Abstract
Background.
The remaining lifetime risk (RLR) is the probability of developing an outcome over the remainder of one’s lifespan at any given age. The RLR for atherosclerotic cardiovascular disease (ASCVD) in three 20-year periods were assessed using data from a single community-based cohort study of predominantly White participants.
Methods.
Longitudinal data from the Framingham Study in three epochs (epoch 1, 1960–1979; epoch 2, 1980–1999; epoch 3, 2000–2018) were evaluated. The RLR of a first ASCVD event (myocardial infarction, coronary heart disease death, or stroke) from age 45 (adjusting for competing risk of death) in the three epochs were compared overall, and in the following strata: sex, body mass index (BMI), BP and cholesterol categories, diabetes, smoking, and Framingham Risk Score (FRS) groups.
Results:
There were 317,849 person-years of observations over the three epochs (56% women, 94% White) and 4855 deaths occurred. Life expectancy rose by 10.1 (men) to 11.9 (women) years across the three epochs. There were 1085 ASCVD events over 91,330 person-years in epoch 1, 1330 ASCVD events over 107,450 person-years in epoch 2, and 775 ASCVD events over 119, 069 person-years in epoch 3. The mean age at onset of a first ASCVD event was higher in the third epoch by 8.1 (men) to 10.3 (women) years compared with the first epoch. The RLR of ASCVD from age 45 declined from 43.7 percent in Epoch 1 to 28.1 percent in Epoch 3 (p<0.0001), a finding that was consistent in both sexes (RLR Epoch 1 vs. Epoch 3: 36.3 vs. 26.5 percent (women); 52.5 vs. 30.1 (men); p<0.001 for both). The lower RLR of ASCVD in the last two epochs was observed consistently across BMI, BP, cholesterol, diabetes, smoking, and FRS strata (p<0.001 for all). The RLR of coronary heart disease events and stroke declined over time in both sexes (p<0.001).
Conclusions.
Over the last six decades, mean life expectancy increased and the RLR of ASCVD decreased in the community-based, predominantly White Framingham Study. The residual burden of ASCVD underscores the importance of continued and effective primary prevention efforts with better screening for risk factors and their effective treatment.
Keywords: Cardiovascular disease, lifetime risk, epidemiology, cohort studies, temporal trends
Introduction
Atherosclerotic cardiovascular disease (ASCVD) is a leading cause of morbidity and mortality worldwide,1,2 affecting over 500 million individuals globally and accounting for 19 million deaths annually.3 In the United States, ASCVD afflicts about 26 million people,4 and results in 2 million hospitalizations and 400,000 deaths every year.5 Therefore, preventing ASCVD via effective risk assessment, communication, and management is a public health priority.6
An important ASCVD risk communication tool in this context is the remaining lifetime risk (RLR) statistic, which is the probability that an individual at a given age will experience the risk of a disease over the remainder of their life (until death), conditional on surviving up to that age free of the disease of interest. The RLR metric provides quantitative information to laypersons regarding the absolute risk of developing ASCVD over their life course in an intuitive and relatable way in contrast with indicators such as disease incidence rates that are often difficult for laypersons to understand.7–10 The RLR measure has been advocated as a tool to motivate younger people with adverse risk factor profiles (who may have low short-term ASCVD risk but greater long-term risk) to adopt healthier lifestyles and manage their risk factors effectively.10 Therefore, several investigations have reported on the RLR of ASCVD (and its subtypes: coronary heart disease [CHD] and stroke), typically using data from one or more community-based cohorts or large administrative databases.11–22
However, data with regard to secular trends in the RLR of ASCVD are scanty,18 possibly because such data require serial longitudinal observations of community-based samples over extended periods. Such data may be critical public health indicators for monitoring temporal changes in ASCVD burden and may serve as an indirect yardstick of the effectiveness of primary prevention measures. Leveraging the continuous longitudinal surveillance for ASCVD (defined using the same criteria consistently23) of the community-based Framingham Heart Study (FHS) cohorts over six decades (1960–2018),24 the present investigation evaluated the temporal trends in the RLR of a first ASCVD event from age 45. The following metrics were assessed: temporal changes in the RLR of ASCVD that reflect the balance between factors that can increase (e.g., greater life expectancy, increasing prevalence of high blood pressure [BP]25, excess adiposity,26 and diabetes27) versus those that can reduce ASCVD risk (decline in smoking, and better control of high BP25 and dyslipidemia28); secular trends in RLR of CHD and stroke (components of atherosclerotic ASCVD events) that might track with corresponding trends in the RLR of atherosclerotic ASCVD.
Methods
All data and materials have been made publicly available at the National Heart, Lung, and Blood Institute data repository (Biologic Specimen and Data Repositories Information Coordinating Center) and can be accessed at https://biolincc.nhlbi.nih.gov/studies/fhs/
Study Samples
The FHS includes six different cohorts.29–32 The Original (Generation 1, N=5209) cohort was enrolled in 1948 and the Offspring (Generation 2, N=5124; children of the original cohort and their spouses) cohort in 1971. In 1996, the multi-ethnic Omni-1 (N=507) cohort was recruited. In 2002, the Third Generation (Generation 3, N=4095) cohort, the New Offspring Spouse (NOS, N=103; spouses of the Offspring cohort participants not enrolled in 1971) group, and the multi-ethnic Omni-2 (N=410) cohort were recruited. The three FHS Generation cohorts and the NOS group are predominantly White. Data from all FHS cohorts between 1960 and 2018 were used for the present investigation. The design and sampling schema of the six FHS cohorts have been described.29–32 The FHS cohorts are examined every two (Original cohort) to four years (all other cohorts) when participants undergo a detailed cardiovascular-focused medical history, a brief physical examination (standardized anthropometry, electrocardiography, and BP measurements), and laboratory testing for ASCVD risk factors. All FHS participants provided written informed consent to participate in the core study protocols approved by the Institutional Review Board at the Boston University Medical Center.
Time Windows (Epochs)
Three separate epochs, each bridging 20 years, were considered observation periods. The first epoch spanned from January 1, 1960, to December 31, 1979; the second epoch extended from January 1, 1980, to December 31, 1999; and the third epoch crossed from January 1, 2000, through December 31, 2018, which reflects the maximum available follow-up to date. Thus, Generations 1 and 2 contributed to observations in the first epoch, Generations 1 and 2 and Omni-1 cohorts to observations in the second epoch and all six cohorts contributed observations to the third epoch.
Selection of Study Samples
For all three epochs, participants needed to be at least 45 years of age and no older than 94 to enter the observation period. Follow-up of participants in each epoch began either on the first day of the epoch (for pre-enrolled participants age 45 or more), the date of their first examination (if enrollment occurred during the epoch), or the date within the epoch on which they reached age 45. Individuals with a history of atherosclerotic ASCVD before entering their observation period were excluded. Participants without an ASCVD event during the observation window were censored either at age 94 or at the end of the epoch, whichever occurred first. The age 94 was chosen as the upper limit of follow-up (consistent with previous reports16,17,19,33) because few participants survive beyond this age threshold. Details of how participants provide observations in each epoch that contribute to the estimation of RLR estimates of ASCVD are detailed in Online Expanded Methods and Supplemental Figure 1. Thus, participants between age 45 and 94 contributed information in each epoch; individuals could provide up to 50 years of follow-up for ASCVD incidence after age 45 across serial epochs. For instance, a participant free of ASCVD at age 60 at the beginning of an epoch could contribute up to 20 years of observation during that epoch. If free of ASCVD at the end of that epoch (at age 80), the individual could contribute up to an additional 14 years of observation in the next epoch. Thus, it is possible to estimate the 30-year and the 40-year incidence of ASCVD and the RLR of ASCVD from age 45 within each epoch, even though each epoch is only 20-years long.
For the first epoch beginning in 1960, eligible participants included FHS participants from the Generation 1 and Generation 2 cohorts. For the second epoch starting in 1980, eligible participants were from the Generation 1, Generation 2, and Omni-1 cohorts. For the third epoch beginning in 2000, eligible participants included all available FHS cohorts: Generations 1 to 3, Omni-1, Omni-2, and NOS. For all epochs, individuals were required to have participated in an FHS examination and have follow-up information available during the observation period to be eligible for the present investigation.
Definitions of risk factor strata
RLR estimates for ASCVD vary based on the risk factor profile of individuals.11,16,33 Accordingly, participants were stratified according to levels of the following risk factors (exposure ascertained between the ages of 40–49): sex, body mass index (BMI), BP, total cholesterol (Tc), diabetes mellitus, current smoking, and the Framingham general ASCVD risk score (FRS).34 To maximize sample sizes for these stratified analyses, participants missing data for a single risk factor were excluded only from analyses of that specific stratum. Still, they remained eligible for analyses of the remaining risk factors. The derivation of sample sizes in the three epochs is displayed in Supplemental Figure 2.
BMI (kg/m2) was graded as normal (18.5≥BMI<25), overweight (25≥BMI<30), and obese (BMI≥30).35 BP was categorized as normal (untreated systolic [SBP]<130 and diastolic [DBP]<80 mmHg), intermediate (130≥untreated SBP<140 or 80≥untreated DBP<90 mmHg), and hypertensive (SBP≥140 or DBP≥90 mmHg or receiving antihypertensive therapy).36 This definition of hypertension was prevalent in the epochs evaluated, which antedated current37 BP guidelines. Diabetes mellitus was defined as elevated blood glucose (fasting ≥126 or non-fasting ≥200 mg/dL) or the use of anti-hyperglycemic medications.38 Total cholesterol (Tc) was categorized as normal (untreated Tc<200 mg/dL), intermediate (200 ≥untreated Tc<240 mg/d) and high (Tc≥240 mg/dL or use of lipid-lowering treatment). Smoking status was defined as either current smoker or current non-smoker. FRS tertiles were estimated only for White participants (Generations 1 to 3, and NOS). Blood high-density lipoprotein cholesterol (HDL-C) concentrations were assayed infrequently in the first epoch, so analyses of this lipid fraction were possible only in the second and third epochs. For these secondary analyses, HDL-C was ranked as high (≥60 mg/dL), intermediate (60<HDL-C≤40 mg/dL), and low (<40 mg/dL).
ASCVD Outcomes
All FHS participants are under regular surveillance for new-onset ASCVD events, which are adjudicated by an expert panel of three experienced physicians that reviews available medical records from physician office visits, hospitals, and death certificates. The primary analyses concentrated on trends in RLR of a first ASCVD event (myocardial infarction or fatal coronary heart disease [CHD], and fatal or nonfatal atherothrombotic brain infarction [stroke]); these events are the primary focus of ASCVD risk prediction using the pooled cohort equations,39 and the cornerstone of national ASCVD prevention guidelines. Additional analyses evaluated the trends in RLR of CHD events (myocardial infarction or CHD death), strokes, and total ASCVD events (a composite outcome that includes CHD [myocardial infarction, stable and unstable angina], stroke, transient ischemic attacks, peripheral arterial disease [PAD] and heart failure [HF]). Criteria for atherosclerotic and total ASCVD events have been published previously.23
Statistical Methods
The descriptive features of the study samples in the three epochs at the time of risk factor ascertainment (age 45) were described with means (± standard deviations) for continuous variables and frequencies (percentages) for categorical variables. The overall mean survival (by sex) and the mean age at the onset of a first ASCVD event in each epoch were evaluated. The 30-year and 40-year risk, and RLR of ASCVD in each epoch were estimated separately, and the estimates were compared across the three epochs using z-tests.7
Subsets with available data on at least one risk factor were compiled within each epoch. The 30-, 40-year and RLR estimates were stratified by sex, BMI, BP and Tc categories, smoking and diabetes status, and FRS tertiles in each epoch. The methods detailed by Beiser et al.7 for estimating the cumulative incidence (adjusted for competing risk of death; referred to as adjusted cumulative incidence) of ASCVD at 30-, 40- and 50-years of follow-up (the latter corresponds to the RLR from age 45) were used. Corresponding hazard ratios (HR) and P values from Wald chi-square tests were estimated using Fine-Gray sub-distribution hazards models, adjusted for competing risk of death.40 The HR estimates used age as the time scale and left truncation at the age of entry into the risk set.
The main analyses were repeated using data only from the three generations of White FHS participants to assess if the inclusion of minority groups (Omni cohorts) in the most recent epoch might have influenced the results.
Comparisons of the RLR of ASCVD and hazards ratios for ASCVD associated with risk factor strata within and across epochs
RLR estimates of ASCVD from age 45 within and across the three epochs (overall and by risk factor strata) were compared using z tests.7 Fine-Gray subdistribution hazards regression models were used for comparing ASCVD risk among participants in risk factor strata within an epoch (e.g., risk of ASCVD associated with overweight and obesity in epoch 2 relative to normal BMI in epoch 2), and for comparing ASCVD risk associated with a specific risk factor stratum across the three epochs (e.g., in men, risk of ASCVD in epoch 1 versus epoch 2, and risk in epoch 1 versus epoch 3). A sandwich estimator was used to control for some of the same participants contributing information to more than one epoch. The assumption of proportionality of hazards was met for all models.
Comparisons of 30-year, 40-year, and RLR of CHD, stroke, and total ASCVD across the epochs
Analyses were repeated to assess temporal trends in long-term risk of the components of ASCVD, i.e., CHD and stroke. Trends in long-term risk of total ASCVD, an expanded composite outcome comprised of CHD, stroke, PAD, and HF, were also evaluated.
To explore if a healthy survivor bias influenced the results, given that participants could contribute to more than one epoch, sensitivity analyses evaluating the 15-year mortality-adjusted cumulative incidence of ASCVD in each epoch (overall, and by sex and smoking and BMI strata) were conducted with the constraints that participants between ages 45–50 could contribute observations only in one epoch. Trends in non-ASCVD mortality across the epochs were examined to assess if a general improvement in overall health may influence both trends in ASCVD incidence and non-ASCVD mortality.
Statistical significance
A two-tailed P value less than 0.05 was considered statistically significant in all analyses. All analyses were performed using SAS v9.4 (Cary, NC).
Results
Table 1 shows the midlife (at age 45) characteristics of participants followed for ASCVD incidence in each epoch. All observation windows included more women (~56%) than men, and 94% of the participants were White. Midlife (at age 45) values of mean BMI and fasting blood glucose, the proportions with obesity, diabetes, and the percentage of participants using antihypertensive, LDL cholesterol-lowering, and diabetes medications were higher in the last two epochs relative to the first. The average midlife values of BP, Tc, Tc/HDL-C ratio, FRS, and smoking prevalence were lower in participants contributing observations in the recent epochs (Table 1).
Table 1.
Characteristics at age 40–49 years of participants followed for CVD in the three epochs
| Epoch 1 (1960–1979) N=7014* |
Epoch 2 (1980–1999) N=8269* |
Epoch 3 (2000–2018) N=9331* |
p-value⁑ | |
|---|---|---|---|---|
| Women | 3824 (54.5) | 4706 (57.0) | 5272 (56.5) | 0.007 |
| Age at start of observation, years | 52±7 | 57±12 | 57±13 | <0.001 |
| Women in largest risk factor sample* | 2721 (55.0) | 3673 (56.8) | 3958 (56.9) | 0.08 |
| Age at time of risk factor collection, years | 43±3 | 43±3 | 43±3 | NS |
| Body mass index, kg/m2 | 25.7±4.2 | 25.8±4.6 | 26.5±5.2 | <0.001 |
| Body mass categories | <0.001 | |||
| Obese | 649 (13.3) | 948 (14.8) | 1352 (19.7) | |
| Systolic blood pressure, mmHg | 128±18 | 123±16 | 119±15 | <0.001 |
| Diastolic blood pressure, mmHg | 82±11 | 80±11 | 77±10 | <0.001 |
| Hypertensive medication use | 95 (1.4) | 265 (3.3) | 483 (6.3) | <0.001 |
| Blood pressure categories | <0.001 | |||
| Hypertensive | 1469 (29.7) | 1472 (22.7) | 1264 (18.2) | |
| Blood glucose, mg/dL | 88±21 | 91±19 | 94±17 | <0.001 |
| Diabetes medication use | 35 (0.7) | 45 (0.7) | 65 (0.9) | 0.22 |
| Diabetes mellitus status | <0.001 | |||
| Diabetes mellitus | 43 (0.9) | 80 (1.3) | 127 (1.8) | |
| Total cholesterol, mg/dL | 220±42 | 211±40 | 199±37 | <0.001 |
| Total cholesterol categories | <0.001 | |||
| High | 1332 (28.1) | 1380 (21.8) | 1091 (15.8) | |
| HDL cholesterol, mg/dL | 52±17 | 51±16 | 53±16 | <0.001 |
| HDL cholesterol categories | <0.001 | |||
| Low | 397 (25.6) | 966 (24.4) | 1245 (20.2) | |
| Total/HDL cholesterol | 4.5±1.8 | 4.4±1.7 | 4.1±1.5 | <0.001 |
| LDL cholesterol-lowering medication use | 15 (0.8) | 36 (0.8) | 246 (3.9) | <0.001 |
| Current smoker | 2466 (53.4) | 2643 (42.1) | 1840 (26.5) | <0.001 |
| Framingham risk score (FRS)† | 0.072±0.051 | 0.062±0.046 | 0.055±0.042 | <0.001 |
Values are Mean±SD or n (%). HDL, high-density lipoprotein cholesterol. LDL, low-density lipoprotein cholesterol.
Some available risk factors are less than the total sample size due to a combination of data not collected at a particular exam cycle or participants having missed exams during the eligible age period of 40–49 years. Thus, the largest sample sizes in the three epochs for risk factor analyses were 4947, 6473, and 6955, respectively (see eFigure 1). Of note, for participants age 50 years or older at the beginning of an epoch, the midlife values of risk factors (at age 40–49) may be from examinations attended in an earlier epoch. Also, treatment frequencies for elevated blood pressure, dyslipidemia and diabetes during an epoch may be higher than shown in the Table (which indicates frequencies at age 45) because such treatment increases with age and participants can contribute information between ages 45 and 94 in a given epoch.
Framingham risk score data excludes participants from Omni-1 and Omni-2 cohorts.
ANOVA tests used for continuous variables and chi-squared tests used for categorical variables.
RLR of ASCVD across the epochs
There were 91,330 person-years (53,215 in women), 107,450 person-years (62,264 in women), and 119,069 person-years (68,122 in women) of follow-up (maximum of 20 years in each epoch) during epochs 1, 2, and 3, respectively. Overall, 15.5%, 16.1%, and 8.3% of eligible participants experienced a first ASCVD event in epochs 1, 2, and 3, respectively. Corresponding ASCVD incidence rates (per 1000 person-years) in the at-risk samples were 11.9, 12.4, and 6.5, respectively (data for pooled sexes). The ASCVD incidence rates (per 1000 person-years) were 17.9, 17.6, and 8.1, respectively, in men, and 7.8, 9.0, and 5.4, respectively, in women (p<0.001 for men vs. women in each epoch). The mean age at onset of ASCVD in the three epochs was 64.8, 71.1, and 72.9, respectively, in men and 69.4, 78.0, and 79.7, respectively, in women. Mean life expectancy in the three epochs was 67.9, 75.6, and 78.0 years, respectively, in men and 69.9, 80.0, and 81.8, respectively, in women.
Figure 1 shows the mortality-adjusted cumulative incidence of ASCVD overall (Panel A) and by sex (Panels B–C) in the three epochs. It is evident from Figure 1 that the incidence of ASCVD in the latest epoch peaks later in life in both sexes, extending into the ninth and tenth decades.
Figure 1.
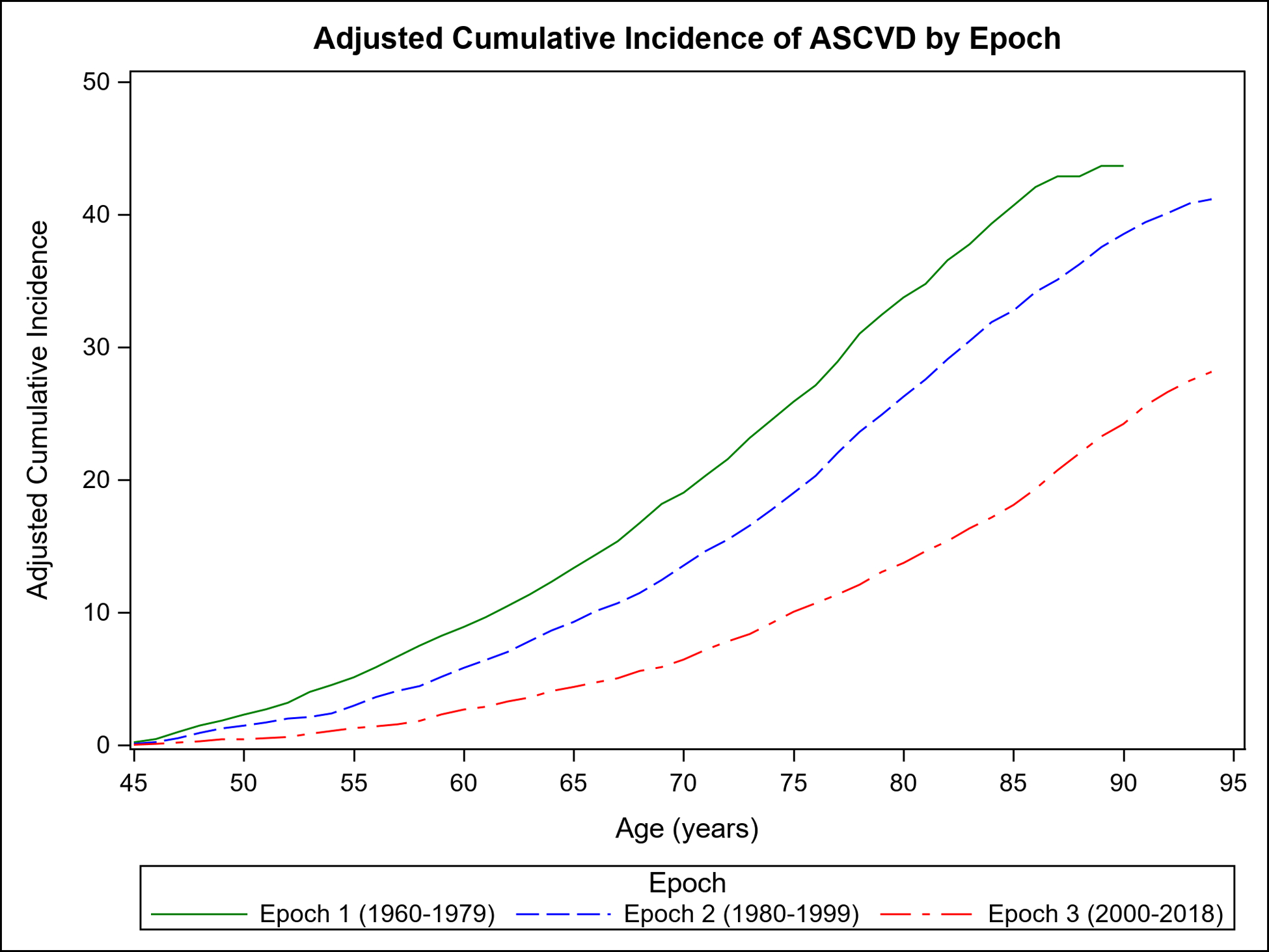
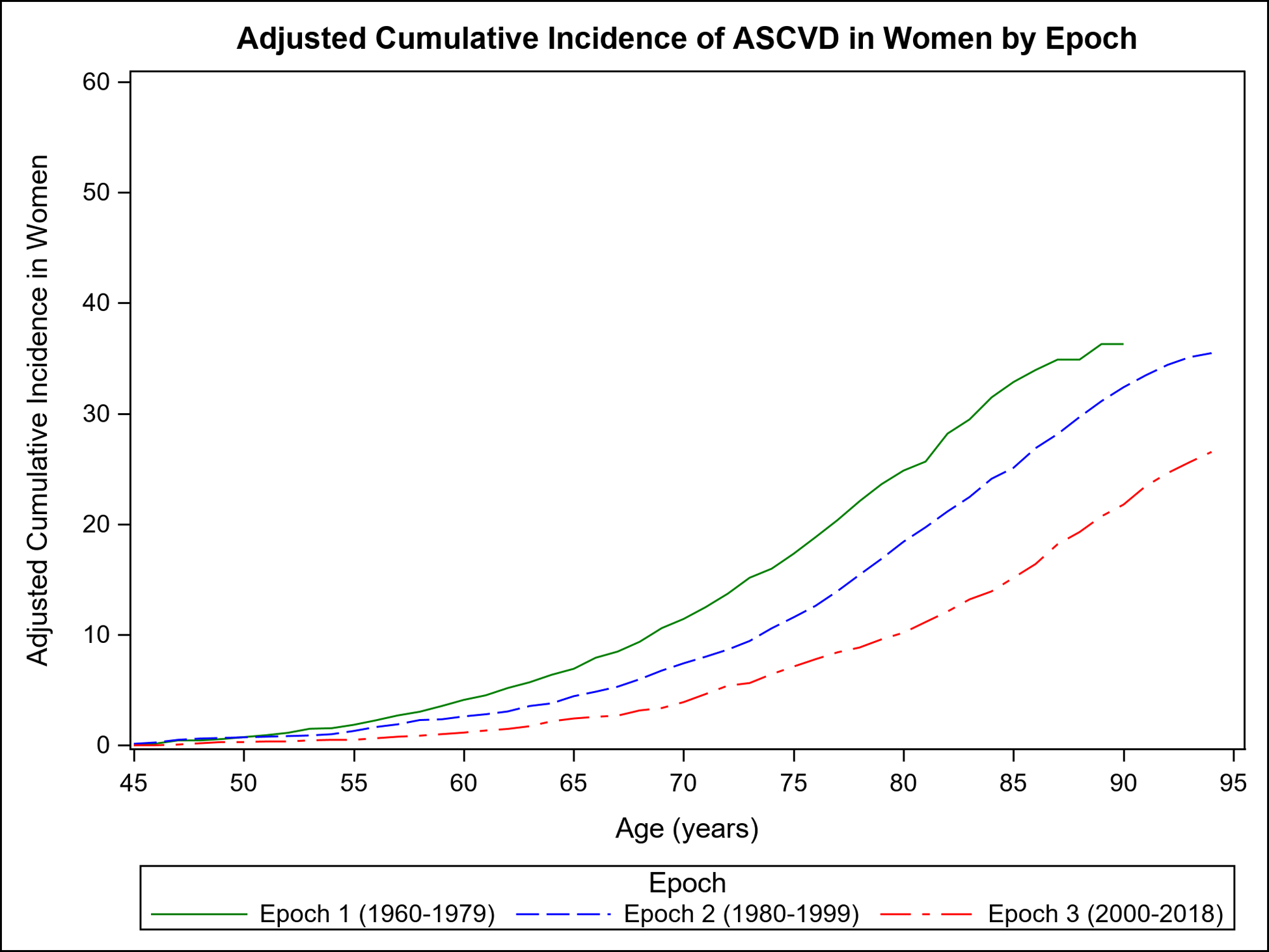
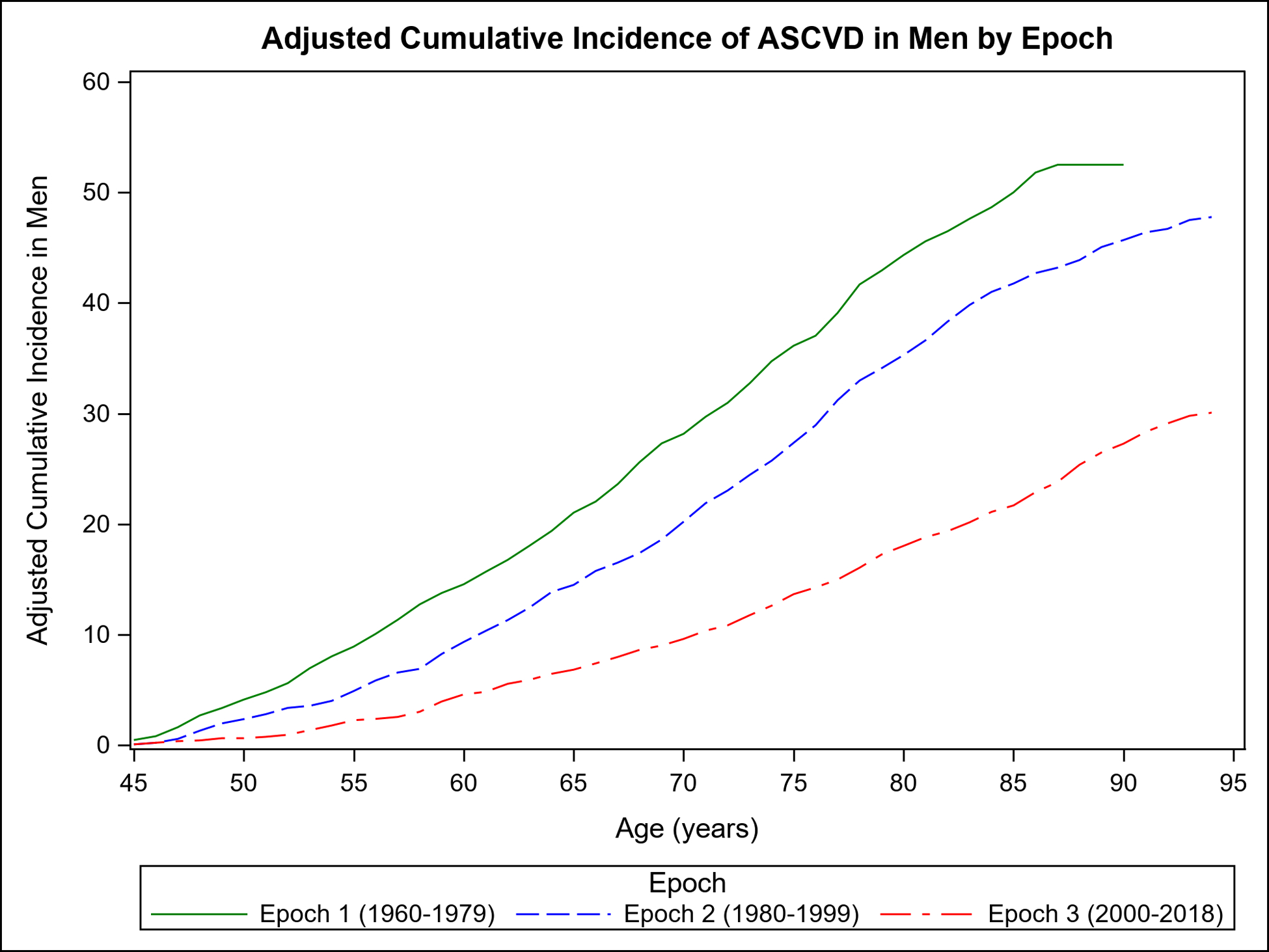
Mortality-adjusted cumulative incidence (%) of ASCVD for participants from age 45 years in each epoch: overall (Panel A) and by sex (Panel B, women; Panel C, men).
Table 2 compares the adjusted cumulative incidence of ASCVD accounting for competing risk of death on follow-up at 30 years, 40 years, and RLR for each epoch. Compared with the first epoch, the 30-year, 40-year risks and RLR of ASCVD were lower across the subsequent epochs overall and in both sexes (p<0.001 for all; Supplemental Table 1); in women, the major decline in RLR of ASCVD occurred between the second and third epochs. Overall, the RLR of ASCVD was 27 (women) to 43 (men) percent lower in the last epoch compared with the first (Supplemental Table 1). The 30-year, 40-year, and RLR of ASCVD remained essentially unchanged in analyses excluding Omni cohort participants (Supplemental Table 2).
Table 2.
Comparison of cumulative incidence of ASCVD from age 45 years across the three epochs, adjusted for competing risk of death
| Mortality-adjusted Cumulative Incidence (95% CI), 1960–1979 | Mortality-adjusted Cumulative Incidence (95% CI), 1980–1999 | Mortality-adjusted Cumulative Incidence (95% CI), 2000–2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30-year | 40-year | Lifetime | 30-year | 40-year | Lifetime | 30-year | 40-year | Lifetime | ||
| ASCVD | ||||||||||
| Pooled sexes | N/PYrs | 827/83436 | 1065/90879 | 1085/91330 | 584/84126 | 1077/101863 | 1330/107450 | 319/95010 | 525/111904 | 775/119069 |
| ACI | 24.5 (23.0–26.0) |
39.3 (37.2–41.5) |
43.7
(40.8–46.6) |
17.8 (16.5–19.1) |
31.9 (30.3–33.5) |
41.2
(39.4–42.9) |
9.2 (8.2–10.2) |
17.2 (15.8–18.5) |
28.1
(26.4–29.9) |
|
| Women | N/PYrs | 293/47995 | 424/52874 | 435/53215 | 186/46258 | 450/58053 | 628/62264 | 120/53215 | 225/63259 | 394/68122 |
| ACI | 16.0 (14.3–17.7) |
31.5 (28.6–34.4) |
36.3
(32.0–40.6) |
10.6 (9.3–12.0) |
24.10 (22.2–26.0) |
35.5
(33.2–37.7) |
6.4 (5.3–7.6) |
13.9 (12.2–15.6) |
26.5
(24.3–28.8) |
|
| Men | N/PYrs | 534/35443 | 641/38007 | 650/38115 | 398/37869 | 627/43811 | 702/45186 | 199/41796 | 300/48646 | 381/50947 |
| ACI | 34.7 (32.3–37.2) |
48.7 (45.5–51.8) |
52.5
(48.8–56.2) |
25.7 (23.6–27.9) |
41.0 (38.5–43.5) |
47.8
(45.2–50.4) |
12.6 (11.0–14.3) |
21.1 (18.9–23.3) |
30.1
(27.4–32.7) |
|
| P-value, Men vs. Women | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.047 | |
ACI= mortality-adjusted cumulative incidence %. N = number of ASCVD events, Pyrs= person-years of observation.
RLR of ASCVD across the epochs within risk factor strata
Supplemental Figure 3 displays the mortality-adjusted cumulative incidence of ASCVD according to categories of BMI (Panels A–C), BP (Panels D–F), diabetes (Panels G–I), cholesterol (Panels J–L), smoking (Panels M–O), and FRS (Panels P–R) in each of the three epochs. The incidence of ASCVD in the third epoch occurs later in life (shifts to the right) in each risk factor stratum.
Table 3 shows the change in 30-year, 40-year, and RLR of ASCVD across the epochs according to risk factor strata; 40-year and RLR estimates for the first epoch could not be estimated because the study sample did not include any participants who lived up to age 94 in that epoch. ASCVD risk estimates within each epoch were higher as the risk factor levels increased (from normal to intermediate to high levels of any given risk factor). Furthermore, compared with the first epoch, 30-year ASCVD risk estimates were lower in all corresponding strata in the second and third epochs. In the third epoch, all three risk estimates (30-year, 40-year, and RLR of ASCVD) were substantially lower (p<0.001 for all) in every risk factor stratum evaluated compared with the second epoch (Table 3). In secondary analyses of HDL-C categories, 30-year ASCVD risk estimates were lower in the third epoch compared with the second epoch (Supplemental Table 3).
Table 3.
Thirty-, Forty-year and Lifetime risk of ASCVD adjusted for death by risk factor strata
| Mortality-adjusted Cumulative Incidence (95% CI), 1960–1979 | Mortality-adjusted Cumulative Incidence (95% CI), 1980–1999 | Mortality-adjusted Cumulative Incidence (95% CI), 2000–2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30-year | 35-year* | Lifetime* | 30-year | 40-year | Lifetime | 30-year | 40-year | Lifetime | ||
| BMI Category | ||||||||||
| Normal | N/PYrs | 195/32237 | 207/32657 | 160/33609 | 346/41750 | 424/43594 | 81/31105 | 152/37538 | 250/40678 | |
| ACI | 17.4 (14.8–19.9) |
25.4 (19.5–31.2) |
12.5 (10.7–14.3) |
25.5 (23.2–27.8) |
36.2
(33.1–39.2) |
7.0 (5.5–8.4) |
14.7 (12.5–16.8) |
25.6
(22.9–28.4) |
||
| Overweight | N/PYrs | 239/24350 | 249/24785 | 211/25664 | 388/30792 | 435/31929 | 97/26375 | 164/31065 | 244/32821 | |
| ACI | 25.2 (22.2–28.2) |
34.0 27.2–40.7) |
21.4 (18.8–24.0) |
38.3 (35.3–41.3) |
46.4
(43.0–49.8) |
10.3 (8.4–12.3) |
19.1 (16.5–21.8) |
31.8
(28.4–35.2) |
||
| Obese | N/PYrs | 101/7662 | 102/7750 | 105/10169 | 151/11426 | 163/11623 | 74/15483 | 102/17147 | 121/17638 | |
| ACI | 35.0 (28.7–41.2) |
37.3 (29.8–44.8) |
26.9 (22.4–31.4) |
41.1 (35.9–46.3) |
48.8
(42.7–55.0) |
15.5 (12.1–18.8) |
24.9 (20.5–29.4) |
33.2
(27.9–38.4) |
||
| P, Overweight vs. Normal | <0.001 | 0.06 | <0.001 | <0.001 | <0.001 | 0.007 | 0.011 | 0.006 | ||
| P, Obese vs. Normal | <0.001 | 0.014 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.013 | ||
| BP Category | ||||||||||
| Normal | N/PYrs | 109/23847 | 113/24082 | 127/32154 | 270/38400 | 307/39611 | 87/42835 | 137/48770 | 234/51410 | |
| ACI | 13.8 (10.8–16.8) |
18.8 (12.8–24.7) |
11.5 (9.6–13.4) |
25.2 (22.5–27.8) |
32.6
(29.2–35.9) |
6.3 (5.0–7.5) |
12.3 (10.3–14.3) |
25.0
(22.1–27.9) |
||
| Intermediate | N/PYrs | 157/22364 | 166/22684 | 172/23378 | 309/28385 | 360/29518 | 86/18327 | 149/22860 | 213/24691 | |
| ACI | 19.5 (16.5–22.5) |
28.0 (20.4–35.6) |
18.5 (16.0–20.9) |
32.4 (29.4–35.4) |
41.3
(37.9–44.8) |
12.0 (9.6–14.3) |
20.6 (17.6–23.5) |
30.6
(27.1–34.0) |
||
| HTN | N/PYrs | 273/19002 | 283/19396 | 179/14682 | 313/18209 | 363/19079 | 80/12734 | 134/15157 | 171/16148 | |
| ACI | 33.0 (29.6–36.4) |
41.6 (34.6–48.6) |
28.2 (24.7–31.7) |
43.2 (39.6–46.8) |
52.2
(48.3–56.1) |
16.2 (12.9–19.5) |
27.7 (23.7–31.7) |
36.0
(31.7–40.4) |
||
| P, Intermediate vs. Normal | 0.008 | 0.06 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.015 | ||
| P, HTN vs Normal | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Diabetes Status | ||||||||||
| Normal | N/PYrs | 493/62124 | 504/62799 | 449/67504 | 848/81770 | 979/84755 | 239/71773 | 400/84108 | 592/89361 | |
| ACI | 21.8 (19.9–23.7) |
26.3 (21.5–31.2) |
17.5 (16.0–19.0) |
32.1 (30.3–33.8) |
41.6
(39.4–43.8) |
9.5 (8.3–10.6) |
17.9 (16.3–19.5) |
29.0
(27.0–31.0) |
||
| DM | N/PYrs | 13/366 | 19/846 | 20/862 | - | 13/1569 | 14/1622 | - | ||
| ACI | 67.5 (45.3–89.8) |
49.2 (32.5–66.0) |
56.2* (36.9–75.4) |
- | 27.3 (13.3–41.4) |
33.1 (16.3–49.8) |
- | |||
| P, DM vs. Normal | <0.001 | <0.001 | - | - | 0.013 | 0.08 | ||||
| Total Cholesterol Category | ||||||||||
| Normal | N/PYrs | 108/20115 | 109/20237 | 112/30035 | 226/35018 | 259/36064 | 94/40704 | 145/46519 | 230/48717 | |
| ACI | 16.5 (13.1–19.8) |
17.4 (13.6–21.1) |
11.2 (9.2–13.2) |
24.9 (22.0–27.8) |
33.7
(29.6–37.7) |
7.1 (5.7–8.5) |
13.3 (11.2–15.4) |
25.9
(22.9–28.9) |
||
| Intermediate | N/PYrs | 184/23931 | 188/24152 | 177/25352 | 323/30795 | 371/31934 | 104/22379 | 175/27093 | 247/29177 | |
| ACI | 20.9 (17.8–23.9) |
23.83 (19.57–28.09) |
17.8 (15.4–20.2) |
31.6 (28.7–34.4) |
40.8
(37.3–44.4) |
12.1 (9.9–14.3) |
21.3 (18.5–24.2) |
31.5
(28.2–34.8) |
||
| High | N/PYrs | 205/18423 | 208/18633 | 186/14069 | 319/17913 | 361/18643 | 54/10499 | 97/12668 | 135/13793 | |
| ACI | 28.0 (24.3–31.6) |
30.1 (25.7–34.5) |
29.5 (25.9–33.1) |
43.7 (40.1–47.4) |
51.9
(47.9–55.8) |
13.9 (10.5–17.4) |
24.9 (20.5–29.2) |
33.1
(28.5–37.7) |
||
| P, Intermediate vs Normal | 0.06 | 0.026 | <0.001 | 0.001 | 0.009 | <0.001 | <0.001 | 0.014 | ||
| P, High vs Normal | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.010 | ||
| Smoking Status | ||||||||||
| Nonsmoker | N/PYrs | 131/26544 | 134/26804 | 188/41289 | 348/48244 | 420/50067 | 140/57487 | 233/66155 | 344/69443 | |
| ACI | 14.9 (12.2–17.6) |
17.9 (13.6–22.2) |
13.8 (11.9–15.6) |
28.0 (25.5–30.6) |
39.6
36.4–42.9) |
7.6 (6.4–8.9) |
15.4 (13.5–17.3) |
27.9
(25.2–30.6) |
||
| Smoker | N/PYrs | 344/33544 | 348/33744 | 278/28325 | 484/35053 | 527/36035 | 113/16397 | 187/20580 | 270/22682 | |
| ACI | 26.7 (23.8–29.6) |
28.4 (25.1–31.8) |
22.3 (20.0–24.6) |
35.1 (32.6–37.7) |
41.7
(38.6–44.7) |
15.3 (12.6–18.0) |
23.7 (20.6–26.8) |
31.2
(28.0–34.4) |
||
| P, Smoker vs Nonsmoker | <0.001 | <0.001 | <0.001 | <0.001 | 0.40 | <0.001 | <0.001 | 0.12 | ||
| FHS Risk Score Tertiles (Omni excluded) | ||||||||||
| Low | N/PYrs | 45/20893 | 50/22436 | 138/27598 | 173/28621 | 20/23523 | 48/27027 | 118/28909 | ||
| ACI | 6.0 (4.0–7.9) |
6.6 (4.8–8.4) |
18.5 (15.7–21.3) |
28.3
(24.3–32.3) |
2.7 (1.5–3.8) |
9.1 (6.6–11.7) |
24.7
(20.8–28.6) |
|||
| Intermediate | N/PYrs | 116/19816 | 117/19989 | 125/22337 | 252/26914 | 296/28037 | 82/22454 | 128/26684 | 194/28508 | |
| ACI | 17.4 (14.1–20.6) |
18.1 (14.6–21.6) |
15.4 (12.9–17.9) |
30.5 (27.4–33.7) |
40.0
(36.2–43.9) |
10.0 (8.0–12.1) |
17.2 (14.5–20.0) |
28.5
(25.0–32.0) |
||
| High | N/PYrs | 302/18138 | 307/18346 | 279/22440 | 427/26163 | 462/26777 | 140/21750 | 228/26354 | 280/27836 | |
| ACI | 37.1 (33.5–40.7) |
39.4 (35.3–43.5) |
28.6 (25.8–31.5) |
42.7 (39.6–45.8) |
48.4
(45.1–51.7) |
16.1 (13.6–18.5) |
25.6 (22.7–28.5) |
33.2
(29.9–36.4) |
||
| P, Intermediate vs Low | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.15 | |||
| P, High vs Low | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | |||
35-year risk, as few participants survived for a more extended period after age 45 to provide stable 40-year risk or RLR estimates
Cells shaded grey had too few observations for risk estimation.
ACI= adjusted cumulative incidence %. N = number of ASCVD events, Pyrs= person-years of observation.
Association of risk factors with ASCVD incidence within and across the three epochs
Figure 2 Panel A demonstrates the results of Fine-Gray subdistribution hazards models relating key risk factors to ASCVD incidence in each epoch. Within each epoch, male sex, overweight or obesity, higher BP, diabetes, and smoking were associated with a higher risk of ASCVD (relative to the referent groups, i.e., women, normal BMI, normal BP, no diabetes, and non-smokers, respectively). Figure 2 Panel B demonstrates that in each epoch, the second and top tertiles of the FRS were associated with higher ASCVD risk relative to the lowest FRS tertile, with point estimates of the hazards ratios being lower in the last epoch.
Figure 2. Association of risk factors with ASCVD incidence using Fine-Gray subdistribution hazards regression models in the three epochs.
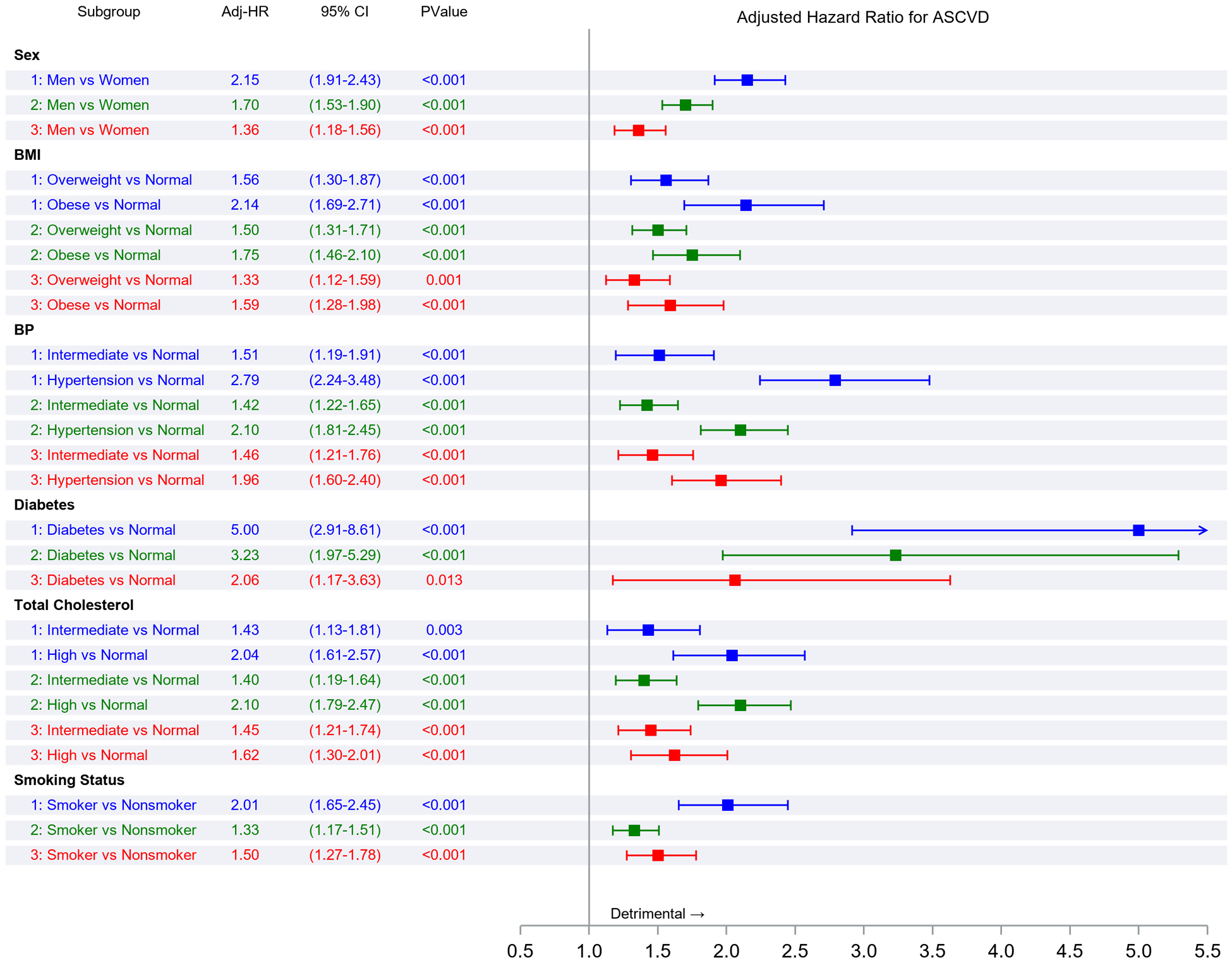
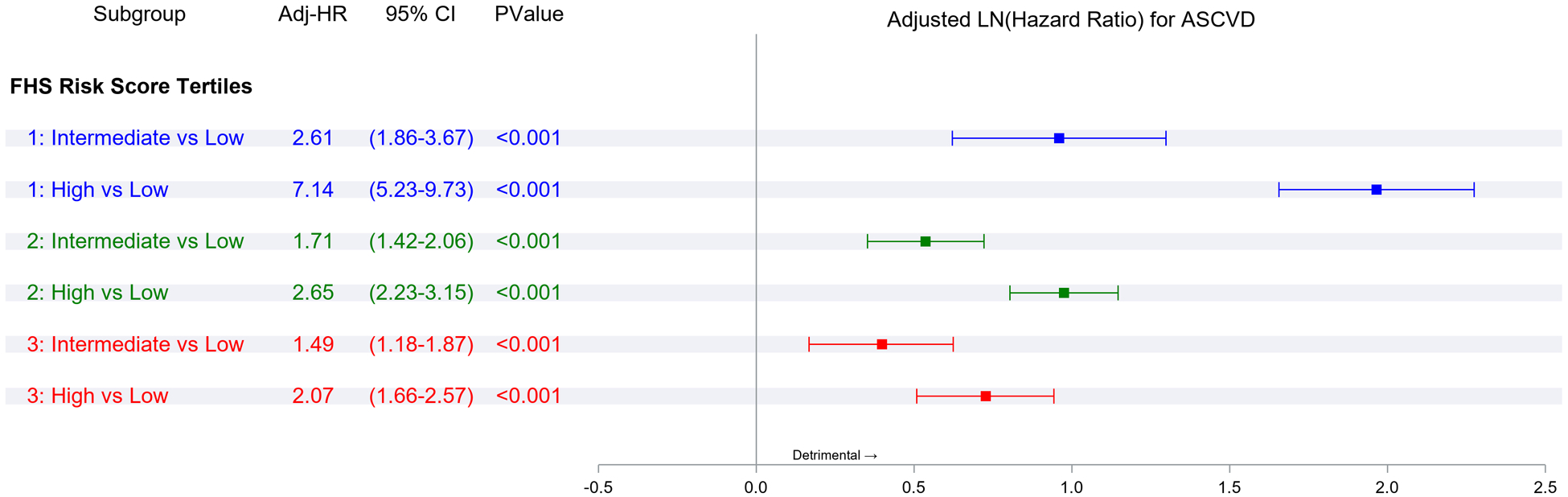
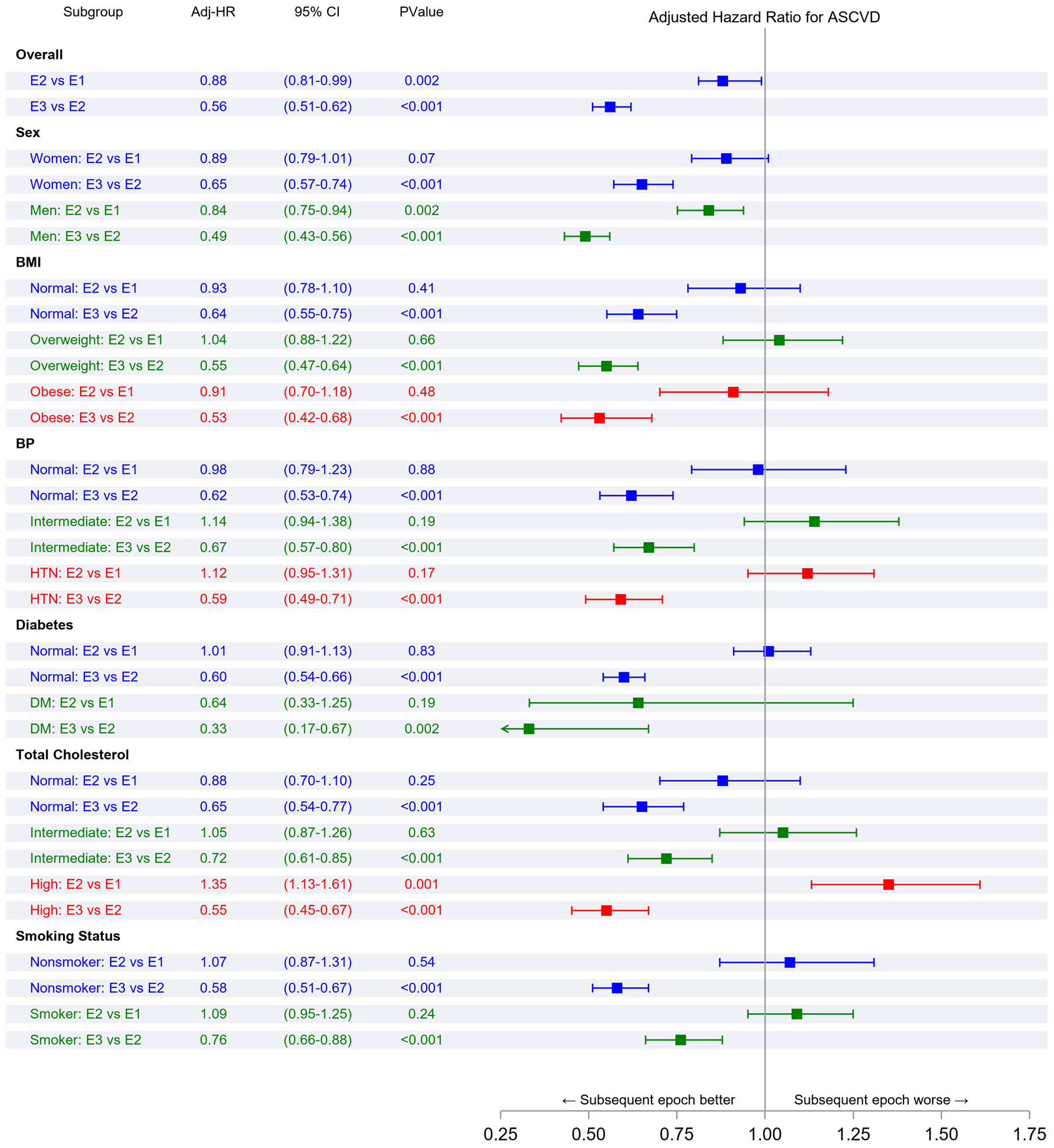
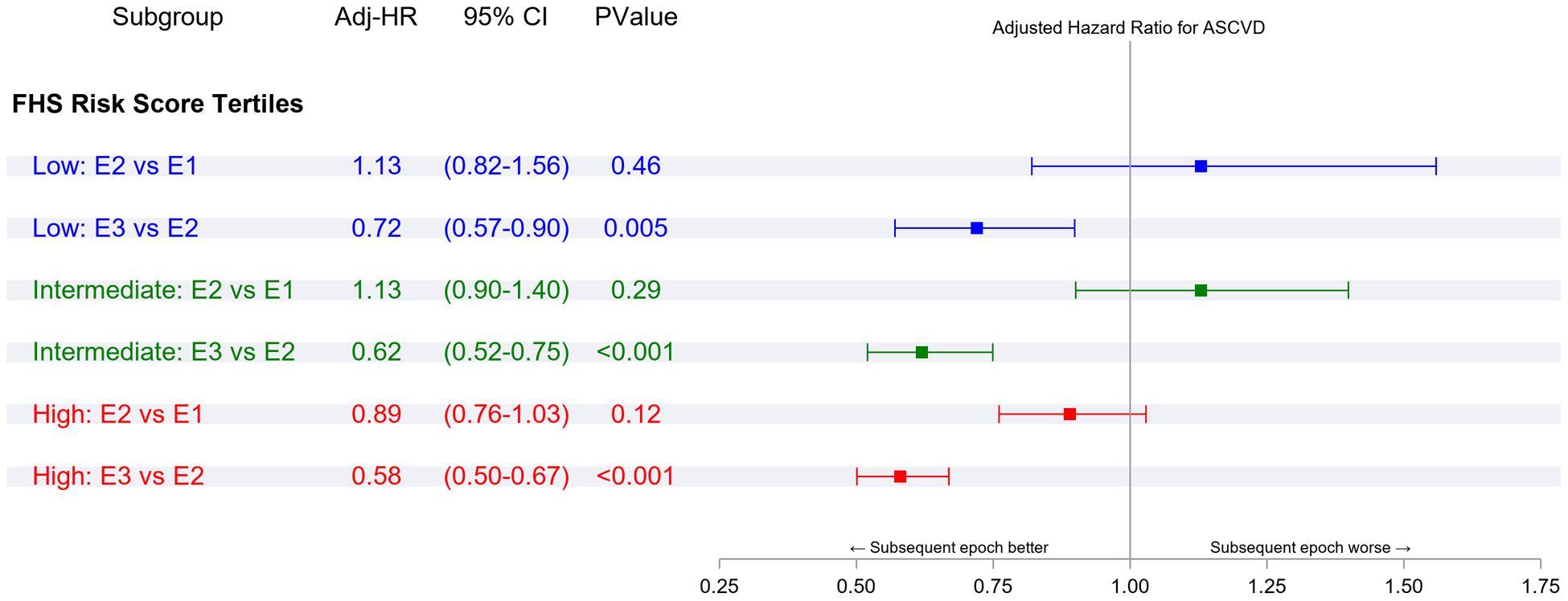
Panel A represents comparisons of risk factor strata associations within each epoch. Panel B represents comparisons of the incidence of ASCVD associated with FRS tertiles within each epoch. Panel C represents comparisons of risk factor strata associations across epochs. Panel D represents comparisons of the incidence of ASCVD associated with FRS tertile across epochs (all expressed as hazards ratios)
Figure 2 Panel C compares ASCVD risk across epochs within each risk factor stratum (e.g., the risk associated with smoking in the first versus the second epoch or the second versus the third epoch). The hazards ratios (for ASCVD) for different risk factor strata were similar for the first and the second epochs. On the other hand, the risks of ASCVD associated with specific strata were much lower (by 24–67%) in the third epoch compared with the second epoch. Figure 2 Panel D demonstrates a similar pattern of observations for the associations of FRS tertiles with ASCVD risk across the three epochs, i.e., no change in hazards ratios between the first and the second epoch, but a much lower hazard in the third epoch (by 28–42%) compared with the second.
Comparisons of 30-year, 40-year risks and RLR of CHD, stroke, and total ASCVD across the three epochs
Supplemental Figure 4 shows the mortality-adjusted sex-specific cumulative incidences of CHD (Panels A–B), stroke (Panels C–D), and total ASCVD (Panels E–F) in each of the three epochs. It is evident from the Figure panels that the incidences of CHD, stroke and total ASCVD fell across the epochs, with the peaks occurring later in life (curves shift to the right) in the third epoch.
Table 4 shows the results of analyses focusing on individual ASCVD events (i.e., CHD and stroke) and total ASCVD (composite outcome defined above). There was a stepwise decline in 30-year and 40-year risk estimates for all three outcomes across the epochs. Comparisons of RLR estimates across the three epochs demonstrated that RLR of CHD (in both sexes), stroke (in women), and total ASCVD (in women) did not change between the first and second epochs. In contrast, RLR for stroke and total ASCVD fell in men from the first to the second epoch (Supplemental Table 4). The RLR of all three outcomes was substantially lower in the third epoch (by 18–52%) in both sexes than in the second epoch (Supplemental Table 4).
Table 4.
Comparison of sex-specific cumulative incidence of CHD vs. Stroke from age 45 years across the three epochs, adjusted for competing risk of death
| Mortality-adjusted Cumulative Incidence (95% CI), 1960–1979 | Mortality-adjusted Cumulative Incidence (95% CI), 1980–1999 | Mortality-adjusted Cumulative Incidence (95% CI), 2000–2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30-year | 40-year | Lifetime | 30-year | 40-year | Lifetime | 30-year | 40-year | Lifetime | ||
| Hard CHD | ||||||||||
| Women | N/PYrs | 170/48511 | 234/53666 | 243/54040 | 119/46597 | 249/58842 | 327/63404 | 76/53519 | 131/63861 | 198/68921 |
| ACI | 9.1 (7.8–10.4) |
17.1 (14.8–19.4) |
21.0
(17.3–24.8) |
6.8 (5.6–7.9) |
13.4 (11.8–14.9) |
18.3
(16.5–20.2) |
4.0 (3.1–4.9) |
7.9 (6.6–9.2) |
12.8
(11.1–14.5) |
|
| Men | N/PYrs | 401/35854 | 466/38551 | 470/38667 | 318/38249 | 485/44516 | 532/45963 | 145/42208 | 205/49292 | 234/51694 |
| ACI | 26.0 (23.7–28.2) |
34.5 (31.7–37.4) |
36.0
(32.9–39.1) |
20.5 (18.5–22.6) |
31.5 (29.2–33.8) |
35.5
(33.1–38.0) |
9.1 (7.7–10.5) |
14.0 (12.2–15.8) |
17.1
(15.0–19.1) |
|
| P, Men vs. Women | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | |
| Stroke | ||||||||||
| Women | N/PYrs | 98/48358 | 152/53300 | 154/53664 | 66/46885 | 181/59119 | 255/63499 | 34/53918 | 82/64459 | 158/69654 |
| ACI | 5.5 (4.4–6.6) |
11.8 (9.8–13.8) |
13.4
(10.2–16.6) |
3.8 (2.9–4.7) |
9.7 (8.4–11.1) |
14.5
(12.8–16.1) |
1.8 (1.2–2.5) |
5.2 (4.1–6.3) |
10.7
(9.1–12.3) |
|
| Men | N/PYrs | 115/37384 | 149/40156 | 155/40267 | 93/40463 | 163/47309 | 178/48843 | 58/43793 | 93/51605 | 112/54316 |
| ACI | 7.7 (6.3–9.1) |
12.8 (10.6–14.9) |
15.7
(12.6–18.7) |
5.9 (4.7–7.0) |
10.5 (8.9–12.0) |
11.9
(10.3–13.6) |
3.6 (2.7–4.5) |
6.3 (5.1–7.6) |
8.2
(6.7–9.7) |
|
| P, Men vs. Women | 0.014 | 0.51 | 0.33 | 0.005 | 0.48 | 0.035 | 0.002 | 0.18 | 0.025 | |
| Total ASCVD | ||||||||||
| Women | N/PYrs | 588/44158 | 745/47889 | 758/48144 | 381/42715 | 696/51957 | 858/54889 | 255/50714 | 421/59009 | 595/62413 |
| ACI | 31.4 (29.2–33.5) |
49.3 (46.3–52.3) |
54.7
(50.7–58.8) |
22.8 (20.8–24.8) |
40.2 (37.9–42.5) |
51.4
(49.0–53.9) |
13.9 (12.3–15.5) |
27.0 (24.7–29.3) |
42.4
(39.7–45.1) |
|
| Men | N/PYrs | 797/31864 | 903/33715 | 911/33783 | 579/34051 | 798/38269 | 852/39148 | 315/39056 | 451/44407 | 531/45860 |
| ACI | 51.3 (48.7–53.9) |
64.5 (61.5–67.5) |
67.8
(64.4–71.2) |
39.5 (37.0–42.1) |
55.8 (53.1–58.4) |
61.2
(58.5–63.8) |
21.0 (18.9–23.1) |
34.1 (31.5–36.8) |
45.6
(42.5–48.7) |
|
| P, Men vs. Women | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.13 | |
ACI= adjusted cumulative incidence %. N = number of ASCVD events, Pyrs= person-years of observation.
Sensitivity analyses
In sensitivity analyses evaluating unique participants between ages 45–60 in each epoch (with the constraint that participants could not contribute to more than one epoch), the 15-year mortality-adjusted cumulative incidence was lower in the second and third epoch relative to the first, a finding that was consistent in both sexes and across smoking and BMI strata (Supplemental Table 5). The incidence of non-CVD mortality was lower in the first epoch (8.9/1000 person-years) compared with the second epoch (16.3/1000 person-years) and then fell modestly in the third epoch (13.1/1000 person-years). These data indicate that a general improvement in overall health may not underlie the lower RLR of ASCVD observed in the last two epochs.
Discussion
The epidemiology of ASCVD has changed over the last five decades due to the escalating burden of risk factors (such as hypertension, excess adiposity, and diabetes),25–27 increasing longevity,41 decline in smoking frequency, and major advances in treating high BP, dyslipidemia, and dysglycemia. As such, the long-term risk of developing ASCVD among individuals may have changed. This premise was examined by evaluating changes in RLR of ASCVD from age 45 over the previous six decades, leveraging data from the community-based FHS cohorts.
Principal Findings
The investigation has four principal findings. First, the RLR of ASCVD from age 45 decreased by 27 (women) to 43 (men) percent over the last six decades in the FHS cohorts. In parallel, the 30-year and 40-year risks of ASCVD also fell markedly over this period in both sexes. The mean age of onset of a first ASCVD event in the last epoch (2000–2018) was approximately 73 years in men and 80 years in women, being about 8 (men) to 10 (women) years higher compared with the first epoch (1960–1979). In parallel, life expectancy rose to 78 (men) to 82 (women) years in the last epoch, an increase of about 10 (men) to 12 (women) years relative to the first epoch. These observations indicate a decrease in the overall risk of ASCVD experienced over the life course and its postponement by almost a decade (i.e., into the eighth decade of life).
Second, the lower 30-year, 40-year risks and the RLR of ASCVD in the most recent epoch (relative to the first and second epochs) was consistent across every risk factor stratum and across FRS tertiles. These observations were corroborated by the Fine-Gray analyses that suggested that the relative risk of ASCVD associated with each risk factor stratum diminished over time across the epochs. Thus, the absolute and the relative risks associated with ASCVD risk factors have declined over the sixty-year observation period in FHS, especially in the last epoch. A striking observation is a decline in the 30-year absolute ASCVD risk associated with the top tertile of FRS (from 37% in the first epoch to 28.6% in the second epoch, to 16% in the third epoch) along with a 42% decrement in the hazard (for ASCVD) in the most recent epoch relative to the second epoch. It is likely that greater use of medications for lowering BP and cholesterol, improvements in the control of diabetes, and a major reduction in smoking prevalence across the three epochs may have contributed to these findings; such a premise is supported by the lower midlife average BP and Tc levels and mean FRS in the last two epochs (relative to the first epoch) in the at-risk samples.
Third, in each of the epochs (including the most recent one), the 30-year ASCVD risk estimate in the stratum with an optimal level of a specific risk factor was up to 50% lower than that for the suboptimal level of that risk factor; corresponding RLR of ASCVD estimates were 10–30% lower for optimal levels of risk factors compared with their suboptimal levels. These findings suggest that even with a lowering of overall RLR of ASCVD in the most recent epoch, striving towards optimal levels of risk factors may further mitigate ASCVD risk.
Fourth, analyses of RLR trends for CHD and stroke demonstrated concomitant declines in risk estimates for both outcomes and in both sexes; the most significant benefit (decrease in RLR) was observed in the most recent epoch, with the relative lowering of RLR being greater for men compared with women. Notably, in the most recent epoch, the RLR of CHD was higher in men than women, but the converse was true for the RLR of stroke, which was higher in women than men. These sex-related differences in ASCVD risk patterns (presentation with CHD versus stroke) have also been reported in the Rotterdam Study.15 There was a similar decrement in RLR of total ASCVD across the three epochs. However, the lingering high estimates in the most recent epoch (42.4 and 45.6 for women and men, respectively) underscore the need for continued and more effective efforts to mitigate the residual risk of total ASCVD.
The decline in RLR of ASCVD over time in the FHS cohorts is likely multifactorial in origin, representing the combined contributions of improved health care access, greater awareness of ASCVD risk, behavioral and lifestyle changes (such as changes in nutrition and smoking cessation), and better screening for and more effective lowering of key risk factors such as high BP, elevated cholesterol and diabetes.42 Additional follow-up studies are warranted to examine if the increased burden of obesity and diabetes in recent decades may offset some of the gains accruing from medical and technological advances that may have lowered ASCVD risk.
Comparison with the literature
Several national and international studies have estimated the RLR of ASCVD and its components (CHD and stroke); Supplemental Table 6 summarizes RLR estimates from these reports.11–22 None of these reports assessed temporal trends in the RLR of ASCVD over the sixty-year period that the current investigation examined. An earlier FHS report18 evaluated trends in RLR of stroke, but follow-up was limited up to 2004. In that report, the RLR of a stroke at age 65 years decreased from 19.5% (1950–1977) to 14.5% (1990–2004) in men and from 18.0% (1950–1977) to 16.1% (1990–2004) in women, but the decrease was not statistically significant in either sex.18 The present report extends those observations into the last two decades of the present century, spanning approximately 60 years.
Studies of temporal trends in the RLR of disease outcomes require extended follow-up of population-based samples with minimal loss and consistent diagnostic criteria and protocols to ascertain and adjudicate disease outcomes over time. These critical features of the FHS cohorts were leveraged to assess trends in RLR of ASCVD from age 45 over the last six decades. It is noteworthy, though, that the RLR estimates for the second epoch (1980–1999) closely approximate those reported in the literature by other studies, including from the Cardiovascular Lifetime Risk Pooling Project.11,12,17 The higher RLR of ASCVD in men than women corroborates prior reports summarized in eTable 5 and is likely multifactorial in origin.28,43 The lesser proportional decline in RLR of ASCVD in women may be related to sex-related differences in presentation of ASCVD,44 and less favorable trends in obesity and cholesterol levels in women.28
Strengths and Limitations
The longitudinal, consistent, and systematic surveillance of the community-based cohorts over a sixty-year observation period (split across three epochs) with excellent long-term follow-up and meticulous adjudication of endpoints are key strengths of the present investigation.24 Additionally, the RLR estimates are based on direct observations of individuals over their entire life course and not derived from modeling incidence rates over shorter periods.
Nonetheless, several limitations of the present investigation must be acknowledged. The utility of RLR estimates versus short-term age-conditional risks has been debated in the literature, with some experts favoring the latter because a short-term time horizon for risk prediction may be more helpful to individuals.45 However, RLR estimates (including trends) are vital from a public health perspective and complement short-term risk estimates. Others46 have emphasized the importance of RLR of total ASCVD (and not just atherosclerotic ASCVD) because ‘soft’ ASCVD outcomes may represent a greater proportion of ASCVD events in women and younger people. The present investigation provides estimates for both types of ASCVD events for this reason. Information regarding some risk factors was missing during the first epoch, and the shorter life expectancy during that period constrained accurate estimates of RLR for that time interval. Analyses of RLR by risk factor strata were contingent on participants having attended an examination between ages 40–49 years, which resulted in the exclusion of approximately a quarter of participants in each epoch for this set of analyses; however, these exclusions did not extend to the overall and the sex-specific RLR estimates that did not require risk factor values. The Fine-Gray models did not adjust for risk factors as time-varying covariates because of methodological challenges described elsewhere.47 Although the FHS used consistent diagnostic criteria over the six decades, technological advances may have led to systematic trends in the ascertainment of ASCVD outcomes in the more recent epochs, which might influence the analysis of RLR trends over such a long period. The use of more sensitive diagnostic tests may contribute to an underestimation of the decline in ASCVD risk over time.48 Additionally, it may be challenging to separate calendar period effects from concomitant birth cohort effects.49 Lastly, the FHS cohorts reside predominantly in the northeastern US, are overwhelmingly White, and their risk factor profiles may not represent that of the entire country or of other countries. RLR of ASCVD may vary with race/ethnicity and geographic location. It is reassuring to note that several reports have emphasized the consistency in estimates of RLR of ASCVD across Whites and Blacks in the United States.11,12,17
Conclusions
The 30-year and 40-year risks and the RLR for atherosclerotic ASCVD from age 45 decreased in community-based Framingham cohorts over the last sixty years. The RLR of ASCVD fell from 1 in 2 in men and 1 in 3 in women during 1960–1979 to less than 1 in 3 in men and about 1 in 4 in women during 2000–2018. The lowering of the long-term risk of ASCVD was observed within every risk factor stratum and consistent for both CHD and stroke outcomes. The higher average age at onset of ASCVD observed across the six decades is possibly the result of a greater life expectancy in recent decades (with a longer ‘at risk’ period) and postponement of morbidity into the later decades of life. The residual burden of total ASCVD risk of almost 4 in 10 men and women underscores the need for continued preventive public health measures.
Supplementary Material
Clinical Perspective.
1). What is new?
The present investigation provides new information regarding temporal trends in remaining lifetime risk (RLR) of atherosclerotic cardiovascular disease (ASCVD) over three twenty-year epochs (Epochs 1–3: 1960–1979, 1980–1999, 2000–2018, respectively) in the community-based, predominantly White Framingham Study cohorts.
Life expectancy rose by 10.1 (men) to 11.9 (women) years across the epochs while the RLR of ASCVD from age 45 declined from 43.7 percent in Epoch 1 to 28.1 percent in Epoch 3 (p<0.0001).
The lower RLR of ASCVD in the last two epochs (compared with the first epoch) was observed consistently across BMI, BP, cholesterol, diabetes, smoking, and Framingham risk score strata.
2). What are the clinical implications?
The RLR of total ASCVD in the most recent epoch remains high at 42.4 and 45.6 for women and men, respectively, underscoring the need for continued and more effective primary prevention efforts with better screening for risk factors and lowering them more effectively.
Additional follow-up studies of multi-ethnic samples are warranted to confirm these findings.
Funding:
This work is supported by Contracts NO1-HC-25195, HHSN268201500001I, and 75N92019D00031 from the National Heart, Lung, and Blood Institute, Bethesda, MD. Additional support includes NS017950 from the National Institute of Neurological Disorders and Stroke.
Dr. Vasan is supported by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine, MA.
Non-standard Abbreviations and Acronyms
- ASCVD
Atherosclerotic cardiovascular disease
- FHS
Framingham Heart Study
- FRS
Framingham Risk Score
- NOS
New Offspring Spouses
- RLR
Remaining lifetime risk
- Tc
Total cholesterol
Footnotes
Disclosures: None.
References
- 1.Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Brown J, Carter A, Casey DC, Charlson FJ, Coates MM, Coggeshall M, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T, Lim S, Abbafati C, Abbas K, Abbasi M and Abbasifard M. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics;2021 Update. Circulation. 2021;143:e254–e743 [DOI] [PubMed] [Google Scholar]
- 5.Ritchey MD, Wall HK, Owens PL and Wright JS. Vital signs: state-level variation in nonfatal and fatal cardiovascular events targeted for prevention by Million Hearts 2022. Morb Mortal Wkly Rep. 2018;67:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J Am Coll Cardiol. 2020;76:2982–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beiser A, D’Agostino RB Sr, Seshadri S, Sullivan LM and Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522 [DOI] [PubMed] [Google Scholar]
- 8.Navar AM, Wang TY, Mi X, Robinson JG, Virani SS, Roger VL, Wilson PWF, Goldberg AC and Peterson ED. Influence of Cardiovascular Risk Communication Tools and Presentation Formats on Patient Perceptions and Preferences. JAMA Cardiology. 2018;3:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sasieni PD and Adams J. Standardized lifetime risk. Am J Epidemiol. 1999;149:869–875 [DOI] [PubMed] [Google Scholar]
- 10.Jaspers NE, Blaha MJ, Matsushita K, Van Der Schouw YT, Wareham NJ, Khaw K-T, Geisel MH, Lehmann N, Erbel R and Jöckel K-H. Prediction of individualized lifetime benefit from cholesterol lowering, blood pressure lowering, antithrombotic therapy, and smoking cessation in apparently healthy people. Eur Heart J. 2020;41:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen N, Berry JD, Ning H, Van Horn L, Dyer A and Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the cardiovascular lifetime risk pooling project. Circulation. 2012;125:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP and Lloyd-Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driver JA, Djoussé L, Logroscino G, Gaziano JM and Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008;337:a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hippisley-Cox J, Coupland C, Robson J and Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leening MJ, Ferket BS, Steyerberg EW, Kavousi M, Deckers JW, Nieboer D, Heeringa J, Portegies ML, Hofman A and Ikram MA. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-Jones DM, Leip EP, Larson MG, d’Agostino RB, Beiser A, Wilson P, Wolf PA and Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798 [DOI] [PubMed] [Google Scholar]
- 17.Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR and Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308:1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB and Wolf PA. Trends in Incidence, Lifetime Risk, Severity, and 30-Day Mortality of Stroke Over the Past 50 Years. JAMA. 2006;296:2939–2946 [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM, Larson MG, Beiser A and Levy D. Lifetime risk of developing coronary heart disease. The Lancet. 1999;353:89–92 [DOI] [PubMed] [Google Scholar]
- 20.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE and Smeeth L. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1· 25 million people. The Lancet. 2014;383:1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB and Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350 [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liu J, Wang W, Wang M, Qi Y, Xie W, Li Y, Sun J, Liu J and Zhao D. Lifetime risk for cardiovascular disease in a Chinese population: the Chinese Multi–Provincial Cohort Study. Eur J Prevent Cardiol. 2015;22:380–388 [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Wolf PA and Garrison RJ. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. Framingham Heart Study, 30 Year Follow-Up. National Institutes of Health Publication No. 87–2703. Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 24.Tsao CW and Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44:1800–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorans KS, Mills KT, Liu Y and He J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J Am Heart Assoc. 2018;7:e008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA and Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KV and Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. The Lancet Diabetes & Endocrinology. 2014;2:867–874 [DOI] [PubMed] [Google Scholar]
- 28.Peters SAE, Muntner P and Woodward M. Sex Differences in the Prevalence of, and Trends in, Cardiovascular Risk Factors, Treatment, and Control in the United States, 2001 to 2016. Circulation. 2019;139:1025–1035 [DOI] [PubMed] [Google Scholar]
- 29.Dawber TR, Meadors GF and Moore FE, Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Pub Health. 1951;41:279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB, Feinleib M, McNamara PM, Garrison RJ and Castelli WP. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290 [DOI] [PubMed] [Google Scholar]
- 31.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr., Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35 [DOI] [PubMed] [Google Scholar]
- 32.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J and Samet JM. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085 [PubMed] [Google Scholar]
- 33.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB and Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24 [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM and Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 35.National Instituites of Health. Clinical guidelines for the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes Res. 1998;6:51S–209S [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S and Wright JT Jr. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571 [DOI] [PubMed] [Google Scholar]
- 37.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483 [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37:S81–S90 [DOI] [PubMed] [Google Scholar]
- 39.Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D and O’Donnell CJ. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73 [DOI] [PubMed] [Google Scholar]
- 40.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509 [Google Scholar]
- 41.Woolf SH and Schoomaker H. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 2019;322:1996–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM, et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circ Res. 2017;120:366–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillis EE and Sullivan JC. Sex differences in hypertension: recent advances. Hypertension. 2016;68:1322–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw LJ, Bugiardini R and Merz CNB. Women and Ischemic Heart Disease: Evolving Knowledge. J Am Coll Cardiol. 2009;54:1561–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson R Lifetime risk: does it help to decide who gets statins and when? Curr Opin Lipidol. 2014;25:247–53 [DOI] [PubMed] [Google Scholar]
- 46.Leening MJ, Berry JD and Allen NB. Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease. JAMA. 2016;315:1449–1450 [DOI] [PubMed] [Google Scholar]
- 47.Austin PC, Latouche A and Fine JP. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med. 2020;39:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H and Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation. 2012;125:1848–57.PMC3341729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bell A and Jones K. The impossibility of separating age, period and cohort effects. Soc Sci Med. 2013;93:163–165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


