Abstract
Background
Telomere dysfunction is associated with idiopathic pulmonary fibrosis (IPF) and worse outcomes following lung transplantation. Telomere dysfunction may impair immunity by upregulating p53 and arresting proliferation, but its influence on allograft-specific immune responses is unknown. We hypothesized that subjects undergoing lung transplantation for IPF would have impaired T-cell proliferation to donor antigens.
Methods
We analyzed peripheral blood mononuclear cells (PBMC) from 14 IPF lung transplant recipients and 12 age-matched non-IPF subjects, before and 2 years after transplantation, as well as PBMC from 9 non-transplant controls. We quantified T cell proliferation and cytokine secretion to donor antigens. Associations between PBMC telomere length, measured by quantitative PCR, and T cell proliferation to alloantigens were evaluated with generalized estimating equation models.
Results
IPF subjects demonstrated impaired CD8+ T cell proliferation to donor antigens pre-transplant (P <0.05). IL-2, IL-7, and IL-15 cytokine stimulation restored T cell proliferation, while p53 upregulation blocked proliferation. IPF subjects had shorter PBMC telomere lengths than non-IPF subjects (P < 0.001), and short PBMC telomere length was associated with impaired CD8+ T cell proliferation to alloantigens (P = 0.002).
Conclusions
IPF as an indication for lung transplant is associated with short PBMC telomere length and impaired T cell responses to donor antigens. However, the rescue of proliferation following cytokine exposure suggests that alloimmune anergy could be overcome. Telomere length may inform immunosuppression strategies for IPF recipients.
Keywords: Telomeres, Idiopathic pulmonary fibrosis, Lung transplantation, Alloimmune response
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and fatal disease characterized by progressive fibrosis of the lung parenchyma.1,2 Disease-modifying therapies may slow progression, but in many cases lung transplantation is used to manage the disease.3
IPF is strongly linked to telomere dysfunction. Telomeres are nucleoprotein caps at the ends of chromosomes that bind in looped shelterin protein complexes to maintain genomic integrity and stability.4 While telomere shortening is a natural consequence of cell division, telomerase proteins can restore telomeric nucleotide regions.5 These telomerase proteins are active in proliferating lymphocytes and malignant cells.6 Studies have found almost 37% of patients with familial pulmonary fibrosis (FPF) have shortened telomeres in their circulating leukocytes.7,8 Telomere-related mutations are found in up to 11.3% of sporadic IPF,9 and even in the absence of known gene mutations, the peripheral blood average telomere length in IPF patient is lower than age-matched controls.8,10,11
While lung transplantation addresses the impact of telomere dysfunction in the lung parenchyma,12 systemic telomere dysfunction may continue to impact the immune system.13 Short leukocyte telomeres are linked to worse outcomes from the use of immunosuppression as a treatment for IPF.14 Post-transplant, short recipient telomere lengths have been variably linked with leukopenia, cytomegalovirus (CMV) infection risk, decreased acute cellular rejection, and impaired chronic lung allograft dysfunction (CLAD)-free survival.15–19 In particular, lung transplant recipients with short telomeres were found to have impaired cytotoxic T cell proliferation to CMV.20 These findings suggest impaired immunity in IPF patients, which could be a consequence of telomere dysfunction in immune cells. However, the effects of IPF on alloimmune responses are unknown. Considering these reports, this study examined the hypothesis that IPF lung transplant recipients with telomere dysfunction would have impaired cytotoxic T cell proliferation to allogeneic antigens.
Materials and Methods
Within a longitudinal cohort of lung transplant recipients consented at the University of California, San Francisco (UCSF, IRB #13–10738), we selected subjects transplanted for IPF and frequency matched to control subjects by recipient age and sex. A convenience sample of healthy controls without immunosuppression or indications for transplant were recruited at UCSF, without matching. Induction immunosuppression included prednisone, basiliximab, and mycophenolate mofetil, while maintenance regimens included tacrolimus, mycophenolate mofetil, and prednisone. We targeted lifelong CMV prophylaxis with valganciclovir, but mycophenolate mofetil and/or valganciclovir dosages were decreased in response to leukopenia or other side effects. Additional details on inclusion and exclusion criteria are in the Supplemental Methods. We examined banked peripheral blood mononuclear cells (PBMC) from pre-transplant and two years post-transplant in mixed lymphocyte reaction with donor-derived stimulated B cell (sBc) alloantigens and quantified proliferative responses by flow cytometry, as described previously21,22, and cytokine secretion by Luminex multiplex assay. Telomere lengths were performed using quantitative PCR as previously described.23 Mixed lymphocyte reactions with KML001 were used to assess effects of p53 telomere disruption.
The proportions of T cells proliferating to alloantigens were compared between IPF and control groups by Student’s t-test. Paired t-tests were used to compare proliferation pre-transplant and at 2 years. Cytokine concentrations were assessed by complete linkage hierarchical cluster analysis and Kruskal-Wallis test between groups. Interaction of CMV or IPF status with memory phenotype distributions were assessed by 2-way ANOVA. The association between proliferation fraction with telomere length was assessed by generalized-estimating equation (GEE) linear models. Further details on the methods and statistical analyses are included in the supplement.
Results
Participant characteristics are shown in Table 1. The two groups were well matched, except that in the control group, donor gender was more commonly male and Lung Allocation Scores at the time of transplant were lower. Post-transplant donor-specific antibodies (DSA) developed more commonly in IPF subjects. CMV viral loads were ≤200 copies/mL except for one subject in the control group with a viral load of 741 copies/mL. There was one case of CMV pneumonitis, also in the control group.
Table 1.
Subject Characteristics and outcomes
| IPF | Controls | P-value | |
|---|---|---|---|
| Total Subjects, N | 14 | 12 | |
| Recipient Age, median [IQR]1 | 63 [54–66] | 64 [55–66] | 0.94 |
| Donor Age, median [IQR]1 | 39 [23–52] | 33 [28–52] | 0.57 |
| Male recipient, N (%) | 10 (71) | 7 (58) | 0.78 |
| Male donor, N (%) | 7 (50.0) | 12 (100) | 0.02 |
| Double lung transplant, N (%) | 14 (100) | 12 (100) | 1.00 |
| CMV serostatus, N (%) | 0.39 | ||
| D+/R− | 5 (36) | 2 (17) | |
| D+/R+ | 2 (14) | 4 (33) | |
| D− | 7 (50) | 6 (50) | |
| Diagnosis group, N (%) | <0.001 | ||
| A (Obstructive) | 0 (0) | 9 (75) | |
| C (Cystic Fibrosis) | 0 (0) | 3 (25) | |
| D (Pulmonary Fibrosis) | 14 (100) | 0 (0) | |
| Recipient Ethnicity, N (%) | 0.18 | ||
| White | 10 (71) | 11 (92) | |
| Black | 0 (0) | 1 (8) | |
| Other | 4 (29) | 0 (0) | |
| Donor Ethnicity, N (%) | 0.98 | ||
| White | 7 (50) | 6 (50) | |
| Black | 1 (7) | 1 (8) | |
| Other | 6 (43) | 5 (42) | |
| Lung allocation score, median [IQR]1 | 72 [44–87] | 36 [33–46] | 0.004 |
| HLA mismatches, median [IQR]1 | 5 [5–6] | 5 [4–6] | 0.25 |
|
| |||
| Panel-reactive antibiodies, median [IQR]1 | 5% [0–12%] | 2% [0–10%] | 0.71 |
|
| |||
| Pre-transplant DSA, N (%) | 1 (7) | 2 (17) | 0.89 |
|
| |||
| De novo DSA, N (%) | 9 (50) | 0 (0) | 0.02 |
|
| |||
| 2-year post transplant data | |||
| On mycophenolate at 2 years, N (%) | 9 (64) | 11 (92) | 0.24 |
| Mycophenolate held for leukopenia | 5 (36) | 1 (8) | 0.24 |
| Mycophenolate dose, median [IQR]1,2 | 625 [0–1000] | 1000 [500–1250] | 0.22 |
| White blood cell count3, mean (SD) | 6.4 (2.1) | 6.5 (2.5) | 0.95 |
| Absolute Neutrophil Count3, mean (SD) | 4.4 (1.8) | 4.3 (1.8) | 0.95 |
| Absolute Lymphocyte Count3, mean (SD) | 1.3 (0.8) | 1.7 (0.8) | 0.27 |
| CMV viremia, N (%) | 2 (14) | 5 (42) | 0.26 |
| Number bronchoscopies, mean (SD) | 11 (2) | 12 (2) | 0.06 |
| Episodes of ≥A1 rejection, mean (SD) | 6 (43) | 3 (25) | 0.59 |
| Episodes of ≥A2 rejection, mean (SD) | 2 (14) | 2 (17) | 1.00 |
| Resulting in antibiotics, mean (SD) | 2.3 (1.6) | 2.0 (1.5) | 0.64 |
| Resulting in steroids, mean (SD) | 0.6 (0.9) | 0.3 (0.5) | 0.31 |
| CLAD-free survival years, restricted mean (SE) | 4.3 (0.5) | 4.3 (0.4) | 0.60 |
denotes non-normal distribution by Shapiro-Wilk test
total daily dose equivalents in milligrams; 180 mg of enteric coated mycophenolic acid was considered equivalent to 250 mg of mycophenolate mofetil.
x 1,000 cells per microliter
N, number; IQR, Interquartile range; CMV, cytomegalovirus; DSA, donor-specific antibodies; D, donor; R, recipient; HLA, human leukocyte antigen; SD, standard deviation; SE, standard error; CLAD, chronic lung allograft dysfunction
The median age was 41 (IQR 40–50) years in the healthy control group, which was younger than for transplant recipient groups (P < 0.001). The healthy control group included 4 men and 5 women (P = 0.48).
IPF subjects had impaired alloreactive CD8+ T cell proliferation pre-transplant.
Proliferative responses for recipient PBMC stimulated with donor-matched or pooled sBc were compared between IPF and control lung transplant recipients pre-transplant. Following stimulation with matched donor alloantigens, we observed a decreased frequency of proliferated CD8+ T cells (Figure 1A and 1B, P = 0.03). Similarly, IPF subjects had decreased CD8+ T cell proliferation in response to a pool of multiple donor alloantigens, selected to cover a broad range of HLA types, when compared with control subjects (Figure 1A and 1B, P = 0.04). There were no statistically significant differences within the non-IPF group between subject with CF and those with obstructive lung disease (P ≥0.74), and chart review of the those with the highest proliferative responses revealed no history of active infection. We did not observe a difference in the donor-specific T cell proliferation frequency for CD8+, conventional CD4+, or regulatory T cells (P ≥0.35) between subjects who developed de novo DSA and those who did not. As expected, there were increases in the Treg, CD4+ Tconv, and CD8+ T cell responses to pooled sBc compared with more antigenically constrained donor sBc stimulation in both cohorts (Supplementary Figure S1, P ≤ 0.003).
Figure 1: Idiopathic pulmonary fibrosis (IPF) subjects demonstrate impaired CD8+ T proliferation to alloantigen pre-transplant.
Proliferation to alloantigens was measured by mixed lymphocyte reaction for IPF and non-IPF lung transplant recipients. Responder PBMC were cocultured with matched donor or pooled stimulated B cells (sBc) for 4 days and harvested for flow cytometric analysis and percent proliferation was defined based on the number of cells with ≥2-fold decreased in CFSE staining intensity. (A) Typical flow plots of proliferated CD8+ T cells for IPF and non-IPF lung transplant recipients. The percentage of proliferating (B) CD8+ Tconv and (C) CD4+ T in response to alloantigen were shown. P-values were calculated by two-tailed unpaired t-test. PBMC = peripheral blood mononuclear cells; sBc = stimulated B cells; Tconv = conventional T cells; pooled sBc = pooled sBc mixture from 6–8 donors. The data were shown as mean ± S.D.
Prior studies suggest greater impacts of IPF on CD8+ T cell proliferation as opposed to CD4+ T cells.20 Accordingly, we did not observe a statistically significant difference in proliferation of CD4+ Tconv or Treg subsets in IPF patients relative to controls (Figure 1C and Supplemental Figure S2).
As shown in Supplemental Figure 2, both IPF and non-IPF lung transplant recipients had impaired CD8+ T cell proliferation to alloantigen when compared with a healthy, relatively younger, non-transplant referent group.
As shown in the representative flow plots (Figure 1A), lymphocytes from IPF subjects had more cells that arrested after 1–3 proliferative events and less proliferation overall. Fewer cells initiated proliferation, as quantified by calculation of allospecific CD8+ T cell precursor frequencies, which were lower in IPF subjects than in non-IPF controls (Supplementary Figure S3, P = 0.02).
IPF subjects had impaired proliferation to pooled HLA, but not donor-specific antigens, at 2 years.
Allograft tolerance could develop following long-term exposure to donor antigens through alloreactive T cells deletion or anergy,24 but such tolerance has not been described in lung transplant recipients for IPF or other indications. At 2 years, IPF subjects had less proliferation to pooled HLA compared with non-IPF subjects, similar to what had been seen in pre-transplant samples to donor antigens. However, we did not observe a statistically significant difference in proliferation to donor-derived sBc for CD4+ Tconv (P = 0.21) or CD8+ T cells (P = 0.08) between IPF and non-IPF subjects (Figure 2A–B). Comparing responses pre- and 2 years post-transplant, donor reactive CD4+ Tconv frequency decreased in IPF patients (P = 0.006, Figure 2D). We did not observe a statistically significant decrease in donor reactive CD8+ T cell proliferation in either group (P = 0.35, Figure 2C). We also did not observe a statistically significant decrease in donor-specific CD4+ T cell responses in non-IPF controls (Figure 2D). This lack of statistical significance for CD4+ Tconv and CD8+ T cells in the non-IPF cohort was attributable to a single subject, who developed early CLAD around the 2-year time point. Together, these data suggest that differences in donor-specific immune responses between IPF and non-IPF subjects may diminish over time in subjects who develop partial tolerance.
Figure 2. Proliferation to donor-specific and pooled HLA at 2 years post-transplant.
PBMC from IPF and non-IPF lung transplant recipients 2 years post-transplant were stimulated with donor-derived matched sBc or pooled sBc from multiple donors in a mixed lymphocyte reaction. Proliferation of (A) CD8+ T cells and (B) CD4+ conventional T cells. Differences between IPF and non-IPF groups were assessed using unpaired Student’s t-test. Changes in (C) CD8+ T cells and (D) CD4+ Tconv are shown over time for the two groups in response to stimulation with matched donor-derived sBc. The dashed line highlights a subject who developed CLAD at the 2-year time point. The percentage of proliferating cells pre- and post-transplant were compared by two-tailed paired Student’s t-test.
Cytokine treatment restores proliferation to alloantigens in IPF lung transplant recipients.
Impaired alloreactivity in IPF patients could be secondary to T cell clonal deletion or anergy. To distinguish between these potential mechanisms, we performed mixed lymphocyte reactions in the presence of a cocktail of IL-2, IL-7, and IL-15 cytokines, which could overcome anergy for some alloreactive T cell precursors but would not increase the precursor frequency if clones are deleted. Proliferation was measured in pre-transplant recipient PBMC stimulated with alloantigens in the presence of this cytokine cocktail (Figure 3A). We found that cytokine cocktail treatment increased proportions of proliferated CD4+ and CD8+ T cells in IPF (P ≤ 0.002) and non-IPF subjects (P < 0.001) (Figures 3B & 3C). Similar results were observed when pooled sBc were used as stimulator to gauge the total alloreactive T cell response.
Figure 3. Cytokine stimulation augments alloreactive T cell responses in both groups.
(A) Typical flow plots showed the portion of proliferated CD4+ Tconv and CD8+ T cells following coculture with alloantigens in the presence or absence of IL-2, IL-7 and IL-15. IPF and non-IPF PBMC pre-transplant (B, C) or 2 years post-transplant (D, E) were stimulated with matched donor antigens or pooled sBc in the presence or absence of cytokines. The data are shown as mean with 95% confidential interval (CI), such that confidence interval not crossing 0 implies a statistically significant increase in proliferation with the addition of cytokines. P-values are shown for comparisons between groups with a P < 0.10 by two-tailed Student’s t-test.
Consistent with the pre-transplant samples data, we observed an increase in alloreactive CD4+ Tconv and CD8+ T cell frequency following stimulation with either donor sBc or pooled sBc in IPF lung transplant recipients after long-term alloantigen exposure (Figure 3D and 3E, P ≤ 0.001). In addition to the fraction of proliferated cells with cytokine stimulation, there were increases in the number of precursors that had undergone any number of proliferation events (precursor frequency) with cytokine stimulation (Supplementary Figure S4, P ≤ 0.003).
Overall, there were no differences between cohorts in terms of the proportion of cells that could be induced to proliferate with cytokine stimulation (P ≥ 0.07). Inspection of the proliferation profiles for CD4+ Tconv and CD8+ T cell cells with cytokine stimulation show that some cells are induced to undergo only one division, while other cells undergo at least three divisions (Figure 3A). The observation that IL-2, IL-7, and IL-15 cytokines restore proliferation capacity suggests that some of the alloreactive IPF T cells are anergic.
Cytokine profiling demonstrates decreased effector function in lymphocytes from IPF lung transplant recipients.
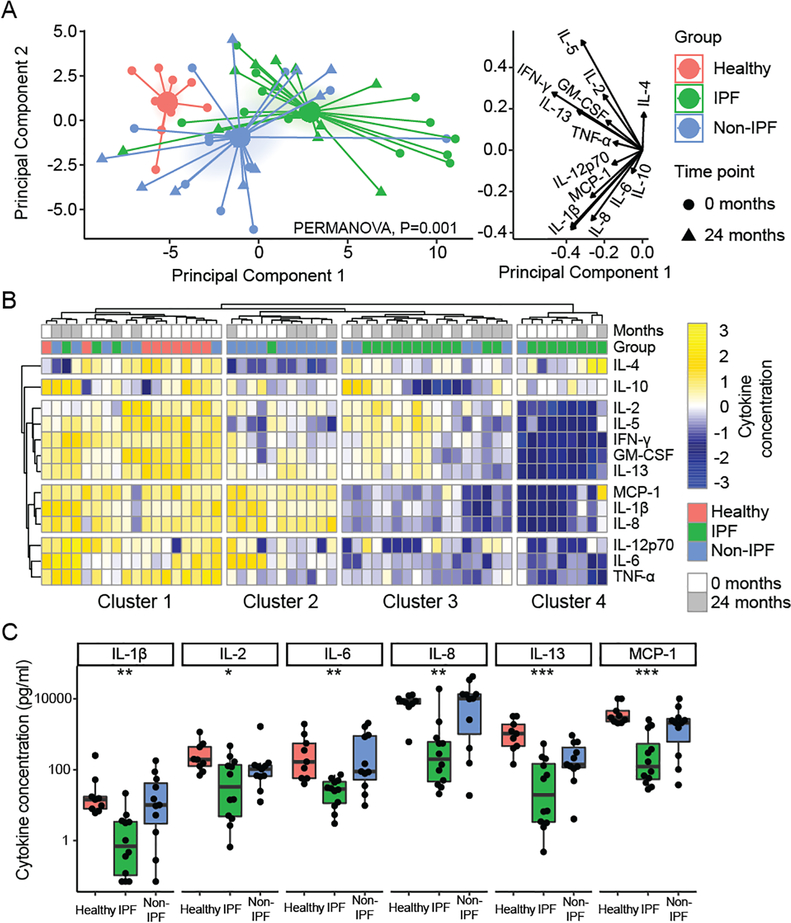
Having observed decreased proliferation to alloantigens in IPF subjects, we asked if there would be a similar decrease in the production of T cell effector cytokines. Concentrations of 13 T-cell cytokines were quantified in supernatants following a mixed lymphocyte reaction with donor stimulated B cells. Principal component analysis (Figure 4A) showed the first principal component to represent overall cytokine production with response to alloantigen stimulation (matched donor for recipients and single donor for non-transplant referents). The second principal component distinguished proliferative cytokines (IL-2, GM-CSF) from activators of innate immune responses (IL-1β and MCP-1). Cytokine production from these IPF, non-IPF, and non-transplant groups were distinct by PERMANOVA. IPF subjects comprised the lower portion of principal component 1 and non-IPF subjects were lower on principal component 2.
Figure 4. Alloreactive T cell cytokine profiling demonstrates decreased cytokine production in IPF subjects.
Cytokines were quantified in supernatant following coculture of pre-transplant or 2-year post-transplant recipient T cells with donor lymphocytes, or non-transplant referents with allogenic lymphocytes. (A) Principal component analysis assessed global differences in cytokine production across samples and showed that the groups were distinct across the first component, with IPF being farthest from normal, particularly for the pre-transplant time point. The groups were distinct as assessed by PERMANOVA (P = 0.001). The cytokine concentration value loadings for these principal components are shown on the right. (B) Four clusters were identified by unbiased hierarchical cluster analysis, within which there was distinct segregation of subjects (Fisher exact test, P < 0.001). Cluster 1 included all non-transplant referents and had robust production of most cytokines, while most IPF subjects were in cluster 3 and 4 and showed decreases in production of multiple cytokine groups. (C) Selected cytokines are shown in at the pre-transplant time-point compared across the three groups by Kruskal-Wallis test (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Unbiased hierarchical clustering was performed to understand differences in cytokine profiles between groups. The four observed clusters of cytokine production segregated by non-transplant, IPF, and non-IPF subject groups (P <0.0001 by Fisher exact test). Non-transplant referents were exclusively in the first cluster and IPF subjects populated the 3rd and 4th clusters. As suggested by principal components analysis, IPF subjects had the lowest production of cytokines in response to alloantigen. Interestingly, IPF subjects demonstrated two patterns of impaired cytokine production with IPF subjects in group 3 displaying a preserved proliferative type 1 cytokine response (IL-2, IFN-γ, GM-CSF). Accordingly, the non-IPF subjects in cluster 2 were distinguished by preserved production of chemotaxis proteins MCP-1, IL-1β, and IL-8.
We examined individual cytokine concentrations at the pre-transplant timepoint in Figure 4C. As suggested by these clustering analyses, the cytokines IL-1β, IL-6, IL-8, and MCP-1 were the most consistently different between IPF, non-transplant referents, and non-IPF subjects. However, the IPF subjects grouped in cluster 4, also displayed significant impairments in proliferative cytokines, such as IL-2.
Memory T cell subtypes in IPF and non-IPF lung transplant recipients
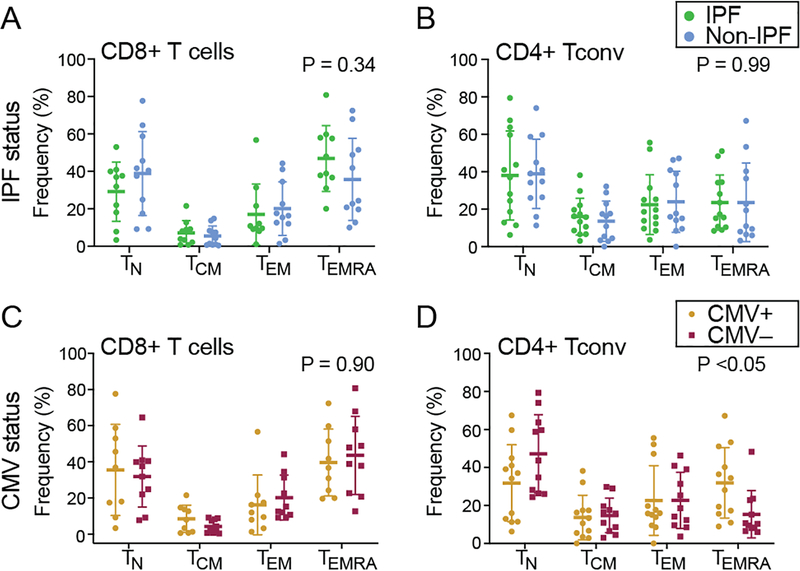
To evaluate differences in memory T cell distribution, CD45RO and CCR7 expression were assessed in the absence of alloantigen stimulation. There were no differences in the memory T cells distribution between IPF and non-IPF lung transplant recipients (two-way ANOVA, Figure 5A and 5B) when T cells were divided into four subsets: naïve T cells (TN, CCR7+CD45RO−); central memory T cells (TCM, CCR7+CD45RO+); effector memory T cells (TEM, CCR7-CD45RO+); and terminally differentiated effector memory T cells (TEMRA, CCR7− CD45RO−).
Figure 5. Similar T cell memory phenotype distributions between IPF and non-IPF subjects.
The memory phenotypes distribution for pre-transplant PBMC in the absence of alloantigen stimulation were analyzed by flow cytometry. The expression of CD45RO and CCR7 were analyzed for IPF and non-IPF PBMC (A-B) and CMV positive or negative recipients PBMC (C-D). Results for naïve T cells (TN: CCR7+CD45RO−), central memory (TCM: CCR7+CD45RO+), effector memory (TEM: CCR7-CD45RO+), and terminally differentiated effector memory (TEMRA: CCR7-CD45RO-) subsets were quantified. P-values shown are for the interaction between cell type and IPF status or CMV status by two-way ANOVA. The data are shown as mean ± S.D.
Next, the distribution of T cell memory phenotypes was analyzed in pretransplant T cells that proliferated in response to matched donor sBc. TCM was the dominant population in both proliferated CD4+ Tconv and CD8+ T subsets (data not shown). Again, there were no differences in the memory phenotype distributions between IPF and non-IPF subjects. These data suggest that loss of naïve T cells does not explain the proliferation differences between these cohorts.
We examined whether recipient’s cytomegalovirus (CMV) exposure had an impact on alloreactive proliferation and memory T cell distributions. Recipients’ pre-transplant proliferative response to matched donor antigen was not different based on CMV serostatus (data not shown). However, lung transplant recipients with CMV exposure had skewed distributions of CD4+ Tconv subsets (Figure 5D, CMV status by cell type interaction P < 0.05). After multiple comparison adjustment, we observed a trend towards increased TEMRA in CMV+ recipients (P = 0.08).
Association between PBMC telomere length and alloimmune responses
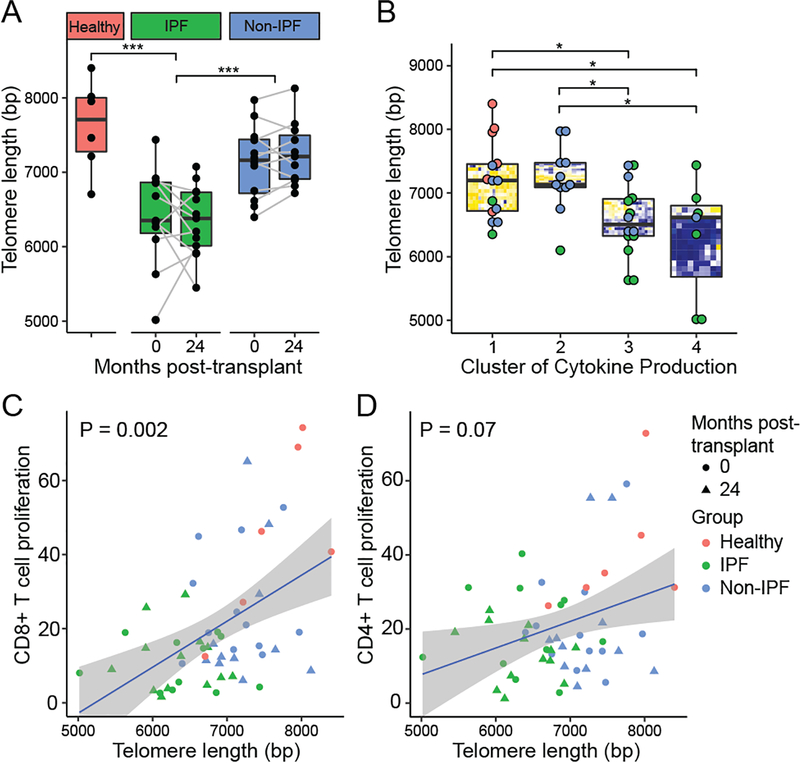
As telomere dysfunction is common in IPF subjects, we suspected that telomere dysfunction might explain the observed impairments in alloimmunity. As shown in Figure 6A, these IPF subjects had significantly shorter PBMC telomere lengths than non-IPF subjects (P < 0.001) or non-transplant referents (P < 0.001). Notably, there was also a trend towards reduced telomere length in non-IPF lung transplant recipients versus healthy controls (P = 0.09). Telomere length measurements at the pre-transplant and 2-year time points were correlated (Pearson’s product moment correlation = 0.80, P < 0.001), and there was no difference in telomere length observed in serial samples over time (P = 0.81).
Figure 6. Short peripheral blood telomere length is associated with impaired alloimmune responses.
Telomere length was measured in PBMC DNA using quantitative PCR and shown for IPF, non-IPF, and non-transplant referents (healthy) both pre-transplant and at 2 years post-transplant (A). Telomere length was shorter in IPF subjects compared with non-IPF and non-transplant referents as assessed by unadjusted GEE models (P < 0.001). For Non-IPF versus healthy, we observed P = 0.09. (B) Telomere length measurements are shown stratified by cytokine profile cluster, as identified in Figure 4. After multiple comparison adjustments clusters 3 and 4, with impaired cytokine production, were found to have shorter telomere lengths than the clusters 1 and 2. Percent proliferation to matched or single donor alloantigens is shown versus telomere length for CD8+ T cells (C) and CD4+ Tconv (D). P-values represent statistical significance for the association of proliferation with telomere length as assessed by unadjusted GEE models.
We examined the cytokine profile groups derived by hierarchical clustering analysis (Figure 6B). Groups 1 and 2, which included non-transplant referents and non-IPF lung transplant recipients, had significantly longer telomere lengths than Groups 3 and 4, which included mostly IPF subjects. However, there was no difference in telomere length between Groups 3 and 4 (P = 0.43), even though Group 4 displayed the most profound impairment in cytokine production.
To determine the association between PBMC telomere length and T cell proliferation to alloantigens, we employed unadjusted GEE models (Figures 6C & 6D). We observed an association between CD8+ T cell proliferation and PBMC telomere length (P = 0.002, Figure 6C) and a trend for the association with CD4+ T cell proliferation (P = 0.07, Figure 6D).
P53 is upregulated with alloantigen stimulation and proliferative arrest in CD8+ T cells
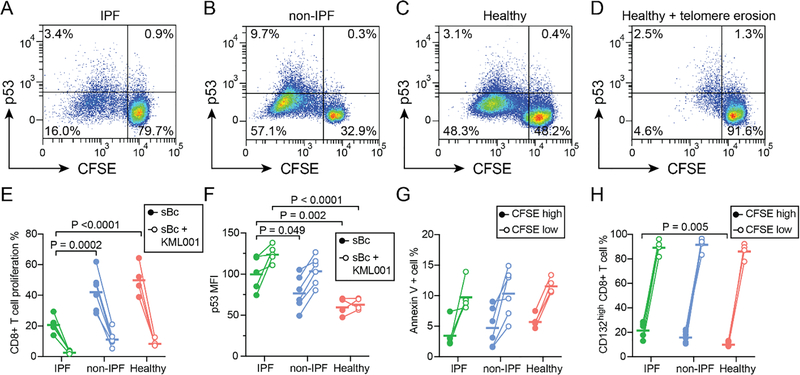
Critical telomere shortening can signal through the ataxia telangiectasia mutated (ATM) kinase to activate p53 and block cell proliferation. To determine if p53 activation occurred in response to allo-stimulation of CD8+ T cells, we quantified p53 expression by flow cytometry following alloantigen stimulation in subjects that had 18 months samples available (N=5, IPF; N=6 non-IPF; N=4, healthy). As was seen in other time points for these subjects, there was decreased proliferation to alloantigens in IPF subjects as compared with non-IPF controls (Figure 7E). P53 expression was upregulated in the subset of CD8+ T cells that proliferated in all three groups (MFI change 183, 95% CI 159–207, P<0.001, Figure 7A–D). Compared with non-IPF or healthy groups, sBc stimulated but non-proliferating (CFSEhigh) CD8+ T cells from IPF subjects had increased p53 expression, consistent with p53-mediated proliferative arrest (Figure 7F).
Figure 7. P53 upregulation is associated with impaired CD8+ T cell proliferation to alloantigens in IPF.
Mixed lymphocyte reactions were performed to detect p53 expression in CD8+ T cells following stimulation with pooled alloantigens. Representative flow plots show p53 expression versus proliferation as measured by CFSE dilution in cells from subjects at 18 months post-transplant for (A) IPF or (B) other indications. P53 thresholds were set based on isotype control staining in the CFSElow population. Responses in healthy referents are shown in (C), as well as responses from healthy subject PBMC after treatment with KML001, a reagent that binds and erodes telomeres (D). (E) CD8+ T cell proliferation was less in IPF subjects than in healthy controls (P <0.0001) or non-IPF subjects (P = 0.0002). KML001 treatment resulted in reduced proliferation for all groups (P <0.0001). (F) Median fluorescence intensity (MFI) for P53 after subtraction of isotype control MFI is shown for non-responsive (CFSEhigh) CD8+ T cells with and without KML001. KML001 treatment resulted in increased p53 levels (P <0.0001), and cells from IPF subjects had increased p53 levels versus non-IPF (P=0.049) and healthy (P=0.002) groups. (G) Annexin V staining, a marker of apoptosis, was increased in proliferated CD8+ T cells versus unstimulated cells (P <0.0001) but not different between groups. (H) The common γ-chain receptor (CD132) was present on 99% of cells, but upregulated in following alloantigen stimulation (P <0.0001). CD132high cells were more common in non-responding (CFSElow) CD8+ T cells from IPF subjects (P = 0.005) as compared with healthy referents. Statistical comparisons were performed using 2-way ANOVA with Dunnett’s post-test.
We then examined whether induced telomere dysfunction could drive a similar phenotype of proliferative impairment as was seen in IPF subjects. KML001 binds to telomere DNA sequences driving telomere erosion.25 As shown in Figure 7D–E, KML001 inhibited CD8+ T cell proliferation to donor antigens and increased p53 expression on CFSEhigh cells. We observed similar proliferative arrest and p53 upregulation in mixed lymphocyte reactions with the compound Nutlin-3a, which stabilizes p53 by blocking its interaction with MDM2 (Figure S5).26
P53 activation can drive apoptosis, so we asked if increased apoptosis might explain this apparent proliferative arrest. Annexin V binds phosphatidylserine that translocates to the cell surface of apoptotic cells. We examined Annexin V binding in CFSE high and low cells after stimulation with donor antigen. Following stimulation, apoptotic cells were increased to around 10% of the total population (P<0.0001), but we did not observe differences in apoptosis across groups (Figure 7G). This finding would favor proliferative arrest, rather than apoptosis, as the major driver of impaired proliferation in IPF subjects.
Given our findings of impaired cytokine production in subjects with IPF (Figure 3), we asked whether impaired proliferation might reflect diminished autocrine stimulation through decreased upregulation of the common γ-chain receptor (CD132). Based on isotype controls <1% of cells were CD132 negative, with no differences between groups. Consistent with prior reports, CD132 was upregulated in response to stimulation.27,28 Among the cells that did not proliferate to alloantigen (CFSEhigh), there was an increase in % CD132high in the IPF group compared with healthy controls. Interestingly, the CD132high fraction of CFSEhigh T cells was also increased following addition of KML001 (10% change in mean from a baseline of 17%, 95% CI 3–16%).
Discussion
Here, we identify impaired immune responses to donor antigens in lung transplant recipients with IPF. These proliferation defects could be overcome with cytokine stimulation, consistent with an anergic phenotype. Cytokine profiling of T cells following response to alloantigen showed decreases in pro-inflammatory cytokines like IL-1β, IL-6, IL-8, and MCP-1 in IPF subjects. Finally, these impairments in immune response correlated with PBMC telomere length, suggesting that telomere dysfunction could be one mechanism for dysfunctional alloimmune responses in IPF subjects.
Mechanistically, short telomeres induce ATM-dependent DNA damage repair pathways, resulting in p53 signaling that arrests cell proliferation and cytokine production.29–31 We found that p53 signaling was induced in T cells that proliferate in response to donor antigens. P53 positivity was 80% higher in CD8+ T cells from IPF patients that were stimulated with donor antigen but did not proliferate (Figure 7F), suggesting that p53 activation might be arresting proliferation of some cells. Similarly, chemical disruption of telomere complexes in healthy or non-IPF cells resulted in p53 and CD132 upregulation and proliferative arrest, mirroring the phenotype of IPF cells. These findings are consistent with reports in T cell lines where critically short telomeres result in proliferative arrest and suppressed gene expression of TNFα and GM-CSF.32
We observed that a combination of IL-2, IL-7, and IL-15 could overcome this proliferation deficiency. Each of these cytokines has been shown to increase lymphocyte telomerase activity and telomere length.33–36 At the same time, there may be associations between immune impairment and IPF unrelated to telomere length, particularly as IPF status was a better predictor of immune dysfunction than telomere length in both proliferation and cytokine profiling studies. Further studies are needed to determine whether impairment in cytokine-mediated induction of telomerase activity is a relevant to the alloimmune impairments in individuals with IPF.
Immune aging is typically linked to a decline in naïve memory T cell populations and an expansion of TEMRA.37 Consistent with prior literature, CMV+ recipients in this study had increased TEMRA and decreased naïve T cells. However, there were no differences between these age matched IPF and non-IPF subjects with respect to T cell memory phenotype distributions. Alloantigen stimulation enriched for T cells with a TCM phenotype, distinct from the terminally differentiated TEMRA phenotype associated with chronic CMV stimulation. These data support the notion that telomere dysfunction may be independent from other mechanisms of immune senescence, as has been reported in aging cohort studies.38,39
There are some important limitations in this study. For example, telomere length was measured by quantitative PCR in mixed populations of cells due to limitations on the number of cells available. Alternative methods, like flow fluorescence in situ hybridization would have the advantage of allowing the measurement of telomere length within specific cell types and address whether they are shortest in CD8+ T cells. It is possible that short telomere length in CD4+ T cells would have a stronger association with CD4+ T cell proliferation, for example. Also, this is a single-center study that was powered for detailed immune phenotyping. Larger studies across multiple centers will be needed to validate these findings and assess their clinical implications. Our control cohort did not include subjects with pulmonary hypertension or non-IPF restrictive lung diseases, and so future studies will be needed to dissect responses across transplant indications. While we did not find clear evidence that infections were driving enhanced proliferative responses, it is possible that patients with CF or COPD could have greater infectious burden that could cause a non-specific enhancement in T cell proliferative responses. Interestingly, we observed decreased alloreactive CD4+ T cell immune response in the IPF cohort, a biological correlate of immune tolerance. A similar pattern was seen in the non-IPF cohort except for a single outlier, for whom increased alloimmune responses may have reflected evolving early-onset CLAD.
Here and in larger cohorts, rates of cellular rejection and CLAD were similar. While de novo DSA were associated with IPF here, larger cohorts have not shown a link between IPF or short telomeres and de novo DSA.40,41 Still, if IPF lung transplant recipients have impaired alloimmune responses, why are they not protected from CLAD? Findings in this study may address this clinical paradox: 1) IPF recipients have difficulty tolerating anti-metabolite immune suppression. Mycophenolic acid has been shown to activate p53 and may synergize with telomere dysfunction to drive leukopenia.42 2) Distinct from the senescence seen in response to CMV, anergic T cells may be more easily activated in the setting of a strong cytokine stimulus, as could happen with acute infection driving rejection. 3) Partial tolerance in many non-IPF recipients may result in post-transplant donor-specific immune responses comparable to that of IPF recipients pre-transplant.43,44
Outside of lung transplant, severe telomere-complex mutations can result in T cell deficiency manifest as enterocolitis and Herpesviridae-associated zoster, encephalitis, and pneumonia. In this population, there is a skewing from naïve T cells to TEMRA, narrowing of the T cell repertoire and increased Fas receptor, but not PD-1 expression.13 However, there are a paucity of data on immune status in adults with IPF, for whom telomere defects are generally less severe. It remains to be determined whether immune dysfunction in IPF patients has clinical implications in the absence of immunosuppressive medications, such as diminished responses to vaccines or greater susceptibility to infections. Finally, an immune phenotype from telomere dysfunction is not necessarily limited to IPF subjects, as telomere dysfunction can have a variety of clinical and radiographic manifestations.45
In summary, IPF as an indication for lung transplant is associated with impaired T cell responses to donor antigens and short telomere length. These findings suggest trials of immunosuppression strategies should consider the unique characteristics of the IPF recipient population.
Supplementary Material
Acknowledgements
The authors thank Chiyo Uchida, Charlene Fong, and their colleagues for assistance collecting blood samples; Daniel Dugger for helpful discussions; Nicholas Kolaitis, Mary Ellen Kleinhenz, Lorianna Leard, Rupal Shah, Aida Venado, and the other members of the lung transplant clinical team for their care of these patients and clinical protocol development. We are thankful for the research participants, the cooperation of Donor Network West, as well as organ and tissue donors and their families.
Financial Disclosure Statement
The authors have no conflicts of interest to disclose relevant to this manuscript. JRG has served on advisory boards for Boehringer Ingelheim, Theravance Biopharma, and Atara Biotherapeutics and has received research funding from Thermo Fisher Scientific, BioFire Diagnostics, and Theravance Biopharma. PJW reports grants from the NIH, Boehringer-Ingelheim, Roche, Sanofi, and Pliant Therapeutics, and consulting fees from Blade Therapeutics, Fate Therapeutics, and Gossamer Bio, outside the submitted work. This work was funded by the Veterans Health Administration Office of Research and Development (CX002011) and the National Institutes of Health (HL151552).
List of non-standard abbreviations:
- IPF
idiopathic pulmonary fibrosis
- PBMC
peripheral blood mononuclear cells
- LTRs
lung transplant recipients
- FPF
familial pulmonary fibrosis
- CLAD
chronic lung allograft dysfunction
- CMV
Cytomegalovirus
- sBc
stimulate B cell
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- MLR
mixed lymphocyte reaction
- Tconv
conventional T cells
- Treg
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. N Engl J Med 2018;378:1811–23. [DOI] [PubMed] [Google Scholar]
- 2.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017;389:1941–52. [DOI] [PubMed] [Google Scholar]
- 3.George PM, Patterson CM, Reed AK, Thillai M. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir Med 2019;7:271–82. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH, Epel ES, Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193–8. [DOI] [PubMed] [Google Scholar]
- 5.Srinivas N, Rachakonda S, Kumar R. Telomeres and Telomere Length: A General Overview. Cancers (Basel) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiyama K, Hirai Y, Kyoizumi S, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol 1995;155:3711–5. [PubMed] [Google Scholar]
- 7.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A 2007;104:7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cronkhite JT, Xing C, Raghu G, et al. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrovski S, Todd JL, Durheim MT, et al. An Exome Sequencing Study to Assess the Role of Rare Genetic Variation in Pulmonary Fibrosis. Am J Respir Crit Care Med 2017;196:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A 2008;105:13051–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuart BD, Lee JS, Kozlitina J, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med 2014;2:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naikawadi RP, Green G, Jones KD, et al. Airway Epithelial Telomere Dysfunction Drives Remodeling Similar to Chronic Lung Allograft Dysfunction. Am J Respir Cell Mol Biol 2020;63:490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner CL, Hanumanthu VS, Talbot CC Jr., et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest 2018;128:5222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newton CA, Zhang D, Oldham JM, et al. Telomere Length and Use of Immunosuppressive Medications in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2019;200:336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tokman S, Singer JP, Devine MS, et al. Clinical outcomes of lung transplant recipients with telomerase mutations. J Heart Lung Transplant 2015;34:1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtwright AM, Lamattina AM, Takahashi M, et al. Shorter telomere length following lung transplantation is associated with clinically significant leukopenia and decreased chronic lung allograft dysfunction-free survival. ERJ Open Res 2020;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton CA, Kozlitina J, Lines JR, Kaza V, Torres F, Garcia CK. Telomere length in patients with pulmonary fibrosis associated with chronic lung allograft dysfunction and post-lung transplantation survival. J Heart Lung Transplant 2017;36:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courtwright AM, Fried S, Villalba JA, et al. Association of Donor and Recipient Telomere Length with Clinical Outcomes following Lung Transplantation. PLoS One 2016;11:e0162409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan AC, Neely ML, Frankel CW, et al. Lung Transplant Outcomes in Patients With Pulmonary Fibrosis With Telomere-Related Gene Variants. Chest 2019;156:477–85. [DOI] [PubMed] [Google Scholar]
- 20.Popescu I, Mannem H, Winters SA, et al. Impaired Cytomegalovirus Immunity in Idiopathic Pulmonary Fibrosis Lung Transplant Recipients with Short Telomeres. Am J Respir Crit Care Med 2019;199:362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putnam AL, Safinia N, Medvec A, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant 2013;13:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenland JR, Wong CM, Ahuja R, et al. Donor-Reactive Regulatory T Cell Frequency Increases During Acute Cellular Rejection of Lung Allografts. Transplantation 2016;100:2090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faust HE, Golden JA, Rajalingam R, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax 2017;72:1052–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salisbury EM, Game DS, Lechler RI. Transplantation tolerance. Pediatr Nephrol 2014;29:2263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phatak P, Dai F, Butler M, et al. KML001 cytotoxic activity is associated with its binding to telomeric sequences and telomere erosion in prostate cancer cells. Clin Cancer Res 2008;14:4593–602. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Maki CG. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr Pharm Des 2011;17:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Au-Yeung BB, Smith GA, Mueller JL, et al. IL-2 Modulates the TCR Signaling Threshold for CD8 but Not CD4 T Cell Proliferation on a Single-Cell Level. J Immunol 2017;198:2445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood 2003;102:2541–6. [DOI] [PubMed] [Google Scholar]
- 29.Komarova EA, Krivokrysenko V, Wang K, et al. p53 is a suppressor of inflammatory response in mice. FASEB J 2005;19:1030–2. [DOI] [PubMed] [Google Scholar]
- 30.Gobbini E, Trovesi C, Cassani C, Longhese MP. Telomere uncapping at the crossroad between cell cycle arrest and carcinogenesis. Mol Cell Oncol 2014;1:e29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou HL, Hoffmann R, Gonzalez-Lopez C, Doherty GJ, Korkola JE, Munoz-Espin D. Cellular senescence in cancer: from mechanisms to detection. Mol Oncol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degerman S, Siwicki JK, Osterman P, Lafferty-Whyte K, Keith WN, Roos G. Telomerase upregulation is a postcrisis event during senescence bypass and immortalization of two Nijmegen breakage syndrome T cell cultures. Aging Cell 2010;9:220–35. [DOI] [PubMed] [Google Scholar]
- 33.Kibe R, Zhang S, Guo D, et al. IL-7Ralpha deficiency in p53null mice exacerbates thymocyte telomere erosion and lymphomagenesis. Cell Death Differ 2012;19:1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3’-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol 2005;174:5261–9. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Zhi W, Wareski P, Weng NP. IL-15 activates telomerase and minimizes telomere loss and may preserve the replicative life span of memory CD8+ T cells in vitro. J Immunol 2005;174:4019–24. [DOI] [PubMed] [Google Scholar]
- 36.Brazvan B, Farahzadi R, Mohammadi SM, et al. Key Immune Cell Cytokines Affects the Telomere Activity of Cord Blood Cells In vitro. Adv Pharm Bull 2016;6:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Epps P, Banks R, Aung H, Betts MR, Canaday DH. Age-related differences in polyfunctional T cell responses. Immun Ageing 2014;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Mitri D, Azevedo RI, Henson SM, et al. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol 2011;187:2093–100. [DOI] [PubMed] [Google Scholar]
- 39.Lustig A, Liu HB, Metter EJ, et al. Telomere Shortening, Inflammatory Cytokines, and Anti-Cytomegalovirus Antibody Follow Distinct Age-Associated Trajectories in Humans. Front Immunol 2017;8:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hachem RR, Kamoun M, Budev MM, et al. Human leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am J Transplant 2018;18:2285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan R, Ghosh S, Madala N, et al. Donor-Specific Antibodies Among Lung Transplant Recipients with Short Telomeres. Am J Respir Crit Care Med 2020;201:A2836. [Google Scholar]
- 42.Sun XX, Dai MS, Lu H. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J Biol Chem 2008;283:12387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong YC, McCaughan GW, Bowen DG, Bertolino P. The CD8 T-cell response during tolerance induction in liver transplantation. Clin Transl Immunology 2016;5:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benseler V, Warren A, Vo M, et al. Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A 2011;108:16735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton CA, Batra K, Torrealba J, et al. Telomere-related lung fibrosis is diagnostically heterogeneous but uniformly progressive. Eur Respir J 2016;48:1710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.