Abstract
Introduction:
Low titer group O whole blood (LTOWB) resuscitation is increasingly common in both military and civilian settings. Data regarding the safety and efficacy of prehospital LTOWB remains limited.
Methods:
We performed a single center, prospective, cluster randomized, prehospital thru in-hospital whole blood pilot trial for injured air medical patients. We compared standard prehospital air medical care including red cell transfusion and crystalloids followed by in-hospital component transfusion to prehospital and in-hospital LTOWB resuscitation. Prehospital vital signs were used as inclusion criteria (SBP ≤ 90 mmHg and HR ≥ 108 bpm) or (SBP ≤ 70 mmHg) for patients at risk of hemorrhage. Primary outcome was feasibility. Secondary outcomes included 28-day and 24 hour mortality, multiple organ failure, nosocomial infection, 24hr transfusion requirements and arrival coagulation parameters.
Results:
Between November 2018 thru October 2020, 86 injured patients were cluster randomized by helicopter base. The trial has halted early at 77% enrollment. Overall, 28-day mortality for the cohort was 26%. Injured patients randomized to prehospital LTOWB (n=40) relative to standard care (n=46) were similar in demographics and injury characteristics. Intent to treat Kaplan-Meier survival analysis demonstrated no statistical mortality benefit at 28 days (25.0% vs. 26.1%, p= 0.85). Patients randomized to prehospital LTOWB relative to standard care had lower red cell transfusion requirements at 24 hours (p<0.01) and a lower incidence of abnormal thromboelastographic measurements. No transfusion reactions during the prehospital or in-hospital phase of care were documented.
Conclusion:
Prehospital through in-hospital LTOWB resuscitation is safe and may be associated with hemostatic benefits. A large-scale clinical trial is feasible with protocol adjustment and would allow the effects of prehospital LTOWB on survival and other pertinent clinical outcomes to be appropriately characterized.
Keywords: Prehospital, Whole Blood, Hemorrhage, low titer type O whole blood, randomized, pilot trial
Introduction
Despite major improvements in trauma resuscitation during the in-hospital phase of care over the last two decades, patients continue to suffer high rates of mortality due to hemorrhage in the first few hours of arrival.1–4 This persistent early mortality highlights the importance of prehospital environment interventions, implemented as close to the time of injury as possible, that result in improved outcome differences for patients at risk of hemorrhagic shock.5–10
Low titer group O whole blood (LTOWB) resuscitation has become increasingly common in both military and civilian settings and is thought to represent the ideal resuscitation intervention.11, 12 Evidence for LTOWB resuscitation safety, when provided in an uncrossmatched fashion, has consistently been demonstrated when initiated during the in-hospital phase of care.13–21 Outcome benefits associated with in-hospital LTOWB resuscitation have been demonstrated.22–26 High level evidence demonstrating the safety and efficacy of prehospital initiated LTOWB resuscitation remains limited.18, 21, 26–29
A large pragmatic clinical trial is needed to definitively establish the efficacy and safety of whole blood resuscitation initiated in the prehospital setting. Only a robust randomized clinical trial will provide essential evidence to justify and provide the impetus for the use of this precious resource early post-injury. Because of the challenges associated with execution of these types of large trials particularly in the prehospital setting,6–8, 30 it is essential to establish feasibility of this approach via a pilot study and provide experience to inform a definitive large, multicenter, prehospital whole blood clinical trial.
The objective of the current Pragmatic Prehospital type O Whole blood Early Resuscitation (PPOWER) pilot trial was to determine the feasibility and most appropriate study population that will lead to a large multicenter clinical trial designed to evaluate the effectiveness of prehospital initiated LTOWB as compared to standard prehospital care in patients at risk of hemorrhagic shock. We hypothesized that prehospital initiated LTOWB resuscitation will be feasible, safe and associated with outcome benefits.
Methods
Design
We performed a pragmatic, prospective, single-center, cluster randomized, prehospital initiated whole blood (LTOWB) pilot trial for injured air medical patients at risk of hemorrhagic shock. We compared standard prehospital air medical care including red cell transfusion (up to two units) followed by in-hospital component transfusion, when indicated, to LTOWB resuscitation initiated in the prehospital phase of care and continued, through the in-hospital phase of care, when indicated. Up to two units of LTOWB were transfused in the prehospital setting, initiated during air medical transport. An in-hospital whole blood resuscitation program (six LTOWB units available from ED refrigerator) had been established at the University of Pittsburgh Medical Center, Presbyterian Hospital, prior to study initiation.16, 17, 31
The prehospital, pragmatic trial was executed using exception from informed consent required for emergency research as outlined in regulation 21CFR50.24 of the Food and Drug Administration (FDA). The trial was designed by the authors, the FDA, the Institutional review board (IRB, STUDY19080344) at the University of Pittsburgh and the external data and safety monitoring board approved the design. The IRB approved all exception from informed consent requirements, after consultation with community members and after public notification regarding the trial took place. Enrolled participants or their legally authorized representatives were notified of enrollment, as soon as feasible, and asked to provide consent for continued participation. The study was registered with Clinical Trials.gov (NCT03477006) and the data and safety monitoring board performed regular safety surveillance throughout the enrollment period. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for pilot or feasibility trials.
Participants
Patients who were transported from the scene of their injury or from an outside referral emergency department to the University of Pittsburgh Medical Center were eligible for enrollment if they had at least one episode of hypotension (systolic blood pressure ≤ 90 mm Hg) and tachycardia (defined as a heart rate > 108 beats per minute) or if they had an episode of severe hypotension (systolic blood pressure ≤ 70 mm Hg), either before the arrival of air medical transport or any time before arrival to the trauma center. Patients were excluded if they were 90 years of age or older or younger than 18 years age, if they had an isolated fall from standing, if they had penetrating brain injury or brain matter exposed, if they had cardiopulmonary resuscitation > 5 minutes without return of spontaneous circulation, if they suffered isolated burns, if their injury was due to isolated hanging or drowning without evidence of injury, if they were a known prisoner or were known to be pregnant, if they were admitted at the referral hospital, if the patient or family voiced an objection to participation in the trial at the scene of injury, or the patient was wearing a PPOWER ‘opt-out’ bracelet.
Randomization
We utilized a single-stage cluster randomization scheme because of the logistics required for a prehospital blood product intervention and to minimize LTOWB wastage. Designated air medical bases at the University of Pittsburgh were block randomized and assigned to the LTOWB arm or standard care arm for 1 month time intervals. The block scheme varied randomly between 2 and 4-month block sizes over the period of enrollment for the trial. Randomization assignments were determined prior to the start of enrollment using standard computerized randomization software. Randomization assignment was unable to be blinded during the prehospital phase or in-hospital phase of care. However, treatment assignments were concealed to personnel who assessed the trial outcomes.
Interventions
Air medical bases that were randomly assigned to the LTOWB arm for the month were provided up to two units of whole blood for eligible patients at risk of hemorrhagic shock based upon storage capabilities. The units were collected from male donors who were RhD-positive and the LTOWB was collected using the FDA approved Terumo Imuflex® WB-SP blood bag system to allow for platelet sparing leukoreduction. LTOWB was stored between 1–6ºC up to 14 days from collection and only units where the titer of anti-A and anti-B antibodies were < 100 were issued for the study. LTOWB units not utilized during the 14-day period were recycled to red cells when feasible. At the initiation of the study RhD-negative LTOWB units were utilized allowing females of child-bearing age to be eligible for enrollment in the prehospital phase of care. An inadequate supply of RhD-negative LTOWB limited capabilities to stock air medical bases and the LTOWB intervention was modified to RhD-positive LTOWB with necessary regulatory approval. At this transition, women of child-bearing age (< 50 years of age) were temporarily excluded from enrollment. Subsequently, standard practice for in-hospital LTOWB resuscitation at our institution changed based upon evolving data and females of child-bearing age were able to receive urgent release RhD-positive LTOWB. At that time the protocol was further amended and women of child-bearing age were eligible for prehospital RhD-positive LTOWB with appropriate regulatory approval.
Air medical bases randomized to the standard care arm for the month carried RhD-negative ‘universal donor’ packed red cells and patients who met all inclusion criteria and no exclusion criteria were resuscitated with crystalloid and red cell transfusion following standard practice. Up to two units of red cells were transfused for eligible patients at risk of hemorrhagic shock.
In-hospital resuscitation followed prehospital randomized assignment and patients randomized to LTOWB were able to receive up to an additional 6 units of LTOWB (available from ED refrigerator) as needed based upon standard indications for transfusion in the first 4 hours from admission and standard care at our trauma center. After a potential maximum of eight units of LTOWB in the prehospital phase of care and first 4 hours in-hospital, standard component therapy was then utilized when indicated. Patients randomized to standard care in the prehospital setting were to receive component transfusion during the in-hospital phase of care as clinically indicated.
Outcomes
The primary outcome for the pilot trial was feasibility measured by enrollment and accrual rate. Secondary performance and feasibility outcomes included eligibility rates and adherence to protocol rates. The principal efficacy and safety outcome for the pilot trial was 28-day mortality. Additional efficacy and safety secondary outcomes included the incidence of 3-hour, 6-hour and 24-hour mortality, multiple organ failure, acute respiratory distress syndrome and nosocomial infection, blood component transfusion volumes at 24 hours from admission, arrival shock severity and the incidence of acute hemolytic transfusion reactions. An interim analysis to assess for safety was planned at 50% planned enrollment (n=56). Laboratory measurements included Prothrombin Time, Parital Thromboplastin Time, International Normalized Ratio and rapid thrombelastography.
Assuming a baseline 30-day mortality risk of 33% in the control arm as was demonstrated in the recently completed PAMPer trial,6 with all cause 28-day mortality as the outcome, enrolling 56 patients in each arm, 112 total patients and using a 2-sided alpha of 0.05, the study as planned had 80% power to detect a 22% (33% to 11%) or greater difference in 28-day mortality.
Statistical Methods
All analyses were performed based on the intent-to-treat principle for the prehospital randomization and analyses include all enrolled patients grouped by randomization assignment. The feasibility of enrollment was evaluated by determining 1) the number of patients that met the eligibility criteria for the trial; 2) the proportion of eligible patients that could be randomized and 3) the proportion of eligible patients who were enrolled in the trial. Adherence rates for each treatment arm of the protocol were ascertained as the proportion of enrolled patients who adhere to the intervention (LTOWB vs. standard care) as assigned in prehospital phase of care.
All efficacy and safety outcomes were exploratory due to the pilot nature of the trial and are not powered for definitive comparison. Kaplan-Meier survival analysis was performed for the 28-day mortality primary outcome. All adverse events were recorded and assigned seriousness and relatedness to the protocol/intervention. Adverse events which were serious, excluding events which were definitively not related, were tabulated. Descriptive statistics were employed to characterize the demographics and injury characteristics of patients across intent to treat randomization groups and for all primary and secondary outcomes. Categorical variables were presented as frequencies and percentages and tested using the Chi-squared test. Continuous variables were expressed as medians and interquartile ranges (IQRs) and were tested using parametric or non-parametric statistics as appropriate. LTOWB for calculating purposes was considered = 1 unit of red cells, 1unit of plasma and 1 unit of random donor platelets. Statistical significance was determined at the p <0.05 level (2-sided). All data were analyzed using SPSS Inc. released 2019, version 26.0
Results
Participant Flow
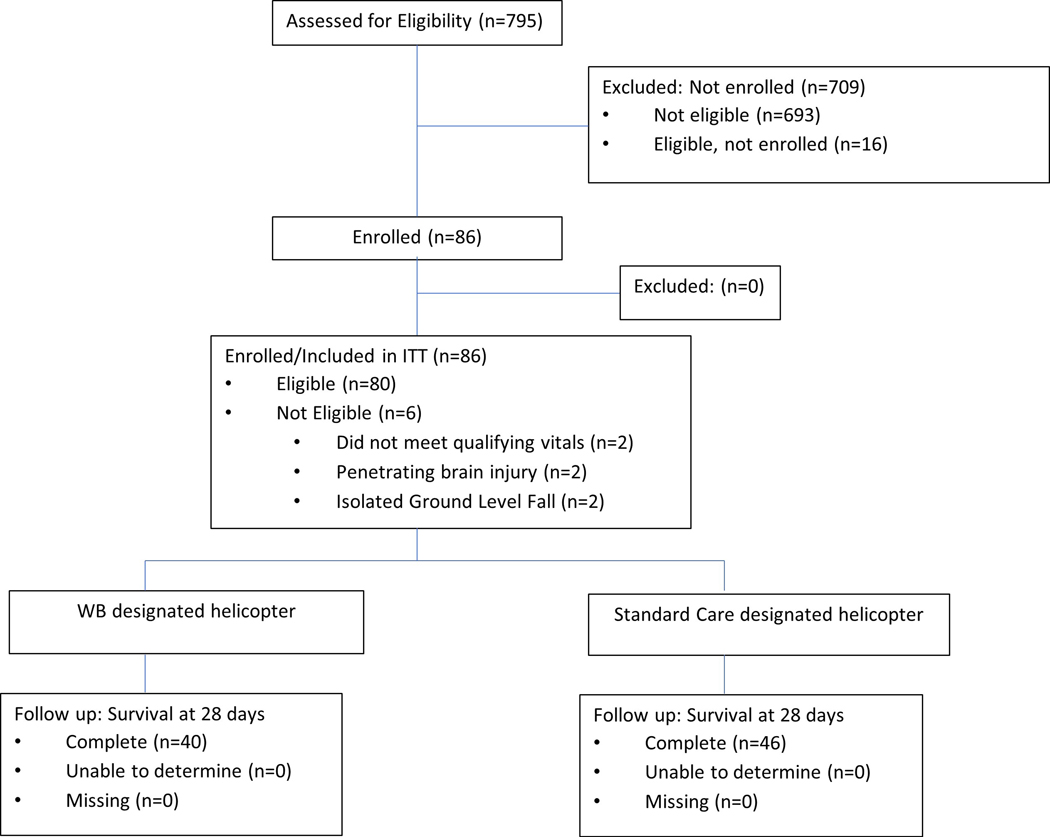
From November 2018 thru October 2020, a total of 795 injured patients who were transported by air medical transport by personnel from up to six air medical bases at any one time were assessed for eligibility. A total of 102 patients were eligible for enrollment in the prehospital setting. Of these patients, 40 patients were transported from air medical bases randomly assigned to the LTOWB group and 46 were transported from bases randomly assigned to the standard care group. These 86 patients met all inclusion criteria and none of the exclusion criteria and compromised the intent-to-treat cohort. (Figure 1.)
Figure 1.
Screening, Randomization, and Follow-up.
Baseline Data
The majority of enrolled patients were men (63%), had a median injury severity score of 16 (IQR 9,22) and an overall 28-day mortality of 25.6%. Over 67% of patients were transported directly from the scene of injury. The LTOWB and standard care groups were similar in demographics, injury characteristics, prehospital vitals, shock severity and incidence of brain injury. (Table 1.)
Table 1.
Demographics and Injury Characteristics Across Randomized Arms
| Standard Care N=46 | LTOWB N=40 | p-value | |
|---|---|---|---|
| Age (years), median (IQR) | 51 (36, 62) | 46 (27, 64) | 0.74 |
| Sex (% Male) | 67.4% | 57.5% | 0.47 |
| Race n, (%N) | |||
| White | 43 (93.5%) | 34 (85.0%) | 0.35 |
| African American | 2 (4.3%) | 4 (10.0%) | 0.30 |
| Other | 1 (2.2%) | 2 (5.0%) | 0.48 |
| Comorbidities n, (%N) | |||
| Alcoholism | 11 (23.9%) | 5 (12.5%) | 0.17 |
| Arrhythmia | 2 (4.3%) | 2 (5.0%) | 0.89 |
| Chronic Obstructive Pulmonary Disease (COPD) | 3 (6.5%) | 3 (7.5%) | 0.86 |
| Coagulopathy | 1 (2.2%) | 0 (0.0%) | 0.35 |
| Congestive Heart Failure (CHF) | 0 (0.0%) | 1 (2.5%) | 0.28 |
| Diabetes mellitus | 4 (8.7%) | 5 (12.5%) | 0.57 |
| History of Myocardial Infarction (MI) | 0 (0.0%) | 4 (10.0%) | 0.03 |
| Hypertension | 15 (32.6%) | 13 (32.5%) | 0.99 |
| Liver Disease | 3 (6.5%) | 3 (7.5%) | 0.86 |
| Renal Dysfunction | 1 (2.2%) | 1 (2.5%) | 0.92 |
| Smoker | 16 (34.8%) | 11 (27.5%) | 0.47 |
| Any Comorbidity n, (N%) | 17 (36.9%) | 14 (35.0%) | 0.85 |
| Blunt Mechanism of Injury n, (%N) | 39 (84.8%) | 34 (85.0%) | 0.99 |
| Mechanism of Blunt Injuries n, (%N); N=73* | |||
| Fall | 10 (25.6%) | 10 (29.4%) | 0.72 |
| Machinery | 1 (2.6%) | 0 (0%) | 0.35 |
| MVC- Occupant, Ejected | 5 (12.8%) | 2 (5.9%) | 0.32 |
| MVC- Occupant, Not Ejected | 12 (30.8%) | 8 (23.5%) | 0.51 |
| MVC- Motorcyclist | 7 (17.9%) | 5 (14.7%) | 0.72 |
| MVC- Pedestrian | 2 (5.1%) | 4 (11.8%) | 0.30 |
| MVC- ATV | 0 (0%) | 3 (8.8%) | 0.06 |
| MVC- Unknown | 1 (2.6%) | 0 (0%) | 0.35 |
| Struck by or Against | 1 (2.6%) | 2 (5.9%) | 0.48 |
| Mechanism of Penetrating Injuries, n (%N); N=17* | |||
| Firearm | 6 (54.5%) | 4 (66.7%) | 0.66 |
| Impalement | 3 (27.3%) | 0 (0.0%) | 0.10 |
| Stabbing | 1 (9.1%) | 1 (16.7%) | 0.92 |
| Other | 1 (9.1%) | 1 (16.7%) | 0.92 |
| Transferred From Outside ED n, (%N) | 17 (37.0%) | 11 (27.5%) | 0.35 |
| ISS, median (IQR) | 17 (9, 25) | 13 (8.5, 22) | 0.69 |
| ISS ≥ 16 n, (%N) | 25 (54.3%) | 17 (42.5%) | 0.27 |
| ISS ≥ 25 n, (%N) | 12 (26.1%) | 7 (17.5%) | 0.34 |
| Head Injury: AIS Head > 0 n, (%N) | 20 (43.5%) | 19 (47.5%) | 0.98 |
| Head AIS, median (IQR) | 0.0 (0, 3) | 0.0 (0, 3) | 0.83 |
| Chest AIS, median (IQR) | 2.0 (0, 3) | 2.0 (0, 3) | 0.66 |
| Abdomen AIS, median (IQR) | 0.0 (0, 2) | 0.0 (0, 2.5) | 0.94 |
| Extremity AIS, median (IQR) | 0.0 (0, 2.8) | 2.0 (0, 3) | 0.25 |
| Prehospital Intubation n, (%N) | 19 (41.3%) | 12 (30.0%) | 0.39 |
| Prehospital Crystalloid (mls), median (IQR) | 675 (0, 1350) | 25 (0, 1000) | 0.11 |
| Prehospital Systolic Blood Pressure, mmHg, median, (IQR) | 71.5 (64, 85) | 70.0 (64, 83) | 0.61 |
| Prehospital Shock Index, median (IQR) | 1.57 (1.4, 1.9) | 1.56 (1.4, 1.9) | 0.67 |
| Received Prehospital Blood Product | 32 (69.6%) | 33 (82.5%) | 0.16 |
| Red Cells | 32 (69.6%) | 5 (12.5%) | <0.01* |
| LTOWB | 0 (0%) | 28 (70%) | <0.01* |
| ED Arrival GCS, median (IQR) | 9 (3, 15) | 13 (3, 15) | 0.44 |
Four patients had combined blunt and penetrating injury
Enrollment for the trial was initiated utilizing two air medical bases (12 months) due to limitations in prehospital LTOWB supply capabilities and competing prehospital interventional trials. Air base participation was expanded to 6 bases during final 8 months of enrollment. The global pandemic of 2020 resulted in cessation of enrollment for a 4-month period when 6 bases were enrolling. We halted the trial early, at 77% of planned enrollment because of financial limitations and slower than expected enrollment for the planned 2-year enrollment pilot trial.
Prehospital providers administered the assigned treatment in 74 of 86 participants (86%). (Table 2.) In the LTOWB randomized group, 12 patients either received red cell transfusion or no blood products prehospital despite meeting all inclusion and no exclusion criteria. Upon trauma center arrival, 9 patients in the standard care randomized group received a single unit of LTOWB before initiating further blood component resuscitation as needed. Overall, there was a protocol deviation in 31/86 (36%) patients.
Table 2.
Enrollment and adherence to protocol For LTOWB and standard care arms
| Enrollment | |
|---|---|
| Months Enrolling | 20 months |
| Enrollment Halted Due to Pandemic | 4 months |
| Air Bases Enrolling | 2 bases-12 months 6 bases- 8 months |
| Number Enrolled | 86 |
| Number Eligible AND Enrolled | 80 |
| Total Patients Entered for Analysis (Intent-To-Treat) | 86 |
| Protocol Adherence | |
| Administration of Assigned Prehospital Randomization Treatment | 74/86 (86.0%) |
| LTOWB randomized group n; (% within randomized group) | |
| Patients did not receive Whole Blood (Prehospital Setting) | 12 (30.0%) |
| - Three patients received packed red cells at outside ED only and stabilized | |
| - Two patients receive packed red cells during transport instead of LTOWB | |
| - Seven patients did not receive LTOWB despite meeting all inclusion criteria | |
| Patients received blood components prior to the planned in-hospital intervention of up to 6 units of LTOWB | 10 (25.0%) |
| Standard Care randomized group n; (% within randomized group) | |
| Patient received In-Hospital Whole Blood (First Four Hours) | 9 (19.6%) |
| - Nine patients received ≤ 1 unit of LTOWB in the ED setting | |
| Total Number of Unique Patients with a Protocol Deviation | 31 (36.0%) |
Outcomes
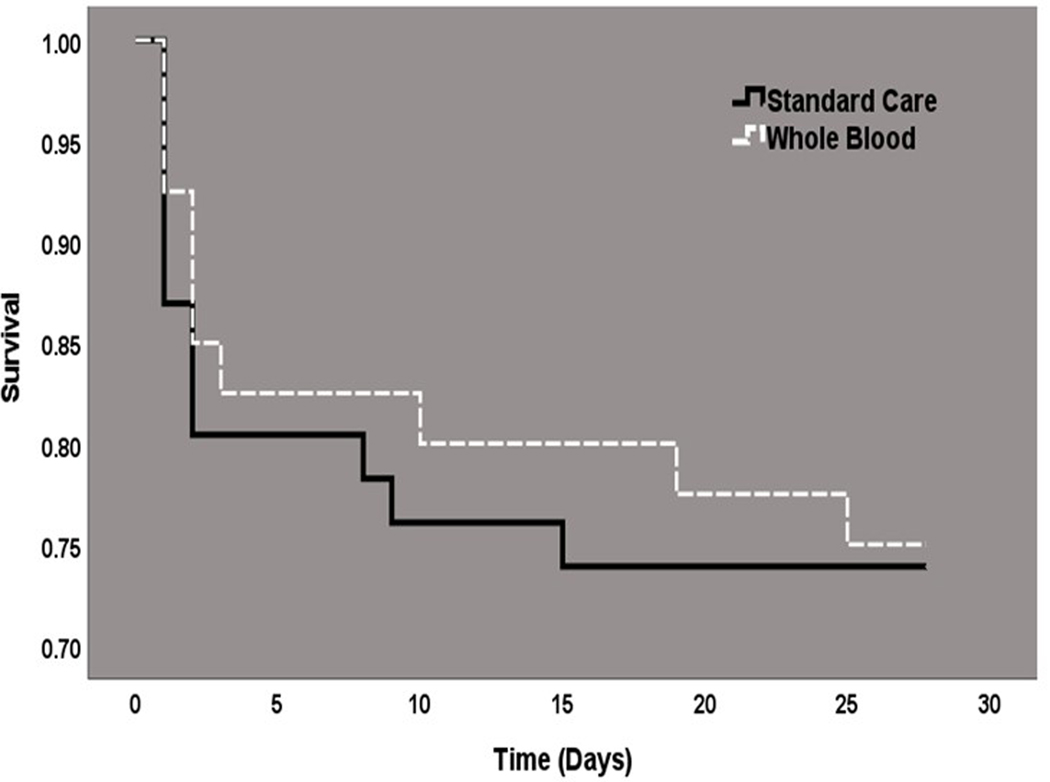
Data for the principal efficacy and safety outcome was available for all 86 patents. At 28-days after randomization, there were 12 deaths in the standard card group and 10 deaths (25.0%) in the LTOWB group (26.1% vs. 25.0%; difference, −1.1%; p=0.91, Table 3.). The Kaplan-Meier survival curves were similar across the standard care and LTOWB groups (log-rank chi-square 0.03, p=0.85, Figure 2.) There were no significant differences in the rate of early mortality (3-hour, 6-hour or 24-hour). (Table 3.) The incidence of multiple organ failure and nosocomial infection were lower in the LTOWB group but this difference did not reach statistical significance. Patients randomized to prehospital LTOWB had significantly lower blood red cell component requirements at 6 hour and 24 hours. Total component transfusion at 24 hours was not statistically different across comparison groups. (1 unit of LTOWB = 1 unit of red cells, 1unit of plasma and 1 unit of random donor platelets for calculation purposes). The number of patients who required red cell transfusion in the first 24 hours of admission was also significantly lower in the LTOWB group, (p = <0.01). Randomized groups had similar indices of shock at admission, the need for operative procedures and ICU and ventilator requirements.
Table 3.
Efficacy and Safety Outcomes
| Standard Care N=46 | LTOWB N=40 | p-value | |
|---|---|---|---|
| Principal Efficacy and Safety Outcome | |||
| 28-day Mortality, n, (%N) | 12 (26.1%) | 10 (25.0%) | 0.91 |
| Secondary Efficacy and Safety Outcomes ‡ | |||
| Early Mortality, n, (%N) | |||
| 3 Hours | 5 (10.9%) | 4 (10.0%) | 0.90 |
| 6 Hours | 6 (13.0%) | 5 (12.5%) | 0.94 |
| 24 Hours | 8 (17.4%) | 6 (15.0%) | 0.76 |
| Acute Respiratory Distress Syndrome n, (%N) | 7 (15.2%) | 7 (17.5%) | 0.73 |
| Multiple Organ Failure n, (%N) | 9 (19.6%) | 4 (10.0%) | 0.23 |
| Nosocomial Infections n, (%N) | 13 (28.3%) | 5 (12.5%) | 0.07 |
| 6-hour In-Hospital Resuscitation Requirements, median (IQR) | |||
| Plasma (units) | 0 (0, 1.3) | 0 (0, 0) | 0.43 |
| Platelets (units) | 0 (0, 0) | 0 (0, 0) | 0.40 |
| Red Cells (units) | 2 (1, 5) | 0 (0, 2.8) | <0.01* |
| Cryoprecipitate (units) | 0 (0, 0) | 0 (0, 0) | 0.78 |
| Whole Blood (Units) | 0 (0, 0) | 0 (0, 1.2) | 0.30 |
| Crystalloids (cc) | 3000 (1462, 4625) | 2227 (1000, 5412) | 0.58 |
| 24-hour In-Hospital Resuscitation Requirements, median (IQR) | |||
| Plasma (units) | 0 (0, 1.3) | 0 (0, 1.8) | 0.91 |
| Platelets (units) | 0 (0, 0) | 0 (0, 0.8) | 0.49 |
| Red Cells (units) | 2.0 (1, 5) | 0.5 (0, 3) | <0.01* |
| Cryoprecipitate (units) | 0 (0, 0) | 0 (0, 0) | 0.71 |
| Whole Blood (units) | 0 (0, 0) | 0 (0, 1.2) | 0.29 |
| Total Blood Component Transfusion (units) | 3.0 (1.8, 7.2) | 1.0 (0, 7.7) | 0.09 |
| Crystalloids (cc) | 3810 (2000, 6442) | 3800 (1250, 7812) | 0.95 |
| Received LTOWB in initial 24 hours n, (%N) | 9 (19.6%) | 11 (27.5%) | 0.38 |
| Received Red Cells in initial 24 hours n, (%N) | 36 (78.3%) | 20 (50.0%) | <0.01* |
| Received Plasma in initial 24 hours n, (%N) | 13 (28.3%) | 11 (27.5%) | 0.93 |
| Received Platelets in initial 24 hours n, (%N) | 8 (17.4%) | 10 (25.0%) | 0.39 |
| Massive Transfusion (10 units in 24 hours) n, (%N) | 4 (8.7%) | 3 (7.5%) | 0.72 |
| Arrival Shock Index, median (IQR) | 0.9 (0.7, 1.4) | 0.9 (0.8, 1.1) | 0.25 |
| Initial Base Deficit, median (IQR) † | 8.0 (5, 11) | 10.0 (4, 13) | 0.36 |
| Initial Lactate Value, median (IQR) †† | 4.1 (2.5, 6) | 4.2 (2.8, 5.5) | 0.94 |
| Operative Procedure in First 24 Hours (%)* | |||
| Thoracotomy/Sternotomy | 1 (2.2%) | 5 (12.5%) | 0.06 |
| Exploratory Laparotomy | 16 (34.8%) | 14 (35.0%) | 0.98 |
| Craniotomy/Craniectomy | 2 (4.3%) | 2 (5.0%) | 0.89 |
| Interventional Radiology/Vascular/Embolization | 4 (8.7%) | 8 (20.0%) | 0.13 |
| Orthopedic | 6 (13.0%) | 6 (15.0%) | 0.79 |
| Other | 15 (32.6%) | 10 (25.0%) | 0.44 |
| ICU days, median (IQR) | 3 (0.2, 9) | 2.5 (1, 8) | 0.63 |
| Ventilator Days, median (IQR) | 2 (1, 6) | 2 (1, 5) | 0.50 |
| Hospital Length of Stay, median (IQR) | 10 (4, 24) | 9 (3, 15) | 0.45 |
All secondary outcome comparisons are exploratory and when adjusting for multiple comparisons no statistical significance is found.
Unavailable in 30 patients-15 from standard care and 15 from LTOWB arm;
Unavailable in 13 patients-7 from standard care are and 6 from LTOWB arm;
Patients can be included in multiple procedure categories;
Figure 2.
Kaplan-Meier 28-day Survival Analysis; (Log-Rank Chi-Square test 0.034; p= 0.85)
Coagulation measurements upon arrival demonstrated a lower INR and lower incidence of acute traumatic coagulopathy (INR > 1.4) in the LTOWB blood group which did not reach statistical significance. (Table 4.) When thromboelastography measurements were compared across groups, no differences were demonstrated. When thromboelastography measurements were dichotomized into abnormal (towards coagulopathy) versus normal, LTOWB patients had a significantly lower rate of abnormal K-time and Maximal Amplitude thromboelastography indices upon arrival. The age of the LTOWB which was transfused during the prehospital phase of the study was 7 days (IQR 4,12).
Table 4.
Arrival Laboratory Measurements of Hemostasis
| Standard Care N=46 | LTOWB N=40 | p-value | |
|---|---|---|---|
| Arrival PT, median (IQR)† | 15.8 (15, 18) | 15.6 (14, 16) | 0.13 |
| Arrival INR, median (IQR)† | 1.3 (1.2, 1.4) | 1.2 (1.1, 1.3) | 0.08 |
| Acute Traumatic Coagulopathy: 6 hour INR > 1.4 (%)† | 22.9% | 6.9% | 0.07 |
| Rapid Thromboelastography Measurements† | |||
| Activated Clotting Time-seconds | 113 (105, 123) | 121 (105, 121) | 0.88 |
| K-time-minutes | 1.5 (1.1, 2.4) | 1.3 (1.2, 1.8) | 0.41 |
| Alpha-angle-degrees | 72.6 (67, 75) | 73.1 (69, 77) | 0.51 |
| Maximal Amplitude-mm | 62.2 (53, 67) | 62.3 (58, 67) | 0.58 |
| LY30-% | 1.0 (0.6, 2.2) | 0.7 (0.3, 1.6) | 0.44 |
| Abnormal (coagulopathic) Rapid Thromboelastography Measurements† | |||
| Activated Clotting Time > 128 seconds (%) | 22.7% | 21.7% | 0.93 |
| K-time > 2.5 minutes (%) | 17.4% | 0.0% | 0.04* |
| Alpha-angle < 60° (%) | 14.8% | 3.8% | 0.17 |
| Maximal Amplitude < 55mm (%) | 25.0% | 4.6% | 0.04* |
| LY30 > 3.0 percent (%) | 6.7% | 5.6% | 0.89 |
Unavailable in 22 patients, 11 from each arm;
Unavailable in 32 patients, 18 from Standard Care and 14 from LTOWB
We observed no documented cases of transfusion reactions or hemolytic complications throughout the enrollment period of the trial. (Table 5) The number of adverse events was similar across the two randomized groups and relatively low despite the magnitude of the injury severity, further demonstrating the safety profile of LTOWB.
Table 5.
Adverse Events/Complications, n (%N) which were designated serious while excluding those events that were definitively not related to trial participation.
| Standard Care N=46 | Whole Blood N=40 | |
|---|---|---|
| Transfusion/Hemolytic Reactions | 0 (0%) | 0 (0%) |
| Acute Respiratory Distress Syndrome | 1 (2.2%) | 3 (7.5%) |
| Cardiac Arrest | 6 (13%) | 5 (12.5%) |
| Cerebral Infarction | 3 (6.5%) | 1 (2.5%) |
| Deep Vein Thrombosis | 3 (6.5%) | 1 (2.5%) |
| Pulmonary Embolus | 1 (2.2%) | 1 (2.5%) |
| Rhabdomyolysis | 2 (4.3%) | 1 (2.5%) |
| Upper Gastrointestinal Bleeding | 1 (2.2%) | 0 (0%) |
| Hyponatremia | 1 (2.2%) | 0 (0%) |
| Patients with an Adverse Event | 15 (32.6%) | 12 (30.0%) |
Discussion
Trauma resuscitation has significantly evolved over the last two decades with a focus on prevention of coagulopathy through minimization of crystalloid-based resuscitation and initiation of early ratio-based ‘damage control resuscitation’ once a patient arrives at definitive trauma care.3, 32, 33 Despite these improvements, patients continue to suffer high rates of mortality due to hemorrhage in the first few hours of arrival.1–4 Increased attention on improving the consequences of hemorrhage and reducing the early deaths from hemorrhage is highlighted by a recent call for zero preventable deaths by the National Academies of Sciences, Engineering, and Medicine.34 Whole-blood transfusion for the treatment of hemorrhagic shock and coagulopathy has a long history in military medicine. Whole blood resuscitation has increasingly been utilized in the civilian setting over the last five years and in-hospital low-titer anti-A and anti-B group O whole blood is now considered standard care at over 70 high-volume trauma centers across the Uni ted States.12, 19, 24
Providing whole blood in the prehospital setting for hemorrhagic shock is appealing and a logical extension of the survival benefits demonstrated for individual blood components when provided early, close to the time of injury, in the prehospital setting.5, 6 The current high-level data regarding the safety and efficacy of whole blood in the prehospital setting is limited and a definitive trial is needed to justify the use of this precious resource early post-injury.
The results of the current whole blood pilot study provide important insights regarding the difficulties of prehospital interventional trials in patients at risk of hemorrhagic shock yet highlights the potential for outcome benefits for this severely injured cohort with appropriate trial execution. A host of mitigating factors resulted in a slower than planned study enrollment including changes in standard care at our institution and a limited whole blood supply, competing prehospital trials and a halt in enrollment due to the global pandemic. The current prehospital inclusion and exclusion criteria employed for the study were based upon identical criteria used from a recent trial which focused on prehospital plasma resuscitation.6 Identical and possibly more robust education and training of prehospital personnel was performed for the current study. Despite these similarities, the current pilot study was complicated by a low rate of protocol adherence in the prehospital setting. These results suggest that initiation of prehospital blood transfusion as compared to prehospital plasma transfusion may necessitate a higher threshold of patient acuity or injury/shock severity in the eyes of prehospital personnel. Of equal significance, continuation of randomized assignment during the in-hospital phase of care, particularly for those assigned to the standard care group, was also limited by poor protocol adherence due to a well engrained early LTOWB resuscitation program at our hospital. This occurred even though ample training and education of surgical care providers was performed. Despite the randomization assignment crossover, the current results do suggest a lower rate and volume of red cell transfusion and improved hemostatic metrics for patients who were randomized to whole blood. The results suggest trends toward lower nosocomial infection and acute traumatic coagulopathy for those who were randomized to prehospital whole blood. These clinical outcome differences are exploratory in nature and are not powered for definitive comparison. It may be that cohort or injury severity differences are responsible for these transfusion volume differences. It is interesting that with improved protocol adherence and randomization assignment, these results could possibly be magnified. (Supplemental Table 1.) Importantly, the current pilot trial results provide no evidence for safety concerns associated with prehospital whole blood with no documented hemolytic/transfusion reactions and no differences in the rate of adverse events.
There are limitations to the current pilot clinical trial. First, it is a single institution cluster randomized pilot trial that was not adequately powered for definitive clinical outcome comparison. Prehospital and early hospital resuscitation practices vary across the country and the generalizability of any study findings is limited. Due to the small sample size and lower than expected protocol adherence, comparison arm differences may exist which may confound any outcome differences demonstrated. The study suffered from slower than expected enrollment and any outcome differences may be the result of confounding due to poor protocol adherence and small sample size. Single institutional protocols and practice may limit the generalizability and applicability at other institutions. Due to the pilot design of the study we did not correct for multiple comparisons and all secondary outcomes are exploratory and would not reach statistically significance if multiple comparisons were adjusted for. Differences in prehospital resuscitation such as reduced crystalloid relative to LTOWB may be an important factor driving hemostatic differences seen and outcomes in future trials. Blood and blood component comparisons or other clinical outcomes in the current pilot trial are not appropriately powered or appropriate for definitive conclusions and should be considered speculative. The low protocol adherence further confounds any outcome differences demonstrated. Transfer origin (scene versus referral emergency department) may be associated with survival biases and may confound any conclusions formulated. (Supplemental Table 2.) The study sample size limits the ability to account for or appropriate adjust for survival bias. The results do provide insight into the feasibility of a larger prehospital initiated whole blood trial. Based upon the current results, a future trial would necessitate additional inclusion criteria including the requirement for the initiation/need of prehospital blood transfusion whether it be whole blood or standard care. Additionally, the prehospital through in-hospital randomization resulted in additional crossover. Future clinical trials may benefit from a prehospital intervention alone as increasing evidence suggests that early prehospital interventions may have the most robust effect.9, 10, 35
In conclusion, prehospital through in-hospital LTOWB resuscitation is safe and may possibly associated with hemostatic benefits. A large-scale prehospital clinical trial is feasible with appropriate design adjustments and would allow the effects of whole blood on survival and other pertinent clinical outcomes to be appropriately characterized. A large definitive prehospital whole blood trial would justify and provide the impetus for whole blood incorporation into standard prehospital care when feasible.
Supplementary Material
Acknowledgments
Funding: This work was funded by NIH NHLBI R34HL135224, UL1 TR001857, KL2 TR001856, TL1 TR001858
Footnotes
Supplemental Digital Content: Per protocol abbreviated group comparison; Outcomes across scene and referral emergency department comparison; CONSORT for Pilot and Feasibility Trials Checklist
Conflicts of Interest: The authors have no conflicts of interest to declare
Level of Evidence- II
Cluster randomized pilot trial
References
- 1.Fox EE, Holcomb JB, Wade CE, Bulger EM, Tilley BC, Group PS. Earlier Endpoints are Required for Hemorrhagic Shock Trials Among Severely Injured Patients. Shock. 2017;47(5):567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvin JA, Wray CJ, Steward J, Lawless RA, McNutt MK, Love JD, Moore LJ, Wade CE, Cotton BA, Holcomb JB. Control the damage: morbidity and mortality after emergent trauma laparotomy. Am J Surg. 2016;212(1):34–9. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee P, Joseph B, Pandit V, Aziz H, Vercruysse G, Kulvatunyou N, Friese RS. Increasing trauma deaths in the United States. Ann Surg. 2014;260(1):13–21. [DOI] [PubMed] [Google Scholar]
- 5.Shackelford SA, Del Junco DJ, Powell-Dunford N, Mazuchowski EL, Howard JT, Kotwal RS, Gurney J, Butler FK Jr., Gross K, Stockinger ZT Association of Prehospital Blood Product Transfusion During Medical Evacuation of Combat Casualties in Afghanistan With Acute and 30-Day Survival. JAMA. 2017;318(16):1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018;379(4):315–26. [DOI] [PubMed] [Google Scholar]
- 7.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, Bulger EM, Idris AH, Christenson J, Morrison LJ, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA. 2020;324(10):961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li SR, Guyette F, Brown J, Zenati M, Reitz KM, Eastridge B, Nirula R, Vercruysse GA, O’Keeffe T, Joseph B, et al. Early Prehospital Tranexamic Acid Following Injury Is Associated With a 30-day Survival Benefit: A Secondary Analysis of a Randomized Clinical Trial. Ann Surg. 2021;274(3):419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruen DS, Guyette FX, Brown JB, Okonkwo DO, Puccio AM, Campwala IK, Tessmer MT, Daley BJ, Miller RS, Harbrecht BG, et al. Association of Prehospital Plasma With Survival in Patients With Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial. JAMA Netw Open. 2020;3(10):e2016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler FK Jr., Holcomb JB, Shackelford S, Barbabella S, Bailey JA, Baker JB, Cap AP, Conklin CC, Cunningham CW, Davis M, et al. Advanced Resuscitative Care in Tactical Combat Casualty Care: TCCC Guidelines Change 18–01:14 October 2018. J Spec Oper Med.18(4):37–55. [DOI] [PubMed] [Google Scholar]
- 12.Leeper CM, Yazer MH, Neal MD. Whole-Blood Resuscitation of Injured Patients: Innovating from the Past. JAMA Surg. 2020;155(8):771–2. [DOI] [PubMed] [Google Scholar]
- 13.Dishong D, Cap AP, Holcomb JB, Triulzi DJ, Yazer MH. The rebirth of the cool: a narrative review of the clinical outcomes of cold stored low titer group O whole blood recipients compared to conventional component recipients in trauma. Hematology. 2021;26(1):601–11. [DOI] [PubMed] [Google Scholar]
- 14.Morgan KM, Yazer MH, Triulzi DJ, Strotmeyer S, Gaines BA, Leeper CM. Safety profile of low-titer group O whole blood in pediatric patients with massive hemorrhage. Transfusion. 2021;61 Suppl 1:S8–S14. [DOI] [PubMed] [Google Scholar]
- 15.Yazer MH, Freeman A, Harrold IM, Anto V, Neal MD, Triulzi DJ, Sperry JL, Seheult JN. Injured recipients of low-titer group O whole blood have similar clinical outcomes compared to recipients of conventional component therapy: A single-center, retrospective study. Transfusion. 2021;61(6):1710–20. [DOI] [PubMed] [Google Scholar]
- 16.Harrold IM, Seheult JN, Alarcon LH, Corcos A, Sperry JL, Triulzi DJ, Yazer MH. Hemolytic markers following the transfusion of uncrossmatched, cold-stored, low-titer, group O+ whole blood in civilian trauma patients. Transfusion. 2020;60 Suppl 3:S24–S30. [DOI] [PubMed] [Google Scholar]
- 17.Seheult JN, Anto V, Alarcon LH, Sperry JL, Triulzi DJ, Yazer MH. Clinical outcomes among low-titer group O whole blood recipients compared to recipients of conventional components in civilian trauma resuscitation. Transfusion. 2018;58(8):1838–45. [DOI] [PubMed] [Google Scholar]
- 18.Clements T, McCoy C, Assen S, Cardenas J, Wade C, Meyer D, Cotton BA. The prehospital use of younger age whole blood is associated with an improved arrival coagulation profile. J Trauma Acute Care Surg. 2021;90(4):607–14. [DOI] [PubMed] [Google Scholar]
- 19.McCoy CC, Cotton BA, Brenner M, Roberts D, Ferrada P, Horer T, Kauvar D, Khan M, Kirkpatrick A, Ordonez C, et al. Back to the Future: Whole Blood Resuscitation of the Severely Injured Trauma Patient. Shock. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahbar E, Cardenas JC, Matijevic N, Del Junco D, Podbielski J, Cohen MJ, Cotton BA, Holcomb JB, Wade CE, Early Whole Blood I. Trauma, Time, and Transfusions: A Longitudinal Analysis of Coagulation Markers in Severely Injured Trauma Patients Receiving Modified Whole Blood or Component Blood Products. Shock. 2015;44(5):417–25. [DOI] [PubMed] [Google Scholar]
- 21.Williams J, Merutka N, Meyer D, Bai Y, Prater S, Cabrera R, Holcomb JB, Wade CE, Love JD, Cotton BA. Safety profile and impact of low-titer group O whole blood for emergency use in trauma. J Trauma Acute Care Surg. 2020;88(1):87–93. [DOI] [PubMed] [Google Scholar]
- 22.Cotton BA, Podbielski J, Camp E, Welch T, del Junco D, Bai Y, Hobbs R, Scroggins J, Hartwell B, Kozar RA, et al. A randomized controlled pilot trial of modified whole blood versus component therapy in severely injured patients requiring large volume transfusions. Ann Surg. 2013;258(4):527–32; discussion 32–3. [DOI] [PubMed] [Google Scholar]
- 23.Assen S, Cardenas J, George M, Wang YW, Wade CE, Meyer D, Cotton BA. Hemostatic potential of cold-stored non-leukoreduced whole blood over time: An assessment of platelet function and thrombin generation for optimal shelf life. J Trauma Acute Care Surg. 2020;89(3):429–34. [DOI] [PubMed] [Google Scholar]
- 24.Pivalizza EG, Stephens CT, Sridhar S, Gumbert SD, Rossmann S, Bertholf MF, Bai Y, Cotton BA. Whole Blood for Resuscitation in Adult Civilian Trauma in 2017: A Narrative Review. Anesth Analg. 2018;127(1):157–62. [DOI] [PubMed] [Google Scholar]
- 25.Shea SM, Staudt AM, Thomas KA, Schuerer D, Mielke JE, Folkerts D, Lowder E, Martin C, Bochicchio GV, Spinella PC. The use of low-titer group O whole blood is independently associated with improved survival compared to component therapy in adults with severe traumatic hemorrhage. Transfusion. 2020;60 Suppl 3:S2–S9. [DOI] [PubMed] [Google Scholar]
- 26.Vanderspurt CK, Spinella PC, Cap AP, Hill R, Matthews SA, Corley JB, Gurney JM. The use of whole blood in US military operations in Iraq, Syria, and Afghanistan since the introduction of low-titer Type O whole blood: feasibility, acceptability, challenges. Transfusion. 2019;59(3):965–70. [DOI] [PubMed] [Google Scholar]
- 27.Nadler R, Tsur AM, Yazer MH, Shinar E, Moshe T, Benov A, Glassberg E, Epstein D, Chen J. Early experience with transfusing low titer group O whole blood in the pre-hospital setting in Israel. Transfusion. 2020;60 Suppl 3:S10–S6. [DOI] [PubMed] [Google Scholar]
- 28.Spinella PC, Gurney J, Yazer MH. Low titer group O whole blood for prehospital hemorrhagic shock: It is an offer we cannot refuse. Transfusion. 2019;59(7):2177–9. [DOI] [PubMed] [Google Scholar]
- 29.Braverman MA, Smith A, Pokorny D, Axtman B, Shahan CP, Barry L, Corral H, Jonas RB, Shiels M, Schaefer R, et al. Prehospital whole blood reduces early mortality in patients with hemorrhagic shock. Transfusion. 2021;61 Suppl 1:S15–S21. [DOI] [PubMed] [Google Scholar]
- 30.Holcomb JB, Weiskopf R, Champion H, Gould SA, Sauer RM, Brasel K, Bochicchio G, Bulger E, Cotton BA, Davis D, et al. Challenges to effective research in acute trauma resuscitation: consent and endpoints. Shock. 2011;35(2):107–13. [DOI] [PubMed] [Google Scholar]
- 31.Seheult JN, Bahr M, Anto V, Alarcon LH, Corcos A, Sperry JL, Triulzi DJ, Yazer MH. Safety profile of uncrossmatched, cold-stored, low-titer, group O+ whole blood in civilian trauma patients. Transfusion. 2018;58(10):2280–8. [DOI] [PubMed] [Google Scholar]
- 32.Harris T, Davenport R, Mak M, Brohi K. The Evolving Science of Trauma Resuscitation. Emerg Med Clin North Am. 2018;36(1):85–106. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–10. [DOI] [PubMed] [Google Scholar]
- 34.Berwick DM, Downey AS, Cornett E, National Academies of Sciences Engineering and Medicine (U.S.). Committee on Military Trauma Care’s Learning Health System and Its Translation to the Civilian Sector. A national trauma care system : integrating military and civilian trauma systems to achieve zero preventable deaths after injury. Washington, DC: The National Academies Press; 2016. xxxix, 490 pages p. [PubMed] [Google Scholar]
- 35.Gruen DS, Brown JB, Guyette FX, Vodovotz Y, Johansson PI, Stensballe J, Barclay DA, Yin J, Daley BJ, Miller RS, et al. Prehospital plasma is associated with distinct biomarker expression following injury. JCI Insight. 2020;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.