Abstract
Background:
Intracerebral hemorrhage (ICH) is the deadliest form of stroke. In observational studies, Lower serum magnesium has been linked to more hematoma expansion and intracranial hemorrhage, implying that supplemental magnesium sulfate is a potential acute treatment for patients with ICH and could reduce hematoma expansion. FAST-MAG, a clinical trial of magnesium sulfate started prehospital in patients with acute stroke within two hours of last known well enrolled, including several hundred patients with acute ICH. In this ancillary analysis, we assessed the effect of magnesium sulfate treatment upon hematoma expansion in patients with acute ICH.
Methods:
We retrospectively analyzed data that were prospectively collected in the FAST-MAG study. Patients received intravenous magnesium sulfate or matched placebo within two hours of onset. We compared hematoma expansion among patients allocated to intravenous magnesium sulfate or placebo with a Mann-Whitney U. We used the same method to compare neurologic deficit severity (NIH Stroke Scale) and global disability (modified Rankin Scale) at 3 months.
Results:
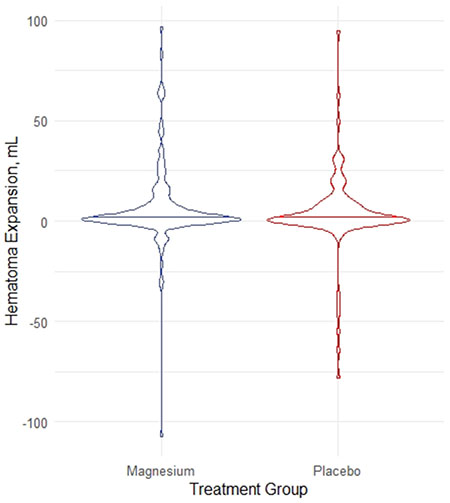
Among 268 ICH patients meeting study entry criteria, mean 65.4 +/− 13/4 years, 33% were female, and 211 (79%) had a history of hypertension. Initial deficit severities were median [interquartile range] 4 [3 – 5] on the Los Angeles Motor Scale in the field and NIH Stroke Scale 16 [9.5 – 25.5] early after hospital arrival. Follow-up brain imaging was performed a median of 17.1 [11.3 – 22.7] hours after first scan. The magnesium and placebo groups did not statistically differ in hematoma volume on arrival, 10.1 [5.6 – 28.7] vs. 12.4 [5.6 – 28.7] mL (P=0.6), or hematoma expansion, 2.0 (0.1 – 7.4) vs. 1.5 (−0.2 – 8) mL (P=0.5). There was no difference in functional outcomes (modified Rankin Scale 3-6), 59% vs. 50% (P=0.5).
Conclusions:
Magnesium sulfate did not reduce hematoma expansion or improve functional outcomes at 90 days. A benefit for patients with initial hypomagnesemia was not addressed.
Clinical Trial Registration:
Graphical Abstract
Intracerebral hemorrhage (ICH) often leads to dependence or death at follow-up. Early hematoma expansion (HE) occurs in up to one-third of ICH patients and is detected by hematoma volume growth from initial to follow-up brain imaging.1 More HE leads to more disability and death at follow-up.2 Reducing HE is a biologically plausible strategy to improve patient outcomes. Clinical trials intended to reduce HE have had mixed results, and several antihypertensive and hemostatic agents have had marginal effects on HE.3–6 Additional safe and effective treatments to reduce HE are needed.
In observational studies, lower serum magnesium levels have been linked to more HE and intracranial hemorrhage.7,8 Magnesium sulfate could reduce HE by hemostatic effects and blood pressure lowering.7,8 Magnesium sulfate would be a potentially attractive pharmacologic treatment for ICH because it is well tolerated and inexpensive.
The FAST-MAG randomized clinical trial of magnesium versus placebo began study infusion in paramedic ambulances prehospital in patients with acute stroke symptoms. As study treatment was started prior to brain imaging, it was anticipated that both ischemic and hemorrhagic stroke patients would be enrolled. In addition to primary analysis in the overall study population, separate analyses for magnesium clinical outcome effects in patients with ischemic stroke and intracerebral hemorrhage were pre-specified secondary analyses.9 We tested the hypothesis that magnesium sulfate reduces HE in patients with acute ICH.
METHODS
Details of the multicenter FAST-MAG trial have been previously published.9 In brief, patients with an acute neurological deficit suggestive of stroke were enrolled by one of 315 ambulances. Study treatment with magnesium sulfate or placebo started prehospital. Patients were randomized 1:1 by sequential assignment in permuted-block sequence to magnesium (4 gm in 54 mL normal saline, then an additional 16 gm in 240 mL normal saline) over 24 hours, or matching normal saline. For data access, contact JLS.
All patients underwent acute brain imaging upon hospital arrival (which occurred after start of study agent). In patients with ICH on initial imaging, follow-up imaging was frequently obtained per the standard clinical policies of each receiving hospital. This post-hoc study analyzed the subset of ICH patients in whom follow-up brain imaging was obtained within the first 24 hours after arrival. Hematoma volumes were calculated using the A*B*C/2 method10 by an expert diagnostic neuroradiologist at a central imaging core lab. Our primary endpoint was HE (final minus diagnostic hematoma volume).
Normally distributed data are presented as mean ± SD, while non-normally distributed data are presented as median [Q1 – Q3]. Hematoma volumes are not normally distributed, and so were compared between groups (magnesium sulfate or placebo) using the Mann-Whitney U. We explored correlations between measured post-study treatment serum magnesium concentration and HE using Spearman correlation coefficient.
RESULTS
A total of 72.8% (268/383) ICH patients met study criteria of undergoing follow-up brain imaging within 24 hours after initial brain imaging (Figure S1). Baseline and workflow characteristics of the patients by treatment group are shown in Table 1. Overall age was mean 65.4 ± 13.4 years, 33% were female, 211 (79%) had history of hypertension, pretreatment deficit severity on the Los Angeles Motor Scale was median 4 [3 – 5], and the initial NIH Stroke Scale early after hospital arrival was 16 [9.5 – 25.5].
Table 1.
Baseline and Workflow Patient Characteristics
| Variable | Magnesium (N=145) |
Placebo (N=123) |
|---|---|---|
| Age, years | 65.1 ± 13.1 | 64.5 ± 13 |
| Female | 53 (37) | 36 (29) |
| Hispanic or Latinx | 49 (34) | 40 (33) |
| Race, White Black Asian Pacific Islander American Indian/Alaskan Native |
116 (80) 11 (8) 15 (10) 3 (2) 0 |
96 (78) 12 (10) 13 (11) 1 (1) 1 (1) |
| Historical hypertension | 116 (80) | 95 (77) |
| Historical hyperlipidemia | 54 (37) | 47 (38) |
| Historical diabetes | 25 (17) | 23 (19) |
| Previous stroke | 14 (10) | 4 (3) |
| Systolic Blood Pressure, prehospital | 176 ± 25 | 175 ± 26 |
| Diastolic Blood Pressure, prehospital | 100 ± 20 | 100 ± 18 |
| Prehospital motor deficit (LAMS score) 1 2 3 4 5 10 |
2 (1) 14 (10) 25 (17) 34 (23) 69 (48) 1 (1) |
4 (3) 4 (3) 32 (26) 30 (24) 52 (42) 1 (1) |
| Time from last known well to study agent start (mins) | 44 (35-59) | 43 (36-61) |
| Time from last known to 1st brain imaging (mins) | 82 (69-100) | 83 (70-102) |
| Time from 1st to follow-up brain imaging (hrs) | 18.0 (15.2-22.7) | 18.1 (15.1-22.7) |
Allocation to magnesium vs placebo was not associated with statistically significant differences in initial ICH volume, hematoma expansion, or 90 day neurologic deficit and functional independence outcomes (Table 2). In the 199 patients with a serum magnesium level measured clinically after study infusion start, there was no correlation between the initial post-serum magnesium level and HE (rho = 0.004, P=0.9).
Table 2.
Imaging and Clinical Outcomes
| Variable | Magnesium (N=145) |
Placebo (N=123) |
P values |
|---|---|---|---|
| Initial hematoma volume, mL | 10.1 (5.6 – 28.7) | 12.4 (5.6 – 28.7) | 0.60 |
| Follow-up hematoma volume, mL | 15.3 (8.3 – 37.7) | 14.7 (6.3 – 34.4) | 0.56 |
| Hematoma Expansion, mL | 2 (0.1 – 7.4) | 1.5 (−0.2 – 8) | 0.49 |
| NIH Stroke Scale at 90 days | 6 (2 – 24) | 5 (2 – 21) | 0.56 |
| Modified Rankin Scale at 90 days 0, no symptoms 1, no motor deficit 2, mild disability 3, moderate disability, independent 4, moderate severe disability 5, bed bound 6, dead |
2 (2) 10 (7) 26 (18) 22 (22) 23 (16) 30 (21) 32 (22) |
1 (1) 6 (5) 36 (30) 16 (13) 15 (12) 21 (17) 26 (21) |
0.46 |
| Functional Independence, mRS 0 - 2 | 38 (26) | 43 (35) | 0.16 |
DISCUSSION
We found that treatment with magnesium sulfate was not associated with reduced initial hematoma volume, hematoma expansion volume, or functional outcomes at 3 months.
The findings of this study contrast with observational data that suggested an association of higher serum magnesium with improved HE and clinical outcomes.8 There are several potential explanations to explain the discrepancy. One possibility is that in observational ICH studies patients with higher serum magnesium levels tend to be healthier. Unfortunately, pre-treatment magnesium levels were not available. It is also possible that HE occurs primarily in patients with abnormally low rather than normal serum magnesium levels and that fewer such patients were enrolled in FAST-MAG. It is possible that as a treatment, magnesium sulfate is not potent enough to correct any deficiencies in hemostasis compared to other treatments that have reduced HE (e.g., tranexamic acid, Factor VII).3–5 While other biomarkers of hemostasis (platelet activity,11 thromboelastography12) predict HE, these were not collected in FAST-MAG and might have been helpful to select a cohort of patients more likely to have HE. FAST-MAG was not powered to reduce hematoma volume, and may be under-powered to detect an effect. Finally, the observation may have been due to chance.
In conclusion, we found no effect of early magnesium sulfate therapy on HE and clinical outcomes at 90 days in patients with acute ICH. While observational data associated HE and serum magnesium, the administration of magnesium sulfate seems unlikely to be an effective treatment for acute ICH.
Supplementary Material
Figure S1, Flowchart of patients included in the study
Acknowledgements:
All those who assisted in the manuscript are listed as an author.
Funding:
FAST-MAG was supported by the NIH, U01 NS044364
Dr. Naidech is supported by the NIH, R01 NS110779 and U01 NS110772
Non-Standard Abbreviations and Acronyms:
- ICH
intracerebral hemorrhage
- HE
hematoma expansion
Footnotes
Disclosures: Dr Liebeskind reports compensation from Genentech for consultant services; compensation from Cerenovus for consultant services; compensation from Medtronic for consultant services; and compensation from Stryker for consultant services.
Dr Sharma reports compensation from AstraZeneca for other services.
REFERENCES
- 1.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T, Investigators ftRAFVIHT. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99 [DOI] [PubMed] [Google Scholar]
- 2.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, Collaboration V. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med. 2008;358:2127–2137. doi: [DOI] [PubMed] [Google Scholar]
- 4.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer M, Skolnick BE, Steiner T. Recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med. 2005;352:777–785. doi: [DOI] [PubMed] [Google Scholar]
- 5.Sprigg N, Flaherty K, Appleton JP, Salman RA, Bereczki D, Beridze M, Christensen H, Ciccone A, Collins R, Czlonkowska A, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018. doi: 10.1016/S0140-6736(18)31033-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Warren AD, Qureshi AI, Morotti A, Falcone GJ, Sheth KN, Shoamanesh A, Dowlatshahi D, Viswanathan A, Goldstein JN. Ultra-Early Blood Pressure Reduction Attenuates Hematoma Growth and Improves Outcome in Intracerebral Hemorrhage. Ann Neurol. 2020;88:388–395. doi: 10.1002/ana.25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liotta EM, Karmarkar A, Batra A, Kim M, Prabhakaran S, Naidech AM, Maas MB. Magnesium and Hemorrhage Volume in Patients With Aneurysmal Subarachnoid Hemorrhage. Crit Care Med. 2020;48:104–110. doi: 10.1097/CCM.0000000000004079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liotta EM, Prabhakaran S, Sangha RS, Bush RA, Long AE, Trevick SA, Potts MB, Jahromi BS, Kim M, Manno EM, et al. Magnesium, hemostasis, and outcomes in patients with intracerebral hemorrhage. Neurology. 2017;89:813–819. doi: 10.1212/WNL.0000000000004249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, Conwit R, Liebeskind DS, Sung G, Kramer I, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372:528–536. doi: 10.1056/NEJMoa1408827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kothari R, Brott T, Broderick J, Barsan W, Sauerbeck L, Zuccarello M, Khoury J. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: [DOI] [PubMed] [Google Scholar]
- 11.Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, Bernstein RA, Alberts MJ, Batjer HH. Reduced platelet activity is associated with early clot growth and worse 3 month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–2401. doi: [DOI] [PubMed] [Google Scholar]
- 12.Kawano-Castillo J, Ward E, Elliott A, Wetzel J, Hassler A, McDonald M, Parker SA, Archeval-Lao J, Tremont C, Cai C, et al. Thrombelastography detects possible coagulation disturbance in patients with intracerebral hemorrhage with hematoma enlargement. Stroke. 2014;45:683–688. doi: 10.1161/STROKEAHA.113.003826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Flowchart of patients included in the study


