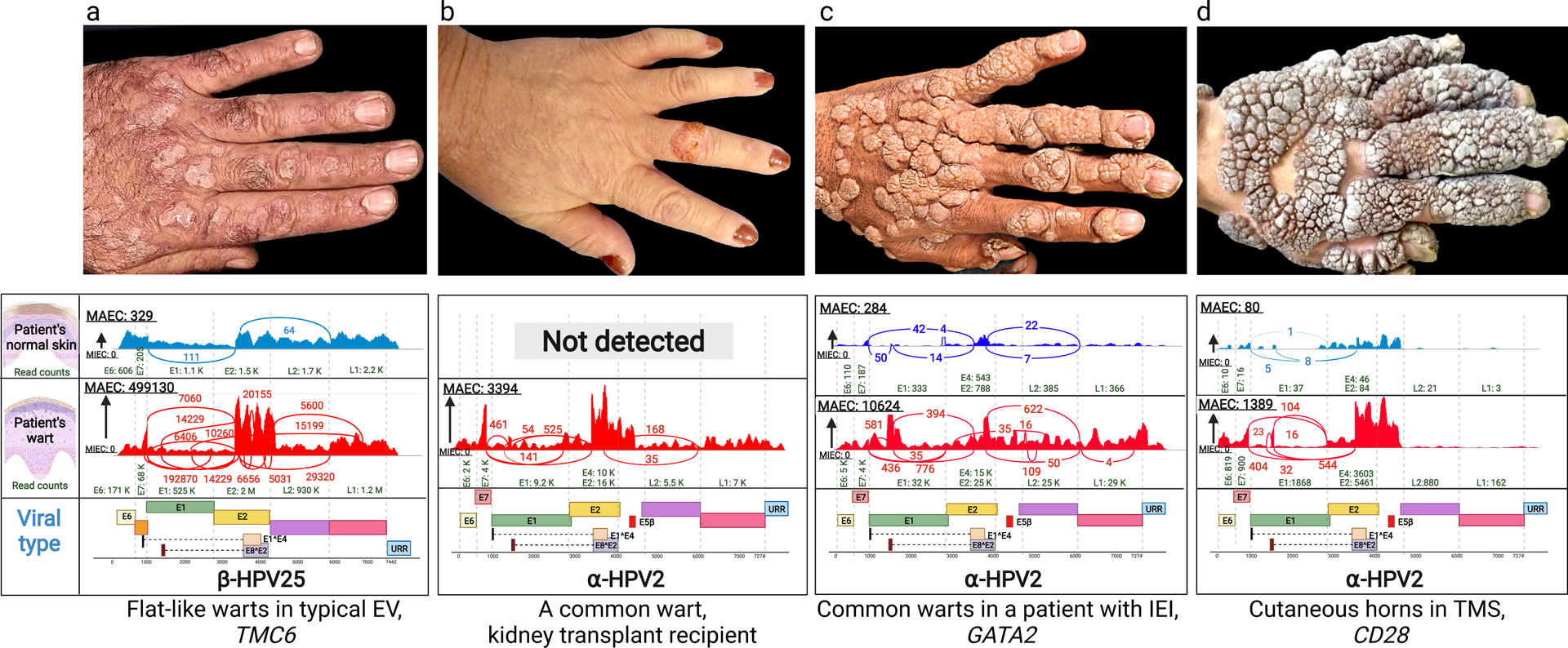
(a) Multiple flat warts in a patient with typical EV caused by mutations in the TMC6 gene. (b) A solitary common wart in the middle finger of a kidney transplant patient. (c) Multiple common warts in a patient with primary inborn error of immunity (IEI) caused by mutations in GATA2. (d) Exophytic warts and cutaneous horns in a patient with TMS due to mutations in CD28. Below each clinical picture, there are representative Sashimi plots derived from whole transcriptome sequencing by RNA-Seq, identifying the types of HPV associated with the lesions shown above or in the normal appearing skin of the same patients. The corresponding genomic structures of HPVs are shown below; note the presence of Early (E1–8), Late (L1 and L2) and Upstream Regulatory Region. The copy number of viruses is very low (a, c and d), or completely absent (b), in normal appearing skin adjacent to the warts. HPV, human papilloma virus; EV, epidermodysplasia verruciformis; IEI, inborn error of immunity; MAEC, Maximum exon coverage; MIEC, Minimum exon coverage; TMS, the tree-man syndrome.
Table 1:
Nosology of HPV types associated with warts with different clinical presentations*
| Cutaneous lesions** | Mucosal lesions | |||
|---|---|---|---|---|
| HPV genera | EV | TMS | Recalcitrant warts-Non genital | Recalcitrant warts-Oral/Anogenital |
| α | 2 | 2, 3, 7, 10, 26, 27, 28, 29, 57, 78, 91, 94, 117 | 6, 11, 13, 16#, 18#, 27, 30, 31#, 32, 33#, 35#, 39#, 40, 42, 43, 44, 45#, 51#, 52#, 53, 54, 56#, 58#, 59#, 61, 62, 66#, 67, 68#, 70, 71, 72, 73, 74, 81, 83, 84, 85, 86, 87, 89, 90 | |
| β | 5, 8, 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, 36, 37, 38, 47, 49, 50, 75, 76, 77, 80, 93, 96 | |||
| γ | 4 | 4, 48, 60, 65, 88, 95, 126, 128, 129, 130, 131, 132, 133, 134, 142, 147, 148, 149, 154 | 153, 175, 180 | |
| μ | 1, 61 | |||
| ν | 41 | |||
Updated from Orth G., 2006, and Sterling J.C., 2916 (Orth, 2006; Sterling, 2016)
EV, epidermodysplasia verruciformis; HPV, human papilloma virus; TMS, the tree-man syndrome
High-risk oncogenic HPVs (https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer)
Recalcitrant warts as paradigm of human papilloma virus infections
Based on their anatomical locations, CWs can be subcategorized, such as generalized, periungual, plantar, and genital warts. The CWs usually resolve spontaneously, but some of these lesions, defined as RCW, persist despite repeated treatments. In exceptional cases, the warts can also transform into exophytic cutaneous lesions, giant horns, resulting in TMS (Beziat et al., 2021). In some cases, there is a familial susceptibility to development of warts, as in EV, a heritable disorder characterized by the presence of numerous flat warts in generalized distribution (de Jong et al., 2018; Vahidnezhad et al., 2019).
The persistence of HPV-related warts suggests the presence of impaired immunity to cutaneous HPVs, although in many cases these patients do not have an obviously impaired immune system. Nevertheless, in some cases the proliferation of HPV, a common pathogen, is not efficiently controlled by the patients’ immune system, and it has been suggested that deficient host cell immunity, including T cell activities, are involved in immune response selectively against HPVs. In support of this notion, inborn errors of immunity (IEI), associated with RCWs, have been identified, highlighting the role of T lymphocytes in the cutaneous anti-HPV immune response (Beziat et al., 2021). In some cases, as illustrated by typical EV, the development of warts is permitted by deficient keratinocyte-intrinsic immunity in otherwise healthy individuals (de Jong et al., 2018; Beziat et al., 2021)
The tree-man syndrome, a disease of exophytic warts and cutaneous horns with aberrant immunity
In the spectrum of cutaneous HPV infections, TMS presents with the most extensive warts developing into cutaneous horns, which can be giant and generalized (Figure 1). These lesions start as CWs slowly spreading over the hands and feet before transforming into cutaneous horns characteristic of the TMS phenotype. This condition is extremely rare, and only a few cases have been reported, essentially all of them being sporadic with no family history. Due to paucity of reported cases, it is unclear if these lesions in TMS have malignant potential. We have recently analyzed an extended multiplex family with three affected individuals, one of them with characteristic TMS phenotype while two others had multiple RCWs (Beziat et al., 2021). Utilizing whole exome sequencing, we identified a homozygous, previously unreported missense mutation (c.52G>A; p.Gly18Arg) in the CD28 gene. No CD28 protein on their T cells was detected, with the exception of a small contingent of revertant memory CD4+ T cells. CD28 has a major costimulatory role of T-cell receptor signaling when engaged by CD80 or CD86 expressed on antigen-presenting cells. The development of T cells was not affected, but the patient’s T cells did not respond to CD28 co-stimulation. CD28 deficient mice were also shown to be susceptible to cutaneous infections with mouse papillomavirus MmuPV1. Collectively, these results suggested that the control of HPV2 and HPV4 in keratinocytes is dependent on the T cell CD28 co-activation. However, the patients with CD28 deficiency, besides the cutaneous HPV infection, were otherwise healthy, indicating that human CD28 is largely dispensable for protective immunity against other pathogens.
Careful examination of the TMS pedigree revealed that the autosomal recessive CD28 deficiency resulted in susceptibility to cutaneous α- and γ-HPVs, but only one of the patients had extensive giant horns characteristic of TMS resulting from a long-term α-HPV2 infection, whereas the other two patients had disseminated RCWs caused by γ-HPV4. These findings attest to the diversity of HPV types in causing cutaneous lesions, and they provide new insights into the context of host-HPV interactions. These observations, in combination with the demonstration of CD28 deficiency in the patient with TMS, indicate that IEIs can underlie the development of TMS. In contrast to HPV2+ RCWs, TMS lesions were associated with aberrant HPV2 oncogene expression, and deregulated viral cycle (Beziat et al., 2021). It remains unclear, however, why most patients with IEI underlying severe HPV2-driven warts do not develop TMS.
Genetic basis of epidermodysplasia verruciformis (EV)
Early evidence for genetic susceptibility to warts was provided by studies on EV, an autosomal recessive disorder with propensity for development of numerous flat warts, which usually first appear during the childhood. Later in life, these warts can evolve into protruding hyperproliferative lesions and verrucous tumors which, particularly on the sun-exposed areas, have propensity for non-melanoma skin cancer formation, mostly squamous cell carcinomas (Becerril et al., 2021). The heritable forms EV have been divided into two categories, typical and atypical, and some sporadic cases with EV-like phenotypes are secondarily associated with immune suppression, such as HIV infection. In typical EV, the clinical manifestations are limited to the skin, the affected individuals being otherwise healthy with no evidence of other infections. In atypical forms of inherited EV, the cutaneous findings are invariably associated with other infections and hematological malignancies, such as the Hodgkin’s lymphoma (Youssefian et al., 2019b). Next generation sequencing has identified mutations in a number of IEI-associate genes, including RHOH, LCK, MST1, CORO1A, IL7, RASGRP1, CARMIL2, TPP2, DCLRE1C, GATA2, and DOCK8. These inborn errors often cause early-onset, life threatening T cell primary immunodeficiency, and consequently, the atypical forms of EV are genetically linked to impaired T cell immunity. While the underlying pathomechanisms of EV in typical and atypical cases can be different, the clinical features are similar with respect to cutaneous β-HPV infection (Figure 1). Thus, in the absence of evidence for other infections or overt systemic malignancies, classification of families as having typical versus atypical EV can be difficult before appropriate genotyping of viral and host genomes has been a performed.
The early genetic studies on typical forms of EV identified mutations in TMC6 and TMC8, which encode EVER1 and EVER2, two endoplasmic reticulum plasma membrane proteins, respectively (Ramoz et al., 2002; Youssefian et al., 2019a). Loss of function of these proteins results in proliferation of HPV infected keratinocytes and in replication of the virus in terminally differentiating host cells. More recently, we identified another gene, CIB1, which can also harbor mutations in approximately one-third of the families with typical EV (de Jong et al., 2018; Vahidnezhad et al., 2019). The mechanism for cell proliferation as a result of mutated EVER1 and EVER2 was initially suggested to involve intracellular zinc transport, however, subsequent to identification of CIB1, it was demonstrated that these three proteins, EVER1, EVER2 and CIB1, form a tertiary complex providing keratinocyte-intrinsic immunity to β-HPVs. Thus, mutations in any of the three genes encoding these subunits can result in aberrant, keratinocyte-intrinsic immunity manifesting with typical cutaneous lesions of EV due to HPVs, and the patients do not display infections due to other pathogens.
Unifying concepts of HPV-associated cutaneous manifestations
Collectively, infections of cutaneous keratinocytes caused by a number of different HPV types can results in a spectrum of phenotypic lesions, including CWs, specifically associated with the 14 α-, 17 γ-, two μ-, and one ν-HPV types (Table 1). In relatively few cases the clinical phenotype can proceed to TMS with extensive protruding lesions with cutaneous horns. This progression to TMS phenotype is apparently predicated on immunological deficiency limited to α-HPV2, as demonstrated in the family with the T cell CD28 deficiency (Figure 1) as well as in other HPV-typed TMS patients reported to date. Another HPV-associated cutaneous disorder, EV, is characteristically associated with β-HPV infections (Table 1). Thus, EV and TMS are clearly distinct entities with characteristic cutaneous manifestations and different associated HPV types. Overall, the spectrum of cutaneous manifestations due to HPV infections is determined by the type of underlying genetic mutations in different immune-associated genes, expressed either in keratinocytes or in T cells, with distinct consequences on cutaneous immunity, intersecting with the HPV types causing the infection.
Conclusions and therapeutic implications
Precise understanding of the immunological mechanisms allowing pathogenic propagation of the infection by HPVs, which are commonly present at low, subclinical levels in the skin of populations at large, should provide new information on potential targets for therapy development for these, currently intractable disorders. However, immunization with multipotent anti-HPV vaccines, such as Gardasil 9, has been generally unsuccessful in improving the skin lesions in patients with EV or TMS (Donaldson et al., 2019; Maor et al., 2018). It should be noted, however, that these vaccines do not specifically target the HPV types responsible for EV or TMS. In patients with atypical EV or RCWs due to T cell deficiency as a result of mutations in critical immune genes, with hematological malignancies, bone marrow transplantation has been shown to be curative also of some skin lesions (Bruzzese et al., 2020; Aydin et al., 2019).
In summary, recent genetic observations on HPV-associated skin disorders, including RCW, EV and TMS, emphasize the control of proliferation of HPV infected keratinocytes by the underlying immunological factors. Loss of immune control, either T cell-based or keratinocyte-intrinsic, can be elicited by genetic loss-of-function mutations in immune-associated genes, leading to susceptibility to infections by HPV and transformation of resident keratinocytes into clinical lesions in the spectrum of HPV-associated cutaneous disorders at the intersection of genetic variability of viral and human genomes.
ACKNOWLEDGEMENTS
These studies were approved by the Institutional Review Board of the Pasteur Institute of Tehran. Carol Kelly assisted in manuscript preparation.
Funding:
National Institutes of Health grant R01AI143810, R01AR080754, and DEBRA International
Abbreviations:
- CW
cutaneous warts
- EV
epidermodysplasia verruciformis
- HPV
human papilloma virus
- RCW
recalcitrant cutaneous warts
- TMS
the tree-man syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: J.-L. C serves on the Scientific Advisory Boards of ADMA Biologics, Inc., Kymera Therapeutics, and Elixiron Immunotherapeutics. The other authors have no conflict of interest.
DATA AVAILABILITY STATEMENT
No new datasets were generated for this Perspective article. The data on the authors’ original work, including FASTQ files, are available from the corresponding author upon reasonable request.
REFERENCES
- Aydin SE, Freeman AF, Al-Herz W, Al-Mousa HA, Arnaout RK, Aydin RC, et al. Hematopoietic stem cell transplantation as treatment for patients with DOCK8 deficiency. J Allergy Clin Immunol Pract 2019;7:848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerril S, Corchado-Cobos R, Garcia-Sancha N, Revelles L, Revilla D, Ugalde T, et al. Viruses and skin cancer. Int J Mol Sci 2021;22:5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziat V, Rapaport F, Hu J, Titeux M, Bonnet des Claustres M, Bourgey M, et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 2021;184:3812–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzese A, Leardini D, Masetti R, Strocchio L, Girardi K, Algeri M, et al. GATA2 related conditions and predisposition to pediatric myelodysplastic syndromes. Cancers (Basel) 2020;12:2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology 2015;476:341–4. [DOI] [PubMed] [Google Scholar]
- de Jong SJ, Matos I, Crequer A, Hum D, Gunasekharan V, Lorenzo-Diaz L, et al. The human CIB1-EVER1-EVER2 complex governs keratinocyte-intrinsic immunity to beta-papillomaviruses. J Exp Med 2018;215:2289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004;324:17–27. [DOI] [PubMed] [Google Scholar]
- Donaldson SL, Purnell JC, Pavlidakey PG, Atkinson TP, Kissel R. Epidermodysplasia verruciformis in a young adult with activated PI3Kdelta syndrome. JAAD Case Rep 2019;5:195–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor D, Brennand S, Goh MS, Fahey V, Tabrizi SN, Chong AH. A case of acquired epidermodysplasia verruciformis in a renal transplant recipient clearing with multimodal treatment including HPV (Gardasil) vaccination. Australas J Dermatol 2018;59:147–8. [DOI] [PubMed] [Google Scholar]
- McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol 2021; 10.1038/s41579-021-00617-5. [DOI] [PubMed] [Google Scholar]
- Orth G Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin Immunol 2006;18:362–74. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, Favre M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 2002;32:579–81. [DOI] [PubMed] [Google Scholar]
- Sterling JC (2016) Viral Infections. In: Rook’s Textbook of Dermatology (Griffiths C, Barker J, Bleiker TO, Chalmers R, & Creamer D, ed) Vol. 571–666: John Wiley & Sons. [Google Scholar]
- Vahidnezhad H, Youssefian L, Saeidian AH, Mansoori B, Jazayeri A, Azizpour A, et al. A CIB1 splice-site founder mutation in families with typical epidermodysplasia verruciformis. J Invest Dermatol 2019;139:1195–8. [DOI] [PubMed] [Google Scholar]
- Youssefian L, Vahidnezhad H, Mahmoudi H, Saeidian AH, Daneshpazhooh M, Kamyab Hesari K, et al. Epidermodysplasia verruciformis: Genetic heterogeneity and EVER1 and EVER2 mutations revealed by genome-wide analysis. J Invest Dermatol 2019a;139:241–4. [DOI] [PubMed] [Google Scholar]
- Youssefian L, Vahidnezhad H, Yousefi M, Saeidian AH, Azizpour A, Touati A, et al. Inherited interleukin 2-inducible T-Cell (ITK) kinase deficiency in siblings with epidermodysplasia verruciformis and Hodgkin lymphoma. Clin Infect Dis 2019b;68:1938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new datasets were generated for this Perspective article. The data on the authors’ original work, including FASTQ files, are available from the corresponding author upon reasonable request.


