Abstract
Background
Pristimerin, a natural flavonoid compound, has potential anti-tumor activities. These activities have been illustrated in various cancer cell lines, including MDA-MB-231 cells. MDA-MB-231 cells are a representative mesenchymal subtype of triple negative breast cancer (MES-TNBC) cell line. Currently, the main treatment for patients with advanced MES-TNBC is cytotoxic chemotherapy. We tried to examine the role and effect of pristimerin on epithelial–mesenchymal transition (EMT) in MDA-MB-231 cells.
Methods
The effects of pristimerin on the proliferation of MDA-MB-231 cells were investigated by cloning formation growth assay. In vitro transwell and adhesion assays were performed for cell invasion and adhesion. The expression levels of EMT markers in E-cadherin and N-cadherin were examined by western blotting. We also established overexpressed- and silenced-integrin β3 cell lines to evaluate the role of integrin β3 in mediating the EMT reversion events in MDA-MB-231 cells.
Results
Pristimerin inhibited cell proliferation, and its inhibitory effect was dose-dependent. We demonstrated that pristimerin reserved EMT by upregulating E-cadherin and downregulating N-cadherin expression. Meanwhile, we revealed that pristimerin inhibited mRNA and protein expression of integrin β3, which is a key heterodimeric transmembrane receptor associated with EMT. These inhibitory effects and reversion of EMT were enhanced when integrin β3 was knockdown in MDA-MB-231 cells, while the overexpression of integrin β3 attenuated these effects. In vivo studies using xenograft mouse model demonstrated that pristimerin inhibited tumor growth.
Conclusions
Our findings provide important insights into the effects of pristimerin on inhibiting cancer progression and EMT reversion by suppression of integrin β3.
Keywords: Pristimerin, MDA-MB-231 cells, Epithelial-mesenchymal transition, Integrin β3
At a glance of commentary
Scientific background on the subject
Pristimerin is a natural flavonoid compound with potential anti-tumor activities. These activities have been illustrated in various cancer cell lines, including MDA-MB-231 cells. However, it is unclear what effect pristatine has on the epithelial-mesenchymal transition (EMT) in MDA-MB-231 cells.
What this study adds to the field
In this study, we demonstrated that pristimerin inhibited mRNA and protein expression of integrin β3, which is a key heterodimeric transmembrane receptor associated with EMT. When integrin β3 is knocked down, these inhibitory effects and the reversal of EMT are enhanced.
Triple negative breast cancer (TNBC) is categorized into four sub-types: Basal-like immunosuppressed (BLIS), basal-like immune-activated (BLIA), mesenchymal (MES), and luminal androgen receptor (LAR). Clinically, the mesenchymal sub-type of TNBC (MES-TNBC) has a higher frequency of distant metastases and has been associated with a poorer prognosis [1]. At present, the main treatment option for patients with advanced MES-TNBC is cytotoxic chemotherapy, because anti-Estrogen Receptor (anti-ER) or anti-human epidermal growth factor receptor-2 (anti-HER-2) target therapy is ineffective for this subtype of breast cancer. Although chemotherapy can prolong the survival rate of patients with advanced diseases, due to frequent reports of excessive toxicity, clinically obvious side effects may reduce their efficacy [2]. Therefore, the discovery of low-toxic agents and a deeper understanding of molecular mechanisms will be of great significance in the management of MES-TNBC patients.
Pristimerin, a naturally occurring triterpenoid, has recently attracted more attention, especially for its potential anticancer activities [3]. These activities have been illustrated in various cancer cell lines, including MDA-MB-231 cells. MDA-MB-231 cells is a representative MES-TNBC cell line. Cevatemre et al., found that pristimerin can induce apoptosis and autophagy via activation of ROS/ASK1/JNK pathway [4]. It has been demonstrated that the anticancer effects of pristimerin are exerted by inhibiting migration via suppressing proteasomal activity and increasing the levels of RGS4 and regulating MMP-2 [5]. Furthermore, recent evidence indicated that MDA-MB-231 breast cancer cells were more sensitive to the treatment of pristimerin than the non-tumorigenic human mammary epithelial cell line MCF-10A [6]. Although it has been demonstrated that pristimerin exhibits various biological processes in MDA-MB-231 cells, the potential anti-tumor mechanisms remains to be fully elucidated.
EMT has been demonstrated to play a key role in the development and invasiveness of cancer [7]. In addition, a large number of pre-clinical and clinical studies have also reported that MDA-MB-231 MES-TNBC is enriched in EMT markers [8]. EMT pathways enact main targets for novel drug development because it is extremely critical and complex [9]. Therefore, it is of great clinical significance to analyze whether EMT is affected by pristimerin for MES-TNBC. Integrins directly bind to the components of the extracellular matrix (ECM) and provide the necessary traction for the movement and invasion of cancer cell [10]. Integrin β3 (a member of integrins) is closed related to EMT in breast cancer cells [11]. However, the interaction between EMT and integrin β3 is still very complex and needs further study.
In this study, we sought to examine the effects of pristimerin on MDA-MB-231 cells in vivo and in vitro, focusing on the effects and molecular mechanism of pristimerin on the EMT. To the best of our knowledge, this is the first report showing that the EMT was greatly reversed by pristimerin, which was associated with integrin β3 in MDA-MB-231.
Materials and methods
Cell culture, drug treatment, proliferation
MDA-MB-231 breast cancer cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in L-15 medium (Sigma, USA). Medium was supplemented with 10% fetal bovine serum (FBS; Every Green) and 1% penicillin/streptomycin (Sigma, USA) at 37 °C without CO2. Pristimerin (Purifa, China) were reconstituted in 0.1% Dimethyl sulfoxide (DMSO, Sigma, USA). Cells were treated with different concentrations pristimerin (0.1, 0.2, 0.5, 1 μM) and times. After drug treatment, cell proliferation was monitored using a Cell Counting Kit-8 (CCK-8, Biosharp) according to the manufacturer's instructions.
Plate colony formation assays
MDA-MB-231 cells were seeded on 3-cm dishes at a density of 200 cells/dish. After overnight culture, the cells were treated with different concentrations of pristimerin (0.1, 0.2, 0.5 or 1 μM) or with 0.1% DMSO. After 14 days of selection, colonies were stained with 0.1% crystal violet (Sigma–Aldrich) and counted.
Transwell invasion assay
Cell invasion assays were performed using Matrigel-coated (invasion assay) 24-well Transwell plates (8.0 μm pore size; Corning, USA). Briefly, MDA-MB-231 cells (2 × 104 cells/well) in the upper chambers were treated with different concentrations of pristimerin (0.1, 0.2, or 0.3 μM) and 700 μL L-15 media containing 5% FBS was added to the bottom chambers. After 24 h of culture, the cells that did not traverse the filter were removed using cotton swabs, and the cells that traversed the filter were fixed using 4% paraformaldehyde and dyed using 1% crystal violet.
Cell adhesion assay
MDA-MB-231 cells were seeded into 6-well plates for 24 h, followed by treatment with 0.1% DMSO or pristimerin (0.1, 0.2, or 0.3 μM) and incubated at 37 °C for 24 h. The attached cells were trypsinized, counted and seeded into gelatin-coated 96-well plates at approximately 5 × 10 4 cells per well for 1 h. The medium was discarded, and the cells were washed twice PBS to remove the non-adherent cells. The cells that had been subjected to Rose Bengal staining were then photographed to assess the adhesion activities. Finally, the cells were fixed, stained, and solubilized for absorbance measurements at 570 nm using a VersaMax microplate reader (Molecular Devices) and Softmax Pro Software.
Western blotting
MDA-MB-231 cells were cultured in the absence or presence of pristimerin or 0.1% DMSO for 24 h, harvested, pelleted, and resuspended in RIPA buffer (sigma). The protein concentrations of the cell lysates were determined using a BCA kit (Beyotime Biotechnology, China). Samples were resolved by SDS-PAGE (BioRad, US) and analyzed by Western blot antibodies (rabbit monoclonal antibodies against E-cadherin, Claudin, N-cadherin, Vimentin, Slug, Snail, β-actin, purchased from Absin). Integrin β3 antibody was purchased from Cell Signaling Technology.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
MDA-MB-231 cells total mRNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using 2 μg total RNA via PrimeScript RT reagent kit (Takara, China). qRT-PCR was performed using a PCR kit (Takara, China) and the following primers on a Bio-Rad CFX Connect instrument (Bio-Rad): E-cadherin, 5′-AGGCCAAGCAGCAGTACATT -3′/5′-GGATGTGATTTCCTGGCCCA-3′; N-cadherin, 5′-AATCAGTGGC-GGAGATCCTAC-3′/5′-TTGACCACGGTGACTAACCC-3′; Integrinβ3, 5′-TGCTCAGAGGAGGACTATCG-3′/5′-GTACTTGCCCGTGATCTTGC-3′; Claudin, 5'--GGCAGATCCAGTGCAAAGTC-3'/5′- TTCTGCTACTCCACGTCTTCT-3'; Vimentin, 5′-AGATGGCC-CTTGACATTGAG-3'/5′-TGGAAGAGGCAGAGAAATCC-3'; Snail, 5′-AAGATGCACATCCGAAGCCA-3'/5′-CAAAAACCCACGCAGACAGG-3'; Slug, 5′-CTTCCTGGTCAAGAAGCA-3'/5′-GGGAAATAATCACTGTATGTGTG-3'; β-Actin: 5′-TTGCCGACAGGATGCA-GAA-3'/5′-GCCGATCCACACGGAGTACT-3′.
Transfection of cells with small interfering RNA (siRNA) and cDNA
Integrin β3 was knocked down using specific siRNA-integrin β3 (human integrin β3, sense [5′–3′]: GCUCAUCUGGAAACUCCUCAUCACC; antisense [5′–3′]: GGUGAUGAGGAGUUUCCAGAUGAGCUC). The plasmid containing integrin β3 cDNA was purchased from Bioeagle (Wuhan, China). MDA-MB-231 cells were seeded into 6-well plates and incubated until reaching approximately 80% confluence. Cells were then transfected with siRNA and a plasmid containing integrin β3 according to the manufacturer's instructions. After 48 h of transfection, cells were treated with 0.3 μM pristimerin and the levels of integrin β3, E-cadherin, and N-cadherin were analyzed by western blotting.
TNBC xenograft model
For in vivo mice experiments, animal care and experimental procedures adhered to the protocol approved by the Institutional Animal Care and Use Committee of Xiangyang No. 1 people's hospital (Project code: DW2018-002; approved date: April 2018).
BALB/C nude mice (4–5 weeks of age) were obtained from the Hunan SJA Laboratory Animal Co. Ltd (China) and housed in a specific pathogen-free laboratory environment. All animals experiment was approved by the ethics committee of Xiangyang No. 1 people's hospital. The animal experiment was carried out as follows. Female nude mice were injected with MDA-MB-231 cells (1 × 106 cells/mouse) in the breast pad. When the tumors reached 100 mm3 in volume, mice were divided randomly into the DMSO-treated group and pristimerin-treated group (1 mg/kg). Tumorigenesis in nude mice was observed, and mice were treated with the appropriate drug or control every 2 days by po (oral gavage); body weights were also determined every 2 days. After 6 weeks, all mice were sacrificed under anesthesia, and body weights, tumor weights, and tumor volumes were measured. Tumors were photographed, and liver, kidney, and lung tissues of nude mice were excised and fixed in methanol. Hematoxylin and eosin (HE) staining and immunohistochemistry were performed for the samples, as described below.
HE staining and immunohistochemistry
HE and IHC staining were performed as previously described [12]. Briefly, samples of the tumors, liver, kidneys, and lungs were fixed, stained with HE, and visualized under a microscope (OLYMPUS, IX73). HE staining enabled determination of the morphological features of the tumors. Slides were deparaffinized, dehydrated, blocked. The slides were blocked with rabbit serum for 30 min and incubated with E-cadherin antibody (1:500), N-cadherin antibody (1:500), integrinβ3 antibody (1:500) were performed at 4 °C overnight. Next, the slides were incubated with the second antibody, stained with diaminobenzidine substrate and counterstained with hematoxylin. Lastly, all samples were analyzed via optical microscope.
Statistical analysis
Data analyses were conducted using Graph Pad software (San Diego, CA, USA) and statistically analyzed using one-way ANOVA with post-hoc Tukey HSD Calculator or Student t-test. A p-value of less than 0.05 was considered statistically significance.
Results
Pristimerin inhibits cell proliferation, invasion, and adhesion
To investigate the effects of pristimerin on the proliferation of cells, MDA-MB-231 cells were treated with different concentrations of pristimerin for 48 h. As shown in Fig. 1A, pristimerin significantly inhibited cell proliferation when the concentration reached 0.5 μM, and the cell viability was wholly decreased as the incubation time increased [Fig. 1B].
Fig. 1.
Pristimerin inhibits cell proliferation, invasion and adhesion of MDA-MB-231 cells. (A) and (B) Pristimerin inhibits MDA-MB-231 cells proliferation. (C) Pristimerin inhibits colony formation ability of MDA-MB-231 cells. (D) The cell invasion and (E) the cell adhesion were inhibited in MDA-MB-231 cells after pristimerin treatment. The data shown as mean ± SD and represent 3 independent experiments, and ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 compared with NC treatment only.
In long-term (14-day) clonogenic growth assays, it significantly inhibited MDA-MB-231 cells growth, as shown by greatly reduced colony formation [Fig. 1C]. In addition, in vitro transwell (Matrigel) and adhesion assay were performed to further investigate the effect of pristimerin on invasion and adhesion. Interestingly, the results showed that treatment with 0.1–0.3 μM pristimerin for 24 h significantly reduced the numbers of cells invasion and adhesion, and its inhibitory effects were dose-dependent [Fig. 1D and E]. These results suggest that pristimerin inhibited MDA-MB-231 cells proliferation, adhesion and invasion, while relatively low levels of pristimerin significantly inhibited the adhesion and invasion in a dose-dependent manner.
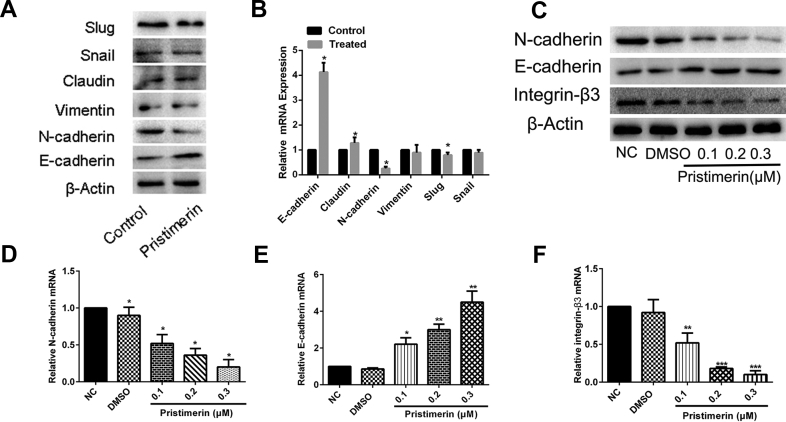
Pristimerin reversed the EMT and inhibited integrin β3 expression
Our results showed that even a relatively low concentration of pristimerin could dramatically inhibit the invasion and adhesion. Considering that the EMT was remarkably correlated with invasion, and adhesion [13,14], we hypothesized that pristimerin might inhibit the MDA-MB-231 cell invasion and invasion by reversing the EMT. Therefore, we decided to examine the effects of pristimerin on the expression of EMT marker in MDA-MB-231 cells. As shown Fig. 2A and B, western blotting and qRT-PCR demonstrated that treatment with 0.3 μM pristimerin had significant effect on E-cadherin and N-cadherin expression, while the expression of Claudin, Snail, Slug, and Vimentin at least showed a minor changed. Thus, we later focus on the change in E-cadherin and N-cadherin of EMT marker. Further experiments showed that this effect was concentration-dependent.
Fig. 2.
Pristimerin reversed the EMT and inhibited integrin β3 expression. (A) and (B) The expression of EMT marker and integrin β3 were examined by western blotting and qPCR after pristimerin treatment (0.3 μM). (C) The expression of EMT proteins (E-cadherin, N-cadherin) and integrin β3 were examined by western blotting after different pristimerin treatment (0.1, 0.2, or 0.3 μM). (D–F) cells were subjected to qPCR for detection of the indicated EMT-related mRNAs. The data shown as mean ± SD and represent 3 independent experiments, and ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 compared with NC treatment only.
Given the fact that pristimerin effectively inhibited the cell invasion and adhesion and the key role of ECM-related genes in cell motility, we hypothesized that ECM-related gene may involve in the process of pristimerin inhibition. It has been reported that integrin β3 was associated with EMT and played an important role in the development of tumor invasion and metastasis [11], so we speculated on whether pristimerin regulated the EMT by inhibiting the expression of integrin β3. To verify that, the mRNA and protein expression of integrin β3 was examined after the treatment of pristimerin. As shown in Fig. 2C, pristimerin can significantly suppress the expression of integrin β3. Similar results were observed at the mRNA expression [Fig. 2D–F]. Taken together these results indicate that pristimerin reversed the EMT and integrin β3 may participate in the EMT reversion events in MDA-MB-231.
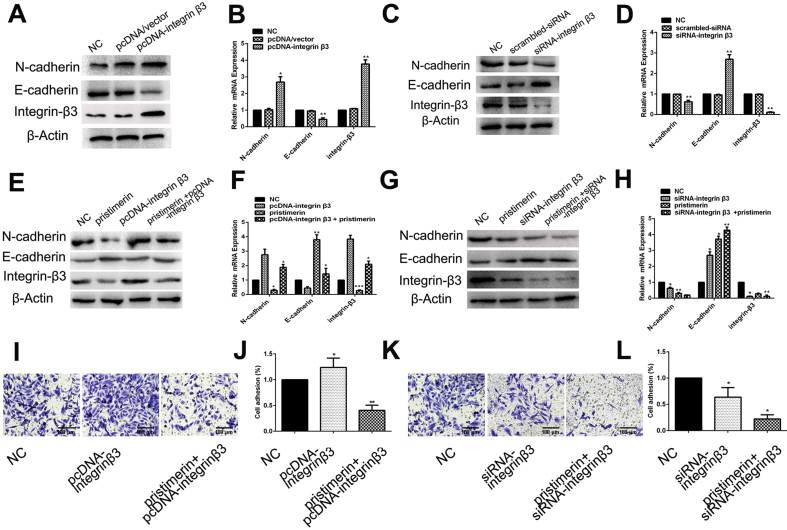
Integrin β3 regulated the EMT
We further evaluated the role of integrin β3 in mediating the EMT by assessing the expression of the E-cadherin and N-cadherin. We established overexpressing or silencing integrin β3 cell lines using MES-TNBC cell MDA-MB-231. Western blot and qRT-PCR showed integrin β3 silencing and overexpression efficiency. As shown in Fig. 3A and B, the expression of N-cadherin was upregulated after integrin β3 overexpression, while E-cadherin was reduced in integrin β3-overexpressing cells. On the contrary, knockdown of integrin β3 using siRNA significantly increased E-cadherin expression and decreased N-cadherin expression [Fig. 3C]. Similar results were observed using qRT-PCR for mRNA levels [Fig. 3D]. Thus, these results further corroborated the above finding that integrin β3 was involved in the EMT in MDA-MB-231.
Fig. 3.
Integrin β3 regulated the EMT and involved in MDA-MB-231 cells progression. Effects of integrinβ3 overexpression (A) and silencing (C) on N-cadherin and E-cadherin protein levels in MDA-MB-231 cells. Effects of integrin β3 overexpression (B) and silencing (D) on N-cadherin and E-cadherin mRNA levels in MDA-MB-231 cells. (E) and (F) Western blot and qRT-PCR analysis of the expression levels of EMT markers in MDA-MB-231 cells treated with control (DMSO), pristimerin, siRNA-integrin β3, or pristimerin + siRNA-integrin β3. (G) and (H) Western blot analysis and qRT-PCR were used to detect the expression levels of EMT markers in MDA-MB-231 cells treated with control (DMSO), pristimerin, pcDNA-integrin β3, or pristimerin + pcDNA-integrin β3. (I) and (J) overexpression of integrin β3 increased the invasion and adhesion of MDA-MB-231. (K) and (L) Knockdown of integrin β3 inhibited the invasion and adhesion of MDA-MB-231. The data shown as mean ± SD and represent 3 independent experiments, and ∗p < 0.05; ∗∗p < 0.01 compared with NC treatment only.
Pristimerin reversed EMT by integrin β3
To further validate whether pristimerin reversed EMT through regulating integrin β3 in MDA-MB-231, we assessed the expression of the EMT marker in response to treatment with pristimerin in overexpressing or silencing integrin β3 cells. As shown in Fig. 3E and F, the inducing of EMT was markedly suppressed in integrin β3-overexpressing cells incubated with 0.3 μM pristimerin. Conversely, a significant inactivation of EMT were observed in integrin β3-silencing cells treated with pristimerin [Fig. 3G and H]. These data indicated that pristimerin reversed EMT via integrin β3.
To testify whether the downregulation or upregulation of integrin β3 affect invasion and adhesion, invasion and adhesion assay were performed. Our data showed that when the integrin β3-overexpression cells were treated by 0.3 μM pristimerin, upregulated integrin β3 partially reversed the inhibiting effects of pristimerin on invasion and adhesion [Fig. 3I and J]. Opposite results were found in the integrinβ3-knockdown cell line [Fig. 3K and I].
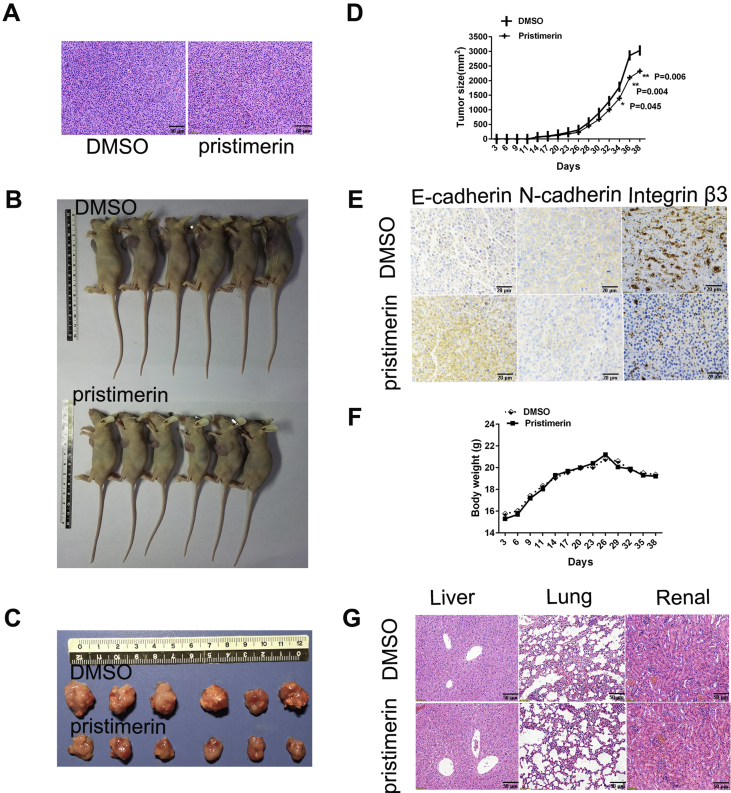
Pristimerin inhibited tumor growth and reversed the EMT pathway in a mouse xenograft model
To determine whether pristimerin could inhibit tumor growth and reverse the EMT in vivo, tumors were evaluated by HE staining and IHC. HE staining [Fig. 4A] showed that the morphological characteristics of the tumor tissue were consistent with the pathological morphological characteristics of MES-TNBC, indicating that the in situ transplantation of the MES-TNBC tumor-bearing mouse model was successful [Fig. 4B]. As expected, pristimerin caused substantial growth retardation of tumors compared with the untreated mice [Fig. 4C and D]. To further address the mechanisms underlying the inhibition of tumor progression, xenograft tumors were analyzed by IHC. The results revealed significantly increased expression of E-cadherin, and decreased expression of N-cadherin and integrin β3 in the pristimerin group, compared with the control group [Fig. 4E]. Of note, no significant loss of body weight were observed [Fig. 4F] and Fig. 4G also revealed that there were no significant differences in the morphological or pathological features of the tissues (liver, kidney, lung) between the control and drug-treated group, suggesting that no obvious adverse response. These results clearly suggest that pristimerin can reverse the EMT through reduced integrin β3 expression in vivo and exerts anti-tumor effect without toxicity to the animals.
Fig. 4.
Pristimerin inhibited tumor growth and reversed the EMT in a mouse xenograft model (A) Pathological HE staining of breast tumors from nude mice. (B) Representative image of the morphology of tumor xenografts after treatment with pristimerin compared with that in the control (treated with DMSO). (C) Tumors in tumor-bearing nude mice. (D) Growth of tumor xenografts over time in nude mice. (E) Immunohistochemical staining of E-cadherin, N-cadherin and integrin β3 in tumor tissues (400×). (F) Changes in body weights in tumor-bearing nude mice. (G) Pathological HE staining of liver tissues, lung tissues, and kidney tissues; original magnification: 200×. The data shown as mean ± SD and represent 3 independent experiments, and ∗p < 0.05; ∗∗p < 0.01 compared with 0.1% DMSO treatment only.
Discussion
In recent years, more and more efforts have focused on identifying new anti-cancer compounds. Pristimerin, a traditional Chinese medicine (TCM) and antitumor agent, has attracted interest because of its antitumor effects and low toxicity [15]. Nevertheless, there are no reports to address the effect of pristimerin on EMT in MDA-MB-231 cells. In the present study, our findings clearly show that overexpression of integrin β3 can enhance the process of EMT, while its silence markedly reversed the EMT. Pristimerin inhibited the growth, migration and invasion of MDA-MB-231 cell through negatively regulated integrin β3 expression. Furthermore, the overexpression of integrin β3 impaired the inhibitory effect of pristimerin on EMT, adhesion, and invasion, but the opposite results were found when integrin β3 knocked down. In vivo studies in mice showed that pristimerin significantly reduced tumor burden without toxic effects. Interestingly, Pristimerin seems to have a smaller effect on reducing tumor size (volume) in the TNBC xenograft model than the inhibition on the cell growth in vitro. It is possible that the difference in the bioavailability of pristimerin in vitro and vivo. In vitro experiments involve cells that directly interact with the pristimerin. In experimental animals in vivo, after oral gavage, the drugs are absorbed through the blood and eventually produce inhibitory effects. However, the specific mechanism is unclear, and further pharmacokinetic studies will be conducted in the future. Similarly, as shown by immunohistochemistry, pristimerin reversed EMT, while integrin β3 mediated the EMT process. In summary, the data from this study provide strong evidence that pristimerin exerts anti-tumor activity against MDA-MB-231 MES-TNBC by downregulating integrin β3 to reverse EMT.
The result of this study is that low concentrations of pristimerin has a significant inhibitory effect on the invasion and adhesion of MDA-MB-231 cells. Previous study have demonstrated that EMT is the main factor contributing to this invasive ability of TNBC patients [16]. Cells undergoing EMT displayed reduced expression of epithelial cell markers (such as E-cadherin, Claudin), while increased expression of mesenchymal molecules (such as N-cadherin, Vimentin, Snail, Slug) [17,18]. To date, only one previous study reported that pristimerin reversed EMT by inhibiting the expression of N-cadherin, fibronectin, Vimentin, ZEB1 in PC-3 cells. Interestingly, we observed that after pristimerin treatment, the changes of these EMT makers also different with a significant increase in E-cadherin level and a significant decrease in N-cadherin level but no significant change in the level of Claudin, Vimentin, Snail, Slug. These results are consistent in vitro. The regulation of EMT marker is complex and may be affected by many other factors, including the influence of the tumor microenvironment, posttranslational modifications, and protein–protein interactions [19]. The cause of this phenomenon may be related to the sensitivity of pristimerin.
These latest studies showed that integrin β3 is closely related to EMT, but little is known about its specific contributions to EMT in tumor progression [20,21]. Here, we observed that pristimerin significantly inhibited the expression of integrin β3 in MDA-MB-231. Therefore, we examined whether integrin β3 is involved in the regulation of EMT after the pristimerin intervention. The results showed that overexpression of integrin β3 in MDA-MB-231 cells led to enhance invasion, adhesion, and EMT induction, including E-cadherin and N-cadherin conversion. In addition, knockdown of integrin β3 enhanced the pristimerin-mediated suppression of cell adhesion, invasion and EMT, while overexpression of integrin β3 attenuated these effects. These results imply that integrin β3 plays a crucial role in anti-cancer therapy mediated by pristimerin.
Conclusion
In conclusion, the anti-tumor effect of pristimerin was demonstrated by in vitro and in vivo experiments. The mechanism of its anti-tumor effect was that pristimerin reversed EMT by negatively regulating integrin β3 in MDA-MB-231 MES-TNBC. This study is the first to provide a study in which pristimerin can reverse the EMT by downregulation of integrin β3, which will provide new scientific evidence for the clinical application of pristimerin. Integrin β3 may become a new anticancer target of MES-TNBC. In addition, it is necessary to use integrin β3 knockout animals for further research in the future.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Acknowledgements
We thank the Ming Sang, Xiaodong Sun, and Lixia Xie of Central laboratory of No. 1 People's Hospital and Zhongshi He and Xiaohong Liu for the technical assistance, advice for the study and critical reading of the manuscript.). This research was funded in part by the National Natural Science Foundation of China (No. 81703015) to Pengchao Hu, and Hubei Natural Science Foundation (No. 2019CFB105) to Ying Wang and Foundation of Hubei Xiangyang No. 1 People's Hospital of Youth programs (No.2018.05) to Bo Hu. Some of the research was financially supported in part by grants from the Ministry of Science and Technology, Taiwan (107-2622-B-182-002-CC2) and the Chang Gung Memorial Hospital Research Fund (CMRPD11K0251, CMRPD1F0281-2 and CMRPD1H0321-3) to RW.
Footnotes
Peer review under responsibility of Chang Gung University.
Contributor Information
Jing Wang, Email: aiyouyou5858@163.com.
Robert YL. Wang, Email: yuwang@gap.cgu.edu.tw.
References
- 1.Burstein M.D., Tsimelzon A., Poage G.M., Covington K.R., Contreras A., Fuqua S.A., et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortes J., Vahdat L., Blum J.L., Twelves C., Campone M., Roche H., et al. Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2010;28:3922–3928. doi: 10.1200/JCO.2009.25.8467. [DOI] [PubMed] [Google Scholar]
- 3.Yousef B.A., Hassan H.M., Zhang L.Y., Jiang Z.Z. Anticancer potential and molecular targets of pristimerin: a mini- review. Curr Cancer Drug Targets. 2017;17:100–108. doi: 10.2174/1568009616666160112105824. [DOI] [PubMed] [Google Scholar]
- 4.Cevatemre B., Erkisa M., Aztopal N., Karakas D., Alper P., Tsimplouli C., et al. A promising natural product, pristimerin, results in cytotoxicity against breast cancer stem cells in vitro and xenografts in vivo through apoptosis and an incomplete autopaghy in breast cancer. Pharmacol Res. 2018;129:500–514. doi: 10.1016/j.phrs.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Mu X.M., Shi W., Sun L.X., Li H., Wang Y.R., Jiang Z.Z., et al. Pristimerin inhibits breast cancer cell migration by up- regulating regulator of G protein signaling 4 expression. Asian Pac J Cancer Prev. 2012;13:1097–1104. doi: 10.7314/apjcp.2012.13.4.1097. [DOI] [PubMed] [Google Scholar]
- 6.Wu C.C., Chan M.L., Chen W.Y., Tsai C.Y., Chang F.R., Wu Y.C. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–1285. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- 7.Elzamly S., Badri N., Padilla O., Dwivedi A.K., Alvarado L.A., Hamilton M., et al. Epithelial-mesenchymal transition markers in breast cancer and pathological responseafter neoadjuvant chemotherapy. Breast Cancer (Auckl) 2018;12 doi: 10.1177/1178223418788074. 1178223418788074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianchini G., Balko J.M., Mayer I.A., Sanders M.E., Gianni L. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das V., Bhattacharya S., Chikkaputtaiah C., Hazra S., Pal M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019;234:14535–14555. doi: 10.1002/jcp.28160. [DOI] [PubMed] [Google Scholar]
- 10.Desgrosellier J.S., Cheresh D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J., Yao J.F., Deng X.F., Zheng X.D., Jia M., Wang Y.Q., et al. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin alphavbeta3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. 2018;37:23. doi: 10.1186/s13046-018-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z.M., Yang X.L., Jiang F., Pan Y.C., Zhang L. Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. J Cell Biochem. 2020;121:2467–2477. doi: 10.1002/jcb.29469. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Zheng H., Yuan S., Zhou B., Zhao W., Pan Y., et al. Overexpression of ANLN in lung adenocarcinoma is associated with metastasis. Thoracic Cancer. 2019;10:1702–1709. doi: 10.1111/1759-7714.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang R.Y., Guilford P., Thiery J.P. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–4422. doi: 10.1242/jcs.099697. [DOI] [PubMed] [Google Scholar]
- 15.Yadav V.R., Prasad S., Sung B., Kannappan R., Aggarwal B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2:2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaled Bidet. New insights into the implication of epigenetic alterations in the EMT of triple negative breast cancer. Cancers. 2019;11:559. doi: 10.3390/cancers11040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeisberg M., Neilson E.G. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 19.Thiery J.P., Sleeman J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 20.Hill B.S., Sarnella A., Capasso D., Comegna D., Del Gatto A., Gramanzini M., et al. Therapeutic potential of a novel alphavbeta(3) antagonist to hamper the aggressiveness of mesenchymal triple negative breast cancer sub-type. Cancers. 2019;11:139. doi: 10.3390/cancers11020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvani J.G., Gujrati M.D., Mack M.A., Schiemann W.P., Lu Z.R. Silencing beta3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-negative breast cancer. Cancer Res. 2015;75:2316–2325. doi: 10.1158/0008-5472.CAN-14-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]