Abstract
Background
Neuromyelitis optica spectrum disorder (NMOSD) is a rare neuroimmunology disorder predominantly affecting the East Asia population, the reason for this preference remains unknown. Genetic factors such as polymorphisms in human leukocyte antigen (HLA) and interleukins (IL) genes have been reported. Although the familial occurrence of NMOSD is rare, it supports that genetic factors may play a role.
Methods
Whole exome sequencing (WES) study was performed on the affected mother and daughter, as well as the unaffected father in a Taiwanese family with NMOSD. A cohort of 19 sporadic patients with aquaporin 4 antibody (AQP4-Ab) positive NMOSD was also recruited; all fulfilled the 2015 International NMOSD Diagnosis Criteria. Sanger sequencing was performed on exon 4 of the CD33 gene on the sporadic NMOSD cohort.
Results
WES study revealed a 19 base pair deletion in exon 4 of the CD33 gene, resulting in frameshift premature truncating protein, which segregated with the affected status. CD33 was the most likely candidate gene due to its known function in immune regulation. A total of 19 sporadic NMOSD patients were tested using Sanger sequencing, including 3 patients with other concomitant autoimmune disorders. Two additional NMOSD patients were found to have the same CD33 frameshift variant, which accounts for 19.04% of all NMOSD patients, and 15% following correction for the familial cases; compared to 2% in Taiwanese population controls.
Conclusion
In this study, we identified a 19 base pair deletion in the CD33 gene may be a potential risk locus for NMOSD, which is predicted to cause loss of function of CD33. The loss of CD33 inhibitory function may affect the regulation of the immune system in NMOSD patients. This finding requires further larger cohorts of NMOSD patients and functional study to corroborate.
Keywords: Neuromyelitis optica, AQP4, CD33, Siglec-3
At a glance of commentary
Scientific background on the subject
Neuromyelitis optica spectrum disorder (NMOSD) is a rare neuroimmunology disorder predominantly affecting the East Asia population, the reason for this preference remains unknown. Genetic factors such as polymorphisms in human leukocyte antigen (HLA) and interleukins (IL) genes have been associated with NMOSD. Familial occurrence of NMOSD is rare but it supports that genetic factors may play a role.
What this study adds to the field
We identified a 19 base pair deletion in the CD33 gene in a family of NOMSD and later a cohort of sporadic NMOSD patients. Our study identified that CD33 may be a potential genetic risk locus. The variant is predicted to cause loss of function, which may affect the regulation of the immune system in NMOSD patients. This study provides a new insight with regard to the pathogenesis of NMOSD.
Neuromyelitis optica spectrum disorder (NMOSD) is an immune-mediated inflammatory disorder of the central nervous system (CNS), characterized by the presence of aquaporin 4 antibodies (AQP4-Ab) [1]. AQP4 is the primary water channel in the brain mainly located in the optic nerve, ependymal cells of the cerebrum, cerebellum and spinal cord [2]. NMOSD and multiple sclerosis (MS) are both inflammatory demyelinating diseases with different underlying pathophysiology and clinical features [3,4]. In contrast to MS, NMOSD is more prevalent in Asian, Indian and Black populations compared with Caucasians [5]. The prevalence of NMOSD ranged from 0.52 to 4.4 per 100,000 individuals around the world and was reported as 17.2 per 100,000 individuals in Taiwan [[6], [7], [8]]. The reason why Asian populations are at a higher risk for NMOSD remains unclear. It is hypothesized that ethnicity-specific genetic backgrounds may play a role.
Most reported cases of NMOSD are sporadic, although a few familial cases have been described [9,10]. NMOSD is likely a complex disease with interactions between the host genetic and environmental factors [5]. The genetic factors underlying NMOSD have been previously investigated, especially the human leukocyte antigen (HLA) complex. The HLA-DPB1∗0501 allele was significantly higher in the Asian population (44.9–73.1%), including the Southern Han Chinese and the Japanese [11,12], than in Caucasians (2.6–5.3%) [11] among patients with AQP4-Ab positive NMOSD. The HLA-DRB1∗03:01 allele was associated with NMOSD in French, Brazilian and Mexican populations, but did not influence the severity of NMOSD and laboratory features [13]. A recent whole-genome sequence study found that genetic variants in the major histocompatibility complex (MHC) region contributed to the etiology of AQP4-Ab positive NMOSD, which is genetically more similar to systemic lupus erythematosus (SLE) than MS [14]. The association between NMOSD and other non-HLA genetic factors has also been reported, including TNFSF4, TPMT∗3C, and IL2RA [[15], [16], [17]].
In this study, we used whole-exome sequencing (WES) technology to study a family with NMOSD; and then validated the finding in a cohort of sporadic NMOSD patients.
Materials and methods
Subjects and diagnostic criteria
A previously reported Taiwanese NMOSD family with affected mother–daughter pair was included in the present study [10]. A cohort of 19 sporadic patients with AQP4-positive NMOSD was subsequently recruited. These patients all fulfilled the 2015 International Panel NMOSD Diagnostic Criteria [18]: 1) at least one core clinical characteristic; 2) AQP4-IgG–positive status; and 3) exclusion of alternative diagnoses. Core clinical characteristics were as follows: 1) optic neuritis, 2) acute myelitis, 3) area postrema syndrome, 4) acute brainstem syndrome, 5) symptomatic narcolepsy or acute diencephalic clinical syndrome with NMOSD-typical diencephalic magnetic resonance imaging lesions, and 6) symptomatic cerebral syndrome with NMOSD-typical brain lesions.
Patients who were negative for AQP4-IgG were excluded. Most patients also received a systemic autoimmune serological work-up, including antinuclear antibody (ANA), antibodies to extractable nuclear antigens (antiENA), anti-Sjögren's-syndrome-related antigen A antibody (anti-SSA) and double stranded-DNA. Patients with concomitant autoimmune disorders were not excluded because of the co-existence of AQP4-Abs. The diagnosis of concomitant autoimmune disorders was based on the relevant diagnostic criteria [19]. Patients with positive systemic autoimmune antibodies who did not fulfill the relevant clinical diagnostic criteria were not diagnosed as having concomitant autoimmune disorders.
WES study of the NMOSD family
WES was performed on the affected mother and daughter as well as the unaffected father. In brief, genomic DNA was sonicated and captured using an Agilent SureSelect All Exon V6 reagent kit (Agilent, Santa Clara, CA, USA). The captured library was indexed and sequenced using an Illumina NovaSeq 6000 Sequencing system with a 150-bp pair-end reagent kit with 50× coverage (Welgene Biotech, Taiwan). Subsequent bioinformatics analysis was done using the web-based open-source Galaxy platform [20]. Fastq reads were mapped to the reference human genome (GRCh37) using BWA-MEM followed by the Genome Analysis Tool Kit (GATK) best-practice pipeline, including small insertion and deletion (indels) realignment and quality score recalibration. Single nucleotide variants (SNVs) and indels were called using haplotype-based FreeBayes and annotated by ANNOVAR as previously described [21]. The analysis was limited to frameshift, stop gain and splicing variants. As for nonsynonymous changes, only variants that were predicted by multiple in-silico prediction algorithms were included: SIFT not tolerated, PolyPhen-2 not benign, MutationTaser not neutral and a CADD Phred Score >20. VarElect was subsequently used to prioritize candidate genes that were associated with the term: “immune” in the function and pathway sections [22].
Sanger sequencing of exon 4 of the CD33 gene
Genomic DNA was extracted using a QIAamp Gentra Puregene Blood kit according to the manufacturer's protocol (QIAGEN, Germany). Primers for exon 4 of the CD33 gene were designed using Primer3Plus as follows: CD33_E4 forward: AGGGGAAATGCCTACCCTTA and CD33_E4 reverse: GCAAGGGGGAAGTTGCTAGT. All sporadic AQP4-Ab positive NMOSD patients were sequenced using an ABI3730XL DNA analyzer (Applied Biosystems, USA). An in-house WES database containing 300 healthy individuals was used as the controls, all were without known autoimmune or neurological disorders.
Statistical analysis
Fisher's exact test was used to compare the difference between NMOSD patients and the controls. A P-value <0.05 was considered to indicate a statistically significant difference. Statistics were calculated in all NMOSD patients, as well as the proband of the familial cases, plus sporadic cases to avoid potential sampling bias. All statistics were calculated using EZR version 1.38 [23].
Results
WES identified CD33 as a candidate risk gene for NMOSD
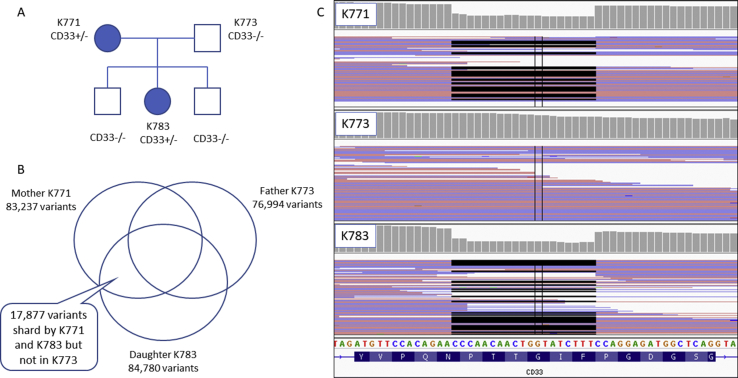
WES of the NMOSD family trio revealed 17,877 variants that were shared by both affected individuals (mother and daughter) but were not inherited from the unaffected father [Fig. 1]. Among them, 1926 variants did not exist in the 12 WES controls. After filtering, 5 frameshift variants, 4 splicing variants, 1 stop gain and 53 predicted pathogenic nonsynonymous variants remained [Table 1].
Fig. 1.
Whole exome sequencing of the neuromyelitis optica spectrum disorder (NMOSD) family. A shows the pedigree of the NMOSD family. B reveals the results of the whole-exome sequencing study. C demonstrates the inherited 19 bp deletion on the Integrated Genome Viewer.
Table 1.
Candidate genes selected from the NMOSD family.
| Types of variants | Gene names |
|---|---|
| Stop gain (n = 1) | EFCAB6 |
| Frameshift (n = 5) | CRIPAK, USP35, ZIC5, GDPGP1, CD33 |
| Splicing (n = 4) | AADACL4, MARC1, ACOXL, ATG16L2 |
| Predicted pathogenic nonsynonymous (n = 53) | VPS13D, GALE, PTPRF, TTC22, SYDE2, TAGLN2, RABGAP1L, CTSE, BECN1P1, DUSP28, C3orf20, LAMB2, LMOD3, DRD5, ARAP2, FRYL, LRBA, SDHA, NIM1K, IL6ST, N4BP3, PTK7, NCF1, MAK16, ZFHX4, TMEM2, OR13C8, PPP1R26, NCOA4, FAM178A, INSC, PTPN5, MADD, AHNAK, SPTBN2, KCTD14, LOH12CR1, ADAMTS20, MYO16, FAM189A1, CES5A, TSR1, DNAH2, DHRS7C, BECN1, KIF2B, FBF1, ANKRD12, COL5A3, NPHS1, TMEM145, UBOX5, DMD |
Abbreviation: NMOSD: neuromyelitis optica spectrum disorder.
VarElect was used for variant prioritization and the submission of all 63 filtered genes where “immune” was used as the keyword term for the “function” and “pathway” sections. There were 9 direct related genes identified in the “function” section: LRBA, SDHA, NCF1, NPHS1, DMD, GALE, CD33, CTSE and IL6ST, and 10 directly related genes identified in the “pathway” section: IL6ST, NCF1, CD33, SPTBN2, CTSE, KIF2B, UBOX5, LAMB2, BECN1, and PTK7. A total of 4 genes were identified by both analyses: NCF1, CD33, CTSE, and IL6ST. Among them, the immunomodulatory receptor gene CD33 (also called sialic-acid-binding immunoglobulin-like lectin [Siglec-3]) was the most likely candidate gene due to its known function within the immune system. The 19 base pair (bp) deletion variant (chr19:51729579–51729597delCCAACAACTGGTATCTTTC) located in exon 4 of CD33, resulted in a frameshift mutation with premature truncation (c.712-730delCCAACAACTGGTATCTTTC, p.Pro238Glnfs∗38), which was confirmed by Sanger sequencing. This change was absent in the 2 unaffected brothers of the proband [Fig. 1]. This frameshift mutation is likely to cause nonsense-mediated decay or the translation of a shorter protein, which lacks 30 C-terminal amino acids.
Validation of CD33 in a cohort of sporadic NMOSD patients
A total of 19 sporadic NMOSD patients were collected, three of which had other concomitant autoimmune disorders (all primary Sjögren's syndrome) [Table 2]. Exon 4 of the CD33 gene was screened using Sanger sequencing. Two additional NMOSD patients were identified with the same CD33 frameshift mutation, which accounts for 19.04% (4/21) of all NMOSD patients and 15% (3/20) when corrected for the familial cases. Both of the sporadic NMOSD patients who were positive for the CD33 mutation had NMOSD without concomitant autoimmune disorders. This variant was previously reported in the gnomAD and ExAc databases to have a minor allele frequency of 2% in East Asian populations, however, it was absent or very rare in other populations [24]. An in-house database of 300 Taiwanese whole genome sequences (from the Genomic Core Laboratory) was also examined for the allele frequency of the CD33 frameshift variant. A total of 7 (2.3%) controls were found to have the same variant (p = 0.0033, odds ratio (OR) = 9.69 for all NMOSD cases and p = 0.019, OR = 7.28 adjust for familial cases).
Table 2.
Clinical characteristics of the patients with neuromyelitis optica spectrum disorders.
| Onset age/Gender | Clinical presentation | Core features | MRI finding | Laboratory data | Autoimmune disease | |
|---|---|---|---|---|---|---|
| Family cases | ||||||
| I | 39/F | Right side limbs progressive weakness and numbness, left side trunk and limb sensation loss and gait disturbance | Acute myelitis | Low C to T8 level LETM, Hyperintense T2WI at left parietal periventricle white matter, corpus callosum and cerebellum Ill-defined bright signal on T2WI in cord C to T spine |
AQP4 Ab(+) | – |
| II | 22/F | Nausea, hiccups and vomiting Numbness over both palms, abdomen and both lower limbs |
Acute myelitis Area postrema syndrome |
High T2WI signal lesion noted over medulla C3-6 levels myelitis |
AQP4 Ab(+) | – |
| Sporadic cases | ||||||
| 1 | 46/F | Sudden onset left eye blurred vision Nausea |
Optic neuritis Area postrema syndrome Acute brainstem syndrome |
Medullar-C1 junction hyperintense lesion Abnormal signal change over bilateral optic nerves, much more severe on the left side |
AQP4 Ab(+) | – |
| 2 | 38/F | Acute onset left eye blurred vision Bilateral lower limb tightness and numbness; back tightness |
Optic neuritis Acute myelitis |
T2 to 5 level LETM | AQP4 Ab(+) | – |
| 3 | 27/F | Acute onset of right blurred vision Episodic transient bilateral limbs weakness and numbness Nausea and poor appetite Frequent hiccup |
Optic neuritis Acute myelitis Area postrema syndrome Acute brainstem syndrome |
Focal hyperintensity at the lower medulla Right optic nerve with T2WI hyperintense signal change C4-5 and C7 level spinal cord |
AQP4 Ab(+) | – |
| 4 | 62/F | Sudden onset left eye blurred vision Posterior nuchal stiffness Dry mouth and dry eyes |
Optic neuritis | High T2WI signal change over C5-6 spinal cord | AQP4 Ab(+) Anti-SSA/Ro(+) |
– |
| 5 | 58/F | Progressive right eye blurred vision Progressive paresthesia from bilateral upper limbs to legs; both lower limbs weakness Dry mouth and dry eyes |
Optic neuritis Acute myelitis |
Multiple intramedullary hyperintense changes in lower cervical and thorax | AQP4 Ab(+) Anti-SSA/Ro(+) Anti-SSB/La(+) Unstimulated whole saliva flow rate test:(+) |
PSS |
| 6 | 36/F | Numbness of both hands; both legs weakness and right leg soreness Nausea Acute onset of binocular diplopia |
Acute myelitis Area postrema syndrome Acute brainstem syndrome |
C4-7, T5-6 high T2WI signal lesion Right posterior pons lesion Hyperintense changes in bilateral optic nerves |
AQP4 Ab(+) | – |
| 7 | 38/F | Leg numbness and tightness with heavy sensation Right visual field defect Gait disturbance |
Optic neuritis Acute myelitis Area postrema syndrome |
High T2WI signal lesion over C3-5, C7-T8, corpus callosum and bilateral F–P regions | AQP4 Ab(+) | – |
| 8 | 54/F | Both eyes visual acuity declined Both legs numbness and mild right side weakness Acute onset of nausea, vomiting |
Optic neuritis Acute myelitis Area postrema syndrome |
Hyperintensity in optic and medulla, focal enhancement right optic chiasm left periventricle and upper C spine to T4 | AQP4 Ab(+) | – |
| 9a | 22/F | Blurred vision in the left eye Bilateral lower limb ascending numbness from the feet to above the knee |
Optic neuritis Acute myelitis |
Cervical and thoracic spinal cords LETM High signal lesion in corpus callosum |
AQP4 Ab(+) | – |
| 10 | 43/F | Persistent hiccup and nausea Acute onset of double vision Left limb numbness and hyperesthesia Unsteady gait |
Area postrema syndrome Acute brainstem syndrome |
Enhanced lesion over C4-7 and medulla to upper C-spine | AQP4 Ab(+) | – |
| 11 | 43/F | Beside left forearm numbness with weakness Hiccup, nausea and vomiting Progressive dysarthria and dysphagia |
Acute myelitis Area postrema syndrome Acute brainstem syndrome |
Central spinal canal lesion, left side dominant Hyperintense lesion over dorsal medulla |
AQP4 Ab(+) ENA screening(+) HCV |
– |
| 12a | 32/F | Acute onset both eyes blurred vision Left side weakness and numbness |
Optic neuritis Acute myelitis; |
Periventricular regions and right cervical spinal cord lesion | AQP4 Ab(+) | – |
| 13 | 31/F | Progressive left eye blurred vision Bilateral distal limbs numbness Bilateral leg weakness |
Optic neuritis Acute myelitis |
Increased perioptic nerve hyperintense Small periventricular lesion |
AQP4 Ab(+) | – |
| 14 | 48/F | Blurred vision in the right eye Bilateral lower limb weakness and numbness Hiccup |
Optic neuritis Acute myelitis Area postrema syndrome |
T5-8 LETM High T2WI signal lesion noted over bilateral pons, basal ganglia, periventricular, and F–P regions |
AQP4 Ab(+) | – |
| 15 | 56/F | Blurred vision Pain and numbness mainly localized at the right side and below T10 level Bilateral lower limb weakness |
Optic neuritis Acute myelitis; |
Multiple focal lesions over T4-5, high T2WI signal lesion at dorsal and central part of spinal cord | AQP4 Ab(+) | – |
| 16 | 45/F | Right hand weakness and numbness Acute onset of double vision and bilateral ptosis Insidious onset of changes in consciousness and quadriparesis |
Optic neuritis Acute myelitis Area postrema syndrome |
T2WI high signal: right temporal horn ependymal lining of periventricular region, optic tract, midbrain and the periaqueductal area Intramedullary lesion C2-5 |
AQP4 Ab(+) Anti-SSA/Ro(+) Schirmer's test: 0 mm/5 min on both eyes Unstimulated whole saliva flow rate test(+) |
PSS |
| 17 | 37/F | Blurred vision in the left eye Numbness of both lower legs Nausea, vomiting then hiccup |
Optic neuritis Acute myelitis Area postrema syndrome |
High T2WI signal change in medulla C1-3 and T4-9, high T2WI signal lesion over periventricular area |
AQP4 Ab(+) Anti-SSA/Ro(+) |
– |
| 18 | 39/F | Deterioration of vision in the right eye Both legs numbness, ascending type then unsteady gait Dry eyes and skin |
Optic neuritis Acute myelitis |
High T2WI signal lesion over C4-T1 and periventricular area | AQP4 Ab(+) Anti-SSA/Ro, SSB/La(+) Schirmer's 0 mm/5 min on both eyes |
PSS |
| 19 | 27/F | Recurrent visual loss in both eyes Acute onset of ascending numbness and left lower limb weakness with progression around the T5-6 region |
Optic neuritis Acute myelitis Acute brainstem syndrome |
Increased cord signal over T2-T7/8 levels | AQP4 Ab(+) | – |
2 sporadic cases identified by Sanger sequencing with positive finding of CD33 frameshift mutation. Abbreviations: C: C spine; T: T spine; T2WI: T2 weighted image; AQP4 Ab: antibodies to aquaporin-4; LETM: longitudinally extensive transverse myelitis; PSS: primary Sjögren's syndrome; F–P region: frontoparietal region; Unstimulated whole saliva flow rate test positive: <0.1 ml/min, case 5: 0.57 ml/15 min, case 16: <0.1 ml/min; ENA screening: extractable nuclear antigen; anti-SSA/Ro: Anti-Sjögren's-syndrome-related antigen A antibody; anti-SSB/La: Anti-Sjögren's-syndrome-related antigen B antibody; Schirmer's test cut off: ≤5 mm/5 min.
Discussion
Using WES of the NMOSD family, a potential risk associated 19 bp deletion frameshift allele was identified. This finding was further validated by Sanger sequencing a cohort of sporadic NMOSD patients. The CD33 risk allele was presented in nearly 20% of all patients with NMOSD, which means it was significantly more prevalent in patients with NMSOD compared with the controls (OR ∼7.3–9.7). Interestingly, the variant was also prevalent in East Asian populations (2–3%) but rarely found in other populations in the gnomAD database. This coincides with the observation that NMOSD is known to have a higher prevalence in East Asian populations compared with Caucasian populations.
CD33 encodes for immune cell surface antigen CD33, also known as p67 or Siglec-3. Siglecs are type I transmembrane proteins, which belong to the immunoglobulin superfamily. Some studies have suggested that the CD33 molecule is not only a myeloid-specific inhibitory receptor but can also be found in some lymphoid lineage cells [25]. CD33 itself is believed to play a potential regulatory role in cellular proliferation and/or differentiation, by modulating the inflammatory and immune responses. In vitro studies have demonstrated that CD33 constitutively suppresses the production of pro-inflammatory cytokines, such as interleukin-1 beta, tumor necrosis factor-alpha and interleukin-8 by human monocytes in a sialic acid ligand-dependent and suppressor of cytokine signaling-3(SOCS3)-dependent manner [26]. CD33 recruits phosphatases, such as Src homology-2-containing tyrosine phosphatase-1 (SPH-1) and SPH-2, that regulate downstream signaling, to inhibit myeloid cell differentiation [27].
The ethnicity-specific frameshift mutation of CD33 has two possible consequences: First, it is predicted to evoke a nonsense-mediated decay mechanism that reduces the expression of CD33. Alternatively, it could still translate the prematurely truncated CD33 protein that lacks the 30 C-terminal cytoplasmic amino acids, which contains the important inhibitory immunoreceptor tyrosine-based inhibitory motifs (ITIM) domain. Theoretically, both mechanisms would result in the loss of its regulatory ability on the immune system. However, this hypothesis needs to be further examined in an animal model.
NMOSD is a severe autoimmune disease that leads to astrocyte-destructive, neutrophil-dominated inflammatory lesions in the CNS. These lesions are initiated by the binding of pathogenic AQP4-Abs to astrocytes and the subsequent complement-mediated lysis of these cells [28]. A recent study demonstrated that AQP4-Ab producing cells can be derived from autoreactive naïve early pre-germinal center B cells without the presence of antigen. This suggests that early defects in B cell tolerance lead to the emergence of circulating AQP4-secreting B Cells [29]. CD33 has also been reported as being expressed in lymphoid cells in addition to myeloid cells [25]. One possible explanation is that the loss of CD33 inhibitory function contributes to the loss of immune tolerance checkpoints for early B cells. Alternatively, the loss of CD33 leads to the over-production of pro-inflammatory cytokines, which may facilitate the production of AQP4 auto-Abs.
The study has some limitations: the case numbers included in the current study were suboptimal and potential selection bias may be introduced by the inclusion of familial cases. Furthermore, we only sequence the exon 4 of the CD33 gene in the sporadic cohort, therefore other loss of function variants in the CD33 gene may be missed, which could also contribute to the increased risk of developing NMOSD. Further large-scale studies are required to consolidate or refute the association between CD33 and NMOSD.
Conclusions
In the present study, a potential risk allele in the CD33 gene was identified as being associated with familial and sporadic NMOSD patients, particularly in cases of NMOSD without concomitant systemic autoimmune disorders. We hypothesize that loss of CD33 inhibitory function may play a role in the pathogenesis of NMOSD. However, this needs to be further investigated in an animal model or in vitro studies. Further advancements in the understanding of the molecular mechanisms behind how CD33 dysfunction leads to the loss of B cell tolerance, may provide great insights into the development of effective therapy for NMOSD.
Funding
The study was approved by the local institutional human research ethics committee of Kaohsiung Chang Gung Memorial Hospital (201700506A3C0501). The genetic study of this work was supported by grants of Chang Gung Medical Foundation (CMRPG8G0252, CMRPG8J0041) and the Ministry of Science and Technology, Taiwan (MOST107-2314-B-182A-057-MY3).
Conflicts of interest
All authors reported no conflicts of interest associated with this publication and there has no financial support for this work that could influence the study outcome.
Acknowledgments
We wish to thank the family and patients for participating in the study.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Oh J., Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. 2012;2012:460825. doi: 10.1155/2012/460825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarei S., Eggert J., Franqui-Dominguez L., Carl Y., Boria F., Stukova M., et al. Comprehensive review of neuromyelitis optica and clinical characteristics of neuromyelitis optica patients in Puerto Rico. Surg Neurol Int. 2018;9:242. doi: 10.4103/sni.sni_224_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lennon V.A., Wingerchuk D.M., Kryzer T.J., Pittock S.J., Lucchinetti C.F., Fujihara K., et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 4.Wingerchuk D.M., Hogancamp W.F., O'Brien P.C., Weinshenker B.G. The clinical course of neuromyelitis optica (Devic's syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 5.Pereira W.L., Reiche E.M., Kallaur A.P., Kaimen-Maciel D.R. Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: a review. J Neurol Sci. 2015;355:7–17. doi: 10.1016/j.jns.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Bruscolini A., Sacchetti M., La Cava M., Gharbiya M., Ralli M., Lambiase A., et al. Diagnosis and management of neuromyelitis optica spectrum disorders – an update. Autoimmun Rev. 2018;17:195–200. doi: 10.1016/j.autrev.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Mori M., Kuwabara S., Paul F. Worldwide prevalence of neuromyelitis optica spectrum disorders. J Neurol Neurosurg Psychiatry. 2018;89:555–556. doi: 10.1136/jnnp-2017-317566. [DOI] [PubMed] [Google Scholar]
- 8.Etemadifar M., Nasr Z., Khalili B., Taherioun M., Vosoughi R. Epidemiology of neuromyelitis optica in the world: a systematic review and meta-analysis. Mult Scler Int. 2015;2015:174720. doi: 10.1155/2015/174720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matiello M., Kim H.J., Kim W., Brum D.G., Barreira A.A., Kingsbury D.J., et al. Familial neuromyelitis optica. Neurology. 2010;75:310–315. doi: 10.1212/WNL.0b013e3181ea9f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.J., Tsai M.H., Lien C.Y., Huang Y.J., Chang W.N. Intra-family phenotype variations in familial neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2019;30:57–62. doi: 10.1016/j.msard.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Wang H., Dai Y., Qiu W., Zhong X., Wu A., Wang Y., et al. HLA-DPB1 0501 is associated with susceptibility to anti-aquaporin-4 antibodies positive neuromyelitis optica in southern Han Chinese. J Neuroimmunol. 2011;233:181–184. doi: 10.1016/j.jneuroim.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita T., Matsuoka T., Isobe N., Kawano Y., Minohara M., Shi N., et al. Association of the HLA-DPB1∗0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders. Tissue Antigens. 2009;73:171–176. doi: 10.1111/j.1399-0039.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- 13.Alvarenga M.P., Fernandez O., Leyva L., Campanella L., Vasconcelos C.F., Alvarenga M., et al. The HLA DRB1∗03:01 allele is associated with NMO regardless of the NMO-IgG status in Brazilian patients from Rio de Janeiro. J Neuroimmunol. 2017;310:1–7. doi: 10.1016/j.jneuroim.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Estrada K., Whelan C.W., Zhao F., Bronson P., Handsaker R.E., Sun C., et al. A whole-genome sequence study identifies genetic risk factors for neuromyelitis optica. Nat Commun. 2018;9:1929. doi: 10.1038/s41467-018-04332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lian Z., Liu J., Shi Z., Chen H., Zhang Q., Feng H., et al. Association of TNFSF4 polymorphisms with neuromyelitis optica spectrum disorders in a Chinese population. J Mol Neurosci. 2017;63:396–402. doi: 10.1007/s12031-017-0990-1. [DOI] [PubMed] [Google Scholar]
- 16.Gong X., Mei S., Li X., Li X., Zhou H., Liu Y., et al. Association between TPMT∗3C and decreased thiopurine S-methyltransferase activity in patients with neuromyelitis optica spectrum disorders in China. Int J Neurosci. 2018;128:549–553. doi: 10.1080/00207454.2017.1401621. [DOI] [PubMed] [Google Scholar]
- 17.Ainiding G., Kawano Y., Sato S., Isobe N., Matsushita T., Yoshimura S., et al. Interleukin 2 receptor alpha chain gene polymorphisms and risks of multiple sclerosis and neuromyelitis optica in southern Japanese. J Neurol Sci. 2014;337:147–150. doi: 10.1016/j.jns.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 18.Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T., et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., et al. 2016 American College of Rheumatology/European League against Rheumatism Classification Criteria for primary Sjogren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afgan E., Baker D., Batut B., van den Beek M., Bouvier D., Cech M., et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018;46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai M.H., Chan C.K., Chang Y.C., Yu Y.T., Chuang S.T., Fan W.L., et al. DEPDC5 mutations in familial and sporadic focal epilepsy. Clin Genet. 2017;92:397–404. doi: 10.1111/cge.12992. [DOI] [PubMed] [Google Scholar]
- 22.Stelzer G., Plaschkes I., Oz-Levi D., Alkelai A., Olender T., Zimmerman S., et al. VarElect: the phenotype-based variation prioritizer of the GeneCards Suite. BMC Genomics. 2016;17:444. doi: 10.1186/s12864-016-2722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alfoldi J., Wang Q., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Caselles T., Martinez-Esparza M., Perez-Oliva A.B., Quintanilla-Cecconi A.M., Garcia-Alonso A., Alvarez-Lopez D.M., et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol. 2006;79:46–58. doi: 10.1189/jlb.0205096. [DOI] [PubMed] [Google Scholar]
- 26.Lajaunias F., Dayer J.M., Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur J Immunol. 2005;35:243–251. doi: 10.1002/eji.200425273. [DOI] [PubMed] [Google Scholar]
- 27.Orr S.J., Morgan N.M., Elliott J., Burrows J.F., Scott C.J., McVicar D.W., et al. CD33 responses are blocked by SOCS3 through accelerated proteasomal-mediated turnover. Blood. 2007;109:1061–1068. doi: 10.1182/blood-2006-05-023556. [DOI] [PubMed] [Google Scholar]
- 28.Ratelade J., Verkman A.S. Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies. Int J Biochem Cell Biol. 2012;44:1519–1530. doi: 10.1016/j.biocel.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson R., Makuch M., Kienzler A.K., Varley J., Taylor J., Woodhall M., et al. Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain. 2018;141:1063–1074. doi: 10.1093/brain/awy010. [DOI] [PMC free article] [PubMed] [Google Scholar]