Key Teaching Points.
-
•
Atrial tachyarrhythmias are common in patients with pulmonary hypertension, but there are limited data to inform rhythm control and catheter ablation strategies in this population.
-
•
Catheter ablation may serve as a meaningful component of the management strategy for atrial tachyarrhythmias in patients with pulmonary hypertension.
-
•
Special considerations must be made for pulmonary hypertension patients regarding anesthesia and periprocedural management.
Introduction
In patients with pulmonary hypertension (PH), there is a high incidence of atrial tachyarrhythmias (ATAs), reported to be 10%–25%.1, 2, 3 Observational studies have shown an association between the development of ATAs and worsening heart failure or death in patients with primary PH.1,2 For these reasons, ESC/ERS guidelines for the management of PH promote a rhythm control strategy with cardioversion and antiarrhythmic drugs for some PH patients with ATAs; however, current catheter ablation (CA) guidelines provide limited guidance regarding ablation of ATAs in PH patients.4, 5, 6 Further, the existing literature pertains almost exclusively to the ablation of typical right atrial flutter (AFL) (Table 1), with a scarcity of data related to left atrial (LA) ablation.1,7 Existing reports are also lacking in procedural details and longitudinal follow-up, with limited exploration of the risks and benefits of CA. Accordingly, we present the first case series describing patients with a PH diagnosis invasively confirmed by right heart catheterization (RHC) who were treated with LA ablation for the management of ATAs.
Table 1.
Published cohorts of catheter ablation of atrial tachyarrhythmias in pulmonary hypertension
| Author (year) | Cohort | Intervention | Outcomes |
|---|---|---|---|
| Showkathali et al (2011) | 22 patients with PH and typical AFL | Typical AFL ablation | 100% procedural success 14% recurrence at 3 months |
| Luesebrink et al (2012) | 38 patients with PH and typical AFL | Typical AFL ablation | 100% procedural success |
| Bradfield et al (2012) | 12 patients with PH and typical AFL | Typical AFL ablation | 86% procedural success 7% procedural death 50% recurrence at 3 months |
| Kanmanthareddy et al. (2014) | 43 patients with PH and ATAs | Typical AFL ablation (n = 20) AF ablation (n = 10) AVN ablation (n = 13) |
Procedural success not reported 85% recurrence one year after AFL ablation 100% recurrence one year after AF ablation |
| Bandorski et al (2014) | 32 patients with PH and ATAs | AFL ablation (n = 14) AT ablation (n = 13) AVN ablation (n = 1) AVNRT ablation (n = 4) |
86% procedural success with AFL ablation 31% procedural success with AT ablation 100% procedural success with AVNRT ablation |
| Małaczyńska-Rajpold et al (2016) | 7 patients with PH and ATAs | Typical AFL ablation (n = 2) AT ablation (n = 8) |
100% procedural success with AFL ablation 63% procedural success with AT ablation |
AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia; ATA = atrial tachyarrhythmia; AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; PH = pulmonary hypertension.
Methods
Cases
All patients undergoing CA of ATAs at our institution are encouraged to participate in a prospectively maintained registry. Similarly, all patients who follow longitudinally in our Pulmonary Hypertension Clinic are encouraged to participate in a prospectively maintained registry. Using these 2 registries, we performed a retrospective review of patients with a diagnosis of PH invasively confirmed by RHC who underwent LA ablations for ATAs at our center between 2003 and 2015. Data related to clinical history, imaging characteristics, ablation findings and strategies, and clinical outcomes were collected. Both registries comply with the Declaration of Helsinki and were approved by the Johns Hopkins University Institutional Review Board. Informed consent was obtained from all participants.
Definitions
Only patients with PH invasively confirmed by RHC who underwent LA ablations for ATAs were included. We used criteria for PH as defined by the 2018 World Symposium on Pulmonary Hypertension, which requires both mean pulmonary artery pressure >20 mm Hg and pulmonary vascular resistance >3 Wood units to represent pulmonary vascular disease.8 Recurrence of ATAs was defined in relation to the standard 3-month recovery period following ablation.5 Early recurrence is that which occurs 0–3 months after ablation. Recurrence is defined as that which occurs 3–12 months after ablation, and late recurrence is defined as occurring >12 months after ablation.
Cases report
Case 1
Patient 1 had a history of obstructive sleep apnea treated with continuous positive airway pressure and was diagnosed with persistent atrial fibrillation (AF) at the age of 70 when she presented to the emergency department with palpitations and dyspnea (Table 2). During the following 5 years, she underwent 8 cardioversions for AF and was started on amiodarone, as she initially expressed a desire to avoid CA. She was referred to the Johns Hopkins Pulmonary Hypertension Clinic owing to transthoracic echocardiogram with estimated right ventricular systolic pressure of 75 mm Hg. She underwent RHC, which confirmed PH (Table 3).
Table 2.
Characteristics of left atrial tachyarrhythmias
| Characteristic | Patient |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age at first left atrial ablation | 76 | 49 | 43 |
| Atrial fibrillation | Yes | No | Yes |
| Atrial flutter | No | Yes | Yes |
| Atrial tachycardia | No | Yes | No |
| Atrial fibrillation type | Persistent | - | Paroxysmal |
| Time from onset to ablation | 5 years | 4 years | 5 years |
Table 3.
Characteristics of pulmonary hypertension as confirmed by right heart catheterization
| Characteristic | Patient |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| WHO group for classification of PH | III | III | V |
| Right heart catheterization measurements | |||
| RAP (mm Hg) | 5 | 6 | 10 |
| mPAP (mm Hg) | 40 | 31 | 51 |
| PCWP (mm Hg) | 18 | 10 | 14 |
| CI (L/min/m2) | 3.3 | 2.0 | 3.6 |
| PVR (Wood units) | 3.9 | 6.0 | 3.9 |
| Pulmonary vasodilatory therapy | |||
| PDE-5 inhibitor | Sildenafil | Sildenafil | Sildenafil |
CI = cardiac index; mPAP = mean pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PDE-5 = phosphodiesterase-5; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; RAP = right atrial pressure; WHO = World Health Organization.
Five years after her AF diagnosis, she opted to undergo CA, which consisted of successful radiofrequency (RF) pulmonary vein isolation (PVI) with additional roof, posterior wall, and mitral isthmus lesions. During voltage mapping, it was apparent that the patient had extensive LA scarring. At her 3-month follow-up appointment, she was experiencing frequent episodes of symptomatic AF, and was directly admitted for initiation of dofetilide. Holter monitoring at 7 months postablation showed normal sinus rhythm without AF, while Holter monitoring at 10 months postablation captured recurrent paroxysmal AF. Because this represented overall improvement, dofetilide was continued. Two years after her ablation, Holter monitoring revealed 100% AF, and dofetilide was stopped. Repeat ablation was deferred owing to extensive LA scarring and patient preference. She was managed thereafter with metoprolol for rate control. Her PH was treated with sildenafil.
Case 2
Patient 2, with a history of scleroderma, was 45 years old when she was diagnosed with AFL with variable block on Holter monitoring (Table 2). The scleroderma caused group III PH owing to restrictive lung disease, as confirmed by RHC (Table 3).
Because her dyspnea worsened without progression of her lung disease, symptoms were attributed to AFL, and the decision was made to proceed with CA. During the procedure, she was found to have cavotricuspid isthmus (CTI)-dependent AFL, which was successfully ablated. Two microreentrant left atrial tachycardias (ATs) were also identified, 1 on the roof outside the left common pulmonary vein (PV) ostium and the other located just inferior to the left inferior PV ostium. The ATs were successfully ablated with RF energy.
She experienced early recurrence of AT and underwent repeat ablation 3 months later. She was found to have a focal AT arising from the superior aspect of the ridge between the LA appendage and the left common PV. RF ablation was successful. Persistent bidirectional block at the CTI was confirmed from the previous ablation. Her response to the second ablation was durable, and she remained arrhythmia free for 5 years until ultimately dying of complications of right heart failure. Her PH was managed with sildenafil.
Case 3
Patient 3 was referred for arrhythmia management at age 43 owing to worsening symptoms from paroxysmal AF and AFL (Table 2). He was born with a double-inlet single ventricle, L-transposition of the great arteries, and parachute mitral valve requiring pulmonary artery banding in infancy. He subsequently underwent creation of an atrial septal defect to relieve his left atrioventricular valve obstruction along with a right-sided maze procedure and tightening of the pulmonary artery band. At the time of referral, he was managed medically with propafenone, dofetilide, and digoxin, and had required multiple cardioversions. RHC revealed findings consistent with PH (Table 3).
His first 2 CAs were unsuccessful. During the first, he was found to have both CTI-dependent AFL and atypical AFL with concealed entrainment at several locations in the left atrium. Linear RF lesions were created at the CTI and at a small isthmus of tissue in the left atrium for the treatment of typical and atypical AFL. At his second procedure, bidirectional block across the prior CTI line was confirmed, and mapping of the AFL demonstrated concealed entrainment from several sites in the left atrium, where RF ablation was performed without termination of the arrhythmia.
The patient continued to have AFL alternating with more frequent AF on Holter monitoring and underwent a third CA with successful RF PVI. Owing to a macroreentrant AFL discovered traversing both right and left atrium, RF lesions were subsequently applied to the CTI, resulting in termination of the arrhythmia. The patient remained in sinus rhythm for approximately 4 years based on symptoms and in-office ECGs.
Four years after the third CA, he had recurrence of AF and underwent cardioversion followed by a fourth CA. The patient was found to have evidence of PV potentials, and RF PVI was successfully performed. There was also the finding of atypical AFL involving the left-sided PVs, where additional lesions were placed. After 11 months, the patient had recurrent paroxysmal AF and underwent a final successful PVI, with both cryoballoon and RF energy. AF recurred 2 years later, and he is currently managed medically with dofetilide. His PH is managed with sildenafil.
Discussion
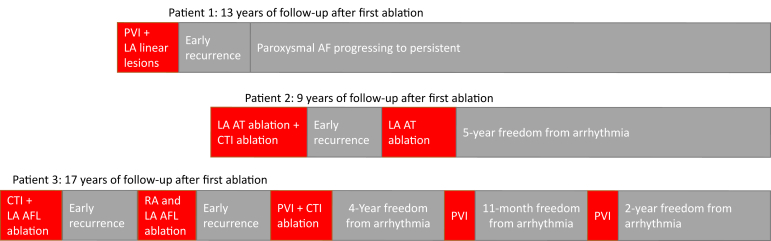
To our knowledge, this is the first case series detailing LA ablation procedures for the management of ATAs among patients with a diagnosis of PH confirmed by invasive measurements. All patients had elevated pulmonary vascular resistance in addition to elevated pulmonary artery pressures, identifying them as a distinct high-risk group with pulmonary vascular disease on vasodilator therapy with sildenafil. The patients presented with diverse etiologies of PH, as is expected in clinical practice. Our experience indicates that recurrence of ATAs is common in PH patients after LA ablation (Figure 1). In our small series, no procedural complications were observed, acute procedural success was common, and long-term freedom from arrhythmia was achieved in 2 of 3 cases.
Figure 1.
Outcomes and follow-up for patients with pulmonary hypertension receiving left atrial ablation. AF = atrial fibrillation; AFL = atrial flutter; AT = atrial tachycardia; CTI = cavotricuspid isthmus; LA = left atrial; PVI = pulmonary vein isolation; RA = right atrial.
Despite the established relationship between PH and ATAs, there are limited data to inform management. There may be hesitation to treat these patients with CA owing to concerns about procedural safety and recurrence of ATAs in this potentially high-risk population. At our center, periprocedural anesthesia care is provided by the cardiac anesthesiology service. Patients with PH are particularly susceptible to right ventricular ischemia if hypotensive. Maintenance of adequate systemic arterial pressure, and specifically diastolic pressure, in the setting of chronic PH and resultant right ventricle hypertrophy is critical to the successful perioperative management of these patients. This can usually be accomplished with the judicious use of intravenous vasopressors. In addition, it is essential to avoid stimuli, such as hypoxemia, hypercarbia, and acidosis, that are recognized to precipitate pulmonary vascular constriction with subsequent worsening of pulmonary vascular resistance.
The need for repeat procedures was a feature of our cohort. In the general population, recurrence rates and need for repeat procedures vary by arrhythmia type. For CA of AF, 12-month success rates have historically ranged from 59% to 89%.5 Following ablation of CTI-dependent AFL, 10% of patients may have recurrent AFL and up to 33% may develop AF.6 The need for repeat procedures in our cohort likely represents the complexity of the disease substrate in PH leading to multiple arrhythmias, particularly AF and atypical AFL.
Existing data regarding CA in PH pertain predominantly to typical AFL (Table 1) in pulmonary arterial hypertension, previously known as primary PH. Luesebrink and colleagues9 reported 100% acute procedural success in patients with PH and typical AFL but did not describe longitudinal outcomes. Bradfield and colleagues10 reported 86% acute success in PH patients receiving ablation for typical AFL, with 7% incidence of procedural death and only 50% arrhythmia-free survival at 3 months. Another group reported 100% procedural success after typical AFL ablation and 14% recurrence within 3 months.11
PVI is mentioned in only a handful of studies of PH patients, but procedural details and longitudinal follow-up are lacking. This gap in knowledge is clinically significant because only a portion of ATAs in the PH population are typical AFL. Fingrova and colleagues7 found that, in a large cohort of pre–capillary PH patients, 66% of ATAs were AF, suggesting that the left atrium is heavily involved in arrhythmogenesis, even in a select subgroup of PH patients with pulmonary capillary wedge pressure <15 mm Hg, who are expected to have less LA remodeling. Unfortunately, ablation outcomes were not reported in their series. Kanmanthareddy and colleagues,12 in their study of ATAs in PH patients, included 10 patients who underwent AF ablation; however, procedural details were not provided. Of note, all 10 of those patients had recurrence within 1 year. Two studies have described AT ablations, with 1 reporting 31% procedural success and the other reporting 63% procedural success.13,14
In our series, all patients had RHC-confirmed PH, based on elevated pulmonary vascular resistance in addition to elevated pulmonary artery pressures. In our high-risk group of patients, there were 8 LA ablation procedures, predominantly for AF and atypical AFL, and involving both PVI and linear lesions. Acute procedural success was achieved in 6 of 8 (75%) cases. Periods of long-term freedom from arrhythmia were achieved in 2 cases, though 1 patient with complex congenital heart disease required 5 ablations. Our findings are limited by a small number of patients. One reason for the small sample is that we used strict consensus definitions, including mean pulmonary artery pressure >20 mm Hg and pulmonary vascular resistance >3 Wood units to identify pulmonary vascular disease not simply reflecting left heart failure.8 Additionally, owing to the inherent limitations of a retrospective investigation, patients meeting these inclusion criteria may have been inadvertently missed. Larger, prospective studies are needed to comprehensively characterize the safety and efficacy of LA ablation for the management of ATAs in patients with PH.
In conclusion, our findings indicate that, despite the complexity of atrial arrhythmias in patients with invasively confirmed PH, CA may serve as a meaningful component of the management strategy of ATAs in this patient population at experienced centers.
Footnotes
Funding Support: Funding for this research was provided in part by the Edward St. John Fund for AF Research, the Roz and Marvin H. Weiner and Family Foundation, the Dr Francis P. Chiaramonte Foundation, the Marilyn and Christian Poindexter Arrhythmia Research Fund, Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund, and the Mr & Mrs Larry Small AF Research Fund.
Disclosures: SCM has relationships with Actelion (clinical trial steering committee), United Therapeutics (data safety and monitoring board, consulting), and Theravance (consulting). The authors have no other conflicts of interest to declare.
References
- 1.Mercurio V., Peloquin G., Bourji K.I., et al. Pulmonary arterial hypertension and atrial arrhythmias: incidence, risk factors, and clinical impact. Pulm Circ. 2018;8 doi: 10.1177/2045894018769874. 2045894018769874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsson K.M., Nickel N.P., Tongers J., Hoeper M.M. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol. 2013;167:2300–2305. doi: 10.1016/j.ijcard.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Tongers J., Schwerdtfeger B., Klein G., et al. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007;153:127–132. doi: 10.1016/j.ahj.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N., Humbert M., Vachiery J., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. doi: 10.1093/europace/eux274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page R.L., Joglar J.A., Caldwell M.A., et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471–e505. doi: 10.1161/CIR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 7.Fingrova Z., Havranek S., Ambroz D., Jansa P., Linhart A. The left atrial substrate plays a significant role in the development of complex atrial tachycardia in patients with precapillary pulmonary hypertension. BMC Cardiovasc Disord. 2019;19:157. doi: 10.1186/s12872-019-1142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonneau G., Montani D., Celermajer D.S., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luesebrink U., Fischer D., Gezgin F., et al. Ablation of typical right atrial flutter in patients with pulmonary hypertension. Heart Lung Circ. 2012;21:695–699. doi: 10.1016/j.hlc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Bradfield J., Shapiro S., Finch W., et al. Catheter ablation of typical atrial flutter in severe pulmonary hypertension. J Cardiovasc Electrophysiol. 2012;23:1185–1190. doi: 10.1111/j.1540-8167.2012.02387.x. [DOI] [PubMed] [Google Scholar]
- 11.Showkathali R., Tayebjee M.H., Grapsa J., et al. Right atrial flutter isthmus ablation is feasible and results in acute clinical improvement in patients with persistent atrial flutter and severe pulmonary arterial hypertension. Int J Cardiol. 2011;149:279–280. doi: 10.1016/j.ijcard.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 12.Kanmanthareddy A., Reddy Y.M., Boolani H., et al. Incidence, predictors, and clinical course of atrial tachyarrhythmias in patients with pulmonary hypertension. J Interv Card Electrophysiol. 2014;41:9–14. doi: 10.1007/s10840-014-9928-5. [DOI] [PubMed] [Google Scholar]
- 13.Bandorski D., Schmitt J., Kurzlechner C., et al. Electrophysiological studies in patients with pulmonary hypertension: a retrospective investigation. Biomed Res Int. 2014;2014:617565. doi: 10.1155/2014/617565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malaczynska-Rajpold K., Komosa A., Blaszyk K., et al. The management of supraventricular tachyarrhythmias in patients with pulmonary arterial hypertension. Heart Lung Circ. 2016;25:442–450. doi: 10.1016/j.hlc.2015.10.008. [DOI] [PubMed] [Google Scholar]