Abstract
Background
A. gratissima is a shrub used in folk medicine as analgesic and sedative. However, studies on its antinociceptive activity are scarce. This research aimed to evaluate the antinociceptive effect of a supercritical carbon dioxide (SCCO2) extract of A. gratissima leaves (EAG) in mice.
Methods
A. gratissima leaves were subjected to extraction with supercritical CO2 (60 °C, 200 bar). The chemical composition of EAG was determined by gas chromatography–mass spectrometry (GC–MS). The antinociceptive profile of the extract (1, 10 and 30 mg/kg, p.o.) was established using acetic acid-induced abdominal contraction tests and formalin-induced paw-licking tests. The open field and rota-rod tests were used to evaluate a possible interference of EAG on mice motor performance. The contribution of the opioid system and adenosine triphosphate (ATP) sensitive K+ channels in the mechanism(s) of EAG action was evaluated by specific receptor blockers. EAG's acute toxicity was investigated using OECD 423 guideline.
Results
The GC–MS revealed the presence of sesquiterpenes (guaiol and pinocamphone) in the EAG. Doses of 10 mg/kg and 30 mg/kg significantly reduced the number of abdominal writhes and paw licking time in mice in the formalin test. The EAG did not affect the locomotor activity and motor coordination of the mice. The antinociceptive effect of the EAG was prevented by glibenclamide in the mice formalin test, unlike naloxone pre-treatment. The acute administration of EAG caused no mortality.
Conclusion
A. gratissima leaves possess antinociceptive effect, mediated by K+ channels sensitive to ATP.
Keywords: Aloysia gratissima, Verbenaceae, Supercritical carbon dioxide, Antinociceptive, Potassium channels, Opioid system
At a glance of commentary
Scientific background on the subject
Aloysia gratissima aerial parts are traditionally used as sedative and for the treatment of headache. The essential oil from A. gratissima presents sesquiterpenes, which are related to the pharmacological action of plants.
What this study adds to the field
Our study demonstrated the presence of sesquiterpenes (guaiol and pinocamphone) in the supercritical CO2 extract of Aloysia gratissima leaves and confirmed its antinociceptive activity in mice. This effect is mediated by K+ channels sensitive to ATP.
The scientific interest in researching compounds derived from plant extracts is associated with the knowledge of folk medicine [1]. The secondary metabolites present in these natural compounds can have their biological activity evaluated through in vivo experimental tests, so that their functionalities as drugs, food and cosmetics can be proven [2,3]. Among the species popularly known for their pharmacological actions, are the ones from the Aloysia genus, belonging to the Verbenaceae family, native to South America [4].
In Brazil, the species Aloysia gratissima (Gillies & Hook) Tronc. is commonly grown as an ornamental plant, being known as "Alfazema do Brasil" [5]. Aerial parts of this plant are used for the treatment of headache, bronchitis, as well as anxiety, depression and digestive disorders [6,7]. The essential oil from A. gratissima presents more than 70% of hydrocarbons, from which more than half are sesquiterpenes. This is particularly interesting, since the pharmacological action of plants is related to the presence of metabolites such as alkaloids, terpenes, flavonoids and phytosterols [5].
The use of natural products in the food and pharmaceutical industry, provides alternative results to current needs [8]. Additionally, obtaining compounds through pressurized fluids, such as carbon dioxide and propane, have been presented as alternatives, due to the use of non-toxic, non-flammable and low-cost solvents [8]. In this context, the extraction and study of the compounds present in these plant matrices through supercritical fluids is an interesting approach [8].
The presence of terpenes in plants, such as caryophyllene, caryophyllene oxide, guaiol, pinocamphone and spathulenol is described by several authors as the possible reason for the antinociceptive action of different extracts that present these compounds [[9], [10], [11], [12]]. Furthermore, it has been demonstrated that terpenes are able to exert antinociceptive effects by opening K+ channels [13], which leads to K+ efflux and cell membrane hyperpolarization [14]. This effect, therefore, reduces the excitability of peripheral neurons and the release of neurotransmitters (e.g. substance P) in the spinal cord [[15], [16], [17]]. Different types of K+ channels are involved in antinociception: the adenosine triphosphate (ATP)-sensitive K+ channels, activated via several different drugs (including terpenes such as caryophyllene oxide and guaiol [11]) in both the central and the peripheral nervous system; and the G-protein-regulated inwardly rectifying K+ channels, that open through the G-protein activation in neurons [18]. These kinds of K+ channel activations are both involved in opioid induced antinociception [14]. Interestingly, some terpenes exert their antinociceptive activity by activating opioid receptors [[19], [20], [21], [22]], thus reducing neurons’ excitability and the release of neurotransmitters.
Considering the presence of terpenes in A. gratissima, it becomes important to investigate, for the first time, the effect of this plant species on animal models of nociception and its mechanisms of action. The present work evaluated the antinociceptive activity of a supercritical CO2 extract of A. gratissima leaves in models that use different stimuli in mice, and also investigated the mechanisms of action involved in its antinociceptive activity.
Materials and methods
Chemicals
Carbon dioxide (99.9% non-liquid purity phase) was purchased from Air Liquide©. Acidic acid and formaldehyde were purchased from Merck©. Ketorolac and glibenclamide were obtained from EMS Sigma Pharma©. Naloxone and morphine were purchased from Cristália© and indomethacin was obtained from Sigma©.
Plant material
A. gratissima leaves were collected in December 2017 (summer), in the municipality of Erval Grande, RS, South Brazil (27°23′14.3S, 52°33′49 'W). This region presents a humid subtropical mesothermic climate. The plant specimen was deposited in the Herbarium of the Community University of Chapecó (Herbário Unochapecó, SC, Brazil) under the accession number UNO 3700. After collection, the leaves were manually separated and dried at room temperature for five days. Afterwards, they were packed in plastic bags, identified and stored at 7 °C until extraction process.
Extraction of the A. gratissima leaves by supercritical CO2 (SCCO2)
The experimental extraction apparatus and procedure have been described in detail in other studies of the research group [23]. A. gratissima leaves were extracted with SCCO2 at 60 °C and 200 bar (density 724 kg/m3). Approximately 11.03 ± 0.09 g of A. gratissima leaves were placed into the extraction vessel. Then, CO2 was pumped into the bed, which had two 300 mesh wire disks at both ends and was held in contact with the sample array to allow the system to stabilize at the same condition as the experiment by 30 min. After stabilization, the extract from A. gratissima was then collected by opening the micrometric valve with 2 h of extraction time.
GC/MS analysis
Extract of Aloysia gratissima leaves (EAG’s) chemical composition was analyzed by Agilent GC/MS (7890B) gas chromatography coupled to a quadripolar mass spectrometer (5977A) (Agilent Technologies, Palo Alto, CA, USA). GC/MS system's experimental condition were described by Scapinello et al. (2018) [24] with some modifications. Briefly, the system conditions were: Agilent 19091S ca-pillary column, dimension: 30 m × 250 μm × 0.25 μm. The mobile phase flow (carrier gas: He) was adjusted to 1.0 mL min−1. The GC temperature program was 40.0 °C at 4 min to 240.0 °C at a rate of 10 °C min−1 and up to 300.0 °C at a rate of 40.0 °C min−1 (maintained for 5 min). The injector temperature was 280.0 °C, sample injection volume 1 μL, split ratio 1:20. The MS transferline temperature was set to 150.0 °C and the source of ions temperature was set at 230.0 °C. For GC–MS detection, an electron ionization system was used with ionization energy set at 70 eV, and mass range atm/z 40–400. The chemical components presented in the extract were identified by comparison with the equipment library (Agilent P/N G1033A). The relative amounts of each individual component were calculated using their respective peak areas in the chromatogram. The extract was solubilized in dichloromethane to be analyzed.
In vivo experiments
Animals
Male Swiss mice (25–35 g) bred at the Community University of Chapecó Region (Unochapecó) bioterium were used in all pharmacological experiments. The animals were kept in a controlled environment under standard conditions (22 ± 2 °C) with a light/dark cycle of 12 h (lights on at 6:00 a.m. to 6:00 p.m.), fed standard laboratory feed and water ad libitum. Animal care and experiments were conducted in accordance with the animal research ethical principles, approved by the Ethics Committee of the university (Approval number 004–18), in accordance with Brazilian law No. 11794 and Council of the European Communities; Directive of 24 November 1986 (86/609/EEC). The animals were fasted for a period of 2 h (no water restriction) prior to administration of any test substance.
Mice were treated with volumes of 10 mL/kg, according to their weight, through oral gavage (p.o.) or intraperitoneal (i.p.) routes and with 20 μL by intraplantar (i.pl.) injection, according to each experiment's specific protocol. Solubilization of the extract and substances was performed in saline (NaCl 0.9%) with the aid of 1% Tween 80 (v/v) and ultrasound. The extract's tested doses were chosen based on the study of Zeni et al. (2013) [4].
Acute toxicity
The acute toxicity study was based on Guideline 423 (2001) of the Organization for Economic Cooperation and Development (OECD). According to OECD 423, the study in female mice is recommended for the possible sensitivity to the acute toxic effects of the chemicals used in the research [25]. Female mice (n = 6) were orally treated with EAG at 2000 mg/kg. The control group (n = 3) received the administration of vehicle (0.9% NaCl, 1% Tween 80, 10 mL/kg). Thereafter, the animals were observed with special attention during the first 4 h after treatment and daily for 14 days. The occurrence of mice death, and signals such as piloerection, hypothermia, palpebral ptosis, abdominal writhing, muscle tone, shacking, hind paw paralysis, salivation, bronchial secretion and seizures were registered. Additionally, body weight and food consumption were recorded for 13 days. At the end of the experiment, the macroscopic aspect of the organs (liver, kidneys, adrenal glands, spleen, lungs, heart and brain) as well as their relative weight (%) were registered.
Acetic acid-induced writhing response
The abdominal writhing test induced by acetic acid has been used as a screening tool to evaluate analgesic or anti-inflammatory agents, as acetic acid induces an inflammatory response in the abdominal cavity, with subsequent activation of nociceptors [26]. In this test, mice writhing is a response to the pain characterized by episodes of arching of the back, extension of hind limbs and contraction of abdominal musculature [27]. Mice (n = 6–8/group) were treated with EAG (1, 10 and 30 mg/kg, p.o.) or vehicle (0.9% NaCl + 1% Tween 80, p.o., control group) 1 h before the injection (i.p.) of acetic acid (0.6%, 10 mL/kg). Immediately after the injection, the animals were gently placed in a transparent apparatus (20 cm diameter glass box) and the number of abdominal contortions was counted cumulatively over a period of 20 min [28] by a human observer blind to the treatment. The positive control was the non-steroid anti-inflammatory indomethacin (10 mg/kg, p.o.). The dose that presented the best result in this experiment was chosen to be used in the other tests of nociception.
Open-field test
The open-field test was performed in order to evaluate the possible EAG effects on locomotor and exploratory activities of mice. The experimental protocol was based on the method described by Müller et al. (2012) [29]. The animals (n = 6/group) received orally the minimal effective dose (10 mg/kg, p.o.) of EAG that was able to reduce the nociceptive behavior in the acetic-acid writhing test, 1 h before being exposed to the open-field arena. The control group was treated with vehicle (0.9% NaCl + 1% Tween 80, p.o.). The arena consisted in an acrylic box (40 × 30 × 30 cm), with the floor divided into 24 equal squares. After the habituation (5 min), the number of squares crossed with the four paws (crossing), rearings and groomings was recorded for 10 min. The number of fecal bolus was counted after the test.
Rota-rod test
The rota-rod test was used to evaluate the EAG effects on mice motor coordination. This test was performed as described by Neves et al. (2010) [30] with minor modifications. The apparatus was a cylinder (4 cm of diameter), rotating at 3 rpm. Animals were individually habituated to the apparatus for 5 min. Twenty-four hours later, they were trained for 5 min and only the ones that were able to stay 90 s on the rotating rod were selected for testing. Immediately after, mice (n = 7/group) were orally treated with vehicle (NaCl 0.9% + 1% tween 80), indomethacin (10 mg/kg) or EAG (10 mg/kg). One hour later, mice performance in the rota-rod was evaluated considering the longest time of permanence on the apparatus and the number of falls, in a 5 min period.
Formalin test
The experimental procedure was similar to the one described by Santos and Calixto (1997) [31]. Briefly, the animals (n = 6/group) were orally treated with vehicle (0.9% NaCl + 1% Tween 80) or EAG (10 mg/kg) 1 h before the injection of 2% formalin (20 μL/paw, ipl) in the right hind paw. Indomethacin (administered orally 1 h before the behavioral test, 10 mg/kg) was the positive control. Immediately after the formalin injection, the time spent licking, biting or lifting the injected hind paw (nociceptive behavior) was registered during the first (0–5 min, neurogenic phase) and the second phases (15–30 min, inflammatory phase) of the test.
Involvement of the opioid system and K+ channels sensitive to ATP
In order to assess the opioid system's involvement in the antinociceptive action mechanism of the EAG, mice were pre-treated with naloxone (a non-selective opioid receptor antagonist; 2 mg/kg, i.p.) or vehicle (0.9% NaCl, i.p.) [32,33]. After 15 min, the animals (n = 4–7/group) were treated with EAG (10 mg/kg, p.o.), morphine (opioid receptor agonist, positive control, 5 mg/kg, s.c.) or vehicle (0.9% NaCl, 1% Tween 80, p.o.).
The involvement of the ATP-sensitive K+ channels in EAG's antinociceptive effect was evaluated according to Zapata-Morales et al. (2017) [34]. Briefly, the animals (n = 4–7/group) were pre-treated with glibenclamide (K+ channels sensitive to ATP blocker; 20 mg/kg, i.p.) or vehicle (0.9% NaCl, i.p.). The EAG (10 mg/kg, p.o.), ketorolac (non-steroid anti-inflammatory that presents antinociceptive effects mediated by ATP-sensitive K+ channels [35], positive control, 20 mg/kg, i.p.) or vehicle (0.9% NaCl + 1% Tween 80, p.o.) were administered to the animals 15 min after the pretreatment.
One hour after the treatments, the nociceptive behavior was evaluated in the formalin test, immediately after the i.pl. injection of formalin 2%.
Statistical analysis
Statistical analyses were carried out using Graph Pad Prism 5.0 for Windows (GraphPad Software, San Diego, California, USA). Data were analyzed by one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. Results from the investigation of the mechanism of action were analyzed by two-way ANOVA followed by Student-Newman-Keuls test. Rota-rod test results and toxicity data were analyzed by two-way repeated measures ANOVA. The relative weight of the organs in the toxicity test was analyzed by Unpaired t test. The results are expressed as the mean ± S.E.M. of n animals per group. Values of p less than 0.05 (p < 0.05) were considered significant.
Results
Table 1 shows the extract's chemical composition. The GC/MS analysis revealed that several terpene compounds are present in the EAG. The major compounds found in EAG were guaiol (18.50%) and pinocamphone (11.40%), in addition to the presence of other compounds such as pinocarvil, (−)-trans-pinocarvilacetate, γ-elemene, bunesol, caryophyllene, caryophyllene oxide, (−)-spathulenol, myrtenol, isopinocamphone, β-cubebene, in smaller quantities.
Table 1.
Chemical composition of the A. gratissima leaves supercritical CO2 extract.
| Chemical compound | Area of each compound (%) |
|---|---|
| Pinocarvil | 5.31 |
| Pinocamphone | 11.4 |
| Isopinocamphone | 3.52 |
| Myrtenol | 3.24 |
| (−)- trans-pinocarvila acetate | 10.5 |
| Caryophyllene | 7.63 |
| γ – Elemene | 7.28 |
| Humelene | 2.97 |
| (−)- Spathulenol | 6.17 |
| Caryophyllene oxide | 6.76 |
| β- Cubebene | 8.21 |
| Guaiol | 18.5 |
| Bunesol | 4.67 |
| Total | 96.1 |
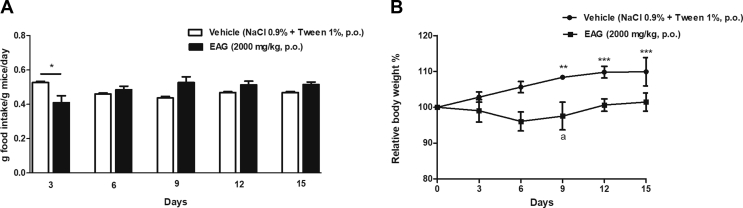
The administration of EAG (2000 mg/kg, p.o.) induced an intense sedation in the mice, which lasted 45 min. After recovery, mice fed normally, with no locomotor activity changes. Food intake [Fig. 1A] of EAG-treated mice was significantly (p < 0.05) lower than of vehicle-treated mice at the 3rd day after treatment. Mice treated with vehicle presented a significant increase in the body weight [Fig. 1B] at the 9th (p < 0.01), 12th (p < 0.001) and 15th (p < 0.001) days of observation in comparison to initial weight. There were no changes in the body weight of EAG-treated animals during the period of observation. Also, no death was recorded.
Fig. 1.
Effect of the A. gratissima leaves supercritical extract (EAG) acute treatment (2000 mg/kg, p.o.) on food intake (A) (g food intake/g mice/day) and relative body weight (%) (B) of female Swiss mice. Data are expressed as mean ± S.E.M. (n = 3–6 mice/group). Two-way repeated measures ANOVA followed by Student-Newman-Keuls. ∗∗p < 0.01 and ∗∗∗p < 0.001 represent differences in relation to the first measure (Day 0) in the same group treatment. ∗p < 0.05 different from the vehicle-treated group at the same day of treatment.
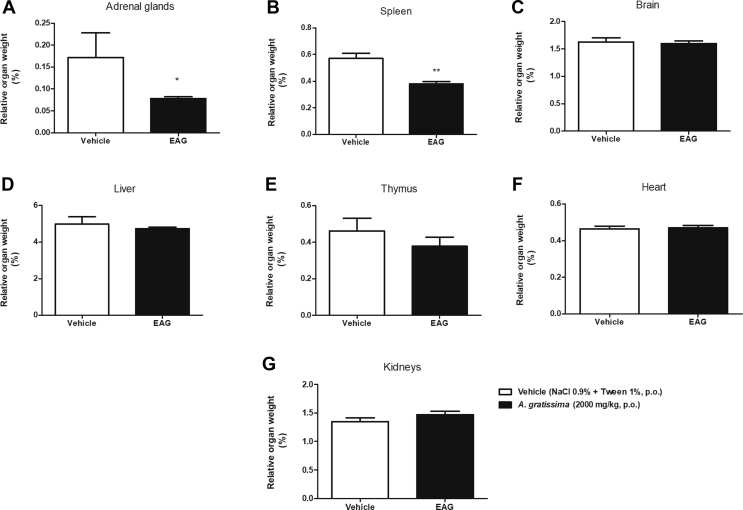
Mice treatment with EAG (2000 mg/kg, p.o.) did not cause changes in the relative weight (%) of the brain, liver, kidneys, lung, heart and thymus [Fig. 2]. However, there was a significant decrease in the relative weight of the adrenal glands (p < 0.01) and spleen (p < 0.05) of EAG-treated mice in comparison to vehicle-treated mice.
Fig. 2.
Effect of A. gratissima leaves supercritical extract (EAG) on the relative weight of female Swiss mice organs (%) in the acute oral toxicity test. (A) adrenal glands; (B) spleen (C) brain; (D) liver; (E) thymus; (F) heart and (G) kidneys. The mice (n = 3–6) were orally treated with vehicle (NaCl 0.9% + tween 1%, 10 ml/kg) or EAG (2000 mg/kg). Unpaired t test, ∗p < 0.05, ∗∗p < 0.01.
The acetic acid injection evokes abdominal contractions in rodents, since it induces the peripheral production of several pro-inflammatory mediators. The EAG at 10 and 30 mg/kg (p < 0.05) and indomethacin at 10 mg/kg (p < 0.01) reduced the number of abdominal writhes provoked by the injection of acetic acid when compared to the vehicle group. On the other hand, the EAG at the lowest dose (1 mg/kg) was not effective in reducing acetic acid-induced writhes [Fig. 3]. The abdominal contortion test was applied to investigate the lowest effective dose, in order to continue the other tests.
Fig. 3.
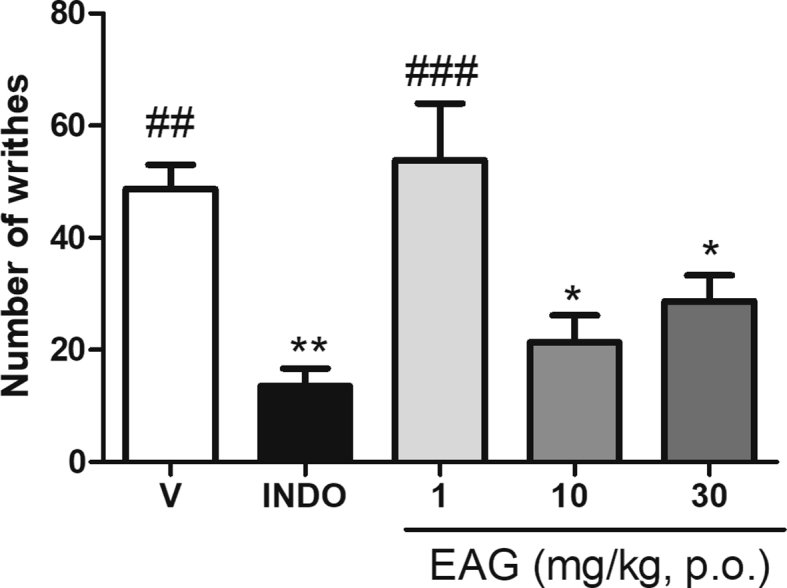
Effect of a supercritical CO2 extract of Aloysia gratissima leaves (EAG) in the acetic acid-induced abdominal writhing test in mice. V: vehicle treated group (0.9% NaCl + 1% Tween, 10 mL/kg p.o., n = 8). INDO: indomethacin (10 mg kg p.o., n = 6) or EAG (1, 10 and 30 mg/kg p.o., n = 6–8), 1 h prior to acetic acid administration. Each column represents the mean ± S.E.M. One-way ANOVA followed by the Student-Newman-Keuls test, ∗∗p < 0.01 and ∗p < 0.05 different from the vehicle group; ###p < 0.001 and ##p < 0.01 different from the INDO group.
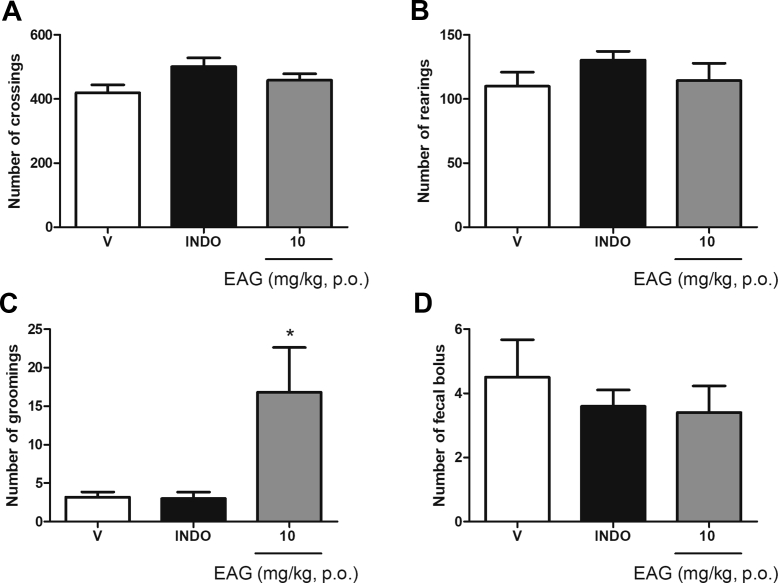
Considering that a non-specific effect of the EAG on animals’ locomotion could influence the results of the antinociceptive tests and, therefore cause false positive or negative results, we investigated the effects of the minimal effective dose of EAG (10 mg/kg, p.o.) in the open field test. Treatment with EAG and indomethacin (10 mg/kg, p.o.) did not alter the numbers of crossings [Fig. 4A], rearings [Fig. 4B] and the number of fecal bolus [Fig. 4D]. The grooming number [Fig. 4C] for EAG-treated animals was significantly (p < 0.05) higher when compared to the groups treated with indomethacin or vehicle.
Fig. 4.
Effect of a supercritical CO2 extract of Aloysia gratissima leaves (EAG) on mice locomotor activity (open field test). A: number of crossings. B: number of rearings. C: number of groomings; D: number of fecal bolus. V: vehicle treated group (0.9% NaCl + 1% Tween, p.o., n = 6). INDO: indomethacin (10 mg/kg, n = 6). EAG (10 mg kg, p.o., n = 6). Each column represents the mean ± S.E.M. One-way ANOVA followed by the Student-Newman-Keuls test, ∗p < 0.05 different from the vehicle group.
Additionally, the treatment with EAG (10 mg/kg, p.o.) and indomethacin (10 mg/kg, p.o.) did not change mice motor coordination, compared to the vehicle group (NaCl 0.9%, p.o.) [Table 2].
Table 2.
Effect of a supercritical CO2A. gratissima leaves extract (EAG) on mice motor coordination (assessed by the Rota–Rod test). The animals (n = 7/group) were orally treated with vehicle (NaCl 0.9% + 1% tween 80), indomethacin (10 mg/kg) or EAG (10 mg/kg) 1 h before the test. Data are expressed as Mean ± S.E.M. Results were analyzed by Two-Way repeated measures ANOVA.
| Group | Length of stay (s) | Number of falls |
|---|---|---|
| Vehicle | 300.0 | 0.0 |
| Indomethacin | 300.0 | 0.0 |
| EAG | 299.6 ± 0.3 | 0.11 ± 0.03 |
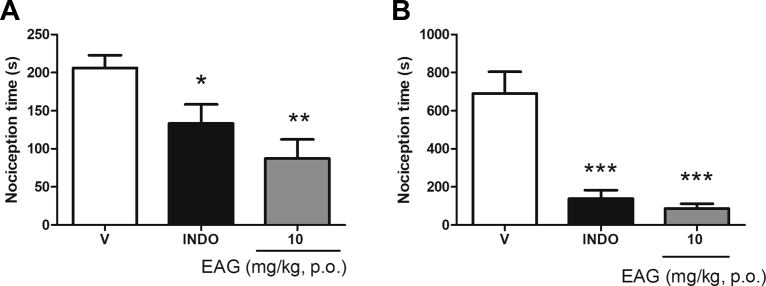
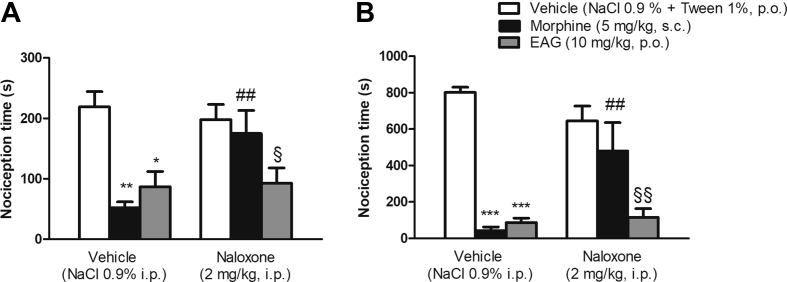
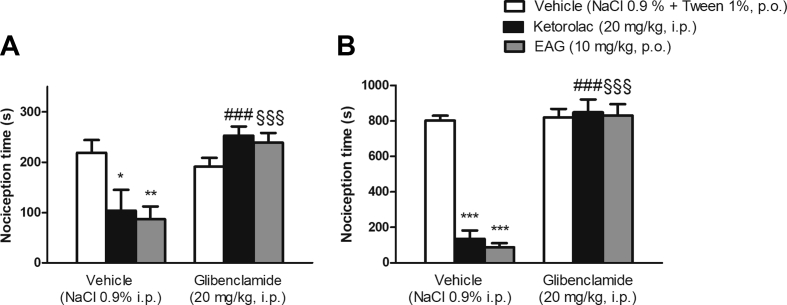
The EAG at 10 mg/kg as well as indomethacin produced antinociception in the first phase (p < 0.01 and p < 0.05, respectively) – the neurogenic phase [Fig. 5A] - and in the second phase (p < 0.001) - inflammatory pain phase [Fig. 5B] of the formalin test. The investigation of the EAG action mechanism revealed that mice underwent pre-treatment with naloxone did not reverse the EAG antinociceptive activity in the two phases of formalin test [Fig. 6A and B], showing that the opioid system is unlikely to be involved in antinociceptive action. However, EAG's effect was prevented by mice who had pre-treatment with glibenclamide in the two formalin phases [Fig. 7A and B], thus suggesting that the K+ channels sensitive to ATP are involved in the mechanism of antinociceptive effect of EAG.
Fig. 5.
Effect of a supercritical CO2 extract of Aloysia gratissima leaves (EAG) on the formalin test. Nociceptive behavior was considered as the time (s) of elevation, biting or licking of the paw in the first phase (A: 0–5 min) and second phase (B: 15–30 min) of the test. Mice (n = 6 per group) were treated with vehicle (V: NaCl 0.9% + 1% tween 80, 10 ml/kg), Indomethacin (INDO: 10 mg/kg) or EAG (10 mg/kg) 1 h prior to administration of formalin 2% i.pl. One-way ANOVA followed by Student-Newman-Keuls: ∗p < 0.05; ∗∗p < 0.01 and ∗∗∗p < 0.001 are different from the vehicle group. Results expressed as mean ± S.E.M.
Fig. 6.
Effect of mice pre-treatment with naloxone (2 mg/kg, i.p.) on the antinociceptive effect of a supercritical CO2 extract of Aloysia gratissima leaves (EAG) (10 mg/kg) on the formalin test. Nociceptive behavior in the first phase (A, 0–5 min) or second phase (B, 15–30 min) of the test. Morphine (5 mg/kg, s.c.) was used as positive control. Results expressed as mean ± S.E.M. (n = 4–7 mice/group). Two-Way ANOVA followed by Student-Newman-Keuls test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 compared to the vehicle plus vehicle-treated group. ##p < 0.01 compared to the vehicle plus morphine-treated group; §p < 0.05; §§p < 0.01 compared to the vehicle plus naloxone-treated group and morphine plus naloxone-treated group.
Fig. 7.
Effect of mice pre-treatment with glibenclamide (20 mg/kg, i.p.) on the antinociceptive effect of a supercritical CO2 extract of Aloysia gratissima leaves (EAG) (10 mg/kg, p.o.) on the formalin test. Nociceptive behavior in the first phase (A, 0–5 min) or second phase (B, 15–30 min) of the test. Ketorolac (20 mg/kg, i.p.) was used as positive control. Each column represents the mean ± S.E.M. (n = 4–7 mice/group). Two-Way ANOVA followed by the Student-Newman-Keuls test, ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 compared to vehicle plus vehicle-treated group. ###p < 0.001 compared to the vehicle plus kerotolac-treated group; §§§p < 0.01 compared to vehicle plus EAG-treated group.
Discussion
The solubility of chemical components obtained in the supercritical fluid is related to the fluid density. When CO2 is used in the extraction of oils, it demonstrates good capacity for solubilization due to low polarity, without altering the chemical composition and the extract produced is free of solvent contamination [36]. The chemical composition of the A. gratissima leaves supercritical extract analyzed by GC/MS revealed the presence of terpene compounds, being guaiol and pinocamphone the major ones. These compounds are commonly found in species from the Aloysia genus, and their antibacterial and antitumor activities have already been demonstrated [37,38]. Terpenes have been known to show a wide range of pharmacological activities, including effects on the central nervous system, antinociceptive, anti-inflammatory, antimicrobial and antitumor properties [39].
Mice treatment with the EAG (2000 mg/kg, p.o.) did not change the weight of the brain, liver, kidneys, lung, heart and thymus. However, there was a significant decrease in the relative weight of the spleen and adrenal glands in EAG-treated mice. Moreover, EAG-treated animals did not gain weight as vehicle-treated ones. This may suggest a sign of toxicity, which should be further investigated in a repeated dose toxicity test. The decrease in food intake of EAG-treated animals at the 3rd day after administration may be due to the sedation caused by the extract [6,7]. Zeni et al. (2013) [13] studied the toxicological effects of a hydroalcoholic extract of A. gratissima aerial parts and demonstrated that it induces hepatic toxicity in male mice at 2000 mg/kg. However, in the present study, we demonstrated the acute toxicity of supercritical A. gratissima leaves extract against mice adrenal glands and spleen. The differences between both findings may be due to the gender of mice and the type of extraction used in the experiments. Nonetheless, both extracts from the same vegetal species have proven to be harmless, since no deaths occurred after the oral administration at 2000 mg/kg. Our results show that the EAG could be included in Category 5 of the Harmonized Global Classification System (OECD Guideline 423, 2001) [25], since its LD50 is above 2000 mg/kg.
It is known that the intraperitoneal acetic acid injection in rodents evokes abdominal writhing, since it induces the peripheral production of several pro-inflammatory mediators, such as prostaglandins, bradykinin, substance P, prostacyclin and other cytokines, which, therefore, excite the nociceptors in the nerve endings [40]. The protective effect of substances against the noxious chemical stimulus may be an indication for a decreased production of these mediators, thus causing a reduction in the number of writhes [41]. The EAG at 30 mg/kg reduced abdominal writhing (on average 41%) compared to the vehicle group, while the dose of 10 mg/kg resulted in an average reduction of 56%, both doses being similar to the group that received indomethacin, the positive control. These results may be related to the presence of pinocamphone and guaiol associated to caryophyllene oxide and spathulenol in EAG's chemical composition [42,43].
The acetic acid model is a preliminary test used to screen new analgesic drugs and was applied in our study to investigate the minimal antinociceptive dose of EAG, in order to continue the other tests. The lowest effective dose was used because of lower adverse effect's possibility. In order to investigate EAG's false-positive results in the nociception tests, mice behavior in the open field and rota-rod tests were evaluated [44].
The results obtained in the open field tests demonstrate that EAG (10 mg/kg) did not induce undesirable effects on the animals' locomotor activity and is not sedative or hyperstimulant. The number of groomings was the only behavior changed in comparison to the vehicle group. Considering that the grooming behavior has been used to measure pharmacologically induced anxiolytic-like effects in rodents [44,45], we may infer that the increase in mice grooming elicited by the EAG might be related to its anxiolytic property [6,7].
In order to investigate EAG's effect on mice motor coordination, we used the rota-rod test. The results of indomethacin, vehicle and EAG treated animals were not different between them in the rota-rod test, suggesting that EAG does not impair the motor coordination and, therefore does not induce false-positive results in other behavioral tests.
The formalin test aims to explore the analgesic effect of substances through central and peripheral pain mechanism [46]. The antinociceptive activity may occur in two distinct phases. In the first phase, neurological pain is induced by the chemical stimulation of afferent sensory fibers, particularly C fibers, whereas in the late phase, pain is caused by inflammatory mediators’ production such as: prostaglandins, histamine, bradykinin, and serotonin [47].
The EAG elicited antinociception both in the first and second phases of the formalin test in mice and therefore, we can suggest that the EAG is effective in the treatment of neurogenic as well as inflammatory pain. Indeed, EAG's antinociceptive and anti-inflammatory activities are probably related to its main constituents. The presence of guaiol and spathulenol may be responsible for the EAG anti-inflammatory effect, as suggested by the study of Apel et al. (2010) [10], with Myrciaria tenella leaves extract, enriched in these compounds.
A number of authors evaluated the antinociceptive and anti-inflammatory activities of plant extracts that present the caryophyllene oxide as one of the main constituents. De Oliveira Júnior et al. (2017) [11] reported that Croton conduplicats’ extract obtained by hydrodistillation showed antinociceptive and anti-inflammatory activities, being the caryophyllene and caryophyllene oxide the main constituents of the extract. Caryophyllene oxide is also the main component of Myrcia pubiflora DC leaves essential oil, obtained by hydrodistillation, and its antinociceptive and anti-inflammatory activities have been proven in in vivo experimental models [12].
Opioids act at the cellular level by binding to the opioid receptors present throughout the central nervous system, so the ultimate effect is the reduction of neuronal excitability, resulting in reduced neurotransmission of nociceptive impulses [48]. Pure opioid agonists (such as morphine) have high affinity for opioid receptors. To verify whether opioid receptors mediate EAG's antinociceptive effect, mice were pretreated with naloxone, an opioid antagonist. Naloxone did not prevent the EAG's antinociceptive activity in the two phases of the formalin test, suggesting that the opioid system is unlikely to be involved in its antinociceptive action.
Herein, we also investigated the involvement of ATP-sensitive K+ channels in the mechanism of EAG's effect, by pretreating mice with glibenclamide (K+ channel blocker). Several studies support the hypothesis that the opening of the K+ channels mediate antinociception by inducing neurons hyperpolarization [49]. Therefore, K+ channels, especially those sensitive to ATP, are particularly involved in the nociceptive responses [49]. Several G protein coupled receptors (including opioid receptors) that stimulate the opening of K+ channels are involved in antinociception [49]. Our results demonstrate that the combined administration of glibenclamide (K+ channel blocker) and EAG blocked the antinociceptive action of EAG in the two phases of the formalin test. In this context, our findings strongly suggest that the peripheral antinociceptive action of EAG might be related to the activation of K+ channels sensitive to ATP. Nevertheless, this activation appears to occur independently from the stimulation of opioid receptors, since naloxone did not prevent the EAG's antinociceptive activity. Indeed, the effect of terpenes as activators of K+ channels sensitive to ATP is well known [13,50]. Corroborating our results, De Oliveira Júnior et al. (2017) [11] demonstrated that the antinociceptive effect of Croton conduplicatus essential oil - which contains caryophyllene oxide and guaiol - is mediated by K+ channels sensitive to ATP.
Conclusion
Taken together, our results demonstrated the antinociceptive action of supercritical extract of A. gratissima leaves in mice, which is mediated by ATP-sensitive K+ channels.
Fundings
This work was supported by the Community University of Chapecó Region [Artigo 170 CE, grant number 008/2018].
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Lang G., Buchbauer G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr J. 2011;27:13–39. [Google Scholar]
- 2.Bersan S.M., Galvão L.C., Goes V.F., Sartoratto A., Figueira G.M., Rehder V.L., et al. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement Altern Med. 2014;14:451. doi: 10.1186/1472-6882-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wink M. In: Encyclopedia of evolutionary biology. Kliman R.M., editor. Academic Press; Oxford: 2016. Secondary metabolites, the role in plant diversification of; pp. 1–9. [Google Scholar]
- 4.Zeni A.L., Zomkowski A.D., Maraschin M., Tasca C.I., Rodrigues A.L. Evidence of the involvement of the monoaminergic systems in the antidepressant-like effect of Aloysia gratissima. J Ethnopharmacol. 2013;148:914–920. doi: 10.1016/j.jep.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Franco A.L.P. Avaliação da composição química e atividade antibacteriana dos óleos essenciais de Aloysia gratissima (Gillies & Hook) Tronc. (Alfazema), Ocimum gratissimum L. (Alfavaca-cravo) e Curcuma longa L. (Açafrão) Rev Eletr Farm. 2007;4:208–220. [Google Scholar]
- 6.Wiest JM. Atividade anti-bacteriana de Aloysia gratissima (Gill et Hook) Tronc. (Garupá, Erva-santa), usada na medicina tradicional no Rio Grande do Sul – Brasil. Biology 2007;88025585.
- 7.Zeni A.L., Zomkowski A.D., Dal-Cim T., Maraschin M., Rodrigues A.L., Tasca C.I. Antidepressant-like and neuroprotective effects of Aloysia gratissima: investigation of involvement of l-arginine-nitric oxide-cyclic guanosine monophosphate pathway. J Ethnopharmacol. 2011;137:864–874. doi: 10.1016/j.jep.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Corso M.P., Fagundes-Klen M.R., Silva E.A., Cardozo Filho L., Santos J.N., Freitas L.S., et al. Extraction of sesame seed (Sesamun indicum L.) oil using compressed propane and supercritical carbon dioxide. J Supercrit Fluids. 2010;52:56–61. [Google Scholar]
- 9.Costa W.K., de Oliveira JRS, de Oliveira AM, da Silva Santos I.B., da Cunha RX, de Freitas AFS, et al. Essential oil from Eugenia stipitata McVaugh leaves has antinociceptive, anti-inflammatory and antipyretic activities without showing toxicity in mice. Ind Crops Prod. 2020;144:112059. [Google Scholar]
- 10.Apel M.A., Lima M.E., Sobral M., Young M.C., Cordeiro I., Schapoval E.E., et al. Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm Biol. 2010;48:433–438. doi: 10.3109/13880200903164386. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira Júnior R.G., Ferraz C.A.A., Silva J.C., de Oliveira A.P., Diniz T.C., e Silva M.G., et al. Antinociceptive effect of the essential oil from Croton conduplicatus Kunth (Euphorbiaceae) Molecules. 2017;22:900. doi: 10.3390/molecules22060900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrade G.S., Guimarães A.G., Santana M.T., Siqueira R.S., Passos L.O., Machado S.M.F., et al. Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Rev Bras Farmacogn. 2012;22:181–188. [Google Scholar]
- 13.Zeni A.L.B., Albuquerque CAC de, Gonçalves F., Latini A., Tasca C.I., Podestá R., et al. Phytochemical profile, toxicity and antioxidant activity of Aloysia gratissima (Verbenaceae) Quim Nova. 2013;36:69–73. [Google Scholar]
- 14.Marker C.L., Luján R., Loh H.H., Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids. J Neurosci. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reshef A., Sperling O., Zoref-Shani E. Opening of ATP-sensitive potassium channels by cromakalim confers tolerance against chemical ischemia in rat neuronal cultures. Neurosci Lett. 1998;250:111–114. doi: 10.1016/s0304-3940(98)00458-3. [DOI] [PubMed] [Google Scholar]
- 16.Soares A.C., Leite R., Tatsuo M.A., Duarte I.D. Activation of ATP-sensitive K(+) channels: mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur J Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbacher J.C., Taylor C.P., Vasko M.R. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–141. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 18.Kweon T.D., Kim J.Y., Kwon I.W., Choi J.B., Lee Y.W. Participation of K(ATP) channels in the antinociceptive effect of pregabalin in rat formalin test. Korean J Pain. 2011;24:131–136. doi: 10.3344/kjp.2011.24.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohbuchi K., Miyagi C., Suzuki Y., Mizuhara Y., Mizuno K., Omiya Y., et al. Ignavine: a novel allosteric modulator of the μ opioid receptor. Sci Rep. 2016;6:31748. doi: 10.1038/srep31748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman R.B., Murphy D.L., Xu H., Godin J.A., Dersch C.M., Partilla J.S., et al. Salvinorin A: allosteric interactions at the mu-opioid receptor. J Pharmacol Exp Ther. 2007;320:801–810. doi: 10.1124/jpet.106.113167. [DOI] [PubMed] [Google Scholar]
- 21.Cruz A., Domingos S., Gallardo E., Martinho A. A unique natural selective kappa-opioid receptor agonist, salvinorin A, and its roles in human therapeutics. Phytochemistry. 2017;137:9–14. doi: 10.1016/j.phytochem.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Butelman E.R., Kreek M.J., Salvinorin A. A kappa-opioid receptor agonist hallucinogen: pharmacology and potential template for novel pharmacotherapeutic agents in neuropsychiatric disorders. Front Pharmacol. 2015;6:190. doi: 10.3389/fphar.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capeletto C., Conterato G., Scapinello J., Rodrigues F.S., Copini M.S., Kuhn F., et al. Chemical composition, antioxidant and antimicrobial activity of guavirova (Campomanesia xanthocarpa Berg) seed extracts obtained by supercritical CO2 and compressed n-butane. J Supercrit Fluids. 2016;110:32–38. [Google Scholar]
- 24.Scapinello J., Aguiar G.P.S., Dal Magro C., Capelezzo A.P., Niero R., Dal Magro J., et al. Extraction of bioactive compounds from Philodendron bipinnatifidum Schott ex Endl and encapsulation in PHBV by SEDS technique. Ind Crops Prod. 2018;125:65–71. [Google Scholar]
- 25.Organisation for Economic Co-operation and Development . 2019. Guideline 423: Acute oral toxicity – acute toxic class method. https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf/ [Google Scholar]
- 26.Gawade S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J Pharmacol Pharmacother. 2012;3:348. doi: 10.4103/0976-500X.103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fish R., Danneman P., Brown M., Karas A. Academic; 2nd ed. Amsterdam; London: 2008. Anesthesia and analgesia in laboratory animals. [Google Scholar]
- 28.Koster R., Anderson M., De-Beer E.J. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–417. [Google Scholar]
- 29.Müller L.G., Salles L.A., Stein A.C., Betti A.H., Sakamoto S., Cassel E., et al. Antidepressant-like effect of Valeriana glechomifolia meyer (valerianaceae) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:101–109. doi: 10.1016/j.pnpbp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Neves G., Menegatti R., Antonio C.B., Grazziottin L.R., Vieira R.O., Rates S., et al. Searching for multi-target antipsychotics: discovery of orally active heterocyclic N-phenylpiperazine ligands of D2-like and 5-HT1A receptors. Bioorg Med Chem. 2010;18:1925–1935. doi: 10.1016/j.bmc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Santos A.R., Calixto J.B. Further evidence for the involvement of tachykinin receptor subtypes in formalin and capsaicin models of pain in mice. Neuropeptides. 1997;31:381–389. doi: 10.1016/s0143-4179(97)90075-5. [DOI] [PubMed] [Google Scholar]
- 32.Trevisan G., Rossato M.F., Hoffmeister C., Müller L.G., Pase C., Córdova M.M., et al. Antinociceptive and antiedematogenic effect of pecan (Carya illinoensis) nut shell extract in mice: a possible beneficial use for a by-product of the nut industry. J Basic Clin Physiol Pharmacol. 2014;25:401–410. doi: 10.1515/jbcpp-2013-0137. [DOI] [PubMed] [Google Scholar]
- 33.Scapinello J., Müller L.G., Schindler M.S.Z., Anzolin G.S., Siebel A.M., Boligon A.A., et al. Antinociceptive and anti-inflammatory activities of Philodendron bipinnatifidum Schott ex Endl (Araceae) J Ethnopharmacol. 2019;236:21–30. doi: 10.1016/j.jep.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 34.Zapata-Morales J.R., Alonso-Castro A.J., Domínguez F., Carranza-Álvarez C., Isiordia-Espinoza M., Hernández-Morales A., et al. The antinociceptive effects of a standardized ethanol extract of the Bidens odorata Cav (Asteraceae) leaves are mediated by ATP-sensitive K+ channels. J Ethnopharmacol. 2017;207:30–33. doi: 10.1016/j.jep.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Lázaro-Ibáñez G.G., Torres-López J.E., Granados-Soto V. Participation of the nitric oxide-cyclic GMP-ATP-sensitive K(+) channel pathway in the antinociceptive action of ketorolac. Eur J Pharmacol. 2001;426:39–44. doi: 10.1016/s0014-2999(01)01206-7. [DOI] [PubMed] [Google Scholar]
- 36.Shi L.K., Zheng L., Liu R.J., Chang M., Jin Q.Z., Wang X.G. Chemical characterization, oxidative stability, and in vitro antioxidant capacity of sesame oils extracted by supercritical and subcritical techniques and conventional methods: a comparative study using chemometrics. Eur J Lipid Sci Technol. 2018;120:1700326. [Google Scholar]
- 37.Choudhary M.I., Batool I., Atif M., Hussain S., Atta-Ur-Rahman null Microbial transformation of (-)-guaiol and antibacterial activity of its transformed products. J Nat Prod. 2007;70:849–852. doi: 10.1021/np068052a. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q., Wu J., Luo Y., Huang N., Zhen N., Zhou Y., et al. (-)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget. 2016;7:62585–62597. doi: 10.18632/oncotarget.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Cássia da Silveira e Sá R., Andrade L.N., de Sousa D.P. A Review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18:1227–1254. doi: 10.3390/molecules18011227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franzotti E.M., Santos C.V., Rodrigues H.M., Mourão R.H., Andrade M.R., Antoniolli A.R. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca) J Ethnopharmacol. 2000;72:273–277. doi: 10.1016/s0378-8741(00)00205-1. [DOI] [PubMed] [Google Scholar]
- 41.Mogosan C., Vostinaru O., Oprean R., Heghes C., Filip L., Balica G., et al. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules. 2017;22:263. doi: 10.3390/molecules22020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ascari J., Sens S.L., Nunes D.S., Wisniewski A., Arbo M.D., Linck V.M., et al. Sedative effects of essential oils obtained from Baccharis uncinella. Pharm Biol. 2012;50:113–119. doi: 10.3109/13880209.2011.634423. [DOI] [PubMed] [Google Scholar]
- 43.Benovit S.C., Silva L.L., Salbego J., Loro V.L., Mallmann C.A., Baldisserotto B., et al. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An Acad Bras Cienc. 2015;87:1675–1689. doi: 10.1590/0001-3765201520140223. [DOI] [PubMed] [Google Scholar]
- 44.Kalueff A.V., Wheaton M., Murphy D.L. What's wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav Brain Res. 2007;179:1–18. doi: 10.1016/j.bbr.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 45.Nin M.S., Couto-Pereira N.S., Souza M.F., Azeredo L.A., Ferri M.K., Dalprá W.L., et al. Anxiolytic effect of clonazepam in female rats: grooming microstructure and elevated plus maze tests. Eur J Pharmacol. 2012;684:95–101. doi: 10.1016/j.ejphar.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira-Tintino C.D.M., Pessoa R.T., Fernandes M.N.M., Alcântara I.S., da Silva B.A.F., de Oliveira M.R.C., et al. Anti-inflammatory and anti-edematogenic action of the Croton campestris A. St.-Hil (Euphorbiaceae) essential oil and the compound β-caryophyllene in in vivo models. Phytomedicine. 2018;41:82–95. doi: 10.1016/j.phymed.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S., Chattopadhyay D., Mandal A., Kaity S., Samanta A. Bioactivity guided isolation of antiinflammatory, analgesic, and antipyretic constituents from the leaves of Pedilanthus tithymaloides (L.) Med Chem Res. 2013;22:4347–4359. [Google Scholar]
- 48.Ghelardini C., Di Cesare Mannelli L., Bianchi E. The pharmacological basis of opioids. Clin Cases Miner Bone Metab. 2015;12:219–221. doi: 10.11138/ccmbm/2015.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards G., Weston A.H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- 50.Zheng G.Q., Kenney P.M., Lam L.K. Sesquiterpenes from Clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J Nat Prod. 1992;55:999–1003. doi: 10.1021/np50085a029. [DOI] [PubMed] [Google Scholar]