Abstract
Background:
For patients at high risk for lung cancer, screening using low-dose CT (LCS) is recommended. The purpose of this study was to examine whether screening may serve as a teachable moment for smoking-related outcomes.
Methods:
In a smoking cessation trial, participants (N=843) completed two phone interviews prior to randomization: pre- (T0) and post-lung screening (T1). Using logistic and linear regressions we examined teachable moment variables (perceived risk, lung cancer worry) and outcomes (readiness, motivation, and CPD).
Results:
Participants were 63.7 (SD=5.9) years old, had 47.8 (SD=17.1) pack-years, 35.2% had ≤high school degree/GED, and 42.3% were undergoing their first scan. Comparing the T0 and T1, 25.7% increased readiness to quit, 9.6% decreased readiness, and 64.7% reported no change (p<0.001). Motivation to quit increased (p<0.05) and CPD decreased between assessments (p<0.001), but only 1.3% self-reported quitting. Compared to individuals who reported no lung cancer worry/little worry, extreme worry was associated with readiness to quit in the next 30 days (OR=1.8, 95% CI: 1.1–3.0) and with higher motivation (b=0.83, p<0.001) at T1. Individuals undergoing a baseline (vs. annual) scan were more ready to quit in the next 30 days (OR=1.8, 95% CI: 1.3–2.5).
Conclusions:
During the brief window between registering for LCS and receiving the results, we found that very few participants quit smoking, but that a significant proportion improved on readiness and motivation to quit, particularly among those undergoing their first scan and among those extremely worried about lung cancer. These results indicate that providing evidence-based tobacco treatment can build upon this teachable moment.
Clinical Trial Registration Number:
The trial is registered at clinical trials.gov: NCT03200236.
Keywords: Lung cancer screening, teachable moment, smoking cessation, tobacco, smoking
Precis:
During the brief window between registering for lung cancer screening and receiving the results, we found that very few participants quit smoking, but that a significant proportion improved on readiness and motivation to quit, particularly among those undergoing their first scan and among those extremely worried about lung cancer. These results indicate that providing evidence-based tobacco treatment can build upon this teachable moment.
INTRODUCTION
Low-dose computed tomographic lung cancer screening (lung screening) is recommended annually for individuals at high risk for lung cancer due to their older age and long-term history of tobacco use.1–3 With the recent expansion of the lung screening eligibility criteria3, it is estimated that 14.5 million individuals in the U.S. will be eligible for lung screening and that more than 50% of eligible individuals currently smoke.4 Thus, providing tobacco treatment is a high priority for this population. The Centers for Medicare and Medicaid Services require counseling on the importance of smoking cessation for patients who currently smoke and provision of tobacco cessation treatment as a component of the shared decision-making discussion prior to lung screening referral.5 The American Cancer Society also recommends informing patients of the lung cancer risk associated with continued smoking and that screening is not an alternative to cessation.6
In addition to the public health benefit of combining disease prevention with early detection in this setting7, some prior studies have suggested that patients may be motivated to quit during this “teachable moment” of lung screening,8–14 especially following an abnormal result.15–18 However, other studies have not found an association between lung screening and smoking attitudes and behaviors.19–25 These studies have differed in study design, presence of cessation intervention, sample size, timing of assessments, and measurement. To date, three reviews have come to differing conclusions about the influence of lung screening on smoking. However, all have recommended taking a systematic approach to look for evidence for a teachable moment and to examine the underlying mechanisms to explain these relationships.22,25,26 The teachable moment heuristic posits that a cueing event (e.g., the period shortly following lung screening and the receipt of results) may increase risk perceptions, disease-specific worry, and prompt behavior change (e.g., enhance readiness to quit and smoking cessation).8
The current study is a planned analysis in the Lung Screening, Tobacco and Health (LSTH) trial27, a telephone-based randomized cessation trial, in which participants completed an assessment prior to undergoing the lung screening scan and again shortly following receipt of the scan results, both of which preceded randomization. These assessments were included in order to assess the impact of undergoing lung screening and receiving the lung screening results, in the absence of a cessation intervention. The LSTH trial is one of eight trials in the National Cancer Institute’s Smoking Cessation at Lung Examination collaboration that are examining smoking cessation treatment conducted in the lung screening setting.28
The present study expands upon prior work12,29 that evaluated the impact of lung screening on readiness to quit. We examined changes in readiness to quit, motivation to quit, and cigarettes per day (CPD) from pre-screening to post-receipt of lung screening results and the extent to which the teachable moment domains of perceived risk for lung cancer and lung cancer worry were associated with changes in these smoking-related attitudes and behaviors.
MATERIALS AND METHODS
Participants
Patients scheduled for lung screening at eight clinical sites (Table 1) were enrolled in the LSTH trial between May 2017 and January 2021. We used the National Comprehensive Cancer Network’s (NCCN) Group 1 and Group 2 eligibility criteria for LCS: 1) 50–80 years of age and 2) 20+ pack-year smoking history. Additional eligibility criteria were: 3) smoked within the past 7 days; 4) registered for but not yet completed the lung screening scan; and 5) English or Spanish-speaking. Individuals were not excluded based on having had a prior low-dose CT scan for lung cancer screening, prior or current tobacco treatment, readiness to quit, or major psychiatric conditions.
Table 1.
Demographic, clinical, lung cancer screening, and study administration characteristics (N = 843)
| Demographics & Clinical Information | |
|---|---|
| Age, M (SD), Median | 63.7 (5.9), 63.0 |
| n (%) | |
| 50–59 | 239 (28.4) |
| 60–69 | 446 (52.9) |
| 70–80 | 158 (18.7) |
| Gender, n (%) | |
| Male | 404 (47.9) |
| Female | 439 (52.1) |
| Race, n (%)* | |
| White | 741 (89.6) |
| Black | 63 (7.6) |
| Other | 23 (2.8) |
| Ethnicity, n (%)* | |
| Non-Hispanic/Latino/Spanish Origin | 779 (93.6) |
| Hispanic/Latino/Spanish Origin | 53 (6.4) |
| Language, n (%) | |
| English | 830 (98.5) |
| Spanish | 13 (1.5) |
| Marital Status, n (%) | |
| Married or in Marriage-like Relationship | 419 (49.7) |
| Other | 424 (50.3) |
| Education Level, n (%)* | |
| High School/GED or Less | 295 (35.2) |
| Associate’s Degree/Vocational School | 336 (40.1) |
| Bachelor’s Degree or More | 206 (24.6) |
| Number of Tobacco-Related Comorbid Conditions, n (%)* | |
| 0 | 140 (17.1) |
| 1 | 258 (31.5) |
| 2 | 223 (27.2) |
| 3+ | 198 (24.2) |
| Pack Years M (SD), Median | 47.8 (17.1), 44.0 |
| Lung Cancer Screening Variables | |
| Lung Cancer Screening History, n (%) | |
| Baseline Scan | 357 (42.3) |
| Annual Repeat Scan | 486 (57.7) |
| Lung Cancer Screening Result, n (%) | |
| Lung-RADS 1 | 251 (29.8) |
| Lung-RADS 2 | 506 (60.0) |
| Lung-RADS 3 | 50 (5.9) |
| Lung-RADS 4 | 36 (4.3) |
| Smoking-related Characteristics | |
| Smoking Frequency (past 30 days), n (%)* | |
| On some days | 58 (6.9) |
| Every day | 783 (93.1) |
| Time to First Cigarette, n (%)* | |
| Within 5 minutes | 224 (26.7) |
| 6–30 minutes | 379 (45.2) |
| More than 30 minutes | 235 (28.0) |
| 24h Quit Attempt in the last 7 days, n (%)* | |
| Yes | 67 (8.0) |
| No | 774 (92.0) |
| Study Administration Variables | |
| Lung Cancer Screening Sites, n (%) | |
| Lahey Hospital and Medical Center | 370 (43.9) |
| UnityPoint Health Trinity | 179 (21.2) |
| Hackensack University Medical Center | 87 (10.3) |
| Baptist Health South Florida | 83 (9.8) |
| Hartford Hospital | 39 (4.6) |
| Georgetown University Medical Center | 33 (3.9) |
| MedStar Shah Medical Group | 31 (3.7) |
| Anne Arundel Medical Center | 21 (2.5) |
| Days between Scan and T1 M (SD), Median | 16.7 (11.0), 13.0 |
| Study Outcomes (T0) | |
| Readiness to Quit, n (%)* | |
| Next 6 Months to Not Considering Quitting | 564 (67.1) |
| Next 30 Days to Already Quit | 277 (32.9) |
| Motivation to Quit, M (SD), Median | 6.5 (2.3), 7.0 |
| CPD, M (SD), Median* | 18.2 (9.0), 20.0 |
Less than 5% missing data
Procedures
Study design and procedures are described in detail elsewhere.27 The lung screening site coordinators described the study to eligible individuals after scheduling the screening appointment, obtained verbal consent, and administered the baseline telephone interview (T0) prior to the lung screening scan. Approximately one week after the scan, once participants had received their results, the Georgetown University Medical Center tobacco treatment specialists research staff called to conduct the post-screening interview (T1). Follow-up assessments of cessation rates are ongoing. The analytic sample (N=843) for the present study included participants who completed both phone assessments (Figure 1).
Figure 1.
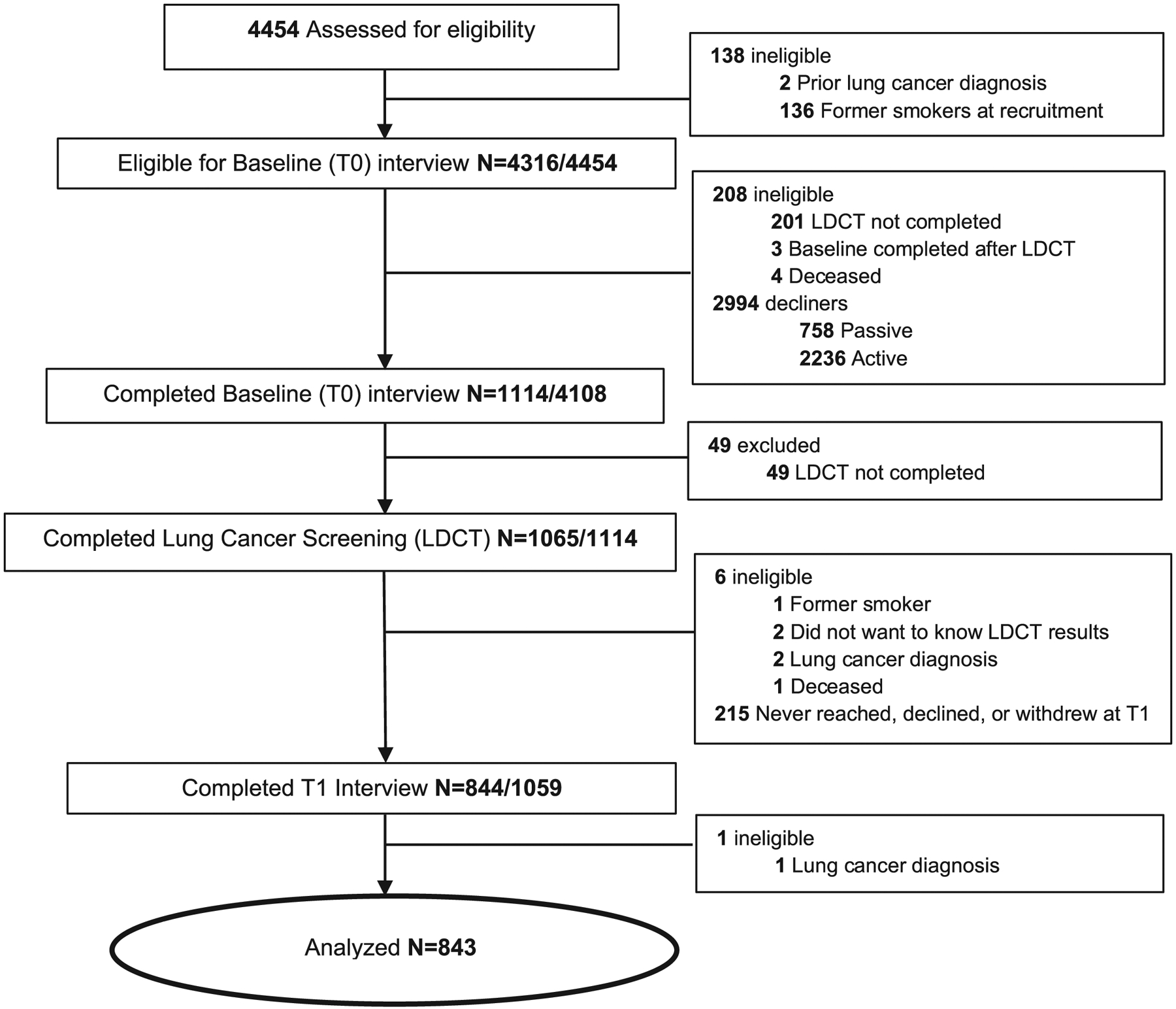
Flow Chart of Analytic Sample from Approach to the T1 Assessment
At the end of the T1 interview, participants were randomized to one of the two study arms: 1) Intensive Telephone Counseling: eight brief telephone sessions + eight weeks of nicotine patches, or 2) Usual Care: three brief telephone sessions + two weeks of nicotine patches.24 Participants did not receive any intervention materials between the T0/T1 assessments. Institutional review board approval was obtained from Georgetown University Medical Center (2016-0651) and Lahey Hospital and Medical Center (2017-022).
Measures
Demographics and Clinical Information.
We collected self-reported age, gender, race, ethnicity, language, marital status, education, tobacco-related comorbidities (e.g., asthma, emphysema/COPD, chronic bronchitis), and smoking history (pack-years).
Lung Cancer Screening Variables.
Using electronic health records, we assessed whether participants were undergoing their first (baseline) vs. an annual repeat lung screening and the Lung-RADS® screening result of the current scan. We included self-reported data when the electronic health record data were unavailable (N=17). The Lung-RADS® system (American College of Radiology Committee on Lung-RADS®) was used by all study sites to report lung screening results. The Lung-RADS® categories are: Lung-RADS® 0 = incomplete; Lung-RADS® 1 = negative result; Lung-RADS® 2 = benign appearance or behavior; Lung-RADS® 3 = probably benign finding(s); and Lung-RADS® 4 = suspicious with recommended follow-up specialist consultation, imaging and/or diagnostic procedures.
Psychological Characteristics.
At both assessments, we assessed lung cancer comparative risk perceptions (‘Compared to smokers, what do you think your chance of getting lung cancer is in your lifetime?’) on a 5-point Likert scale, from ‘much less risk’ to ‘much greater risk’30 and lung cancer worry (‘How worried are you about getting lung cancer in your lifetime?’) on a 4-point Likert scale, from ‘not at all’ to ‘extremely’.12 We also assessed perceptions of one’s overall health (0=worst health to 100=best health).31
Smoking-related Characteristics.
At both assessments, we captured smoking frequency in the past 30 days (some days; every day; not at all), time to first cigarette (Within 5 minutes; 6–30 minutes; More than 30 minutes)32, and occurrence of a 24-hour quit attempt in the past 7 days (Yes; No).
Outcome Variables.
At both assessments, we assessed readiness to quit using the 10-item Contemplation Ladder.33 We measured motivation to quit (10-point scale), ‘Overall, on a scale from 1 to 10 where 1 is not at all motivated and 10 is extremely motivated, how motivated are you to stop smoking?’ and confidence in quitting (10-point scale) (1=lowest to 10=highest).34 To capture current CPD, participants were asked, ‘How many cigarettes per day do you smoke?’.
Statistical Analysis
We calculated descriptive statistics to characterize the study sample (Table 1). Due to skewed distributions, we collapsed 1) perceived risk from a 5-level to a 3-level variable (much less risk/less risk, same risk, greater risk/much greater risk), 2) lung cancer worry from a 4-level to a 3-level variable (not at all/a little, somewhat, extremely), 3) readiness to quit into a 2-level variable (within the next 30 days to already quit vs. in the next six months/not ready to quit). We also collapsed the Lung-RADS from a 4- to a 2-level variable (negative – Lung-RADS® 1, 2 and positive – Lung-RADS® 3, 4). When these variables were included in the models without collapsing the response categories, the findings remained the same. The McNemar test was used to evaluate changes between the T0 and T1 on readiness to quit. Paired t-tests evaluated changes in motivation to quit and CPD.
Next, we examined bivariate associations of the outcomes (T1 assessments of readiness to quit, motivation to quit, and CPD) with the demographic and clinical characteristics, lung cancer screening variables, and psychological variables (Table 2). In multivariable analyses, we included the T0 characteristics that were significantly associated with the outcomes of readiness to quit, motivation to quit, and CPD at T1, adjusting for the T0 assessment of the outcomes (Table 3). Due to the strong association between perceived risk and lung cancer worry, these variables were entered into separate models. Due to associations between pack-years and the other smoking-related characteristics, all models included only pack-years. The models were adjusted for the number of days between the lung screening and the T1 interview. Analyses adjusting for lung screening site did not impact the findings and thus we have presented the regressions without study site. Data were analyzed using SPSS version 27.
Table 2.
Sample characteristics by post-screening readiness, motivation to quit, and CPD (N=843)
| T1 Readiness to Quit | T1 Motivation to Quit | T1 Cigarettes Per Day | ||
|---|---|---|---|---|
| Next 6 Months to Not Considering Quitting | Next 30 Days to Already Quit | |||
| Demographics & Clinical Information | ||||
| Age | M=63.9 (5.8) | M=63.4 (5.9) | r = −0.11** | r = −0.05 |
| Gender | ||||
| Male | 203 (47.2) | 200 (49.1) | M=6.8 (2.2) | M=17.8 (9.3) *** |
| Female | 227 (52.8) | 200 (51.9) | M=6.7 (2.4) | M=15.7 (8.9) |
| Race | ||||
| White | 382 (90.7) | 359 (88.4) | M=6.7 (2.3) | M=17.1 (9.0) *** |
| Black | 26 (6.2) | 37 (9.1) | M=7.2 (2.2) | M=13.4 (10.3) |
| Other | 13 (3.1) | 10 (2.5) | M=6.1 (3.0) | M=16.8 (9.2) |
| Pack Years | M=49.0 (16.9) | M=46.6 (17.2) * | r = −0.11** | r = 0.35 ** |
| Lung Cancer Screening Variables | ||||
| Lung Cancer Screening History | ||||
| Baseline Scan | 156 (36.3) | 201 (48.8) *** | M=7.0 (2.2) *** | M=16.4 (9.5) |
| Annual Repeat Scan | 274 (63.7) | 211 (51.2) | M=6.5 (2.3) | M=16.9 (8.9) |
| Lung Cancer Screening Result | ||||
| Lung-RADS 1/2 | 388 (90.2) | 368 (89.3) | M=6.7 (2.3) | M=16.6 (9.1) |
| Lung-RADS 3/4 | 42 (9.8) | 44 (10.7) | M=6.8 (2.4) | M=17.2 (9.4) |
| Psychological Characteristics | ||||
| T0 Perceived Risk | ||||
| Lower | 53 (12.9) | 54 (13.5) | M=6.4 (2.5) | M=14.9 (9.5) |
| About the Same | 202 (49.3) | 180 (45.1) | M=6.7 (2.2) | M=16.5 (8.6) |
| Higher Risk | 155 (37.8) | 165 (41.4) | M=6.9 (2.3) | M=17.4 (9.5) * |
| T0 Worry about Lung Cancer | ||||
| Not at all/A Little | 114 (26.9) | 86 (21.0) | M=6.1 (2.5) | M=16.3 (8.8) |
| Somewhat | 177 (41.7) | 161 (39.3) | M=6.5 (2.2) | M=17.1 (9.3) |
| Extremely | 133 (31.4) | 163 (39.8) * | M=7.4 (2.1) *** | M=16.9 (8.8) |
| T0 Overall Health (0=worst health, 100=best health) | M=69.7 (19.3) | M=70.7 (18.1) | r = 0.10 ** | r = −0.10** |
| Smoking-related Characteristics (T0) | ||||
| Cigarettes per day | M=19.8 (9.3) | M=16.6 (8.3) *** | r = −0.19** | r = 0.76 ** |
| Smoking Frequency (past 30 days) | ||||
| On some days | 16 (3.7) | 41 (10.0) | M=7.7 (2.0) *** | M=9.6 (7.4) *** |
| Every day | 413 (96.3) | 370 (90.0) *** | M=6.6 (2.3) | M=17.2 (9.0) |
| Time to First Cigarette | ||||
| Within 5 minutes | 116 (27.2) | 108 (26.3) * | M=6.6 (2.4) | M=19.6 (9.9) *** |
| 6–30 minutes | 208 (48.7) | 171 (41.7) | M=6.6 (2.3) | M=17.8 (8.8) |
| More than 30 minutes | 103 (24.1) | 131 (32.0) | M=7.0 (2.2) | M=12.0 (7.1) |
| 24h Quit Attempt in the last 7 days | ||||
| Yes | 25 (5.8) | 41 (10.0) * | M=7.4 (2.4) * | M=12.0 (8.1) *** |
| No | 404 (94.2) | 370 (90.0) | M=6.7 (2.3) | M=17.1 (9.1) |
| Study Administration Variables | ||||
| Days between Scan and T1 | M=17.4 (12.0) | M=15.8 (9.6) * | r = −0.01 | r = −0.06 |
p < 0.05,
p < 0.01,
p ≤ 0.001
Note: Ethnicity, language, marital status, education level, and number of tobacco-related comorbidities were not significantly associated with the outcome variables (data not shown).
Pearson correlation coefficients were used to determine the association between age, pack-years, overall health, cigarettes per day, and days between the scan and T1 and the outcome variables: Motivation to Quit and Cigarettes per Day.
Table 3.
Logistic & Linear Regression Models Predicting Readiness to Quit and Motivation to Quit at the T1 Post-Screening Interview (N=843)
| T1 Readiness to Quit Next 30 Days to Already Quit | T1 Motivation to Quit | |||
|---|---|---|---|---|
| Model 1: Perceived Risk | Model 2: Lung Cancer Worry | Model 3: Perceived Risk | Model 4: Lung Cancer Worry | |
| OR [95% CI] | OR [95% CI] | B (SE) | B (SE) | |
| Age | 0.94 [.71–1.2] | 1.0 [.77–1.3] | −0.02 (.01) | −0.01 (.01) |
| Gender | ||||
| Female (ref) | ||||
| Male | 0.99 [.73–1.4] | 1.1 [.82–1.5] | 0.17 (.14) | 0.26 (.13) * |
| Race | ||||
| White (ref) | ||||
| Black | 1.2 [.65–.2.2] | 1.1 [.64–2.0] | 0.09 (.26) | 0.07 (.25) |
| Other | 0.67 [.23–1.9] | 0.87 [.34–2.3] | −0.14 (.44) | −0.26 (.41) |
| Pack-Years | 0.94 [.86–1.0] | 0.95 [.86–1.0] | −0.008 (.004) | −0.007 (.004) |
| Overall Health | 1.0 [.95–1.1] | 1.1 [.97–1.2] | 0.006 (.004) | 0.005 (.004) |
| Lung Cancer Screening History | ||||
| Baseline Scan | 1.8 [1.3–2.5] *** | 1.8 [1.3–2.4] *** | 0.05 (.15) | 0.07 (.14) |
| Annual Repeat Scan (ref) | ||||
| Lung Cancer Screening Result | ||||
| Lung-RADS 1/2 (ref) | ||||
| Lung-RADS 3/4 | 1.1 [.62–1.8] | 0.97 [.58–1.6] | 0.05 (.23) | 0.08 (.22) |
| T1 Perceived Risk | ||||
| Lower risk (ref) | ||||
| Same risk | 0.52 [.32–.86] ** | n/a | 0.06 (.22) | n/a |
| Higher risk | 0.34 [.23–.65] *** | n/a | −0.25 (.22) | n/a |
| T1 Worry about Lung Cancer | ||||
| Not at all/A Little (ref) | ||||
| Somewhat | n/a | 1.2 [.77–1.8] | n/a | 0.28 (.19) |
| Extremely | n/a | 1.8 [1.1–3.0] * | n/a | 0.83 (.22) *** |
p < 0.05,
p < 0.01,
p ≤ 0.001
Note: The results from models 1 & 2 are adjusted for T0 Readiness to Quit and time between scan and T1.
The results from models 3 & 4 are adjusted for T0 Motivation to Quit and time between scan and T1.
The results from models 1 & 3 are adjusted for T0 Perceived Risk.
The results from models 2 & 4 are adjusted for T0 Worry about Lung Cancer.
Due to associations between pack-years and the other smoking-related characteristics, all models included only pack-years.
The results from models 1 & 2 for age, pack-years, and the overall health score are reported for 10 units increase.
RESULTS
The analytic sample (N=843) included all participants who completed the T0 and the T1 interviews. Exclusions are presented in Figure 1. Participants had a mean age of 63.7 (SD=5.9) years, 52.1% were female, 89.6% were White, 6.4% were of Hispanic/Latino/Spanish origin, 1.5% were Spanish-speaking, 49.7% were married or in a marriage-like relationship, and 35.2% had a high school degree/GED or less (Table 1). About one quarter (24.2%) reported three or more tobacco-related comorbidities and participants had an average of 47.8 (SD=17.1) pack-years. At the T1 interview, N=11 (1.3%) self-reported having quit since the T0 assessment for >7 days.
Participants were undergoing their first (42.3%) or annual (57.7%) low-dose CT scan for lung cancer, and 10.2% received a lung screening result requiring follow-up in < 1 year (Lung-RADS® 3, 4). The median time between the scan and the T1 interview was 13.0 days (range: 2–79 days), with 90.3% reached within 30 days of the scan.
We compared participants’ T0 to T1 self-reported readiness to quit, motivation to quit, and CPD. At the T1 (post-receipt of lung screening results), 25.7% reported increased readiness to quit, 9.6% reported decreased readiness, and 64.7% had no change in readiness (Figure 2; p <0.001). Motivation to quit increased between assessments (M=6.5, SD=2.3 vs. M=6.7, SD=2.3; p <0.05). There was a reduction in CPD between the T0 (M=18.2, SD=9.0) and the T1 (M=16.7, SD=9.1; p <0.001).
Figure 2.
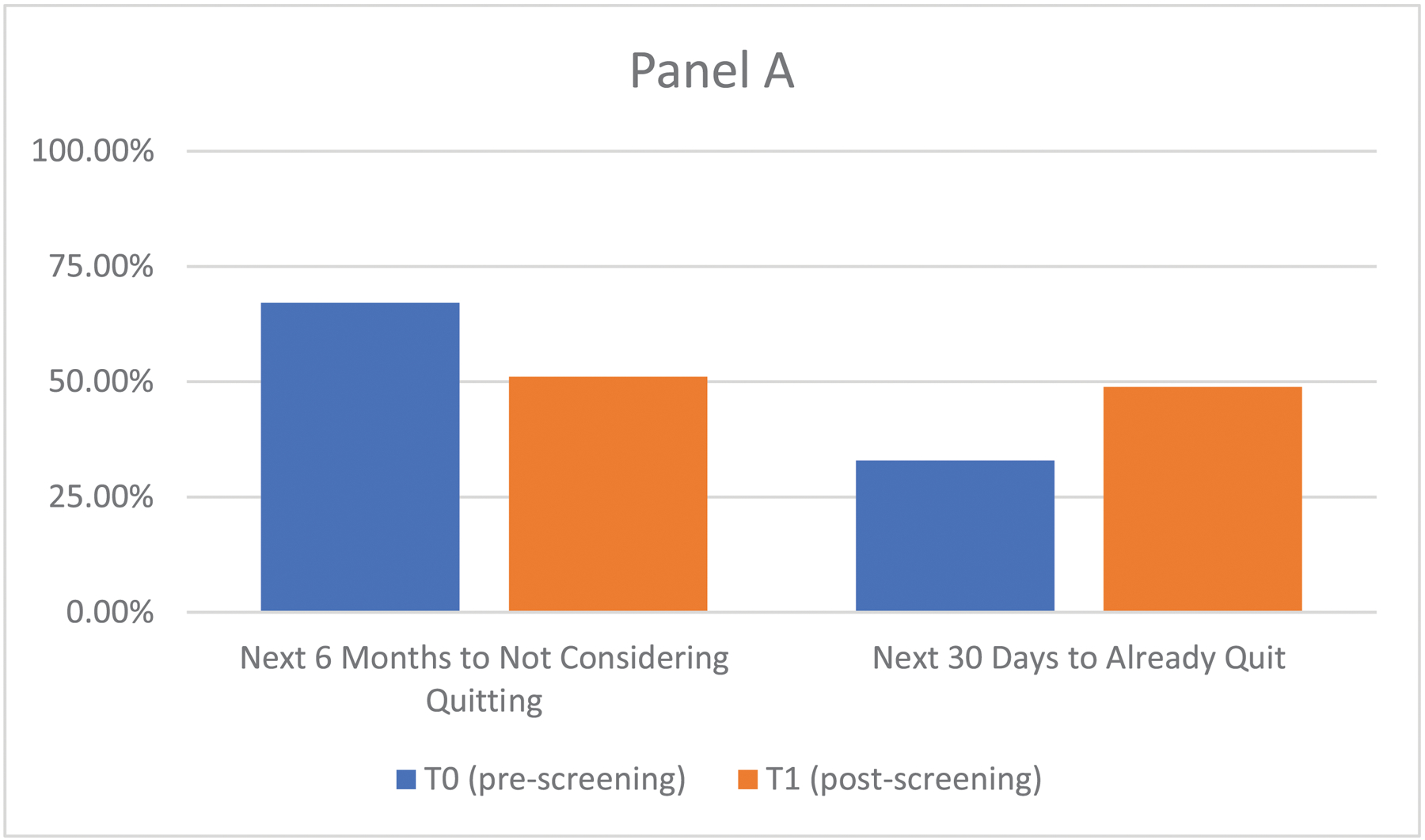
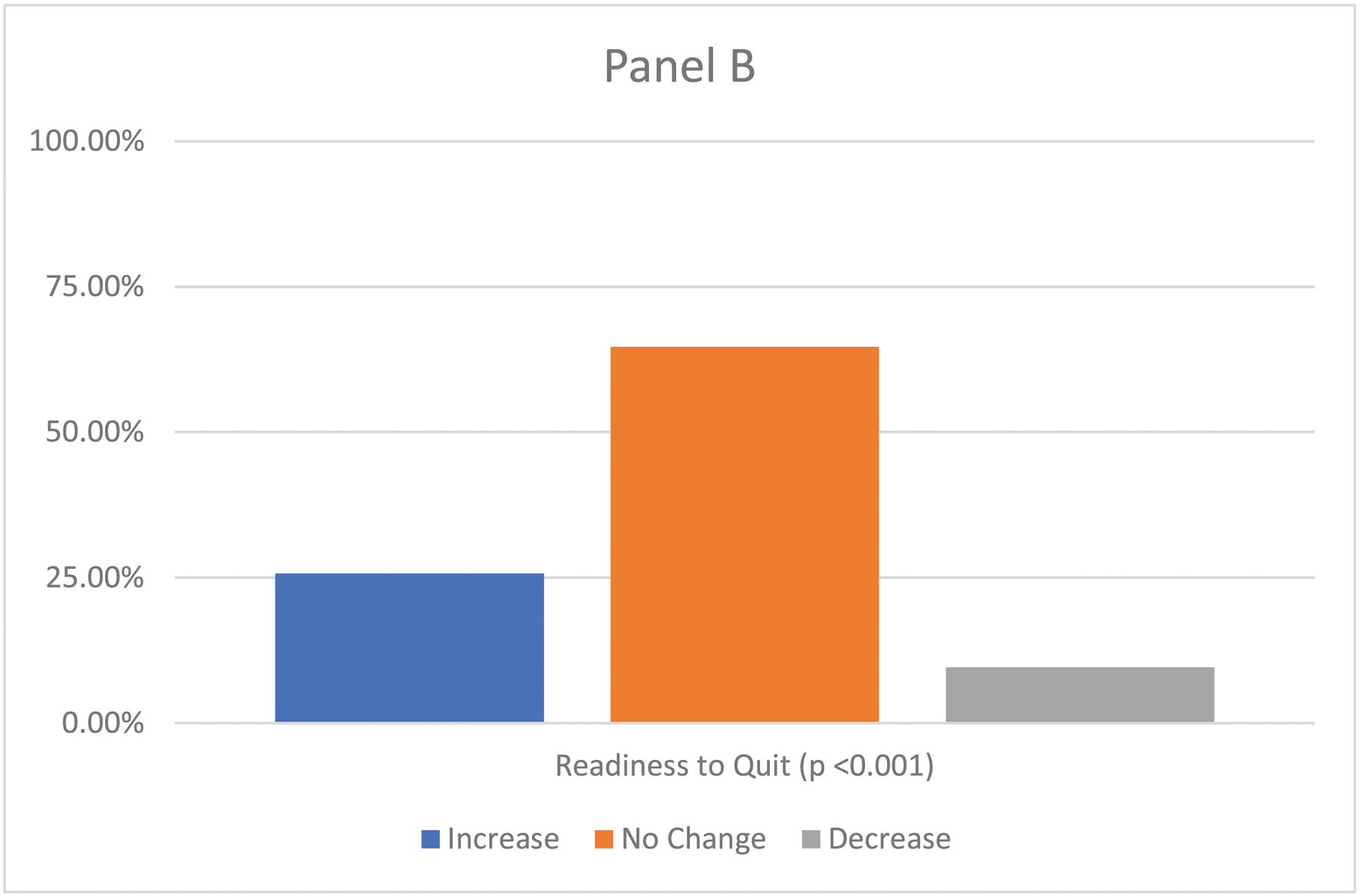
Change from Pre- to Post-Lung Cancer Screening in Readiness to Quit (N=843)
Note: Panel A shows the T0 (pre-screening) and T1 (post-screening) frequency distribution.
Panel B shows changes between the T0 and T1 on readiness to quit using the McNemar test.)
Bivariate analyses are displayed in Table 2. Compared to those not ready to quit in the next 30 days, individuals who were ready to quit in the next 30 days had on average fewer pack-years (p < 0.05), were undergoing their baseline lung screening (p < 0.001), reported extreme lung cancer worry (p < 0.05), were less likely to smoke every day (p < 0.001), and were more likely to have made a 24 hour quit attempt in the last seven days (p < 0.05). Regarding motivation to quit, age and pack-years were negatively correlated with motivation to quit at T1 (p’s < 0.01). Higher motivation to quit was associated with undergoing a baseline lung screening scan, extreme worry, overall good health, smoking on some days, and having made a quit attempt in the past seven days (p’s < 0.05). Regarding T1 cigarettes smoked per day, men smoked more than women (p < 0.001), black individuals smoked fewer CPD compared to other racial groups (p < 0.001), and pack-years were positively associated with CPD (p < 0.01). CPD was significantly higher among those who: considered themselves at high risk for lung cancer (p<0.05), reported poorer overall health (p < 0.01), had higher nicotine dependence (p < 0.001), were daily smokers (p < 0.001), and had not made a recent quit attempt (p < 0.001). The lung cancer screening result was not associated with the outcomes.
Readiness to Quit.
We conducted two logistic regression models predicting T1 (post-screening) readiness to quit, controlling for T0 readiness, T0 perceived risk, T0 worry, and the time between the scan and the T1 assessment (Table 3). In Model 1, compared to individuals who reported lower perceived risk relative to others, individuals reporting the same risk as others (OR=0.52, 95% CI 0.32–0.86) as well as higher risk than others (OR=0.39, 95% CI 0.23–0.65) were less likely to be ready to quit in the next 30 days at the post-screening assessment. In Model 2, compared to individuals who reported no worry/ little worry about lung cancer, those who were extremely worried at T1 were more likely to be ready to quit in the next 30 days (OR=1.8, 95% CI 1.1–3.0). In both models, compared to those undergoing an annual repeat lung screening scan, individuals undergoing a baseline lung screening scan were almost twice as likely to be ready to quit in the next 30 days (p’s < 0.001).
Motivation to Quit.
In linear regression models (Table 3), perceived risk was not significantly associated with post-screening motivation (Model 3). In Model 4, extreme worry about lung cancer was associated with higher motivation to quit post-screening (b=0.83, p < 0.001).
Cigarettes per Day.
In linear regression models, there were no significant associations between CPD and the demographic and clinical characteristics, lung cancer screening history, Lung-RADS, or psychological variables (data not shown).
DISCUSSION
There have been conflicting findings regarding whether undergoing lung screening and receiving the screening result may promote readiness and motivation to quit or a reduction in smoking. Prior to the onset of the intervention component of the LSTH trial, we assessed changes in readiness to quit, motivation to quit, and cigarettes per day to assess the extent to which undergoing lung screening may serve as a teachable moment. This study design allowed for the planned analysis in which we assessed smoking attitudes and behaviors before undergoing the lung screening scan and again following receipt of the scan results, prior to randomization and delivery of the cessation intervention.
In multivariable regression analyses, compared to individuals who reported no worry/little worry, those who were extremely worried about lung cancer at T1 were more likely to be ready to quit in the next 30 days, as well as to report higher motivation to quit. The heightened emotional response observed, as exhibited by high lung cancer worry, was consistent with the teachable moment heuristic. This finding supports previous research regarding the immediate heightened emotional response that may be caused by undergoing cancer screening.35,36
Regarding perceived risk, compared to individuals who reported lower perceived risk, individuals reporting the same risk as well as higher risk were less likely to be ready to quit in the next 30 days at the post-screening assessment. This finding was counter to the conceptual framework guiding this work, that higher perceived risk is associated with being more ready to quit smoking.8,9,36 However, prior research supports the link between higher perceived risk and lower self-efficacy to quit smoking21,37, as individuals who smoke may have high perceived risk for lung cancer while also holding a negative attitude toward quitting. These results suggest that, among patients undergoing lung screening, efforts to increase perceived risk for lung cancer may be counterproductive in increasing readiness and motivation to quit.
In addition to these teachable moment constructs, we found that individuals undergoing a baseline lung screening scan were more likely to report being ready to quit in the next 30 days relative to those undergoing an annual repeat lung screening scan. Although patients should be connected with tobacco treatment at all timepoints in the lung screening process, engaging patients in tobacco treatment may be particularly important among those undergoing their first scan. The lung screening result was not statistically significant in any of the models, which differed from some prior findings,15–18 while also supporting other prior findings.19–23 While the lack of a significant relationship may be due to limited power in our study, this relationship between the lung screening result and tobacco use outcomes is of great interest and requires further research. In the LSTH trial, we plan to assess whether the lung screening result has a moderating impact on the cessation interventions.
Additionally, the multivariable models did not indicate any significant associations between the variables of interest and changes in the amount of smoking. Park37 examined pre-lung screening risk perceptions and found significant associations with quitting intentions, while another study led by Park and colleagues did not find a significant impact of perceived risk on change in smoking behaviors over time.20
In the bivariate analysis, the smoking-related characteristics changed in the expected direction on readiness to quit, motivation to quit, and CPD. However, we are unable to determine the causal relationship. For example, whether an individual becomes ready to quit and then starts smoking less, or whether smoking less results in becoming more ready is unknown and warrants further study.
This study adds to the literature on smoking behaviors among patients undergoing lung screening. Past studies have varied in the timing of assessments with several measuring the impact one year later.14,16,18 A strength of our study is the short-term assessment of smoking attitudes and behaviors following receipt of screening results, with the majority of the sample (90.3%) completing the post-screening assessment within 30 days of the scan. Understanding immediate changes following screening can inform future intervention targets. Some studies that have assessed the impact of screening on smoking behavior have offered a brief smoking cessation intervention.17,19,23 Our study aimed to assess the immediate impact of screening in the absence of a smoking cessation intervention and prior to randomization. Prior research that has evaluated the association between lung screening and smoking have not adjusted for covariates resulting in potentially biased results. We adjusted for potential confounding effects in the multivariable analysis and found other important factors that were associated with the short-term changes reported.
The results should be interpreted in light of the study’s limitations. The majority of study participants were White, reflecting the population of individuals undergoing screening during this period.38 Due to the low percentage of non-White participants, this study is unable to examine whether the associations observed are consistent across other groups. With the expanded lung screening criteria that are expected to increase the number of racial and ethnic minorities eligible and the implementation of more lung screening programs in diverse, community-based settings, future studies should examine these changes by subgroups. The present sample may be a more motivated group relative to the those who did not remain in the trial, because they were retained at the T1 (post-lung screening) interview. In a separate paper, we examined characteristics of those individuals who were retained vs. dropped out between the two assessments.39 In brief, retention was higher among those who smoked <20 cigarettes per day, were more highly educated (more than high school/GED), somewhat worried about lung cancer (vs. not at all worried), undergoing their annual LCS exam (vs. baseline scan), and had Lung-RADS® 2/3 exam results compared to Lung-RADS® 1. Regarding how this sample may compare to a larger population, there has been limited research on smoking attitudes among the population of people undergoing lung screening, but some studies have reported higher quit rates among participants in lung screening programs compared to the general population of people who smoke that may be explained by differences in background demographics and levels of motivation.40–42 A positive screening result has been associated with changes in smoking-related behavior in previous studies15–18,22, however, only 10% of our sample had a positive screening result (Lung-RADS® 3 or 4), which may have limited our power to detect this association. The post-lung screening perceived risk and lung cancer worry variables were assessed at the same time point as the outcomes, which limits our ability to conclude that these variables are in the causal path of readiness, motivation to quit, and CPD. In the larger trial, we will evaluate mediators, including teachable moment factors, on the cessation outcomes at 3-, 6- and 12-months.
In this observational study, the results suggest that the experience of undergoing lung screening was related to changes in readiness and motivation to stop smoking, in part due to increased worry about lung cancer. These findings support the provision of evidence-based tobacco treatment in the context of lung screening, which may be particularly effective when individuals are experiencing a heightened response to screening. Future research needs to examine the impact of the screening result and explore methods of providing information about tobacco-related health risks while also addressing attitudes about quitting. The larger LSTH trial will address whether a smoking cessation intervention can build on these changes in order to help increase abstinence in the lung screening setting.
ACKNOWLEDGEMENTS
The authors are grateful to all of the participants who contributed their time. To the lung cancer screening sites: Georgetown University Medical Center (GUMC), Washington, DC, Lahey Hospital & Medical Center, Burlington, MA, Hackensack University Medical Center (HUMC), Hackensack, NJ, Baptist Hospital of Miami, Miami, FL, Hartford Hospital, Hartford, CT, UnityPoint Health, Des Moines, IA, MedStar Shah Medical Group, Ft. Washington, MD, Anne Arundel Medical Center (AAMC), Annapolis, MD. To the site investigators: Eric Anderson (GUMC), Juan Batlle (Baptist Health), Harry Harper (HUMC), Andrea McKee (Lahey), Brady McKee (Lahey), Vicky Parikh (MedStar Shah), Ellen Dornellas (Hartford), Judith Howell (UnityPoint), Maria M. Geronimo (AAMC). To our tobacco treatment specialists: Claudia Campos, Marisa Cordon, Danielle Deros, Ellie Eyestone, Jennifer Stephens, and Shelby Fallon. To our project coordinators, interviewers, and the entire LSTH study team: Danielle Deros, Shelby Fallon, Jennifer Stephens, Emily Kim, Jen-Yuan Kao, Daisy Dunlap, Sarah Hutchison, Julia Friberg, Lisa Charles, and Ryan Anderson.
Funding:
This study was funded by the National Cancer Institute, Integrating Smoking Cessation Interventions with Lung Cancer Screening Programs: A Randomized Trial (R01CA207228) as part of the NCI’s Smoking Cessation at Lung Examination (SCALE) collaboration.
Footnotes
Prior Abstract Presentation: Williams RM, Yang, F, Charles, L, Cordon, M, Eyestone, E, Smith, L, Luta, G, Davis, K, McKee, B, Regis, S, Stanton, C, Parikh, V, & Taylor, KL. (February 2021). Smoking Attitudes and Behaviors Following Lung Cancer Screening: Assessing Short-Term Changes Among Participants in a Telephone-Based Cessation Trial Prior to Tobacco Treatment. Poster accepted as a Featured Poster Presentation at the 2021 Society for Research on Nicotine and Tobacco Annual Meeting, Virtual.
Declaration of interests: Dr. McKee reports personal fees from UptoDate Inc, AstraZeneca, and serves as a Board Member on Rescue Lung Society, Inc. No other authors have conflicts of interest to report.
REFERENCES
- 1.Aberle DR. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503–513. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 4.Landy R, Young CD, Skarzynski M, et al. Using Prediction-Models to Reduce Persistent Racial/Ethnic Disparities in Draft 2020 USPSTF Lung-Cancer Screening Guidelines. J Natl Cancer Inst. Published online January 5, 2021. doi: 10.1093/jnci/djaa211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Accessed December 24, 2019. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274
- 6.Wender R, Fontham ETH, Barrera E, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–117. doi: 10.3322/caac.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanner NT, Kanodra NM, Gebregziabher M, et al. The Association between Smoking Abstinence and Mortality in the National Lung Screening Trial. Am J Respir Crit Care Med. 2016;193(5):534–541. doi: 10.1164/rccm.201507-1420OC [DOI] [PubMed] [Google Scholar]
- 8.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156 [DOI] [PubMed] [Google Scholar]
- 9.McBride CM, Blocklin M, Lipkus IM, Klein WMP, Brandon TH. Patient’s lung cancer diagnosis as a cue for relatives’ smoking cessation: evaluating the constructs of the teachable moment. Psychooncology. 2017;26(1):88–95. doi: 10.1002/pon.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kathuria H, Koppelman E, Borrelli B, et al. Patient-Physician Discussions on Lung Cancer Screening: A Missed Teachable Moment to Promote Smoking Cessation. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2020;22(3):431–439. doi: 10.1093/ntr/nty254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pistelli F, Aquilini F, Falaschi F, et al. Smoking Cessation in the ITALUNG Lung Cancer Screening: What Does “Teachable Moment” Mean? Nicotine Tob Res Off J Soc Res Nicotine Tob. 2020;22(9):1484–1491. doi: 10.1093/ntr/ntz148 [DOI] [PubMed] [Google Scholar]
- 12.Taylor KL, Cox LS, Zincke N, Mehta L, McGuire C, Gelmann E. Lung cancer screening as a teachable moment for smoking cessation. Lung Cancer. 2007;56(1):125–134. doi: 10.1016/j.lungcan.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 13.Zeliadt SB, Heffner JL, Sayre G, et al. Attitudes and Perceptions About Smoking Cessation in the Context of Lung Cancer Screening. JAMA Intern Med. 2015;175(9):1530–1537. doi: 10.1001/jamainternmed.2015.3558 [DOI] [PubMed] [Google Scholar]
- 14.Balata H, Traverse-Healy L, Blandin-Knight S, et al. Attending community-based lung cancer screening influences smoking behaviour in deprived populations. Lung Cancer Amst Neth. 2020;139:41–46. doi: 10.1016/j.lungcan.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 15.Brain K, Carter B, Lifford KJ, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK Lung Cancer Screening Trial. Thorax. 2017;72(10):912–918. doi: 10.1136/thoraxjnl-2016-209690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tammemägi MC, Berg CD, Riley TL, Cunningham CR, Taylor KL. Impact of lung cancer screening results on smoking cessation. J Natl Cancer Inst. 2014;106(6):dju084. doi: 10.1093/jnci/dju084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsend CO, Clark MM, Jett JR, et al. Relation between smoking cessation and receiving results from three annual spiral chest computed tomography scans for lung carcinoma screening. Cancer. 2005;103(10):2154–2162. doi: 10.1002/cncr.21045 [DOI] [PubMed] [Google Scholar]
- 18.Styn MA, Land SR, Perkins KA, Wilson DO, Romkes M, Weissfeld JL. Smoking behavior 1 year after computed tomography screening for lung cancer: Effect of physician referral for abnormal CT findings. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2009;18(12):3484–3489. doi: 10.1158/1055-9965.EPI-09-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Aalst CM, van den Bergh KAM, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax. 2010;65(7):600–605. doi: 10.1136/thx.2009.133751 [DOI] [PubMed] [Google Scholar]
- 20.Park ER, Gareen IF, Jain A, et al. Examining whether lung screening changes risk perceptions: National Lung Screening Trial participants at 1-year follow-up. Cancer. 2013;119(7):1306–1313. doi: 10.1002/cncr.27925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park ER, Streck JM, Gareen IF, et al. A qualitative study of lung cancer risk perceptions and smoking beliefs among national lung screening trial participants. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2014;16(2):166–173. doi: 10.1093/ntr/ntt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slatore CG, Baumann C, Pappas M, Humphrey LL. Smoking behaviors among patients receiving computed tomography for lung cancer screening. Systematic review in support of the U.S. preventive services task force. Ann Am Thorac Soc. 2014;11(4):619–627. doi: 10.1513/AnnalsATS.201312-460OC [DOI] [PubMed] [Google Scholar]
- 23.Ashraf H, Saghir Z, Dirksen A, et al. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax. 2014;69(6):574–579. doi: 10.1136/thoraxjnl-2013-203849 [DOI] [PubMed] [Google Scholar]
- 24.Golden SE, Ono SS, Melzer A, et al. “I Already Know That Smoking Ain’t Good for Me”: Patient and Clinician Perspectives on Lung Cancer Screening Decision-Making Discussions as a Teachable Moment. Chest. 2020;158(3):1250–1259. doi: 10.1016/j.chest.2020.03.061 [DOI] [PubMed] [Google Scholar]
- 25.Poghosyan H, Kennedy Sheldon L, Cooley ME. The impact of computed tomography screening for lung cancer on smoking behaviors: a teachable moment? Cancer Nurs. 2012;35(6):446–475. doi: 10.1097/NCC.0b013e3182406297 [DOI] [PubMed] [Google Scholar]
- 26.Piñeiro B, Simmons VN, Palmer AM, Correa JB, Brandon TH. Smoking cessation interventions within the context of Low-Dose Computed Tomography lung cancer screening: A systematic review. Lung Cancer. 2016;98:91–98. doi: 10.1016/j.lungcan.2016.05.028 [DOI] [PubMed] [Google Scholar]
- 27.Taylor KL, Deros DE, Fallon S, et al. Study protocol for a telephone-based smoking cessation randomized controlled trial in the lung cancer screening setting: the lung screening, tobacco, and health trial. Contemp Clin Trials. 2019;82:25–35. doi: 10.1016/j.cct.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph AM, Rothman AJ, Almirall D, et al. Lung cancer screening and smoking cessation clinical trials. SCALE (smoking cessation within the context of lung cancer screening) collaboration. Am J Respir Crit Care Med. 2018;197(2):172–182. doi: 10.1164/rccm.201705-0909CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deros DE, Hagerman CJ, Kramer JA, et al. Change in amount smoked and readiness to quit among patients undergoing lung cancer screening. J Thorac Dis. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn EJ, Rayens MK, Hopenhayn C, Christian WJ. Perceived risk and interest in screening for lung cancer among current and former smokers. Res Nurs Health. 2006;29(4):359–370. doi: 10.1002/nur.20132 [DOI] [PubMed] [Google Scholar]
- 31.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy Amst Neth. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 33.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 1991;10(5):360–365. doi: 10.1037//0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- 34.Bertholet N, Gaume J, Faouzi M, Gmel G, Daeppen JB. Predictive value of readiness, importance, and confidence in ability to change drinking and smoking. BMC Public Health. 2012;12:708. doi: 10.1186/1471-2458-12-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borrelli B, McQuaid EL, Tooley EM, et al. Motivating parents of kids with asthma to quit smoking: the effect of the teachable moment and increasing intervention intensity using a longitudinal randomized trial design. Addict Abingdon Engl. 2016;111(9):1646–1655. doi: 10.1111/add.13389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner J, Pond GR, Tremblay A, et al. Risk Perception Among a Lung Cancer Screening Population. Chest. 2021;160(2):718–730. doi: 10.1016/j.chest.2021.02.050 [DOI] [PubMed] [Google Scholar]
- 37.Park ER, Ostroff JS, Rakowski W, et al. Risk perceptions among participants undergoing lung cancer screening: baseline results from the National Lung Screening Trial. Ann Behav Med Publ Soc Behav Med. 2009;37(3):268–279. doi: 10.1007/s12160-009-9112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kee D, Wisnivesky J, Kale MS. Lung Cancer Screening Uptake: Analysis of BRFSS 2018. J Gen Intern Med. Published online September 21, 2020. doi: 10.1007/s11606-020-06236-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim E, Williams RM, Eyestone E, et al. Predictors of attrition in a smoking cessation trial conducted in the lung cancer screening setting. Contemp Clin Trials. 2021;106:106429. doi: 10.1016/j.cct.2021.106429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minnix JA, Karam-Hage M, Blalock JA, Cinciripini PM. The importance of incorporating smoking cessation into lung cancer screening. Transl Lung Cancer Res. 2018;7(3):272–280. doi: 10.21037/tlcr.2018.05.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen JH, Tønnesen P, Ashraf H. Smoking cessation and lung cancer screening. Ann Transl Med. 2016;4(8). doi: 10.21037/atm.2016.03.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer. 2011;73(3):325–331. doi: 10.1016/j.lungcan.2010.12.018 [DOI] [PubMed] [Google Scholar]


