Abstract
Introduction:
Abnormal chloride (Cl−) transport dehydrates airway surface liquid (ASL) in sinonasal epithelium leading to mucus stasis and chronic rhinosinusitis. As an experimental epithelium, rabbit tissue provides an excellent representation of human sinus disease, and the rabbit sinusitis model is established and well suited for therapeutic interventions in vivo. The objective of this study is to evaluate whether ivacaftor reverses the consequences of Pseudomonas aeruginosa-induced acquired CFTR dysfunction.
Methods:
Rabbit nasal cavities were assessed for responsiveness to ivacaftor in vivo (nasal potential difference (NPD) assay). Rabbit nasal epithelial (RNE) cultures were incubated with an ultrafiltrate of P. aeruginosa (PAO1 strain) for 4 hours and tested for acquired CFTR dysfunction. Markers of mucociliary function, including airway surface liquid depth (ASL), periciliary liquid depth (PCL), ciliary beat frequency (CBF) and mucociliary transport (MCT) were measured by micro-optical coherence tomography (μOCT) following PAO1 and/or ivacaftor incubation.
Results:
Ivacaftor resulted in a significant 21.8+/−2.1 mV mean NPD polarization that was significantly greater than low Cl− control (12.9+/−1.3; p = 0.01). PAO1 exposure induced a state of acquired CFTR dysfunction in rabbit nasal epithelium as measured by forskolin-stimulated short-circuit current (ISC) (control, 37.0+/−1.1 μA/cm2 vs PAO1, 24.4+/−1.1 μA/cm2; p < 0.001). RNE cultures exposed to PAO1 had inhibited mucociliary function, whereas co-incubation with ivacaftor restored mucociliary clearance as measured by μOCT.
Conclusion:
In RNE, ivacaftor robustly stimulates CFTR-mediated Cl- secretion and normalizes ASL and CBF in PAO1-induced acquired CFTR dysfunction Preclinical testing of CFTR potentiators as therapy for P. aeruginosa rabbit sinusitis is planned.
Keywords: ivacaftor, Pseudomonas, cystic fibrosis, CFTR, mucociliary clearance, mucociliary transport, sinusitis, chronic sinusitis, chronic rhinosinusitis, micro optical coherence tomography
INTRODUCTION
Cystic fibrosis transmembrane conductance regulator (CFTR) is a cyclic adenosine monophosphate (cAMP)-regulated chloride channel expressed on the apical membrane of epithelial cells in the lung, sinonasal cavities, intestine, and other tissues.1–4 Aberrant vectorial ion transport due to absent or dysfunctional CFTR in patients with cystic fibrosis (CF) results in dehydration of airway surface liquid and impairment of mucociliary transport (MCT) in the upper and lower airways.5,6 Growing evidence indicates that acquired CFTR dysfunction in the absence of genetic mutations is linked to the pathophysiology of other airway diseases characterized by inflammation and infection, including chronic obstructive pulmonary disease and chronic rhinosinusitis (CRS).7–9 CRS tissues from both CF and non-CF individuals have mucin hyperexpression and secretion, mucus accumulation, as well as submucosal gland and goblet cell hyperplasia.10 Furthermore, the presence of impaired MCT, chronic bacterial colonization, and persistent submucosal inflammation in non-CF CRS patients resembles what is observed in CF sinus disease.11 It is now well established that CFTR processing and function can be markedly compromised by environmental perturbations, such as cigarette smoke exposure, hypoxia/hypoxemia, inflammation, and infectious agents (particularly exoproducts of Pseudomonas aeruginosa).12–17 CRS patients have a high rate of P. aeruginosa infections (approximately 25%), and the sinonasal cavities are particularly vulnerable to a vicious cycle of infection and obstruction when MCT is compromised. CFTR-mediated anion transport dysfunction from the combined impact of hypoxia and P. aeruginosa exoproducts, particularly lipopolysaccharide through generation of reactive oxygen species, only further perpetuates progression of the disease.7,12–14,18
Ivacaftor is an oral CFTR potentiator identified by screening over 228,000 small molecules using high throughput analysis and a cell-based fluorescence membrane potential assay.19 The drug was licensed in 2012 both in the United States and Europe for patients with CF aged six years and over who carry at least one copy of the G551D mutation. Ivacaftor is the first CF medication that addresses the primary consequences of CFTR protein dysfunction rather than the downstream sequelae of the disease. Studies in both recombinant cell lines and primary cultures of human bronchial epithelia have demonstrated that ivacaftor promotes Cl− transport by increasing CFTR channel open probability, and augments both ASL height and ciliary beat frequency (CBF).19 Results of two multicenter, randomized, controlled trials unequivocally showed significant improvement in markers of CFTR function (sweat chloride and nasal potential difference (NPD)) and clinical endpoints (lung function (FEV1), body mass index, hospitalization rate, and Pseudomonas burden).20,21 The drug clearly activates Cl− secretion in the nasal as well as lower airways, and so may be a valid therapy for subjects with CRS and impaired sinonasal MCT as it also improves activity of wild type CFTR.22–27 We have also created a ciprofloxacin ivacaftor sinus stent that causes marked reduction in P. aeruginosa infection in vitro and preclinical non-CF rabbit models.22,23 Clinical outcomes are improved in G551D CF CRS with marked improvement in symptoms after starting therapy.28 Because MCT is critical to CRS pathogenesis, it is reasonable to presume that ivacaftor will improve clinical endpoints in non-CF CRS in the setting of acquired CFTR dysfunction. Unfortunately, ivacaftor does not activate CFTR in rodent airways,29 and thus a suitable preclinical animal model for testing the drug in CRS is needed.
As an experimental epithelium, rabbit nasal and sinus tissues provide an excellent resource to study human sinus disease. Our group recently generated a reliable model of rabbit CRS demonstrating dominant pathogenic species (including Pseudomonas), significant infiltration of plasma cells and lymphocytes, increased submucosal glands, and decreased markers of MCT (periciliary liquid and ciliary beat frequency).11,30–33 The objectives of this study are 1) to evaluate the suitability of rabbit nasal epithelia for studies of ivacaftor and 2) to determine whether ivacaftor reverses the consequences of P. aeruginosa-induced acquired CFTR dysfunction.
MATERIALS AND METHODS
Tissue Culture
Investigational Animal Care and Use Committee approval was obtained from the University of Alabama at Birmingham prior to the initiation of the study. We have previously established a model of murine, as well as rat, nasal septal epithelial cultures that recapitulate as well differentiated cell culture models with robust ciliated respiratory epithelium and have all the appropriate ion channels necessary for evaluation of acquired CFTR dysfunction. For this reason, we chose to culture the rabbit nasal septa in this study. Rabbit primary nasal epithelial cells were cultured at an air-liquid interface with slight modifications to previously established protocols.34–37 Rabbit nasal septa of 14 weeks old New Zealand White rabbits were harvested immediately following euthanasia and transferred to pre-warmed dissociation media (minimal essential medium (MEM), 100 units penicillin per ml, and 0.1mg streptomycin per ml, 1.4mg/ml pronase, and 0.1mg/ml DNAse). Septa were placed in dissociation media in a 5% CO2 incubator at 37oC for 1 hour, then 1% FBS (fetal bovine serum) was added to interrupt the enzymatic dissociation. After 2 minutes of incubation, epithelial cells were released by gentle inversion. Collected epithelial cells were washed twice by centrifuge at 1000 rpm for 5 min at room temperature. The cell pellet was resuspended in culture media (5% FBS, 100 units/ml penicillin, 0.1mg/ml streptomycin, and 1% ITS™ universal culture supplement) (BD Biosciences, Bedford, MA) and incubated at 37oC in 100mm Primaria™ culture dishes to reduce fibroblast contamination. After 2 hours, the cell suspension was collected and centrifuged again at 1000 rpm for 5 min. Cell pellets were gently resuspended with culture media, and dissociated rabbit nasal epithelial cells were seeded at the density of 8×105 cells/cm2 to transwell inserts with 200μl culture media and 600μl in the basal compartment. After three days of incubation at confluence, media on the apical surfaces were removed and basal media replaced with 600μl differentiation media (12.5% FBS, 2% Nuserum, 1% ITS, 100 units/ml penicillin, 0.1mg/ml streptomycin, and 60nmol/ml trans-retinoic acid). Basal differentiation medium was changed every other day. Primary cultures grown at an air-liquid interface in this manner reach full differentiation with widespread ciliogenesis at 21 days. Optimization of culture conditions was conducted to achieve approximately 80–90% ciliated cells. Cultures were selected for Ussing Chamber analysis only when fully differentiated with transepithelial resistances > 300 Ω/cm2.
Bioelectric Measurements
Chemicals and solutions.
All chemicals were procured from Sigma Aldrich (Carlsbad, Ca). Bath solution contents consisted of (mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose, with a pH of 7.3–7.4 in a gassed mixture of 95% O2:5% CO2 at 37°C. All studies were conducted in low Cl− (6 mM) mucosal baths. Pharmacologic manipulations consisted of amiloride (100 μM), ivacaftor (10 μM) and/or forskolin (20 μM), and the CFTR inhibitors CFTRinh-172 (10 μM) and GlyH-101 (50 μM).38–42 Amiloride, which inhibits epithelial sodium channels (ENaC), was administered to assure that any changes in short-circuit current (ISC) are independent of effects upon ENaC activity. Ivacaftor was administered at 10μM concentration as optimal dosing in vitro based on Van Goor et al.19 Forskolin was administered to maximally stimulate cAMP-mediated Cl− secretion. The CFTR inhibitors CFTRinh-172 and GlyH-101 permit evaluation of the contribution of CFTR to the overall stimulated potential difference in vivo (mV) or short-circuit current (Isc) in vitro. For the creation of P. aeruginosa (PAO1 strain) ultrafiltrate, bacterial frozen stock was transferred (−80°C) to 50 ml of Luria-Bertani (LB)-Miller broth overnight (37°C) and shaken at 200 rpm. Overnight cultures were transferred to LB-Miller agar plates using the quadrant streaking method to ensure purity. An isolated colony was transferred to 10 ml of LB-Miller broth and incubated (37°C) on a shaker (200 rpm) for 20 hours overnight. The broth was passed through 2 EMD Millipore Millex-GP sterile syringe filters (Fisher-Scientific, Waltham, Ma.), and the subsequent solution streaked onto agar plates to confirm complete sterility.
Measurement of short circuit current and conductance.
Transwell inserts were placed in Ussing chambers for monitoring of ion transport and pharmacologic blockade as previously described.43 Apical monolayers were analyzed in short-circuit conditions following compensation of fluid resistance with automatic VCC 600 voltage clamps (Physiologic Instruments, San Diego, CA). Inserts were subsequently mounted in the aforementioned bath solution at 37°C. Short-circuit measurements were recorded at 1 sample/second under the premise that positive deflections indicate net anion movement from the serosal to the mucosal surface. For experiments designed to assess for PAO1 induced CFTR dysfunction, a time period of 4-hours was chosen based on the previous dose-response experiments with lipopolysaccharide.12
Nasal potential difference assay.
NPD measurements are useful as a diagnostic tool for quantification of CFTR function in the nasal airways. We utilize small amounts of test solutions into the anterior nostril of the rabbit to measure uptake or secretion of Na+ and Cl−. A four-step protocol was used, as described previously.34,44 First, nasal cavities of anesthetized white New Zealand rabbits were perfused with Ringer’s solution containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and amiloride 100 μM (pH 7.3). Next, a low-chloride–containing solution was perfused (mM N-methyl-D-glucamine [NMDG], 6 mM Cl−, pH 7.3). Ivacaftor (10 μM) was then added to the perfusate to evaluate CFTR activation, followed by Glyh-101 (50 μM) and CFTRinh-172 (10 μM) to evaluate the contribution of CFTR to the total transepithelial Cl− secretion. Because of the continuous presence of amiloride (50 μM) and the complete replacement of Na+ with a membrane-impermeant cation (140 NMDG in the perfusion solution), hyperpolarization reflects Cl− secretion rather than cation absorption. The activity was measured from the stable baseline to the highest point of hyperpolarization.
Micro Optical Coherence Tomography
Optical coherence tomography (OCT) creates cross-sectional images through reflectance properties intrinsic to the airways.45 ASL, PCL, and CBF can be assessed simultaneously and non-invasively at the airway surface. Recently, an OCT system called micro-OCT (μOCT) was developed to capture microanatomy with improved precision and 1-micron resolution with unprecedented subcellular detail, not possible with traditional OCT.46 μOCT can measure these functional parameters under a variety of conditions in multiple airway models.
Image acquisition.
RNE filters were evaluated by μOCT under four conditions. An ultrafiltrate of PAO1 or vehicle control was applied to the apical surface of filters with or without ivacaftor (10 μM) and incubated for 4 hours. μOCT imaging of RNE cultures was performed with incident illumination of the apical cell surface to assess microanatomic parameters of ASL in vitro. The imaging optics axis were placed within 10 degrees of normal to the cell plane in order to reduce errors in geometric measurements.46 All imaging was achieved using four regions of interest per well (2 points at 1 mm from the center and another at 1 mm from the edge for 2 different locations) and averaged for a single value per well.
Image analysis.
ASL and PCL were quantitatively evaluated by directly measuring the visible thickness within the image. To account for refractory properties of the liquid, layer thickness measurements were corrected for the index of refraction of the liquid (n=1.33). CBF was assessed using a time series of images and quantitatively measured by ascertaining peak amplitude frequency in the temporal Fourier transform of areas demonstrating oscillatory behavior. Measurements were performed at five uniformly distributed areas of the image. All images were analyzed using ImageJ version 1.50i (National Institutes of Health, Bethesda, MD) and MATLAB® R2016a (The MathWorks, Natick, MA) by a blinded reviewer (SZ).
Statistical Analysis
Statistical analyses used two-tailed, unpaired t-tests for Ussing chamber and NPD data. One way ANOVA with Tukey-Kramer post hoc analysis was utilized for μOCT studies. All values are reported as the mean+/− standard error of the mean [SEM]. A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Ivacaftor activates CFTR-dependent ion transport in rabbit nasal mucosa in vivo and in vitro.
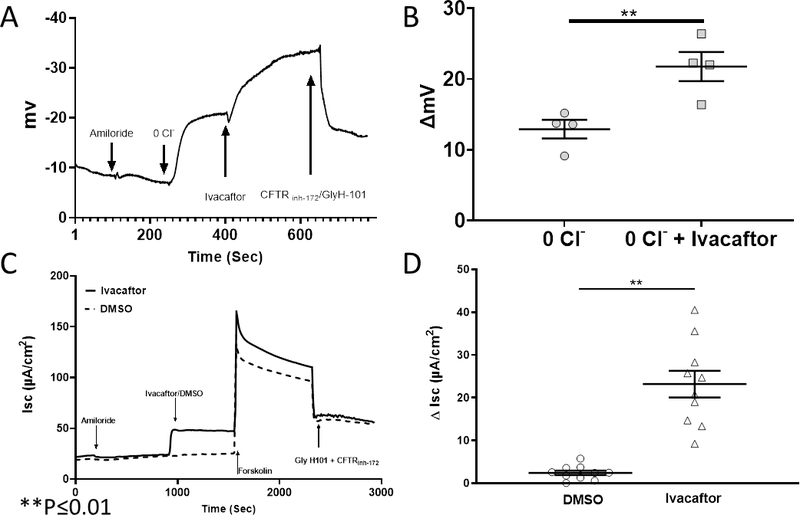
Four rabbits underwent a standardized NPD protocol with the addition of 10 μM ivacaftor in the third perfusion solution. The tracing (Figure 1A) is representative of the hyperpolarization observed when ivacaftor was included in the perfusate. Total Cl− transport was significantly higher with ivacaftor compared to low Cl− alone and resulted in a 21.8+/−2.1 mV mean NPD polarization that was significantly greater than low Cl− control (12.9+/−1.3) (n = 4; p ≤ 0.01) (Figure 1B). When RNE cultures were dissociated and cultured at an air-liquid interface, they were subjected to pharmacologic manipulation in Ussing chambers. Ivacaftor significantly increased CFTR-mediated Cl− transport (change in short circuit current = ΔISC) over vehicle controls (n = 10, ivacaftor 23.2+/−3.1μA/cm2 vs DMSO vehicle controls, 2.4+/−0.5 μA/cm2; p ≤ 0.01).
Figure 1.
(A) Representative tracing from a rabbit in a standardized nasal potential difference (NPD) protocol with sequential administration of amiloride, +low Cl− solution, +ivacaftor (10 μM), + GlyH-101(20μM)/CFTRinh-172 (10 μM). (B) Summary data of studies described above. Ivacaftor perfusion resulted in mean NPD polarization that was significantly greater than low Cl− control (n=4; p≤0.01). (C) Representative Ussing chamber current tracings of RNE. Sequential addition of amiloride, ivacaftor or DMSO control, forskolin, and CFTR inh-172 are shown. (D) Ivacaftor significantly increased CFTR-mediated Cl− transport over vehicle controls (n = 10; p < 0.001).
PAO1 induces an acquired CFTR dysfunction in RNE
RNE cultures were incubated for 4 hours with 100 μl of PAO1 ultrafiltrate or LB broth control at the apical surface (Figure 2). Filters were evaluated with the Ussing chamber (n ≥ 10 per condition) using pharmacologic manipulation with amiloride, forskolin, and CFTR inh-172 / Glyh-101 (Figure 2). Rabbit epithelium was noted to have small changes in ISC with administration of amiloride, indicating ENaC may contribute less to the overall ion transport phenotype and was consistent with what was observed during amiloride perfusion in the in vivo rabbit NPD assay. However, there was a significant difference in amiloride-sensitive ΔISC between controls in LB broth vehicle and PAO1 (3.6+/−3.0 vs. 3.0+/−0.6; p ≤ 0.001). Also, forskolin-stimulated anion transport was significantly greater in the control group compared with the PAO1-exposed RNE cultures (70.2+/−30.0 μA/cm2 vs. 40.3+/−10.6 μA/cm2; p ≤ 0.01), suggesting that PAO1 exposure induces a state of acquired (partial) CFTR dysfunction in rabbit nasal epithelium. The combined CFTR inhibitors (GlyH-101 and CFTR inh-172) led to a significantly larger inhibition in CFTR-mediated Cl− transport in the control group (40.3+/−20.3 vs. PAO1, 20.0+/−5.1; p ≤ 0.001). This also supports the observation that diminished forskolin-stimulated Cl− secretion is secondary to CFTR dysfunction.
Figure 2.
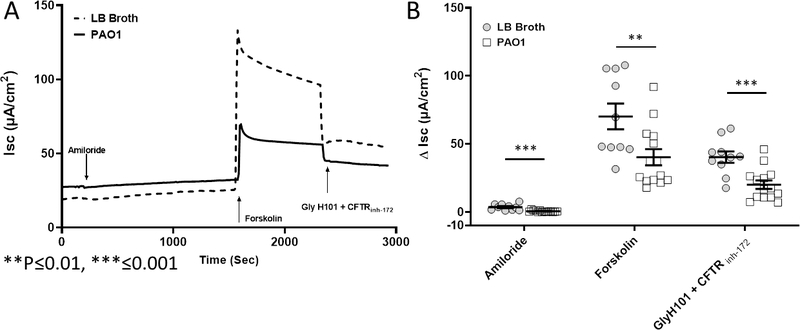
(A) RNE filters were exposed to PAO1 ultrafiltrate or vehicle control for 4 hours. Representative Ussing chamber tracings demonstrate reduced forskolin-dependent and CFTR-inhibited ISC. (B) Summary data demonstrates significant difference in amiloride-sensitive ΔISC, forskolin-dependent ΔISC, and CFTR inhibition. Forskolin-dependent and CFTR inhibited ΔISC were decreased by approximately 40% and 50%, respectively (n ≥ 10 per condition, p ≤ 0.01).
Ivacaftor restores mucociliary function in PAO1-induced acquired CFTR dysfunction
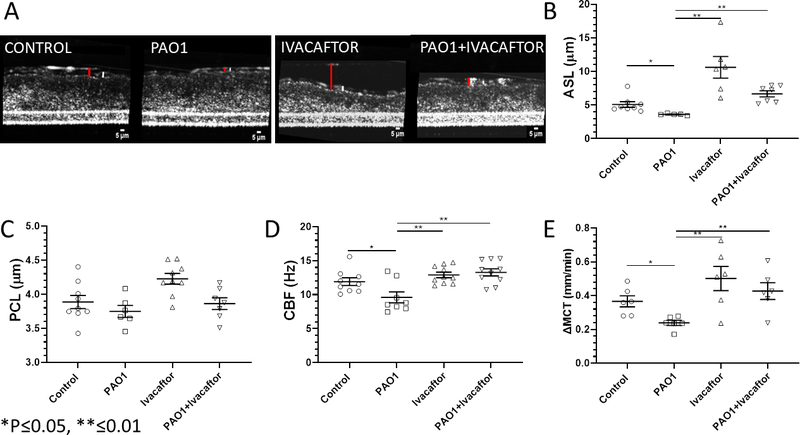
With evidence that ivacaftor stimulates CFTR-mediated Cl− secretion and a 4-hour PAO1 exposure significantly diminishes CFTR function, μOCT was used to assess the impact of PAO1 ultrafiltrate on ASL, PCL, CBF, and MCT; and whether ivacaftor could restore these markers of mucociliary function to normal levels (Figure 3). RNE cultures were exposed for 4 hours to an ultrafiltrate of PAO1 P. aeruginosa supernatant or control vehicle+/−ivacaftor. There were marked differences among groups (n ≥ 6 per all conditions) with significant inhibition of ASL depth (in μm), whereas ivacaftor enhanced ASL in both control and PA01 filters (PAO1, 3.6+/−0.1 vs. control, 5.0+/−0.4 vs. ivacaftor, 10.6+/−1.6 vs. ivacaftor+PAO1, 6.7+/−0.5; p ≤ 0.05 all conditions vs. PAO1). Interestingly, the impact on PCL was negligible and not significantly different among groups. Because ASL depth was so low in PAO1 cultures, ASL and PCL were indistinguishable during these measurements. Mean CBF (in Hz) was also significantly slower in PAO1 cultures (9.6+/−0.8 Hz) compared to all other conditions (control 11.9+/−0.6; ivacaftor 12.9+/−0.4; PAO1+ivacaftor 13.3+/−0.5; p ≤ 0.05 all conditions vs. PAO1) suggesting ivacaftor reestablishes CBF impacted by PAO1 exposure. Ivacaftor also restored MCT in RNE cultures after exposure to PAO1 (PAO1, 0.24+/−0.01 vs. control, 0.37+/−0.03 vs. ivacaftor, 0.50+/−0.07 vs. ivacaftor+PAO1, 0.43+/−0.05; p ≤ 0.05 all conditions vs. PAO1).
Figure 3.
RNE cultures were incubated with PAO1 ultrafiltrate, DMSO control vehicle, ivacaftor, or ivacaftor + PAO1. Selected μOCT images of the conditions (red bar – ASL, white bar – PCL) are shown in (A). (B) PAO1 significantly inhibited ASL depth (n ≥ 6, p ≤ 0.05) whereas ivacaftor restored ASL above levels observed in control and PAO1-incubated cultures. PCL depth (C) was not significantly different between groups. CBF (D) and MCT (E) significantly decreased in PAO1 exposed RNE (n ≥ 6, p ≤ 0.05), but were similar to controls when co-incubated with ivacaftor.
DISCUSSION
Developing a preclinical animal model and corresponding respiratory epithelial cultures that reflect the biologic characteristics of the human sinus is critical to the development and testing of novel CFTR-based therapeutic strategies for CRS. Recently, we developed a rabbit model of CRS that is consistent with findings in human CRS, including shifts in the microbiome to pathogenic microorganisms such as P. aeruginosa, significant infiltration of plasma cells and lymphocytes with increased submucosal glands, and decreased markers of mucociliary clearance.11 The rabbit sinus cavity is large, the nasal airways easily assessed with nasal endoscopy, and the in vivo rabbit CRS model is now established and well suited for therapeutic intervention studies. We evaluated the effect of ivacaftor on CFTR activity in the rabbit nasal cavities in vivo in the current study to afford additional evidence of the similarities between rabbit and human airway epithelium. Ivacaftor significantly activated CFTR-mediated Cl− transport in the nasal cavities of rabbits according to nasal potential difference measurements. These data reveal important molecular similarities between rabbit and human CFTR and highlight the shortcomings of rodent models that do not respond to this CFTR channel potentiator.19
In this study, PAO1 P. aeruginosa ultrafiltrate induced acquired CFTR dysfunction in cultured RNE, and thus has important implications for understanding impaired MCT in numerous upper and lower respiratory infectious illnesses. Measurements of functional airway microanatomy confirmed a corresponding diminishment of ASL depth and CBF. While the mechanisms underlying the PAO1-induced acquired CFTR dysfunction were not evaluated, P. aeruginosa produces a number of inflammatory factors including pyocyanin and lipopolysaccharide that can trigger changes to respiratory epithelial cell function – primarily through endoplasmic reticulum stress and subsequent activation of the unfolded protein response.47 We recently identified lipopolysaccharide as a major perturbation that causes diminished transepithelial Cl− transport and mucociliary function through generation of reactive oxygen species in vitro and in mice in vivo.12 Thus, in the setting of chronic P. aeruginosa bacterial infection in non-CF CRS, restoration of impaired CFTR function with CFTR potentiators represents an important approach to therapy.
Remarkably, we demonstrated that ivacaftor can reverse PAO1-induced CFTR dysfunction and restore functional parameters of MCT in RNE. These findings can be readily adopted to support the evaluation of the drug in the preclinical rabbit model of rhinosinusitis and human clinical trials. Considering the limited medicinal options for CRS, this strategy will provide a new and important intervention, but also a novel therapeutic model for studying other CFTR potentiators currently being developed as part of basic and preclinical investigation. There is also a narrow patient population who now receive benefit from ivacaftor. Therefore, repurposing ivacaftor for therapy of CRS will broaden the use of the treatment to millions of individuals with a serious and debilitating chronic disease.
CONCLUSION
Ivacaftor robustly stimulates CFTR-mediated Cl− secretion in RNE cultures and normalized MCT and other markers of mucociliary function in PAO1-induced acquired CFTR dysfunction. Findings should translate well to testing of ivacaftor in the rabbit model of CRS and provides a basis for targeting CFTR as an innovative therapeutic approach for acquired CFTR dysfunction in CRS.
Funding Support:
This work was supported by the National Institutes of Health (NIH)/National Institutes of Allergy and Infectious disease (K08AI146220-02) to D.Y.C.; NIH/National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-03) to S.M.R.; and NIH/NHLBI (R01 HL133006-05 and K08HL107142-05) to B.A.W.
Footnotes
Disclosures: All authors have read and approved the manuscript. Dr. Bradford A. Woodworth is a consultant for Cook Medical and Smith and Nephew.
Level of Evidence: NA.
REFERENCES
- 1.Lowery AS, Gallant JN, Woodworth BA, et al. Chronic rhino-sinusitis treatment in children with cystic fibrosis: A cross-sectional survey of pediatric pulmonologists and otolaryngologists. International journal of pediatric otorhinolaryngology. 2019;124:139–142 [DOI] [PubMed] [Google Scholar]
- 2.Karanth TK, Karanth V, Ward BK, Woodworth BA, Karanth L. Medical interventions for chronic rhinosinusitis in cystic fibrosis. The Cochrane database of systematic reviews. 2019;10:CD0129796805252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipirneni KE, Woodworth BA. Medical and Surgical Advancements in the Management of Cystic Fibrosis Chronic Rhinosinusitis. Current otorhinolaryngology reports. 2017;5(1):24–345626435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon GM, Frederick C, Zhang S, et al. IP-10 is a potential biomarker of cystic fibrosis acute pulmonary exacerbations. PloS one. 2013;8(8):e723983745468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormick JP, Thompson HM, Cho DY, Woodworth BA, Grayson JW. Phenotypes in Chronic Rhinosinusitis. Curr Allergy Asthma Rep. 2020;20(7):20. [DOI] [PubMed] [Google Scholar]
- 6.Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Current opinion in pulmonary medicine. 2014;20(6):623–6314301682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tipirneni KE, Zhang S, Cho DY, et al. Submucosal gland mucus strand velocity is decreased in chronic rhinosinusitis. International forum of allergy & rhinology. 2018;8(4):509–5126520985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks C, Freeman L, Cho DY, Woodworth BA. Acquired cystic fibrosis transmembrane conductance regulator dysfunction. World journal of otorhinolaryngology - head and neck surgery. 2018;4(3):193–1996251951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho DY, Woodworth BA. Acquired Cystic Fibrosis Transmembrane Conductance Regulator Deficiency. Advances in oto-rhino-laryngology. 2016;79:78–85 [DOI] [PubMed] [Google Scholar]
- 10.Wu X, Peters-Hall JR, Ghimbovschi S, Mimms R, Rose MC, Pena MT. Glandular gene expression of sinus mucosa in chronic rhinosinusitis with and without cystic fibrosis. American journal of respiratory cell and molecular biology. 2011;45(3):525–533PMC3175585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho DY, Mackey C, Van Der Pol WJ, et al. Sinus Microanatomy and Microbiota in a Rabbit Model of Rhinosinusitis. Frontiers in cellular and infection microbiology. 2017;7:5405770360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho DY, Zhang S, Lazrak A, et al. LPS decreases CFTR open probability and mucociliary transport through generation of reactive oxygen species. Redox Biol. 2021;43:101998.PMC8129928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodworth BA. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. The Laryngoscope. 2015;125 Suppl 7:S1–S134579062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blount A, Zhang S, Chestnut M, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. The Laryngoscope. 2011;121(9):1929–19343205413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virgin FW, Azbell C, Schuster D, et al. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. The Laryngoscope. 2010;120(7):1465–14693763501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander NS, Blount A, Zhang S, et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. The Laryngoscope. 2012;122(6):1193–11973548450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Londino JD, Lazrak A, Noah JW, et al. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(7):2712–27254478808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tipirneni KE, Grayson JW, Zhang S, et al. Assessment of acquired mucociliary clearance defects using micro-optical coherence tomography. International forum of allergy & rhinology. 2017;7(9):920–9255671640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18825–18830PMC2773991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nature reviews Disease primers. 2015;1:150107041544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. The New England journal of medicine. 2010;363(21):1991–20033148255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson HM, Lim DJ, Banks C, et al. Antibiotic eluting sinus stents. Laryngoscope Investig Otolaryngol. 2020;5(4):598–607PMC7444760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim DJ, McCormick J, Skinner D, et al. Controlled delivery of ciprofloxacin and ivacaftor via sinus stent in a preclinical model of Pseudomonas sinusitis. International forum of allergy & rhinology. 2020;10(4):481–488PMC7182488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho DY, Zhang S, Lazrak A, et al. Resveratrol and ivacaftor are additive G551D CFTR-channel potentiators: therapeutic implications for cystic fibrosis sinus disease. International forum of allergy & rhinology. 2019;9(1):100–1056318032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho DY, Lim DJ, Mackey C, et al. In-vitro evaluation of a ciprofloxacin- and ivacaftor-coated sinus stent against Pseudomonas aeruginosa biofilms. International forum of allergy & rhinology. 2019;9(5):486–4926491263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho DY, Lim DJ, Mackey C, et al. Ivacaftor, a Cystic Fibrosis Transmembrane Conductance Regulator Potentiator, Enhances Ciprofloxacin Activity Against Pseudomonas aeruginosa. American journal of rhinology & allergy. 2019;33(2):129–1366728449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho DY, Lim DJ, Mackey C, et al. l-Methionine anti-biofilm activity against Pseudomonas aeruginosa is enhanced by the cystic fibrosis transmembrane conductance regulator potentiator, ivacaftor. International forum of allergy & rhinology. 2018;8(5):577–5835918150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick J, Cho DY, Lampkin B, et al. Ivacaftor improves rhinologic, psychologic, and sleep-related quality of life in G551D cystic fibrosis patients. International forum of allergy & rhinology. 2019;9(3):292–2976520980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birket SE, Davis JM, Fernandez-Petty CM, et al. Ivacaftor Reverses Airway Mucus Abnormalities in a Rat Model Harboring a Humanized G551D-CFTR. American journal of respiratory and critical care medicine. 2020;202(9):1271–12827605185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho DY, Skinner D, Hunter RC, et al. Contribution of Short Chain Fatty Acids to the Growth of Pseudomonas aeruginosa in Rhinosinusitis. Frontiers in cellular and infection microbiology. 2020;10:412PMC7431473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho DY, Skinner D, Mackey C, et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. International forum of allergy & rhinology. 2019;9(6):629–6376555666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho DY, Lim DJ, Mackey C, et al. Preclinical therapeutic efficacy of the ciprofloxacin-eluting sinus stent for Pseudomonas aeruginosa sinusitis. International forum of allergy & rhinology. 2018;8(4):482–4895880690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho DY, Hoffman K, Skinner D, et al. Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model. International forum of allergy & rhinology. 2017;7(4):352–3585514418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander NS, Hatch N, Zhang S, et al. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. The Laryngoscope. 2011;121(6):1313–13193100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodworth BA, Zhang S, Tamashiro E, Bhargave G, Palmer JN, Cohen NA. Zinc increases ciliary beat frequency in a calcium-dependent manner. American journal of rhinology & allergy. 2010;24(1):6–10 [DOI] [PubMed] [Google Scholar]
- 36.Bhargave G, Woodworth BA, Xiong G, Wolfe SG, Antunes MB, Cohen NA. Transient receptor potential vanilloid type 4 channel expression in chronic rhinosinusitis. American journal of rhinology. 2008;22(1):7–12 [DOI] [PubMed] [Google Scholar]
- 37.Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. American journal of rhinology. 2007;21(5):533–537 [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Blount AC, McNicholas CM, et al. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PloS one. 2013;8(11):e815893839872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conger BT, Zhang S, Skinner D, et al. Comparison of cystic fibrosis transmembrane conductance regulator (CFTR) and ciliary beat frequency activation by the CFTR Modulators Genistein, VRT-532, and UCCF-152 in primary sinonasal epithelial cultures. JAMA otolaryngology-- head & neck surgery. 2013;139(8):822–8273933974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator-mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. American journal of rhinology & allergy. 2011;25(5):307–3123584334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azbell C, Zhang S, Skinner D, Fortenberry J, Sorscher EJ, Woodworth BA. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2010;143(3):397–4043073343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Fortenberry JA, Cohen NA, Sorscher EJ, Woodworth BA. Comparison of vectorial ion transport in primary murine airway and human sinonasal air-liquid interface cultures, models for studies of cystic fibrosis, and other airway diseases. American journal of rhinology & allergy. 2009;23(2):149–152 [DOI] [PubMed] [Google Scholar]
- 43.Dean N, Ranganath NK, Jones B, et al. Porcine nasal epithelial cultures for studies of cystic fibrosis sinusitis. International forum of allergy & rhinology. 2014;4(7):565–5704088356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Virgin F, Zhang S, Schuster D, et al. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. The Laryngoscope. 2010;120(5):1051–1056 [DOI] [PubMed] [Google Scholar]
- 45.Leung HM, Birket SE, Hyun C, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Science translational medicine. 2019;11(504)6886258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PloS one. 2013;8(1):e54473PMC3553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van ‘t Wout EF, van Schadewijk A, van Boxtel R, et al. Virulence Factors of Pseudomonas aeruginosa Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells. PLoS Pathog. 2015;11(6):e1004946PMC4471080. [DOI] [PMC free article] [PubMed] [Google Scholar]