Abstract
Background:
Current surgical guidelines for the treatment of intra-abdominal sepsis recommend interventional source control as the key element of therapy, alongside resuscitation and antibiotic administration. Past trials attempted to predict the success of interventional source control to assess whether further interventional therapy is needed. However, no predictive score could be developed.
Materials and methods:
We utilized an established murine abdominal sepsis model, the cecal ligation and puncture (CLP), and performed a successful surgical source control intervention after full development of sepsis, the CLP-excision (CLP/E). We then sought to evaluate the success of the source control by characterizing circulating neutrophil phenotype and functionality 24 hours post intervention.
Results:
We showed a significant relative increase of neutrophils and a significant absolute and relative increase of activated neutrophils in septic mice. Source control with CLP/E restored these numbers back to baseline. Moreover, main neutrophil functions, the acidification of cell compartments, such as lysosomes, and the production of Tumor Necrosis Factor alpha (TNF-α), were impaired in septic mice but restored after CLP/E intervention.
Conclusion:
Neutrophil characterization by phenotyping and evaluating their functionality indicates successful source control in septic mice and can serve as a prognostic tool. These findings provide a rationale for the phenotypic and functional characterization of neutrophils in human patients with infection. Further studies will be needed to determine whether a predictive score for the assessment of successful surgical source control can be established.
Keywords: Sepsis, intra-abdominal infection, source control, neutrophil, TNF-α, acidification
Introduction
Despite significant international research efforts to improve sepsis treatment, the mortality rate of sepsis remains unchanged over recent years, while case numbers continue to climb [1]. Multiple studies indicate that abdominal infection persists as a major source of sepsis [2].
If patients develop a complicated intraabdominal infection, characterized by localized or diffuse peritonitis, current guidelines recommend fluid resuscitation, surgical source control and antibiotic therapy [3]. The aim of source control intervention is to debride the source of infection, reduce the bacterial burden and surgically correct end-organ injury to assure physiologic functionality [4]. A prospective observational clinical study revealed that source control in patients with severe sepsis and septic shock significantly reduces mortality, regardless of age and major organ dysfunction [5].
Another current study defined successful sepsis intervention as the lack of evidence of infection in the clinical chart or imaging on day 14, with no further interventions were required [6]. In the study, roughly half of the intensive care unit (ICU) patients with an intrabdominal infection had to undergo multiple interventions before source control was considered successful. The same study found organ failure associated with insufficient source control at day 14 [6]. This highlights the need for a predictive scoring system which evaluates the success of the source control intervention. To date, studies have failed to provide clinical or immunological scores to determine sufficient source control intervention [6,7].
We sought to assess the effectiveness of surgical source control in an established murine sepsis model, the cecal ligation and puncture (CLP), by conducting a surgical debridement, peritoneal wash and the administration of proper antibiotics, a previously validated source-control model termed CLP-excision (CLP/E) [8,9]. We characterized the phenotypic and functional changes of circulating neutrophils to gauge the effectiveness of surgical source control. Neutrophils are key components of the immune defense in the initial sepsis phase [10], as they are the first to migrate to inflamed and infected tissues to eliminate pathogens. Their maturity and functionality can be assessed by the expression of CD54 (Intracellular Adhesion Molecule-1 [ICAM-1}) and CD62L (L-Selectin) [11–13]. Further, Toll-Like Receptor-4 (TLR4)-expressing cells have the ability to produce pro-inflammatory cytokines, such as TNF-α, driven by pathogen-associated molecular patterns (PAMPs), such as Lipopolysaccharide (LPS). We hypothesize that these phenotypical and functional changes of neutrophils can be used to assess the success of the source control in murine abdominal sepsis.
Material and Methods
Animal models
Outbred, male CD-1 IGS mice were purchased from Charles River (Wilmington, MA, USA). All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati (IACUC protocol no: 08–09-19–01).
Cecal ligation and puncture and source control
We performed CLP as described previously [15] by ligating 2/3 of the cecum and puncturing it once with an 18-Gauge needle. The mice were anesthetized using 2.5% isoflurane in oxygen applied via face mask. The abdomen was shaved and disinfected with povidone-iodine and alcohol. An incision was made to access the peritoneal cavity. The cecum was ligated, punctured and replaced intraabdominally, and the incision closed in a two-layer suture. The mice were resuscitated with 1 ml of 0.9% saline solution (Hospira, Lake Forest, IL) subcutaneously and rested on a heating pad for one hour. For those mice that underwent source control by CLP-excision, we disinfected the abdomen as described above, surgically re-opened the applied sutures to access the abdomen after 3 hours. We then completely debrided the ligated portion of the cecum, conducted a peritoneal wash using pre-warmed 0.9% saline solution (Hospira, Lake Forest, IL), administered Imipenem / Cilastatin (Primaxin®) (2.5mg/kg) antibiotics into the peritoneal cavity and closed the incision in a two-layer suture and let the mice rest on the heating pad for one additional hour. Healthy, CLP- and source-controlled CLP/E-mice were euthanized after 24 hours and whole blood was harvested via post mortem cardiac puncture. Blood bacteria was determined using soy tryptic agar plates (BBL™ Stacker Plate™, Becton Dickinson, MD, USA).
Monitoring of the body core temperature post CLP intervention
An Anipill temperature-monitoring implant (Data Science International, New Brighton, MN) was inserted into the abdomen during the CLP surgery. Core temperature was recorded electronically every 15 minutes until the mice were euthanized.
LPS stimulation of bone marrow cells
The femur and tibia were flushed to harvest bone marrow. After counting, two million cells of the suspension were plated per well, stimulated with 100 ng/mL LPS (Escherichia coli 0111:B4, Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37°C at 5% CO2 for 24 hours, before being labelled and analyzed via flow cytometry.
Labeling and characterization of neutrophils using flow cytometry
The whole blood white blood cell (WBC) count with differential was assessed using an automated cell counter (Act10, Beckman Coulter, Brea, CA). Red blood cells were lysed for 5 minutes with 2 mL of Ammonium-Chloride-Potassium (ACK) lysing buffer, washed, and labeled for flow cytometry analysis. Cells were treated with Fc-receptor blockage prior to labeling with CD16/CD32 (Mouse BD Fc Block™) (clone 2.4G2 (RUO), BD Pharmingen, San Jose, CA, USA) and 5% rat serum (Invitrogen, Carlsbad, CA) for 10 minutes. Subsequently, labelling antibodies were incubated for another 20 minutes. The cells were washed and analyzed using an Attune® NxT™ Acoustic Focusing Cytometer (Thermo Fisher Scientific, Waltham, MA).
The following fluorescent-labeled antibodies were used for surface and intracellular labeling: Ly6G (clone: 1A-8), Ly6C (clone: AL-21), CD54 (ICAM-1) (clone: 3E2), L-Selectin (CD62L) (clone: MEL-14) all from BD Biosciences, San Jose, CA. Neutrophil (Ly-6G+/Ly-6C+) subsets were characterized using CD62L (L-Selectin) and CD54 (ICAM-1). The CD54(ICAM-1)-/CD62L+ neutrophil phenotype is considered to characterize naïve neutrophils, whereas the CD54(ICAM-1)+/CD62L- neutrophils are considered activated [11,12].
Functional assessment of neutrophils: acidification of neutrophil cell compartments (pHrodo assay)
Particles labelled with pH-sensitive dyes (pHrodo Green Escherichia coli BioParticles (Invitrogen, MA, USA)) were prepared according to the manufacturers protocol and incubated with neutrophils at 37°C, 5% CO2 for 1 hour. The phagocytic uptake of these particles was then interrupted by placing the cells on ice and fixing them with 1% paraformaldehyde (PFA). Subsequently, cells were kept on ice, washed and labelled for flow cytometry as described above. The acidification of internal cell lysosomes was analyzed via flow cytometry for the median fluorescent intensity (MFI).
Functional assessment of TNF-α production
For the ELISPOT functional assay, 50,000 WBC were plated on capture plates from the manufacturer Cellular Technology Limited (Cleveland, OH). These cells were incubated with or without 100ng / mL LPS and harvested after 24 hours. The production of TNF-α was analyzed according to the manufacturer’s instructions. The spot number indicates how many cells produced TNF-α and the spot size how much TNF-α was produced by each cell.
Statistical analyses
The data was statistically analyzed using GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA). To identify and remove outliers the ROUT method (Q=1%) was used, however, no outliers were found necessary to be eliminated by that method. The groups were tested for normality using the Shapiro-Wilk, the DÀgostino and Pearson normality test. If groups were considered normally distributed, differences were analyzed using a two tailed Student’s t-test comparison of two groups or one-way ANOVA with Tukey post-hoc analysis for comparisons of more than two groups. If the groups were not normally distributed, differences were analyzed using a Mann-Whitney test to compare two groups or Kruskal-Wallis test with Dunn`s multiple-comparison test analysis for comparisons of more than two groups. The data is depicted in bars with the mean ± standard deviation or as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. A p-value of ≤0.05 was considered statistically significant.
Results
Justification for time of surgical source control
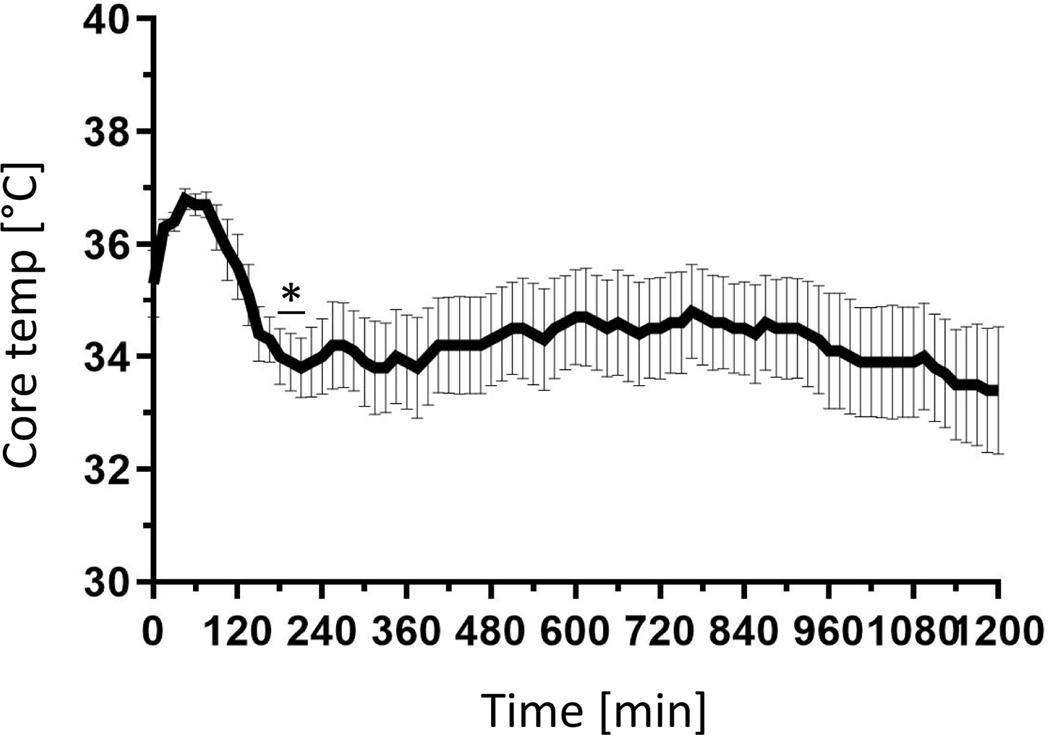
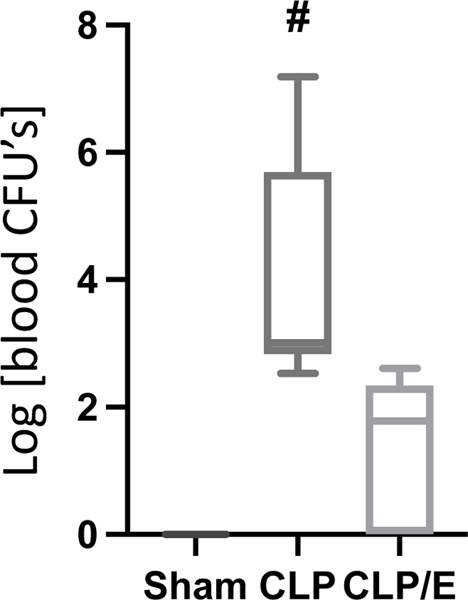
To evaluate the effectiveness of surgical source control, we had to first determine a proper time point at which there are overt signs of sepsis, which in murine populations includes a significantly decreased body temperature [16]. We hypothesized that the core body temperature of the mice dropping significantly after CLP intervention would be an indicator of overt sepsis, and an accurate timepoint marking the onset of sepsis in which to institute source control (CLP/E). After roughly 3 hours (180 – 210 min), we observed a significant decrease of the core body temperature (Fig. 1) and chose this as the timepoint for source control intervention (CLP/E). Secondly, we had to determine if the source control by CLP/E intervention was clinically successful. As the aim of source control is to reduce the bacterial burden [4] we hypothesized that a significant decrease in blood bacteria would be a clinical indication for effective source control in our CLP/E model. After 24 hours, we observed a significant reduction of the systemic bacterial burden. An approximate 3-log decrease in blood colony forming units (CFU’s) represents a 99.9% clearance and effective source control (Fig. 2). Survival studies have been previously conducted to verify source control, but introduce a survivor bias into the septic group, and were not utilized in this model [9].
Figure 1: Hypothermia is observed within three hours of cecal ligation and puncture.
Mice underwent surgical cecal ligation and puncture (CLP) (n=8). A temperature recording implant was placed in the abdomen and the time was recorded electronically every 15 min as described in the methods. The sample size = 8. The data are expressed as mean ± SD. *, p < 0.05 as compared to pre-surgical temperatures.
Figure 2: Source control reduces systemic bacterial load.
Mice underwent sham (n=5) or CLP (n=6) treatment. Source control (CLP/E) was performed after 3 h (n=5) as described in the methods. After 24h the mice were euthanized and CFU from whole blood was determined as described in the methods. Data are presented as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. # p<0.05 vs sham and CLP/E.
Appropriate Source Control reduces activated neutrophils to that of healthy controls
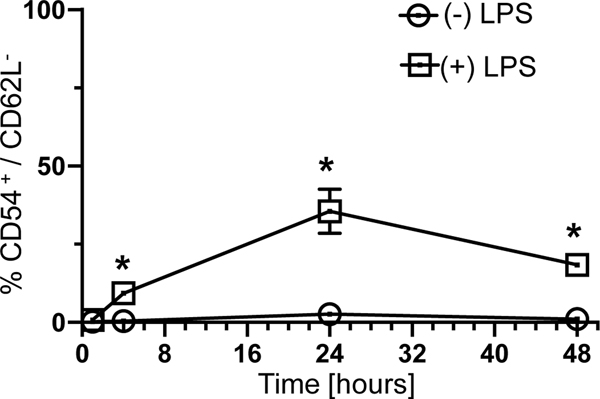
It has been previously published that neutrophils can be divided into subtypes by phenotyping them using the expression of CD54 (ICAM-1) and CD62L (L-Selectin), also reflecting a changed functional behavior of these subtypes [11–13]. CD54(ICAM-1)-/CD62L+ neutrophils can be considered naive and CD54(ICAM-1)+/CD62L- neutrophils, activated [11–13]. We hypothesized that an ex-vivo LPS challenge would lead to an increase of the proportion of activated neutrophils. Measuring the share of CD54(ICAM-1)+/CD62L- from bone marrow derived neutrophils challenged by LPS at 1, 4, 24 and 48 hours revealed a significant relative increase as soon as 4 hours (Fig. 3). We concluded that a phenotypic maturation of neutrophils, noticeable by an increased share of CD54(ICAM-1)high/CD62Llow neutrophils, can be potentially detected as early as 4 hours and is sustained to at least 48 hours.
Figure 3: Lipopolysaccharide stimulation of neutrophils leads to an activated phenotype within four hours.
Bone marrow derived neutrophils were incubated for 1, 4, 24 and 48 hours in cell culture media with or without LPS (1h: n=14, 4h: n=14, 24h: n=14, 48h: n=5). The cells were analyzed for CD54 (ICAM-1) and CD62L (L-Selectin) as described in the methods. Data are presented as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. * p<0.05 vs timepoint 0 min.
Neutrophil phenotype and proportions are indicators of source control
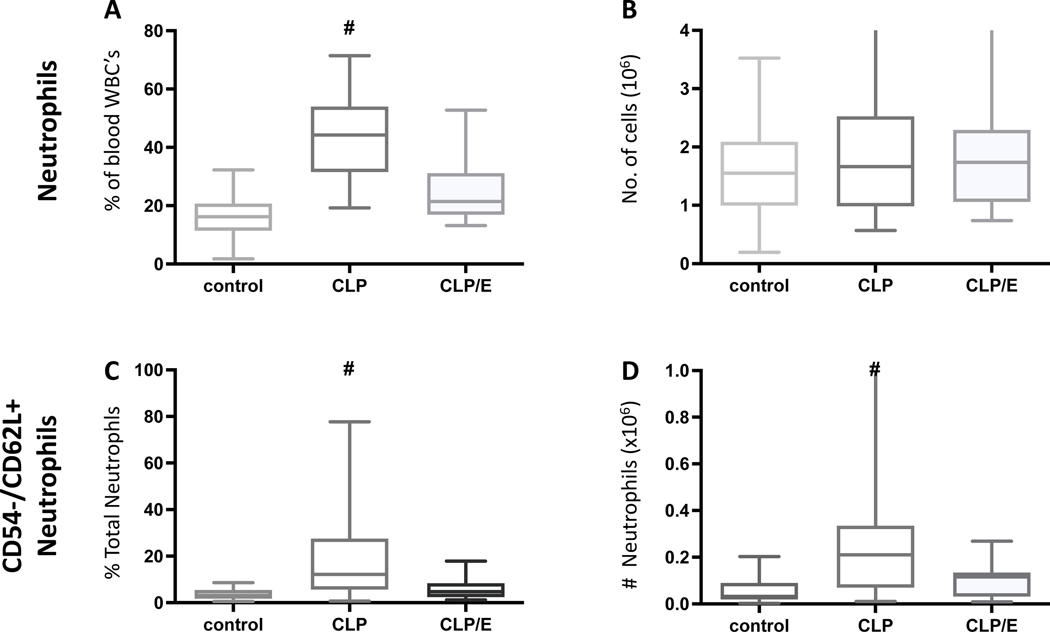
We next hypothesized that analyzing neutrophils from healthy, septic and source-controlled mice may indicate the effectiveness of the source control. First, we observed a significant increase of neutrophil proportions in the whole blood of septic mice. This proportion returned to that of healthy control levels after successful source control 18 hours earlier (Fig. 4A). In contrast, total neutrophil numbers are unchanged (Fig. 4B). The proportion and number of the activated neutrophils significantly increased in septic mice (Fig. 4C,D). Importantly, these increases were not observed in circulating neutrophils that had undergone effective source control.
Figure 4: Changes in neutrophil proportion, numbers and phenotype indicate effective source control.
Mice underwent sham treatment (n=36) or CLP procedure with (n=18) and without (n=33) surgical source control (CLP/E) after 3h as described in the method section (A-D). After 24 hours post CLP intervention, whole blood analyzed by flow cytometry. Neutrophil A) proportion and B) numbers were determined. were identified as Ly6G positive cells and their relative and absolute number was assessed (A, B). The activated neutrophil C) proportion and D) number were determined. Data are presented as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. # p<0.05 vs sham and CLP/E.
Effective Source control enhances cellular functionality
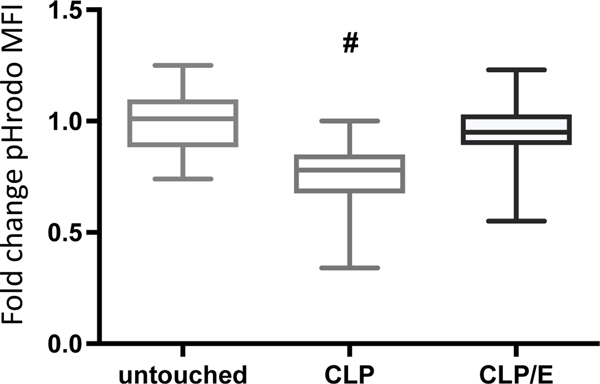
The use of single biomarkers has been noted to be limited to determine the host immune status during sepsis [19,20]. Functional immunologic assays a contribute to a better understanding of the current immunological state of septic patients [21]. We hypothesized that cellular functionality, in addition to phenotypic changes such as those described above, can be utilized as an indicator of source control adequacy. Using a phagocytosis assay that allows for neutrophil specificity, we observed a decreased functionality in neutrophils from septic mice (Fig. 5). However, 18 hours after effective source control, neutrophil phagocytosis was not significantly altered compared to healthy controls.
Figure 5: Neutrophils from septic mice have impair phagocytosis.
Mice underwent sham treatment (n=32), surgical CLP injury (n=25) or CLP injury with subsequent surgical source control (CLP/E) after 3h (n=20). After 24h post injury, whole blood was harvested and the cells were incubated with particles labelled with pH-sensitive dyes, as described in the method section. The acidification of the internal cell compartments was analyzed via flow cytometry. Increased acidification correlates with an increased median fluorescent intensity (MFI) of the ingested pH-sensitive particles. Data are presented as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. # p<0.05 vs sham and CLP/E.
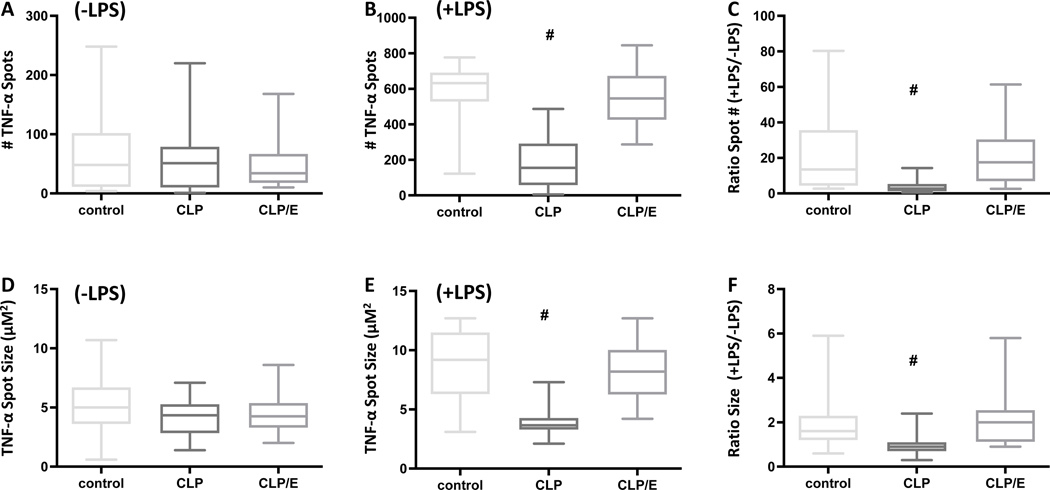
One of the critical cytokines produced by TLR-4 expressing cells is TNF-α. We observed no changes in spontaneous (without exogenously adding LPS) cellular TNF-α production as measured by spot numbers and size amongst the three cohorts analyzed (Fig. 6A, 6D). In contrast, the cellular numbers and quantity that produce TNF-α upon LPS stimulation were significantly decreased in septic mice. However, effective source control ameliorated this effect (Fig. 6B, 6E). Finally, due to the potential heterogeneity between immune systems, we hypothesized that a ratio of spontaneous and LPS-driven TNF-α spot numbers or size would be a useful read-out to determine effective source control. Here, we observed that this ratio, using both spot numbers and size, decreased in cells from septic mice. This decrease was mitigated analyzing cells from mice with effective source control. Altogether, cellular functionality is decreased in septic mice. However, this blunting is reversed as soon as 18 hours after effective source control.
Figure 6: Increased cellular generation of TNF-α production indicates effective source control.
Mice were sham treated (n=23), underwent surgical CLP injury (n=19) or CLP injury following surgical source control (CLP/E) after 3h (n=20). Whole blood was harvested after 24h post injury. Cellular TNF-α spot numbers were analyzed using ELISPOT A) (−) 100 ng / mL LPS, B) (+) 100 ng / mL LPS, and C) as a ratio of +LPS/-LPS. Cellular TNF-α spot size was analyzed using ELISPOT D) (−) 100 ng / mL LPS, E) (+) 100 ng / mL LPS, and F) as a ratio of +LPS/-LPS. Data are presented as a box plot covering the first, second (median), third quartile, and 5th + 95th percentile. # p<0.05 vs sham and CLP/E.
Discussion
Current surgical guidelines for the treatment of complicated intraabdominal infection and abdominal sepsis recommend fluid resuscitation, surgical source control and antibiotic therapy [3,22,23]. The need for complete debridement of necrotic tissue and infectious foci is undisputed [3,22,23]. Inadequate source control is associated with increased mortality in abdominal sepsis [6]. Additionally, a recent prospective, multicenter, randomized controlled study suggested that the antibiotic therapy is less relevant than adequate source control. No differences could be found in the outcome of short and long-term antimicrobial therapy in patients with septic complicated intra-abdominal infection, when adequate source control was conducted [24]. This led us to the assumption that the eradication of pathogens depends more on the ability of the immune system to eradicate bacteria then on the supportive antimicrobial therapy.
It is a pressing clinical challenge to develop prognostic scores to evaluate the success of source control interventions [6,7,22]. A previous trial using clinically established scoring systems, including the Acute Physiology and Chronic Health Evaluation (APACHE)-II score, Simplified Acute Physiology Score (SAPS)-II, Mannheim Peritonitis Index (MPI), Multiple Organ Dysfunction Score (MODS), Sequential Organ Failure Assessment (SOFA) score, and the acute part of the APACHE-II score (APS) have failed to predict ongoing infection in patients with abdominal sepsis after the initial surgical intervention [25]. Other studies followed, but could not establish a reliable scoring system [6,26,27]. We believe the immunological assessment, alone or in combination with physiological markers, provides a new rationale to conduct clinical trials to evaluate effective source control.
With this study, we sought to establish a clinically relevant murine abdominal sepsis with an underlying rationale on when to conduct source control as well as evaluating the effectiveness of the source control. We demonstrate that the CLP-excision (CLP/E) intervention consisting of surgical debridement, peritoneal lavage and antibiotic therapy, conducted upon overt display of sepsis, reduces the bacterial burden by greater than 99%, as previously demonstrated in the literature [9]. We next sought to characterize circulating neutrophils to examine whether changes in phenotype can indicate efficient source control compared to septic mice without source control intervention. We observed that septic mice have a relative increase in the number of neutrophils and an absolute and relative increase of the activated neutrophils. Neutrophils are a major component of the innate immune system to migrate to and eradicate bacteria in inflamed tissue [10]. Moreover, PAMPs are strong activators of neutrophils and drive the development of sepsis [28,29]. We conclude that the characterization of neutrophils might provide insight into the effectiveness of source control, as concentration of PAMPs, such as LPS, drives neutrophil activation and should be significantly decreased or eradicated after successful source control. Of note, the time from blood collection, preparing the cells for flow cytometry, and the FACS analysis could be accomplished in about an hour. Although more studies are necessary, such a timely assay to determine source control efficacy may be of use to surgeons.
Neutrophils from septic mice showed a decreased ability to acidify internal cell compartments such as lysosomes. This functionality was able to be restored after source control. However, the time duration for this phagocytosis assay takes longer than determining the neutrophil phenotype. We did not compare the sensitivity and accuracy of the two different methodologies to determine if one is superior to the other, which is a limitation of this study. In future studies, we suggest characterizing neutrophils from human patients to determine whether these findings translate into human studies and can provide predictive scoring systems for the evaluation of adequate source control in patients with abdominal sepsis.
Enzyme-linked immune absorbent spot (ELISPOT) is a highly sensitive immunoassay that measures the frequency of cytokine-secreting cells at the single-cell level. A key advantage of ELISpot is that the assay has excellent dynamic range. ELISpot can detect as little as 1 cytokine secreting cell in 100,000 cells or as many as several thousand cytokine secreting cells per individual chamber well [30]. In addition to detecting the number of cytokine secreting cells, the relative amount of cytokine that is produced by each cell can be determined by quantitating the area of each spot. Using the TNF-α ELISPOT, we observed significant functional defects in cells obtained from septic mice when compared to healthy or source-controlled mice. We are currently conducting studies in human patients to determine if the murine results can be recapitulated. If this can be accomplished, there are existing College of American Pathologists (CAP) or Clinical Laboratory Improvement Amendments of 1988 certified (CLIA) laboratories that are using ELISPOT testing of functional immune responses. We can envision moving toward using an existing infrastructure to rapidly translate these results into CAP-CLIA-accredited, Food and Drug Administration (FDA)-cleared diagnostic assays. It is realistic to envision a surgeon ordering this assay, blood transported to a CAP CLIA laboratory, with results obtained within 36 hours.
Interestingly, the functional assays reveal that the relevant neutrophil functions of phagocytosis and acidification of internal cell compartments, such as the lysosome, is impaired (Fig. 5). Acidification is necessary to disintegrate phagocytosed bacteria in lysosomes. Another key cell functional of TLR-4 expressing cells is to produce TNF-α, a potent pro-inflammatory cytokine that promotes key anti-microbial functions. We did not see any changes of TNF-α production in the septic mice when examining the spontaneous production of TNF-α (Fig. 6A,D). Yet, when circulating neutrophils are stimulated with LPS, we do see a decrease in their capacity to produce TNF-α (Fig. 6B,E), as well as in the ratio of challenged to spontaneous TNF-α production (Fig. 6C,F). We consider the spontaneous production to reflect how many cells have already encountered an antigen, i.e. PAMP, and accordingly produce TNF-α. On the other hand, the secretion of TNF-α when stimulated by LPS indicates the maximal capacity of the neutrophils to produce TNF-α. Therefore, the decrease of stimulated TNF-α production can be interpreted as cellular de-sensitization during sepsis. Altogether, our data revealed that neutrophil functionality in abdominal sepsis can be restored when source control was performed in as early as 18 hours.
Lastly, we want to address some additional limitations of this study. In the functional analysis of TNF-α production, we did not specifically examine the production of only neutrophils as we used whole blood rather than isolated neutrophils. It is possible that other cells present are responsible for a portion of the TNF-α production [14]. However, the relative share of neutrophils in septic blood, in which we see the significant decrease of TNF-α production, is significantly higher than in the sham or CLP/E mice (Fig. 4A). Consequently, it can be assumed that a significant share of the impaired TNF-α production can be attributed to neutrophils. Secondly, we chose a timely source control model. It has yet to be examined whether neutrophil functionality can be restored if source control is conducted at later timepoints. Additionally, this study was carried out using all male animal subjects. This was done to homogenize our subject pool in order to better discriminate differences between test groups, but may have implications in eventual treatment outcomes for female subjects, and will need to be further investigated in the pre-clinical setting.
We believe that this murine model does provide a rationale to examine human patients in clinical trials by assessing their neutrophil behavior after source control. We suggest that the predictive value might not be limited to abdominal infections but can also be applied to other pathophysiologic conditions in which surgical source control is key to prevent deteriorating inflammation, conversion to a chronic state or re-infection, e.g. in bone infections.
Highlights.
Successful surgical source control in abdominal sepsis can be modeled with the murine CLP-excision model (CLP/E).
Sepsis leads to relative increase of neutrophils in whole blood and relative and absolute increase of activated neutrophils, both of which return to that of the healthy control after source control (CLP/E).
Sepsis also leads to functional impairment of neutrophils (acidification of cell compartments and TNF-α production) from septic mice but not those mice with source control (CLP/E).
The phenotypic and functional characterization of neutrophils in mice can provide a rationale to develop a predictive scoring system to evaluate the success of source control in infected human patients.
Acknowledgements
The authors would like to thank Holly Goetzman for her great help conducting experiments and assistance with the surgeries.
Funding
This work was supported by funding from the Deutsche Forschungsgemeinschaft (German Research Foundation) (BE 7016/1–1) (C.C.B.) and the National Institute of General Medical Sciences (T32 GM08478) (C.E.S.)
Footnotes
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hajj J, Blaine N, Salavaci J, Jacoby D, The “Centrality of Sepsis”: A Review on Incidence, Mortality, and Cost of Care, Healthcare (Basel) 6 (2018). 10.3390/healthcare6030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Muresan MG, Balmos IA, Badea I, Santini A, Abdominal Sepsis: An Update, J Crit Care Med (Targu Mures) 4 (2018) 120–125. 10.2478/jccm-2018-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltran MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, Olaoye I, Omari AH, Ordonez CA, Pereira BM, Pereira Junior GA, Pupelis G, Reis T, Sakakhushev B, Sato N, Segovia Lohse HA, Shelat VG, Soreide K, Uhl W, Ulrych J, Van Goor H, Velmahos GC, Yuan KC, Wani I, Weber DG, Zachariah SK, Catena F, The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections, World journal of emergency surgery : WJES 12 (2017) 29. 10.1186/s13017-017-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marshall JC, Principles of source control in the early management of sepsis, Curr Infect Dis Rep 12 (2010) 345–353. 10.1007/s11908-010-0126-z. [DOI] [PubMed] [Google Scholar]
- [5].Martinez ML, Ferrer R, Torrents E, Guillamat-Prats R, Goma G, Suarez D, Alvarez-Rocha L, Pozo Laderas JC, Martin-Loeches I, Levy MM, Artigas A, Edusepsis Study G, Impact of Source Control in Patients With Severe Sepsis and Septic Shock, Critical care medicine 45 (2017) 11–19. 10.1097/CCM.0000000000002011. [DOI] [PubMed] [Google Scholar]
- [6].van de Groep K, Verhoeff TL, Verboom DM, Bos LD, Schultz MJ, Bonten MJM, Cremer OL, consortium M, Epidemiology and outcomes of source control procedures in critically ill patients with intra-abdominal infection, Journal of critical care 52 (2019) 258–264. 10.1016/j.jcrc.2019.02.029. [DOI] [PubMed] [Google Scholar]
- [7].van Ruler O, Boermeester MA, Surgical treatment of secondary peritonitis : A continuing problem, Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 88 (2017) 1–6. 10.1007/s00104-015-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH, Cecal ligation and puncture, Shock 24 Suppl 1 (2005) 52–57. 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- [9].Kuethe JW, Midura EF, Rice TC, Caldwell CC, Peritoneal wash contents used to predict mortality in a murine sepsis model, The Journal of surgical research 199 (2015) 211–219. 10.1016/j.jss.2015.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L, Update on Neutrophil Function in Severe Inflammation, Frontiers in immunology 9 (2018) 2171. 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Woodfin A, Beyrau M, Voisin MB, Ma B, Whiteford JR, Hordijk PL, Hogg N, Nourshargh S, ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia, Blood 127 (2016) 898–907. 10.1182/blood-2015-08-664995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ivetic HL Hoskins Green, S.J. Hart, L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling, Frontiers in immunology 10 (2019) 1068. 10.3389/fimmu.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sengupta S, Caldwell CC, Nomellini V, Distinct Neutrophil Populations in the Spleen During PICS, Frontiers in immunology 11 (2020) 804. 10.3389/fimmu.2020.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Smedman, Gardlund B, Nihlmark K, Gille-Johnson P, Andersson J, Paulie S, ELISpot analysis of LPS-stimulated leukocytes: human granulocytes selectively secrete IL-8, MIP-1beta and TNF-alpha, Journal of immunological methods 346 (2009) 1–8. 10.1016/j.jim.2009.04.001. [DOI] [PubMed] [Google Scholar]
- [15].Tschop J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschop MH, Caldwell CC, The cannabinoid receptor 2 is critical for the host response to sepsis, Journal of immunology 183 (2009) 499–505. 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Granger JI, Ratti PL, Datta SC, Raymond RM, Opp MR, Sepsis-induced morbidity in mice: effects on body temperature, body weight, cage activity, social behavior and cytokines in brain, Psychoneuroendocrinology 38 (2013) 1047–1057. 10.1016/j.psyneuen.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kushimoto S, Gando S, Saitoh D, Mayumi T, Ogura H, Fujishima S, Araki T, Ikeda H, Kotani J, Miki Y, Shiraishi S, Suzuki K, Suzuki Y, Takeyama N, Takuma K, Tsuruta R, Yamaguchi Y, Yamashita N, Aikawa N, Group JSRS, The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: an analysis from a multicenter, prospective survey of severe sepsis, Critical care 17 (2013) R271. 10.1186/cc13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Drewry AM, Fuller BM, Skrupky LP, Hotchkiss RS, The presence of hypothermia within 24 hours of sepsis diagnosis predicts persistent lymphopenia, Critical care medicine 43 (2015) 1165–1169. 10.1097/CCM.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Taneja, Reddy B, Damhorst G, Dave Zhao S, Hassan U, Price Z, Jensen T, Ghonge T, Patel M, Wachspress S, Winter J, Rappleye M, Smith G, Healey R, Ajmal M, Khan M, Patel J, Rawal H, Sarwar R, Soni S, Anwaruddin S, Davis B, Kumar J, White K, Bashir R, Zhu R, Combining Biomarkers with EMR Data to Identify Patients in Different Phases of Sepsis, Scientific reports 7 (2017) 10800. 10.1038/s41598-017-09766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reinhart K, Bauer M, Riedemann NC, Hartog CS, New approaches to sepsis: molecular diagnostics and biomarkers, Clin Microbiol Rev 25 (2012) 609–634. 10.1128/CMR.00016-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Albert-Vega DM Tawfik S. Trouillet-Assant L. Vachot F. Mallet J. Textoris, Immune Functional Assays, From Custom to Standardized Tests for Precision Medicine, Frontiers in immunology 9 (2018) 2367. 10.3389/fimmu.2018.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Boldingh QJ, de Vries FE, Boermeester MA, Abdominal sepsis, Curr Opin Crit Care 23 (2017) 159–166. 10.1097/MCC.0000000000000388. [DOI] [PubMed] [Google Scholar]
- [23].Hecker A, Reichert M, Reuss CJ, Schmoch T, Riedel JG, Schneck E, Padberg W, Weigand MA, Hecker M, Intra-abdominal sepsis: new definitions and current clinical standards, Langenbeck’s archives of surgery 404 (2019) 257–271. 10.1007/s00423-019-01752-7. [DOI] [PubMed] [Google Scholar]
- [24].Rattan R, Allen CJ, Sawyer RG, Askari R, Banton KL, Claridge JA, Cocanour CS, Coimbra R, Cook CH, Cuschieri J, Dellinger EP, Duane TM, Evans HL, Lipsett PA, Mazuski JE, Miller PR, O’Neill PJ, Rotstein OD, Namias N, Patients with Complicated Intra-Abdominal Infection Presenting with Sepsis Do Not Require Longer Duration of Antimicrobial Therapy, J Am Coll Surg 222 (2016) 440–446. 10.1016/j.jamcollsurg.2015.12.050. [DOI] [PubMed] [Google Scholar]
- [25].van Ruler O, Kiewiet JJ, Boer KR, Lamme B, Gouma DJ, Boermeester MA, Reitsma JB, Failure of available scoring systems to predict ongoing infection in patients with abdominal sepsis after their initial emergency laparotomy, BMC Surg 11 (2011) 38. 10.1186/1471-2482-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kiewiet JJ, van Ruler O, Boermeester MA, Reitsma JB, A decision rule to aid selection of patients with abdominal sepsis requiring a relaparotomy, BMC Surg 13 (2013) 28. 10.1186/1471-2482-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jung B, Molinari N, Nasri M, Hajjej Z, Chanques G, Jean-Pierre H, Panaro F, Jaber S, Procalcitonin biomarker kinetics fails to predict treatment response in perioperative abdominal infection with septic shock, Critical care 17 (2013) R255. 10.1186/cc13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Raymond SL, Holden DC, Mira JC, Stortz JA, Loftus TJ, Mohr AM, Moldawer LL, Moore FA, Larson SD, Efron PA, Microbial recognition and danger signals in sepsis and trauma, Biochimica et biophysica acta (2017). 10.1016/j.bbadis.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hayashi F, Means TK, Luster AD, Toll-like receptors stimulate human neutrophil function, Blood 102 (2003) 2660–2669. 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- [30].Mazer M, Unsinger J, Drewry A, Walton A, Osborne D, Blood T, Hotchkiss R, Remy KE, IL-10 Has Differential Effects on the Innate and Adaptive Immune Systems of Septic Patients, J Immunol 203 (2019) 2088–2099. 10.4049/jimmunol.1900637. [DOI] [PMC free article] [PubMed] [Google Scholar]