Abstract
To evaluate if follow-up mpMRI scans of patients in PI-RADS category 3 are safe enough to omit or delay prostate biopsy in the future and to determine an optimal control interval. This retrospective single center study includes consecutive PI-RADS category 3 patients with one or more follow-up mpMRI (T2WI, DWI, DCE) and subsequent MRI-targeted and systematic TRUS-guided biopsy between 2012 and 2018. Primary study objective was the verification of a significant PI-RADS category upgrade in follow-up mpMRI in patients with subsequent PCA positive biopsy versus patients with negative biopsy. Further objectives were development of the PI-RADS category and clinical parameters between initial and follow-up mpMRI in the context of histopathologic results and time interval. Eighty-nine patients (median PSA 6.6 ng/ml; PSAD 0.13 ng/ml/ml) were finally included (follow-up period 31 ± 18 months). 19 cases had PCA (median PSA 7.8 ng/ml; PSAD 0.14 ng/ml/ml). 4 cases had csPCA (median PSA 5.4 ng/ml; PSAD 0.13 ng/ml/ml) for which there was a significant PI-RADS upgrade after 12–24 months (mean 3.75; p = 0.01) compared to patients without PCA (mean 2.74). Without PCA the mean PI-RADS category decreased after 25–36 months (mean 2.74; p = 0.02). Clinical parameters did not change significantly except a PSAD increase for PCA patients after 24 months. Patients within PI-RADS category 3 may not need prompt biopsy since those with PCA reliably demonstrate a PI-RADS category upgrade in follow-up mpMRI after 12–24 months. PI-RADS 3 patients with negative biopsy do not benefit from follow-up mpMRI earlier than 24 months.
Subject terms: Medical research, Cancer
Introduction
MpMRI of the prostate has meanwhile become the gold standard of prostate cancer diagnostics providing high sensitivity in cancer detection on the one hand and a high negative predictive value to exclude clinically significant prostate cancer on the other hand, thus helping to reduce overtreatment1–8. Nevertheless, cancer detection rates of the PI-RADS can vary widely among different institutions or readers expertise and there is ongoing controversy of whether or not equivocal PI-RADS 3 lesions require early biopsy or not3,9. Indistinct changes induced by chronic inflammation or (atypical) stromal hyperplasia additionally hamper definite cancer visualization, especially in unclear cases10,11. The British NICE guideline and the European EAU guideline still recommend biopsy in PI-RADS 3 lesions despite the well-known disadvantages of overdiagnosis and overtreatment12. Specific management and follow-up recommendations for these equivocal PI-RADS lesions instead of biopsy do not exist. Another unsolved problem is the analysis of the optimal time interval between the initial and follow-up mpMRI, either with or without conducted biopsy, reaching from a few months up to several years13. Some studies suggest PSAD values in addition to the PI-RADS category to trigger or to delay biopsy14,15. The current version of PI-RADS v2.1 does not incorporate management recommendations and does also not provide guiding on evaluation of serial mpMRI.
In this study we analyzed follow-up mpMRI scans of patients with initial PI-RADS category 3 including the development of the PI-RADS category and clinical parameters over time. The aim was to verify that those patients in PI-RADS category 3 who harbour or develop PCA can be detected via PI-RADS upgrade in the follow-up mpMRI. Also, we strived to evaluate an optimal time interval for follow-up MRI scans for PI-RADS 3 cases.
Material and methods
Study design
This retrospective single center study includes consecutive patients with initial PI-RADS category 3 and one or more follow-up mpMRI (T2WI, DWI, DCE) and subsequent MRI-targeted and systematic TRUS-guided biopsy after follow-up mpMRI between 2012 and 2018. The study was approved by the Ethics Committee, Faculty of Medicine, Heinrich-Heine University of Duesseldorf, Germany. All experiments were performed in accordance with relevant guidelines and regulations. Informed consent was obtained from all subjects for the present study. Patients without biopsy or without follow-up mpMRI where excluded (Fig. 1). PI-RADS categories and clinical parameters (PSA, PSAD, prostate volume) between initial and follow-up mpMRI and between patients with or without PCA in subsequent biopsies were compared. Additional subgroup analysis of different time intervals between initial and follow-up examinations sought to determine the optimal time interval for control scans. PI-RADS scoring at baseline and follow-up was performed without knowledge of the histopathologic results at the time of the MRI by the same two readers (T.U., L.S.) with at least 6 years’ experience in prostate MRI. In ambigous or difficult cases, decision was made in consensus between the two readers.
Figure 1.
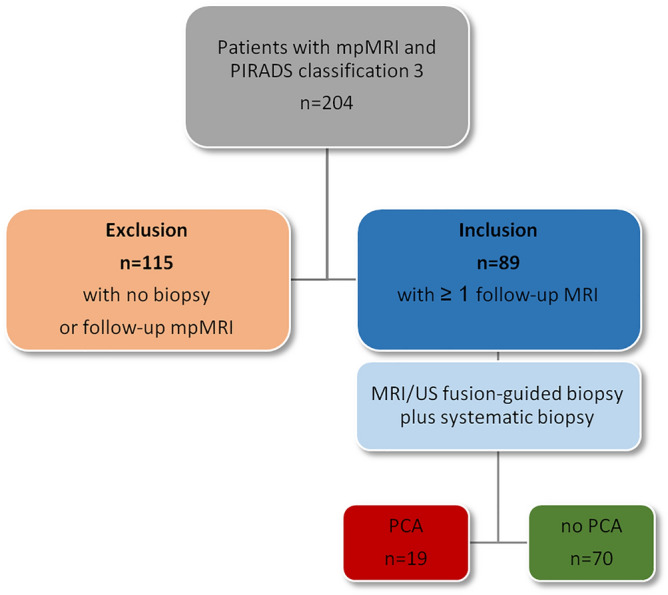
Flowchart of included patient. mpMRI: multiparametric magnetic resonance imaging; PI-RADS: Prostate Imaging Reporting and Data System; PCA: prostate cancer.
Study objectives
Primary study objective was the verification of a significant PI-RADS category upgrade in follow-up mpMRI in patients with subsequent PCA positive biopsy versus patients with negative biopsy. Further objectives were development of the PI-RADS category and clinical parameters between initial and follow-up mpMRI in the context of histopathologic results and depending on the time interval (12, 24, 36 months).
Imaging acquisition
All scans were conducted on 3 T MRI scanners (Magnetom TIM Trio, Prisma, or Skyra; Siemens Healthcare GmbH) using either an 18-channel phased-array surface coil combined with a 32-channel spine coil or a 60-channel phased-array surface coil. MR imaging parameters were chosen according to international recommendations and PI-RADS v2.1 guidelines and contained T2-weighted sequences in 3 planes (T2WI; turbo spin echo, TSE; axial: voxel size 0.5 × 0.5 × 3.0 mm; FOV 130 mm), diffusion-weighted imaging (DWI; ss-EPI and rs-EPI; voxel size 0.9–1.4 × 0.9–1.4 × 3.0 mm; b values 0, 500, 1000 s/mm2 and 1800 s/mm2), and dynamic contrast-enhanced imaging (DCE; T1 vibe; voxel size 0.8–1.5 × 0.8–1.5 × 3.0 mm, scan time 3 min, temporal resolution 7 s). Apparent diffusion coefficient (ADC) parameter maps and high b-values (1800 s/mm2) were calculated by the scanner using the standard monoexponentially model.
Biopsy and histopathology
Targeted MRI/ultrasound fusion-guided biopsy (two targeted cores from each lesion) and subsequent systematic 12-core TRUS-GB were conducted on an MRI/US fusion-guided biopsy system with elastic registration (UroNAV, Invivo, Gainsville, USA). All biopsies were performed by urologists with at least more than 4 years’ experience in MRI-targeted transrectal prostate biopsy (D.M., C.A.). Results from a second histopathologic sample were also considered, i.e., re-biopsy during follow-up. All histopathological findings were analyzed based on the recommendations of the International Society of Urological Pathology to distinguish between csPCA (Gleason score ≥ 7; ISUP grade group 2) and nsPCA (Gleason score 6; ISUP grade 1)16.
Statistical analysis
Statistics were performed using IBM SPSS® Statistics (Version 27 IBM Deutschland GmbH). P-values < 0.05 were defined as statistically significant. Descriptive statistics included mean values and standard deviation for normally distributed variables and median and interquartile range for non-parametric data. Wilcoxon signed rank test was used to compare paired data. Friedman test and ANOVA were used to compare paired data of multiple groups. Post-hoc analysis was conducted to evaluate differences between single groups. Kolmogorov–Smirnov-test and Shapiro–Wilk-test were used to check for normal distribution. Levene-test was applied to check data for homogeneity.
Results
Study population
Two-hundred four patients with overall PI-RADS category 3 in mpMRI of the prostate were initially enrolled. 89 patients (mean age 59 ± 9 years) had a follow-up examination within 15 months after the first scan and subsequent targeted and systematic biopsy and were finally included (Fig. 1). 70 of 89 patients (79%) had negative results in the histopathology, whereas PCA was detected in 19 patients (21%), including 4 with csPCA (4%) (n = 15 ISUP grade 1, n = 3 ISUP grade 2, n = 1 ISUP grade 3). The median PSA value of the entire study population increased significantly from 6.6 ng/ml (IQR 5.1–8.6 ng/ml) to 8.2 ng/ml (IQR 5.4–11 ng/ml; p < 0.001) between the two time points (Table 1). The prostate volume also increased from 51 ml (IQR 39–66 ml) to 60 ml (IQR 45–86 ml; p < 0.001). The median PSAD and PI-RADS did not change significantly for the entire population between initial and follow-up examination.
Table 1.
Baseline characteristics initially and during follow up.
| Initial | FU | p-value* | ||
|---|---|---|---|---|
| Number | n | 89 | 89 | |
| PSA, ng/ml | Median (IQR) | 6.6 (5.1–8.6) | 8.2 (5.4–11) | < 0.001 |
| PSAD, ng/ml/cm3 | Median (IQR) | 0.13 (0.10–0.17) | 0.13 (0.09–0.17) | 0.56 |
| Prostate volume, ml | Median (IQR) | 51 (39–66) | 60 (45–86) | < 0.001 |
| PI-RADS | Median (mean) | 3 (3.0) | 3 (2.89) | 0.11 |
FU: follow up; PSA: prostate specific antigen; PSAD: prostate specific antigen density; PI-RADS: Prostate Imaging Reporting and Data System; IQR: interquartile range.
*Wilcoxon signed rank test.
Significant values are given in bold.
Comparison of patients with and without PCA/csPCA during follow-up
The given mean overall PI-RADS classification in the follow-up mpMRI differed significantly between the subgroups of patients without PCA, without csPCA, with PCA, and with csPCA in the subsequent histopathologic evaluation, respectively (Table 2; p = 0.01). Post-hoc analysis for single group evaluation revealed statistically higher given PI-RADS categories for the group with csPCA (PI-RADS median 4, mean 3.75) compared to patients without PCA (PI-RADS median 3, mean 2.74; p = 0.009). All patients with biopsy proven csPCA received a PI-RADS upgrade in the follow-up mpMRI. The median PSA value, PSAD, and prostate volume did not differ significantly between the follow-up subgroups. Exact values are illustrated in Table 2.
Table 2.
Comparison of patients without and with PCA/csPCA.
| FU without PCA | FU without csPCA | FU with PCA | FU with csPCA | p-value* | |
|---|---|---|---|---|---|
|
Number n |
70 | 85 | 19 | 4 | |
|
PSA Median (IQR), ng/ml |
8.4 (5.5–11) | 8.4 (5.5–11) | 7.8 (5.3–10.6) | 5.4 (4.2–7.9) | 0.36 |
|
PSAD Median (IQR), ng/ml/cm3 |
0.13 (0.09–0.17) | 0.13 (0.09–0.17) | 0.14 (0.12–0.18) | 0.13 (0.12–0.16) | 0.92 |
|
Volume Median (IQR) ml |
68 (47–91) | 62 (45–86) | 52 (38–65) | 44 (32–50) | 0.72 |
|
PI-RADS Median (mean) |
3 (2.74) | 3 (2.85) | 4 (3.56) | 4 (3.75) | 0.01 |
|
ISUP grade group n |
1 | 15 | 15 | 0 | |
| 2 | 0 | 3 | 3 | ||
| 3 | 0 | 1 | 1 | ||
| 4–5 | 0 | 0 | 0 |
FU: follow up; PCA: prostate cancer; csPCA: clinically significant prostate cancer; PSA: prostate specific antigen; PSAD: prostate specific antigen density; PI-RADS: Prostate Imaging Reporting and Data System; ISUP: International Society of Urological Pathology; IQR: interquartile range; n: number.
*Wilcoxon signed rank test.
Significant values are given in bold.
Follow-up of patients without PCA
There was a significant PI-RADS downgrade in cases with negative biopsy over the course of 25–36 months from mean PI-RADS 3.0 to 2.78 (p = 0.02) (Table 3, Fig. 2). The median PSA value, PSAD, and prostate volume did not differ significantly between the different time points among patients without PCA (p = 0.24, 0.06, 0.08, respectively). Exact values are illustrated in Table 3.
Table 3.
Follow up of patients without PCA.
| Initial | ≤ 12 M | 12–24 M | 25–36 M | p-value | |
|---|---|---|---|---|---|
|
Number n |
70 | 25 | 46 | 23 | |
|
PSA Median (IQR), ng/ml |
8.4 (5.5–11) | 7.3 (5.0–10.3) | 8.3 (5.7–12.3) | 8.5 (7.1–13.4) | 0.24 |
|
PSAD Median (IQR), ng/ml/cm3 |
0.13 (0.09–0.17) | 0.14 (0.11–0.17) | 0.11 (0.09–0.21) | 0.13 (0.09–0.2) | 0.06 |
|
Volume Median (IQR), ml |
68 (47–91) | 53 (39–66) | 67 (48–88) | 69 (51–80) | 0.08 |
|
PI-RADS Median (mean) |
3 (3.0) | 3 (2.84) | 3 (2.93) | 3 (2.78) | 0.02 |
|
M mean ± SD |
10 ± 2 | 18 ± 3 | 30 ± 3 | ||
PSA value: prostate specific antigen; PSAD: prostate specific antigen density; PI-RADS: Prostate Imaging Reporting and Data System; IQR: interquartile range; M: months; n: number.
Significant values are given in bold.
Figure 2.
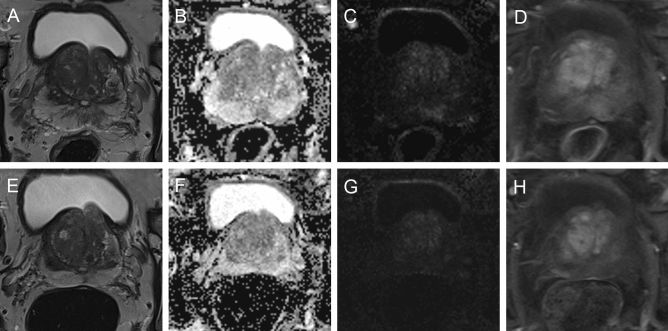
64-year-old patient with rising PSA 12.2 ng/m. Initial MRI examination: Axial T2-weighted image (A) showed a non-circumscribed, rounded, moderate hypointensity in the left lateral peripheral zone with focal discrete hypointense ADC signal (B), focal discrete hyperintense signal on high b-value DWI (C) and discrete correlating contrast enhancement in DCE (D), assessed as PI-RADS category 3. Follow-up MRI after 38 months: Axial T2-weighted image (E) showed only residual changes, no ADC reduction (F), no more hyperintense signal on high b-value DWI (G), and no contrast enhancement (H). The PSA decreased (6.6 ng/mL) and mpMRI was downgraded to PIRADS category 2.
Follow-up of patients with PCA
In the subgroup of patients with proven PCA, the median PSAD increased significantly from 0.13 ng/ml/ml (IQR 0.13–0.18 ng/ml/ml) to 0.18 ng/ml/ml (IQR 0.13–0.22 ng/ml/ml) after 24 months (p < 0.001). Table 4 illustrates the exact values for the distinct time intervals. The mean PI-RADS classification differed significantly between the investigated time intervals (p = 0.02). Post-hoc analysis revealed a significant mean PI-RADS upgrade from 3.0 to 3.88 after 12 -24 months (p = 0.03). The development of PI-RADS in patients with PCA divided by ISUP group is shown in Supplemental Table 1.
Table 4.
Follow up data of patients with PCA.
| Initial | ≤ 12 M | 12–24 M | > 24 M | p-value | |
|---|---|---|---|---|---|
|
Number n |
19 | 7 | 8 | 10 | |
|
PSA Median (IQR) ng/ml |
6 (6–9.2) | 5.5 (5.4–8.2) | 6.9 (5.4–9.1) | 8.5 (6.1–11.1) | 0.52 |
|
PSAD Median (IQR), ng/ml/cm3 |
0.13 (0.13–0.18) | 0.12 (0.09–0.16) | 0.13 (0.11–0.15) | 0.18 (0.13–0.22) | < 0.001 |
|
Volume Median (IQR), ml |
46 (46–65.5) | 48 (46–61) | 50 (31–66) | 57 (38–73) | 0.08 |
|
PI-RADS Median (mean) |
3 (3.0) | 3 (3.43) | 4 (3.88) | 3 (3.30) | 0.02 |
|
M Mean ± SD |
9 ± 2 | 14 ± 2 | 39 ± 13 | ||
PSA value: prostate specific antigen; PSAD: prostate specific antigen density; PI-RADS: Prostate Imaging Reporting and Data System; IQR: interquartile range; M: months; n: number.
Significant values are given in bold.
Discussion
Our results suggest that patients with initial PI-RADS category 3 lesions who receive a biopsy positive for PCA over the course of the follow-up, reliably show a PI-RADS upgrade in follow-up mpMRI after 12–24 months. In contrary, patients with PI-RADS 3 lesions without PCA positive biopsies during follow-up, receive a PI-RADS downgrade after 25–36 months. Consequently, follow-up mpMRI of PI-RADS category 3 lesions after 12–24 months seems useful and may justify a delay or even waiver of biopsy.
The overall detection of csPCA in patients with PI-RADS 3 lesions in our collective is very low (4%), which stands in line with previously published data5,17–20. We did not observe the detection of PCA with ISUP grade ≥ 3 at all in our patient cohort. The decision whether to proceed to biopsy immediately or perform follow-up MRI after a defined period is discussed controversially. Our findings suggest that follow-up via mpMRI instead of prompt biopsy of these patients does not lead to missed csPCA along the way, a strategy that has been proposed many times by other authors10,21–23. The main advantage of follow-up MRI is to avoid unnecessary invasive diagnostic and to ensure higher compliance, as patients often prefer conducting follow-up MRI over biopsy. Besides, information of follow-up MRI is useful to evaluate lesions over time. The comparison to baseline scans can be conducted analogously to the PRECISE criteria in Active Surveillance24. That includes the evaluation of ADC-values, size, and contrast enhancement in the course of time. The overall low risk for metastasis in ISUP grade 1 and 2 (< 10%) supports this approach25. Nevertheless, standardized management recommendations for PI-RADS 3 patients and especially specific follow-up intervals have not been established yet. The time interval of 12–24 months after which a PI-RADS upgrade was seen in our patients with csPCA has already been reported by a study of Steinkohl at al.13. In our cohort all patients with csPCA in subsequent biopsy received a PI-RADS upgrade. However, detection rates are heavily dependent on MRI quality, biopsy, pathology quality, and experience of the respective physicians and some diffuse PCA can be missed by MRI26. Additionally, there are cancers missed by MRI with negative fusion biopsy, but cancer is detected in systematic cores, especially in smaller lesions or in cases where fusion of ultrasound and MRI is insufficient.
Furthermore, if a negative histopathology is already confirmed, our data suggest that follow-up mpMRI after 24 months seems sufficient not to miss csPCA and to reduce the number of unnecessary biopsies13. A possible explanation for the relatively longer time intervals in patients without PCA until a PI-RADS downgrade was observed may be inflammatory changes due to granulomatous or non-bacterial prostatitis or atypical hyperplasia. As long as these diffuse changes persist, they can potentially still mask PCA lesions and therefore PI-RADS category 3 is still justified27–29.
A recently published study by Washington SL et al. discussed the role of MRI based PSAD as a predictor for upgrade of the Gleason score under active surveillance30. This matches with our finding that PSAD significantly increased in patients with PCA between initial and follow-up mpMRI. In cases with PI-RADS 3 lesions and PSAD ≤ 0.2, the rate of ISUP grade ≥ 2 is vanishingly small31. PSAD alone without mpMRI showed poor performance in predicting csPCA in clinical routine over time15. Nonetheless, clinical parameters as PSAD seem to be a valuable tool in combination with mpMRI in uncertain cases to trigger or to postpone biopsy32.
Our study has limitations. First, this retrospective, single center study investigates a heterogeneous collective of patients who received follow-up mpMRI and subsequent biopsies at different time points. However, our study reflects a real-life scenario, and we were able to demonstrate significant changes even under these circumstances. We performed a systematic 12-core and additional MRI-targeted biopsy, taking two cores from each suspicious lesion. This is the standard in-house procedure and a common approach, also in larger studies. Nevertheless, there are different findings in the literature to perform targeted biopsy extracting more biopsy cores to reliably diagnose prostate cancer33–35. Even though other time intervals between the serial mpMRI, different from ours, may be thinkable, the intervals we used are based on guidelines for follow-up of patients under active surveillance and are widely used in clinical practice13. It is possible that different time intervals may have different outcomes. Further research on the optimal time interval is warranted.
In conclusion our results suggest that patients with PI-RADS category 3 may primarily receive follow-up mpMRI 12 to 24 months after the initial MRI scan instead of direct biopsy without missing csPCA. The overall number of csPCA in PI-RADS 3 lesions was very small and all patients with PI-RADS 3 lesions who harbored or developed csPCA over the course of the follow-up showed a PI-RADS upgrade in follow-up mpMRI in our cohort. This strategy may help to prevent overdiagnosis and overtreatment. In patients where the histopathologic results revealed no PCA, there was a significant downgrade in the PI-RADS category at follow-up mpMRI after 24 months. Therefore, patients with PI-RADS category 3 and negative biopsy do not seem to benefit from follow-up mpMRI earlier than after 24 months. In uncertain cases clinical parameters as PSAD may support clinical decision making.
Supplementary Information
Abbreviations
- PCA
Prostate cancer
- csPCA
Clinically significant prostate cancer (ISUP grade ≥ 2)
- nsPCA
Non clinically significant prostate cancer (ISUP grade 1)
- PI-RADS
Prostate Imaging Reporting and Data System
- PSA
Prostate-specific antigen
- PSAD
Prostate-specific antigen density
- TRUS-GB
Transrectal ultrasound-guided prostate biopsy
- IQR
Interquartile range
- mpMRI
Multiparametic prostate MRI
Author contributions
M.B., L.S. and T.U. wrote the main manuscript text, acquired and analyzed the data and calculated statistics. R.A., D.M., C.A. acquired and analyzed the data. F.Z., M.Q. and J.M. prepared figures. All authors reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: M. Boschheidgen and L. Schimmöller.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-10859-9.
References
- 1.Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. [DOI] [PubMed] [Google Scholar]
- 2.Záleský M, Stejskal J, Adamcova V, Hrbáček J, Minarik I, Pavlicko A, et al. Use of prostate specific antigen density combined with multiparametric magnetic resonance imaging improves triage for prostate biopsy. Urol. Int. 2019;103:33–40. doi: 10.1159/000500350. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zeng N, Zhang F, Huang Y, Tian Y. How to make clinical decisions to avoid unnecessary prostate screening in biopsy-naïve men with PI-RADs v2 score ≤ 3? Int. J. Clin. Oncol. 2020;25:175–186. doi: 10.1007/s10147-019-01524-9. [DOI] [PubMed] [Google Scholar]
- 4.Panebianco V, Barchetti G, Simone G, Del Monte M, Ciardi A, Grompone MD, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: What's next? Eur. Urol. 2018;74:48–54. doi: 10.1016/j.eururo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Maggi M, Panebianco V, Mosca A, Salciccia S, Gentilucci A, Di Pierro G, et al. Prostate imaging reporting and data system 3 category cases at multiparametric magnetic resonance for prostate cancer: A systematic review and meta-analysis. Eur. Urol. Focus. 2020;6:463–478. doi: 10.1016/j.euf.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Wysock JS, Mendhiratta N, Zattoni F, Meng X, Bjurlin M, Huang WC, et al. Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12-core biopsy results. BJU Int. 2016;118:515–520. doi: 10.1111/bju.13427. [DOI] [PubMed] [Google Scholar]
- 7.Haffner J, Lemaitre L, Puech P, Haber G-P, Leroy X, Jones JS, Villers A. Role of magnetic resonance imaging before initial biopsy: Comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–E178. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- 8.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer DL, van der Kwast TH, Evans AJ, Sun L, Yaffe MJ, Trachtenberg J, Haider MA. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2—sparse versus dense cancers. Radiology. 2008;249:900–908. doi: 10.1148/radiol.2493080236. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich T, Quentin M, Arsov C, Schmaltz AK, Tschischka A, Laqua N, et al. Risk stratification of equivocal lesions on multiparametric magnetic resonance imaging of the prostate. J. Urol. 2018;199:691–698. doi: 10.1016/j.juro.2017.09.074. [DOI] [PubMed] [Google Scholar]
- 11.Ullrich T, Quentin M, Arsov C, Laqua N, Abrar D, Hiester A, et al. Value of dynamic contrast-enhanced (DCE) MR imaging in peripheral lesions in PI-RADS-4 patients. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2020;192:441–447. doi: 10.1055/a-1020-4026. [DOI] [PubMed] [Google Scholar]
- 12.Hauth E, Jaeger H, Hohmuth H, Beer M. Follow-up MR imaging of PI-RADS 3 and PI-RADS 4 prostate lesions. Clin. Imaging. 2017;43:64–68. doi: 10.1016/j.clinimag.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Steinkohl F, Gruber L, Bektic J, Nagele U, Aigner F, Herrmann TRW, et al. Retrospective analysis of the development of PIRADS 3 lesions over time: When is a follow-up MRI reasonable? World J. Urol. 2018;36:367–373. doi: 10.1007/s00345-017-2135-0. [DOI] [PubMed] [Google Scholar]
- 14.Vourganti S, Rastinehad A, Yerram N, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J. Urol. 2012;188:2152–2157. doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Görtz M, Radtke JP, Hatiboglu G, Schütz V, Tosev G, Güttlein M, et al. The value of prostate-specific antigen density for prostate imaging-reporting and data system 3 lesions on multiparametric magnetic resonance imaging: A strategy to avoid unnecessary prostate biopsies. Eur. Urol. Focus. 2021;7:325–331. doi: 10.1016/j.euf.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 international society of urological pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 17.Schoots IG, Padhani AR, Rouvière O, Barentsz JO, Richenberg J. Analysis of magnetic resonance imaging-directed biopsy strategies for changing the paradigm of prostate cancer diagnosis. Eur. Urol. Oncol. 2020;3:32–41. doi: 10.1016/j.euo.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Osses DF, Arsov C, Schimmöller L, Schoots IG, van Leenders GJLH, Esposito I, et al. Equivocal PI-RADS three lesions on prostate magnetic resonance imaging: Risk stratification strategies to avoid MRI-targeted biopsies. J. Pers. Med. 2020;10:270. doi: 10.3390/jpm10040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer H-P, Wieczorek K, et al. The value of PSA density in combination with PI-RADS™ for the accuracy of prostate cancer prediction. J. Urol. 2017;198:575–582. doi: 10.1016/j.juro.2017.03.130. [DOI] [PubMed] [Google Scholar]
- 20.Israël B, van der Leest M, Sedelaar M, Padhani AR, Zámecnik P, Barentsz JO. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: What urologists need to know. Part 2: interpretation. Eur. Urol. 2020;77:469–480. doi: 10.1016/j.eururo.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Klingebiel M, Ullrich T, Rabenalt R, Albers P, Antoch G, Schimmöller L. Not available. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2018;190:1067–1069. doi: 10.1055/a-0620-8875. [DOI] [PubMed] [Google Scholar]
- 22.Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 2019;2019(76):340–351. doi: 10.1016/j.eururo.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B, et al. Interobserver reproducibility of the PI-RADS version 2 Lexicon: A multicenter study of six experienced prostate radiologists. Radiology. 2016;280:793–804. doi: 10.1148/radiol.2016152542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore CM, Giganti F, Albertsen P, Allen C, Bangma C, Briganti A, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: The PRECISE recommendations-a report of a European school of oncology task force. Eur. Urol. 2017;71:648–655. doi: 10.1016/j.eururo.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Diolombi ML, Epstein JI. Metastatic potential to regional lymph nodes with Gleason score ≤7, including tertiary pattern 5, at radical prostatectomy. BJU Int. 2017;119:872–878. doi: 10.1111/bju.13623. [DOI] [PubMed] [Google Scholar]
- 26.Moldovan PC, Van den Broeck T, Sylvester R, Marconi L, Bellmunt J, van den Bergh RC, Roderick CN, et al. What Is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European association of urology prostate cancer guidelines panel. Eur. Urol. 2017;72:250–266. doi: 10.1016/j.eururo.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Ullrich T, Schimmöller L, Oymanns M, Blondin D, Dietzel F, Kirchner J, et al. Current utilization and acceptance of multiparametric MRI in the diagnosis of prostate cancer. A regional survey. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2018;190:419–426. doi: 10.1055/s-0043-118128. [DOI] [PubMed] [Google Scholar]
- 28.Gold SA, Hale GR, Bloom JB, Smith CP, Rayn KN, Valera V, et al. Follow-up of negative MRI-targeted prostate biopsies: When are we missing cancer? World J. Urol. 2019;37:235–241. doi: 10.1007/s00345-018-2337-0. [DOI] [PubMed] [Google Scholar]
- 29.Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A. Benign conditions that mimic prostate carcinoma: MR imaging features with histopathologic correlation. Radiographics. 2016;36:162–175. doi: 10.1148/rg.2016150030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Washington SL, Baskin AS, Ameli N, Nguyen HG, Westphalen AC, Shinohara K, Carroll PR. MRI-based prostate-specific antigen density predicts Gleason score upgrade in an active surveillance cohort. Am. J. Roentgenol. 2020;214:574–578. doi: 10.2214/AJR.19.21559. [DOI] [PubMed] [Google Scholar]
- 31.Hansen NL, Kesch C, Barrett T, Koo B, Radtke JP, Bonekamp D, et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int. 2017;120:631–638. doi: 10.1111/bju.13711. [DOI] [PubMed] [Google Scholar]
- 32.Venderink W, van Luijtelaar A, Bomers JGR, van der Leest M, Hulsbergen-van de Kaa C, Barentsz JO, et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur. Urol. 2018;73:353–360. doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N. Engl. J. Med. 2020;382:917–928. doi: 10.1056/NEJMoa1910038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bevill MD, Troesch V, Drobish JN, Flynn KJ, Rajput M, Metz CM, et al. Number of cores needed to diagnose prostate cancer during MRI targeted biopsy decreases after the learning curve. Urol. Oncol. 2022;40:7.e19–7.e24. doi: 10.1016/j.urolonc.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Lu AJ, Syed JS, Ghabili K, Hsiang WR, Nguyen KA, Leapman MS, Sprenkle PC. Role of core number and location in targeted magnetic resonance imaging-ultrasound fusion prostate biopsy. Eur. Urol. 2019;76:14–17. doi: 10.1016/j.eururo.2019.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.