Abstract
Bacteriocin production was determined for 218 Enterococcus isolates (Enterococcus faecalis [93] and E. faecium [125]) obtained from different origins (human clinical samples [87], human fecal samples [78], sewage [28], and chicken samples [25]) and showing different vancomycin susceptibility patterns (vancomycin resistant, all of them vanA positive [56], and vancomycin susceptible [162]). All enterococcal isolates were randomly selected except for the vancomycin-resistant ones. A total of 33 isolates of eight different bacterial genera were used as indicators for bacteriocin production. Forty-seven percent of the analyzed enterococcal isolates were bacteriocin producers (80.6% of E. faecalis and 21.6% of E. faecium isolates). The percentage of bacteriocin producers was higher among human clinical isolates (63.2%, 81.8% of vancomycin-resistant isolates and 60.5% of vancomycin-susceptible ones) than among isolates from the other origins (28 to 39.3%). Only one out of the 15 vancomycin-resistant isolates from human fecal samples was a bacteriocin producer, while 44.4% of fecal vancomycin-susceptible isolates were. The bacteriocin produced by the vanA-containing E. faecium strain RC714, named bacteriocin RC714, was further characterized. This bacteriocin activity was cotransferred together with the vanA genetic determinant to E. faecalis strain JH2-2. Bacteriocin RC714 was purified to homogeneity and its primary structure was determined by amino acid sequencing, showing an identity of 88% and a similarity of 92% with the previously described bacteriocin 31 from E. faecalis YI717. The presence of five different amino acids in bacteriocin RC714 suggest that this could be a new bacteriocin. The results obtained suggest that the epidemiology of vancomycin resistance may be influenced by different factors, including bacteriocin production.
Organisms of the genus Enterococcus, and in particular Enterococcus faecium, have become a significant cause of nosocomial infections and usually show multiple drug resistance (45). Resistance to the most commonly used antibiotics for gram-positive bacteria provides these organisms with a selective advantage in the hospital environment (40, 43). In Europe, a number of studies have documented the spread of vancomycin-resistant (Vanr) enterococci in sewage, food, animals, and human fecal samples taken from healthy volunteers (1, 5, 8, 17, 37, 38, 49, 54, 56, 57). Paradoxically, in Europe there is a low incidence of Vanr enterococci among human clinical isolates compared with the United States (45, 55, 58). The factors involved in these epidemiological differences remain unknown.
Bacteriocins are peptides or proteins produced by different bacteria that inhibit the growth of strains and species usually related to bacteriocin-producing bacteria (31). The ability to produce bacteriocins has been shown to confer an ecological advantage (48). In the genus Enterococcus, bacteriocin production has been linked to the same genetic determinant as β-hemolysin synthesis (4, 7, 13, 27, 30), and its production is a pathogenic marker (30, 39). In E. faecalis, the best-characterized inhibitor substances are the pAD1-encoded bacteriocin-hemolysin (or cytolysin) (50, 52) and the peptide AS-48 (24, 26), both encoded by transferable plasmids (3). Other bacteriocins have been characterized in E. faecalis (bacteriocin 31, encoded by a conjugative plasmid [53]) or in E. faecium (enterocin A [2], enterocin I [22], enterocin P [11], enterocin L50A/L50B [12], and enterocin B [9]). A number of less-characterized bacteriocins in Enterococcus spp. have been reported (enterocin EFS2 [41], enterocin 1146 [47], and enterocin 900 [23], among others). The cotransference of bacteriocin production and the pheromone response, together with antibiotic resistance, have been described for Enterococcus strains (28, 39). The purpose of this study was to determine the relationship of bacteriocin production and vancomycin resistance in Enterococcus isolates of different species and origins.
(Part of this work was presented previously [R. del Campo et al., Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C95, 1998].)
MATERIALS AND METHODS
Bacterial isolates and media.
This study included 218 Enterococcus isolates (93 E. faecalis and 125 E. faecium) with different vancomycin susceptibility patterns (162 vancomycin susceptible [Vans] and 56 Vanr) and from different origins (87 human clinical samples, 78 human fecal samples, 28 sewage samples, and 25 chicken samples) (Table 1). Enterococcal isolates from human clinical samples (blood, urine, wounds, etc.) corresponded to consecutive E. faecalis and E. faecium isolates obtained from different patients from San Millán Hospital, La Rioja, Spain (1996). Enterococcal isolates from human fecal samples were recovered from consecutive samples from in- and out-patients in Hospital Clínico, Zaragoza, Spain (1996). Fecal samples were seeded on M-Enterococcus agar (Biomérieux, La Balme, France), and one colony per plate was studied and retained if it belonged to the species E. faecalis or E. faecium. Identification was carried out by the API-20 Strept System (Biomérieux) and by PCR, using specific primers for E. faecium (16) and E. faecalis (18). Enterococcal isolates from chicken samples corresponded to those recovered from chicken feces or chicken products in the La Rioja area (Spain). All Vanr isolates were characterized as having a vanA genotype by PCRs (59) and were included in this study on the basis of vancomycin resistance. A total of 33 isolates of eight different bacterial genera (Enterococcus, Listeria, Staphylococcus, Lactobacillus, Leuconostoc, Pediococcus, Escherichia, and Bacillus) were used as bacteriocin production indicators (Table 2). These isolates were maintained as frozen stocks at −80°C in skim milk (Difco, Detroit, Mich.) and propagated twice in brain heart infusion agar (Difco), with the exception of the strains of the genera Lactobacillus, Leuconostoc, and Pediococcus, which were grown in Man, Rogosa, & Sharpe (MRS) agar (Biomérieux).
TABLE 1.
Enterococcus isolates used for the screening of bacteriocin production
| Species (no.) | Origin (no.) | Phenotype (no.) |
|---|---|---|
| E. faecalis (93) | Human clinical samples (57) | Vanr (8) |
| Vans (49) | ||
| Human fecal samples (19) | Vanr (2) | |
| Vans (17) | ||
| Sewage (10) | Vans (10) | |
| Chicken samples (7) | Vanr (7) | |
| E. faecium (125) | Human clinical samples (30) | Vanr (3) |
| Vans (27) | ||
| Human fecal samples (59) | Vanr (13) | |
| Vans (46) | ||
| Sewage (18) | Vanr (5) | |
| Vans (13) | ||
| Chicken samples (18) | Vanr (18) |
TABLE 2.
Bacterial isolates used as indicators for the screening of bacteriocin production
| Isolate | Resistance phenotypea | Origin/source or referenceb |
|---|---|---|
| E. faecium AR1 | Van (vanA), Ery, Str, Kan | Sewage/LC |
| E. hirae P9 | Van (vanA), Ery | Chicken feces/LC |
| E. faecalis H1 | Van (vanA), Ery | Human feces/LC |
| E. faecium 517 | Van (vanA), Ery, Str, Kan | Human feces/LC |
| E. gallinarum CECT970 | Van (vanC1) | Chicken feces/CECT |
| E. faecalis SF-299 | Van (vanB) | Gold et al. (25) |
| E. faecalis AR4 | Str | Sewage/LC |
| E. faecalis AR6 | Ery, Str, Kan | Sewage/LC |
| E. faecalis AR8 | Sewage/LC | |
| E. faecium AR9 | Ery, Str | Sewage/LC |
| E. faecalis AR13 | Sewage/LC | |
| E. faecium AR18 | Ery, Str, Kan | Sewage/LC |
| E. faecium AR24 | Sewage/LC | |
| E. faecalis AR30 | Ery, Gen, Tob, Kan | Sewage/LC |
| E. faecium AR39 | Str, Kan | Sewage/LC |
| E. faecalis AR42 | Ery, Str, Kan | Sewage/LC |
| E. faecalis AR43 | Ery, Gen, Tob, Kan, Str | Sewage/LC |
| E. faecium AR50 | Ery | Sewage/LC |
| E. faecium AR58 | Ery, Str, Kan | Sewage/LC |
| E. faecalis AR69 | Str | Sewage/LC |
| S. epidermidis S3 | Human clinical sample/LC | |
| S. aureus M892 | Human clinical sample/LC | |
| S. haemolyticus S13 | Human clinical sample/LC | |
| E. coli C228 | Human clinical sample/LC | |
| L. monocytogenes CECT4032 | Food/CECT | |
| L. innocua CECT910 | Animal clinical sample/CECT | |
| L. murrayi CECT942 | Food/CECT | |
| L. grayi CECT931 | Animal feces/CECT | |
| B. subtilis CECT356 | Unknown/CECT | |
| L. paracasei C162 | Van | Human clinical sample/LC |
| L. plantarum C193 | Van | Human clinical sample/LC |
| Leuconostoc sp. C214 | Van | Human clinical sample/LC |
| P. pentosaceus AR63 | Van | Sewage/LC |
Van, vancomycin; Ery, erythromycin; Gen, gentamicin; Tob, tobramycin; Kan, kanamycin; Str, streptomycin. Isolates showed resistance to all the antibiotics listed.
LC, Laboratory collection of the University of La Rioja (Spain); CECT, Spanish Culture Type Collection.
Bacteriocin and β-hemolysin assays.
For qualitative bacteriocin detection, 50 μl of an overnight culture of the indicator isolate was added to 5 ml of molten soft tryptic soy broth (Difco) supplemented with 0.5% yeast extract and 0.7% agar (Difco), mixed, and poured onto a yeast extract-supplemented tryptic soy agar plate (Difco). A single colony of each Enterococcus isolate to be tested for bacteriocin production was transferred with a sterile toothpick to the agar plate seeded with the indicator. Plates were incubated at 37°C for 48 h and then observed for zones of inhibition around the strains. Isolates were considered bacteriocin producers (BAC+) when they showed activity (inhibition zone) against at least 1 of the 33 indicator isolates. This assay does not discriminate between single or multiple bacteriocin production. β-hemolysin detection was performed in tryptic soy agar medium containing 5% horse blood (Biomérieux). A clear zone of β-hemolysis around the isolate growth was considered a positive reaction.
Susceptibility testing and PCR determinations.
The antibiotic resistance phenotype of the enterococcal isolates was determined by agar dilution following the NCCLS standard method (46). For AS-48 bacteriocin and enterocin I detection, PCRs were performed using primers and conditions as described in other studies (22, 36). The pAM401-61 plasmid containing an SphI-BglII fragment of the AS-48 genetic determinant was used as a positive control for AS-48 bacteriocin detection (kindly supplied by M. Martínez-Bueno); E. faecium 6T1a was used as a positive control for enterocin I (22).
Mating experiments.
The transferability of bacteriocin production, as well as Vanr and erythromycin resistance (Eryr) determinants was tested by conjugation using a filter method (19), with E. faecalis strain JH2-2 as recipient (rifampin and fusidic acid resistant, vancomycin and erythromycin susceptible, nonbacteriocin producer [Rifr, Fusr, Vans, Erys, BAC−]). All donor strains were Rifs and Fuss. Vancomycin-resistant transconjugants were first selected onto brain heart infusion agar plates supplemented with rifampin (100 μg/ml), fusidic acid (20 μg/ml), and vancomycin (20 μg/ml); bacteriocin production and Eryr were then analyzed in the Vanr transconjugants obtained.
Pulsed-field gel electrophoresis (PFGE).
Genomic DNA was prepared as previously described (44). A third part of the plug was digested with 10 U of SmaI (Amersham Life Science) for 18 h, and then an additional 10 U was added and the sample was left for another 4 h. Electrophoresis was then carried out (CHEF DR-II; Bio-Rad) in a 1.2% agarose gel with 0.5% Tris-borate-EDTA, and the following settings were applied: 5 to 35 s, 6 V/cm2, and 30 h. The gel was stained with ethidium bromide for UV observation. Isolates were classified as indistinguishable, closely related, possibly related, or different according to previously published criteria for bacterial strain typing (51).
Characterization of the bacteriocin produced by E. faecium RC714.
To perform a preliminary characterization of the bacteriocin activity from vanA-containing E. faecium RC714, a cell-free, filter-sterilized (0.22-μm-pore-size Millex-GV filter; Millipore SA, Molsheim, France), stationary-phase MRS culture supernatant was tested for stability to heat, pH, and proteolytic enzymes. To test for heat sensitivity, 1-ml samples were heated to 80, 90, and 100°C for 5, 10, and 20 min each. To test for pH sensitivity, 1-ml aliquots of active supernatants were adjusted to different pH values (3, 4, 5, 6, 7, 8, 9, 10, and 11) with 1 M NaOH or 0.6 M HCl. After the different treatments, the remaining bacteriocin activity was then tested by spotting a 25-μl aliquot on a plate seeded with E. faecium AR9 as the indicator strain. Plates were incubated at 37°C for 24 h and then observed for inhibition zones.
Active supernatants from E. faecium RC714 were tested for their susceptibility to the following proteolytic enzymes: trypsin, α-chemotrypsin, alkaline protease type XXI, proteinase K, papain, and lysozyme (Sigma, St. Louis, Mo.). All the enzymes (4 mg/ml) were prepared according to the manufacturer's instructions, and an aliquot of 750 μl of this solution was added to 250 μl of the active supernatants. The mixture was then incubated for 24 h, at 37°C for proteinase K, alkaline protease, or lysozyme and at 25°C for the other enzymes. In all cases, the remaining bacteriocin activity was determined by spotting 25 μl onto a plate seeded with the indicator E. faecium strain AR9, and then plates were incubated at 37°C for 24 h. Positive and negative controls were included.
Purification of the bacteriocin produced by E. faecium RC714.
All the purification steps were carried out at room temperature, and all of the chromatographic equipment and media were purchased from Pharmacia Biotech. Bacteriocin was purified from a 2-liter MRS broth culture of the vanA-containing E. faecium RC714 strain, following the method previously described for the bacteriocin plantaricin S (35). Briefly, the supernantant was ammonium sulfate precipitated, desalted through a PD10 column, consecutively applied to a cation-exchange and a hydrophobic interaction column, and finally subjected to C2/C18 reverse-phase chromatography. Fractions showing activity after the C2/C18 reverse-phase column step were pooled and subjected to a second run. Fractions from this second run showing inhibitory activity were stored at −80°C in 30% 2-propanol containing 0.1% trifluoroacetic acid until use.
SDS-PAGE.
C2/C18 reverse-phase column-purified bacteriocin RC714 was analyzed by sodium dodocyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (34) with an 18.5% acrylamide resolving gel. A molecular mass marker (range, 2,512 to 16,946 Da) kit (Pharmacia Biotech) was used for size standards. After electrophoresis, a gel was silver stained (42), and a similar gel was used for detection of antimicrobial activity (6) with E. faecium AR9 as the indicator strain.
N-terminal amino acid sequencing of bacteriocin RC714.
Amino acid sequencing was performed by automated Edman degradation with a Beckman LF3000 sequencer-phenylthiohydantoin amino acid analyzer (System Gold) by F. Canals, Institut de Biologia Fonamental “Vicent Villar Palasí”, Barcelona University, Barcelona, Spain.
RESULTS
Bacteriocin production in Enterococcus isolates.
One hundred and two out of 218 (46.8%) E. faecalis or E. faecium isolates were found to produce an antibacterial substance active against at least 1 of the 33 indicator isolates, thus being considered BAC+. Eighty percent of the E. faecalis isolates were BAC+, whereas only 21.6% of the E. faecium isolates were. The proportion of BAC+ isolates was significantly higher among isolates from human clinical samples (55 of 87 [63.2%]) than from those of human fecal samples (29 of 78 [37.2%]) (P = 0.00041) (Table 3). The frequencies of BAC+ isolates from other origins were as follows: chicken (7 of 25 [28%]) and sewage (11 of 28 [39.3%]). Among the isolates obtained from human clinical samples, Vanr Enterococcus isolates showed a higher proportion of BAC+ isolates (9 of 11 [81.8%]) than did Vans isolates (46 of 76 [60.5%]). This trend to higher bacteriocin production among Vanr isolates from human clinical samples was observed for both E. faecalis and E. faecium isolates (Table 4). However, only 1 out of the 15 (6.7%) vanA-containing Enterococcus isolates from human fecal samples (13 E. faecium and 2 E. faecalis) was BAC+ (E. faecalis H1), while 44.4% of human fecal Vans Enterococcus isolates were BAC+ (58.8% in E. faecalis and 39.1% in E. faecium).
TABLE 3.
Bacteriocin production in 218 Enterococcus isolates from different origins
| Origin | No. of isolates | No. of bacteriocin producers (%) |
|---|---|---|
| Human clinical samples | 87 | 55 (63.2) |
| Human fecal samples | 78 | 29 (37.2) |
| Sewage | 28 | 11 (39.3) |
| Chicken fecal samples | 25 | 7 (28) |
| Total | 218 | 102 (46.8) |
TABLE 4.
Bacteriocin production in Enterococcus isolates from different origins and with different vancomycin susceptibility patterns
| Origin (no. of isolates) | Resistance phenotype (no. of isolates) | No. of bacteriocin producers (%) | Species (no.) | No. of bacteriocin producers (%) |
|---|---|---|---|---|
| Human clinical samples (87) | Vanr (11) | 9 (81.8) | E. faecalis (8) | 8 (100) |
| E. faecium (3) | 1 (33.3) | |||
| Vans (76) | 46 (60.5) | E. faecalis (49) | 42 (85.7) | |
| E. faecium (27) | 4 (14.8) | |||
| Human fecal samples (78) | Vanr (15) | 1 (6.7) | E. faecalis (2) | 1 (50) |
| E. faecium (13) | 0 | |||
| Vans (63) | 28 (44.4) | E. faecalis (17) | 10 (58.8) | |
| E. faecium (46) | 18 (39.1) | |||
| Sewage (28) | Vanr (5) | 2 (40) | E. faecium (5) | 2 (40) |
| Vans (23) | 9 (39.1) | E. faecalis (10) | 8 (80) | |
| E. faecium (13) | 1 (7.7) | |||
| Chicken samples (25) | Vanr (25) | 7 (28) | E. faecalis (7) | 6 (85.7) |
| E. faecium (18) | 1 (5.5) | |||
| Total (218) | Vanr (56) | 19 (33.9) | E. faecalis (17) | 15 (88.2) |
| E. faecium (39) | 4 (10.2) | |||
| Vans (162) | 83 (51.2) | E. faecalis (76) | 60 (78.9) | |
| E. faecium (86) | 23 (26.7) |
Among the vanA-containing E. faecium isolates obtained from sewage, two of five were BAC+, whereas only 1 of 13 Vans E. faecium isolates was found to be BAC+. All 10 E. faecalis isolates from sewage were Vans, and 8 of them were BAC+. All 25 Enterococcus isolates studied from chicken samples were Vanr, and 7 of them were BAC+ (E. faecalis, 6 of 7; E. faecium, 1 of 18) (Table 4). β-hemolytic activity was detected in 9 of 32 Vans BAC+ clinical isolates. Interestingly, this activity was not observed in any of the BAC+ vanA-containing Enterococcus isolates.
These data show that there is a high prevalence of BAC+ isolates among vanA-containing E. faecalis or E. faecium from human clinical samples. To explore the possibility that a number of these isolates could correspond to widely disseminated clones, the PFGE patterns of all the BAC+ and vanA-containing E. faecalis (15 isolates) or E. faecium (4 isolates) strains from different origins included in this study were analyzed. Among the 15 E. faecalis isolates, seven different PFGE patterns were found, and five different patterns were detected among the 8 isolates from human clinical samples. A single pattern was found in four E. faecalis strains obtained from human clinical samples in distant geographic sites. All four BAC+ and vanA-containing E. faecium isolates corresponded to different PFGE patterns.
Spectrum of activity of bacteriocinogenic isolates.
The results of the assay of the inhibitory activity of BAC+ enterococcal isolates against the indicators are summarized in Table 5. In the case of the Vans E. faecalis, Vans E. faecium, and Vanr E. faecium isolates, more than one isolate was used as the indicator, and the results express the average values. In general, the most frequently inhibited indicators were Listeria monocytogenes (48%), vanA-containing E. faecium (46.6%), Vans E. faecium (36.6%), and Vans E. faecalis (32.1%). Pediococcus pentosaceus was the most inhibited of the lactic acid bacteria studied (29.4%). Among vanA-containing Enterococcus indicator strains, E. faecalis and E. hirae were similarly inhibited (20.6%), while vanA-containing E. faecium was inhibited by 46.6% of the BAC+ isolates.
TABLE 5.
Inhibitory activity of BAC+ enterococcal isolates from different origins against each of the bacterial indicators used
| Indicator isolatea | No. (%) of BAC+ isolates with inhibitory activity against indicators
|
||||
|---|---|---|---|---|---|
| Human clinical samples (55 BAC+) | Human fecal samples (29 BAC+) | Sewage (11 BAC+) | Chicken samples (7 BAC+) | All isolates (102 BAC+) | |
| E. faecalis H1 (vanA) | 17 (30.9) | 2 (6.9) | 2 (18.2) | 0 | 21 (20.6) |
| E. faecium (vanA)b | 21.5 (39.1) | 17.5 (60.3) | 4.5 (41) | 4 (57.1) | 47.5 (46.6) |
| E. hirae P9 (vanA) | 15 (27.7) | 4 (13.8) | 2 (18.2) | 0 | 21 (20.6) |
| E. faecalis SF-299 (vanB) | 16 (29) | 8 (27.6) | 6 (54.5) | 3 (42.8) | 33 (32.3) |
| E. gallinarum CECT970 (vanC-1) | 17 (30.9) | 11 (37.9) | 3 (27.3) | 0 | 31 (30.4) |
| E. faecalis Vansb | 20.5 (37.2) | 4.7 (16.3) | 4.2 (38.6) | 3.2 (46.4) | 32.7 (32.1) |
| E. faecium Vansb | 20 (36.3) | 10.8 (37.3) | 3 (27.3) | 4.2 (59.5) | 37.3 (36.6) |
| L. monocytogenes CECT4032 | 20 (36.3) | 16 (55.2) | 6 (54.5) | 7 (100) | 49 (48) |
| L. innocua CECT910 | 14 (25.4) | 4 (13.8) | 5 (45.4) | 6 (85.7) | 29 (28.4) |
| L. murrayi CECT942 | 5 (9) | 11 (37.9) | 1 (9.9) | 1 (14.3) | 18 (17.6) |
| L. grayi CECT931 | 12 (21.8) | 12 (41.3) | 3 (27.3) | 1 (14.3) | 28 (27.4) |
| L. paracasei C162 | 12 (21.8) | 0 | 0 | 0 | 12 (11.7) |
| L. plantarum C193 | 4 (7.2) | 0 | 1 (2.5) | 0 | 5 (4.9) |
| Leuconostoc sp. C214 | 1 (1.8) | 0 | 0 | 0 | 1 (0.9) |
| P. pentosaceus AR63 | 18 (32.7) | 10 (34.5) | 2 (18.2) | 0 | 30 (29.4) |
Indicator isolates correspond to those referred to in Table 2. Results of inhibitory activity against B. subtilis, Staphylococcus spp., or E. coli are not included, because negative results were obtained with all the BAC+ enterococcal isolates tested.
There is more than one isolate in these indicator categories. The results represent the average values.
Interestingly, vanA-containing E. faecalis was inhibited by 30.9% of the BAC+ isolates from human clinical samples but only by 6.9% of human fecal isolates (P = 0.0061). A similar trend was found for vanA-containing E. hirae, which was also more frequently inhibited by human clinical isolates than by human fecal isolates (27.7 and 13.8%, respectively). However, vanA-containing E. faecium was inhibited to a higher degree by human fecal isolates than by human clinical ones. L. monocytogenes was inhibited by all the BAC+ isolates from chicken samples. L. monocytogenes was also more frequently inhibited by BAC+ enterococcal isolates from human fecal samples (55.2%) than by those obtained from human clinical samples (36.3%). None of the BAC+ enterococcal isolates showed inhibitory activity against Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus haemolyticus, or Escherichia coli.
The 10 BAC+ vanA-containing Enterococcus isolates obtained from human samples were tested for antimicrobial activity using all of them as producers and indicators. A group of five isolates (E. faecalis RC715, RC716, RC719, C215, and E337, corresponding to two different PFGE patterns) showed an identical bacteriocin inhibition pattern that was different from the bacteriocin inhibition patterns of the other five BAC+ vanA isolates. A second group of three isolates (E. faecalis RC718, C237, and H1, all three with different PFGE patterns) also had a common pattern of bacteriocin inhibition which was quite different from that in the first group. The remaining two isolates (E. faecalis RC721 and E. faecium RC714) showed two different patterns of bacteriocin inhibition.
Among bacteriocin producers, two types of isolates were considered: (i) isolates producing bacteriocin with a broad interspecific activity, considered as such when members of at least three out of the eight different indicator genera were inhibited by the producers, and (ii) isolates producing bacteriocin with high intraspecific activity, considered as such when they showed antimicrobial activity against at least 10 out of the 20 Enterococcus isolates used as indicators.
Bacteriocin producer isolates with broad interspecific activity and high intraspecific activity were more frequently detected among vanA-containing Enterococcus isolates (44.4 and 88.8%, respectively) than among Vans isolates (13 and 26%, respectively) obtained from human clinical samples. When other origins were considered, the proportion was lower for vanA-containing strains (0 and 40%) and similar for Vans isolates (24.3 and 24.3%) (Table 6). Strains with simultaneously broad interspecific and high intraspecific activities were much more frequently found among vanA-containing isolates (33.3%) than among Vans isolates (6.5%) from clinical samples. The BAC+ E. faecalis isolates showed more frequent high intraspecific activity than the BAC+ E. faecium isolates (29 of 75 [38.6%] and 7 of 26 [27%], respectively).
TABLE 6.
Broad interspecific activitya and high intraspecific activityb of BAC+ enterococcal isolates from different origins and vancomycin resistance phenotypes
| Isolates origin (no. of BAC+ isolates) | Phenotype (no.) | No. (%) of isolates with:
|
||
|---|---|---|---|---|
| Broad interspecific activity | High intraspecific activity | Broad interspecific activity plus high intraspecific activity | ||
| Human clinical samples (55) | Vanr (9) | 4 (44.4) | 8 (88.8) | 3 (33.3) |
| Vans (46) | 6 (13) | 12 (26) | 3 (6.5) | |
| Human fecal samples (29) | Vanr (1) | 0 | 0 | 0 |
| Vans (28) | 8 (28.6) | 5 (17.9) | 2 (7.1) | |
| Sewage (11) | Vanr (2) | 0 | 1 (50) | 0 |
| Vans (9) | 1 (11.1) | 4 (44.4) | 1 (11.1) | |
| Chicken samples (7) | Vanr (7) | 0 | 3 (42.8) | 0 |
| Total (102) | Vanr (19) | 4 (21) | 12 (63.1) | 3 (15.8) |
| Vans (83) | 14 (16.9) | 21 (25.3) | 6 (7.2) | |
Broad interspecific activity, inhibition of at least three of the eight different genera tested as indicators.
High intraspecific activity, inhibition of at least 10 of the 20 different Enterococcus isolates tested as indicators.
Mating experiments.
In all 12 vanA-containing Enterococcus isolates tested (nine E. faecalis and three E. faecium), bacteriocin production was cotransferred together with vancomycin and erythromyein resistance to the recipient E. faecalis strain JH2-2, with a mating frequency ranging from 5 × 10−2 to 6.6 × 10−8. In 8 of these 12 isolates, high-level kanamycin and streptomycin resistance was also cotransferred. In all cases, vancomycin-resistant transconjugants showed the same spectrum of inhibitory activity against indicator isolates as the donors, suggesting cotransference of the same bacteriocin(s) genetic determinant(s).
Bacteriocin RC714 characterization and purification.
The bacteriocin produced by the vanA-containing E. faecium RC714 strain (which was isolated from a human exudate sample) was chosen for further characterization. Bacteriocin RC714 showed inhibitory activity against all E. faecalis (eight Vans and one vanA- and one vanB-containing isolate) and E. faecium (six Vans and two vanA-containing isolates) strains tested as indicators, as well as against L. monocytogenes, Listeria innocua, Listeria murrayi, Listeria grayi, Lactobacillus paracasei, Lactobacillus plantarum, Leuconostoc sp., and P. pentosaceus. However, no inhibitory activity was detected against the vanA-containing E. hirae, vanC-1-containing E. gallinarum, S. epidermidis, S. aureus, S. haemolyticus, E. coli, and B. subtilis. E. faecium RC714 did not show β-hemolysis, and negative PCR results for the previously reported bacteriocins AS-48 and enterocin I were also obtained. The RC714 strain consistently cotransferred vancomycin and erythromycin resistance and bacteriocin production to the E. faecalis strain JH2-2. This bacteriocin was resistant to heat treatment (100°C for 20 min) and was stable in a wide range of pH values (3 to 11). Bacteriocin RC714 was susceptible to the proteolytic activity of trypsin, α-chemotrypsin, papain, alkaline protease, and proteinase K, but it was resistant to lysozyme.
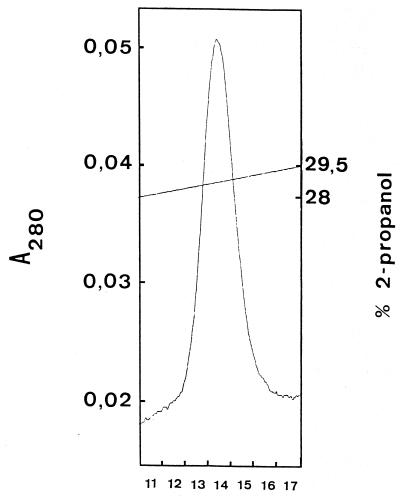
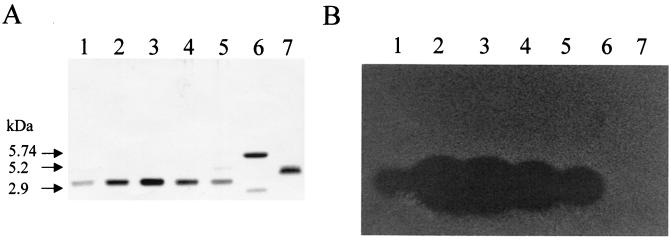
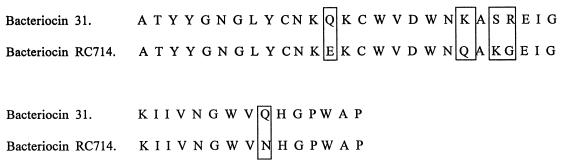
The purification scheme for bacteriocin RC714 is shown in Table 7. After the second reverse-phase chromatography step, a final yield of 1.1% of the initial activity and a 29-fold increase in the specific activity of bacteriocin RC714 was obtained. The overall purification procedure resulted in a single peak upon C2/C18 reverse-phase liquid chromatography (Fig. 1). SDS analysis showed an electrophoretically pure protein with an apparent molecular size of ca. 3,000 Da and with inhibitory activity against E. faecium AR9 (Fig. 2). The N-terminal sequencing of purified bacteriocin RC714 allowed determination of a total of 42 amino acid residues. This sequence showed an identity of 88% and a similarity of 92% with bacteriocin 31 previously described by Tomita et al. (53) in an E. faecalis strain (Fig. 3). A difference of 5 out of 42 amino acids with respect to bacteriocin 31 was observed.
TABLE 7.
Purification of bacteriocin RC714 from E. faecium vanA RC714
| Fraction | Vol (ml) | A280a | Total activity (105 BUb) | Sp actc | Fold increase in sp act | Yield (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 2,000 | 388.5 | 6.4 | 1.6 × 103 | 1 | 100 |
| Ammonium sulfate precipitation (fraction I) | 91.5 | 67.15 | 9.4 | 1.4 × 104 | 8.7 | 147 |
| Binding to SP-Sepharose fast flow (fraction II) | 30.5 | 37.5 | 7.7 | 12.0 × 104 | 12.5 | 120 |
| Binding to phenyl-Sepharose CL-4B (fraction III) | 23 | 7.5 | 0.77 | 1.0 × 104 | 6.25 | 12 |
| FPLCd (C2/C18 reverse-phase chromatography) | ||||||
| First run | 3.5 | 1.21 | 0.5 | 4.1 × 104 | 25.6 | 7.8 |
| Second run | 0.6 | 0.15 | 0.07 | 4.6 × 104 | 29.0 | 1.1 |
Total A280 is the A280 multiplied by the volume in milliliters.
BU, bacteriocin units.
Specific activity is the BU divided by the A280.
FPLC, fast-performance liquid chromatography.
FIG. 1.
C2/C18 reverse-phase chromatography of bacteriocin RC714 (second run). Numbers below the graph indicate the fraction (0.15 ml each) exhibiting bacteriocin RC714 activity.
FIG. 2.
SDS-PAGE of bacteriocin RC714 and detection of antimicrobial activity. (A) Silver-stained gel. (B) Gel fixed in 20% 2-propanol–10% acetic acid and washed in deionized water as described by Bhunia et al. (6). The gel was then placed on an MRS agar plate and overlaid with MRS soft agar containing E. faecium AR9. Lanes 1 to 5, fraction numbers 13 to 17 of C2/C18 second run-purified bacteriocin RC714 (see Fig. 1); lane 6, purified pIS β peptide (35); lane 7, purified enterocin I (22). Size standards are indicated on the left.
FIG. 3.
Comparison of bacteriocin RC714 amino acid sequence with that corresponding to bacteriocin 31 (53). The different amino acids are indicated.
DISCUSSION
Bacteriocin production has been shown to confer an ecological advantage on the producer strain (48). In this study, a higher proportion of BAC+ isolates was detected among E. faecalis (80.6%) than among E. faecium (21.6%) isolates. Similarly, Tomita et al. (53) found that 54% of their E. faecalis isolates were BAC+. Both in feces and in invasive isolates, E. faecalis was more commonly found and was at a higher proportion than E. faecium (43). In accordance with our results, this fact may be explained, at least in part, by the ecological advantage of the BAC+ E. faecalis strains.
Our data indicate that the proportion of BAC+ isolates among human clinical isolates was significantly higher (63.2%) than that among human fecal isolates (37.2%). Among Vanr isolates, bacteriocin production was found in 81.8% of the isolates from human clinical samples and in 6.7% of those from human fecal samples. In addition to the low frequency of bacteriocin production by vanA-containing Enterococcus isolates from human fecal origin, Vans BAC+ enterococcal isolates of fecal origin showed a high inhibitory activity against vanA-containing E. faecium isolates (Table 5). In fact, more than half (60.3%) of all our BAC+ human fecal Enterococcus isolates, all of them Vans, inhibited both clones of vanA-containing E. faecium used as indicators. Only 6.19% of these isolates inhibited the vanA-containing E. faecalis H1 strain used as indicator. Moreover, not only most vanA enterococcal isolates obtained from human clinical samples were BAC+, but their bacteriocin activities showed broad interspecific activity and high intraspecific activity in a higher proportion than BAC+ isolates from the other origins. Most BAC+ and vanA-containing E. faecium and E. faecalis isolates corresponded to different PFGE patterns, thus indicating that the results were not severely biased by the predominance of a particular widespread clone. These observations suggest an ecological advantage of bacteriocinogenic strains for colonization and for invasion, as previously postulated (30, 39).
L. monocytogenes was the Listeria species most inhibited by BAC+ enterococcal isolates of different origins. The activities of BAC+ enterococcal isolates against lactic acid bacteria indicate that P. pentosaceus is the most susceptible strain to this antimicrobial inhibition. The moderate activities of BAC+ isolates against lactic acid bacteria and the absence of activity against Bacillus or staphylococci suggest high bacteriocin specificity, preferentially mediating amensalistic interactions among different enterococcal populations in the intestinal habitat.
The bacteriocins detected in E. faecalis frequently correspond to the bacteriocin/hemolysin encoded by the plasmid pAD1 (32), which usually also confers a sex pheromone response (13, 60). The bacteriocin/hemolysin has been associated with virulence in animal models (10, 29, 33). Nine of our 32 BAC+ tested isolates (eight E. faecalis and one E. faecium) (28%) were β-hemolytic, and all of them were Vans and of clinical origin. None of our BAC+ Vanr enterococcal isolates showed this β-hemolytic activity. Tomita et al. (53) detected a high proportion of BAC+ isolates among their E. faecalis isolates (54%), and 68% of these bacteriocin producers showed β-hemolytic activity. The occurrence of β-hemolysin in clinical isolates of E. faecalis varied from 17 to 60% in different studies (14, 21, 30). Similarly, Coque et al. (15) detected β-hemolysin activity among E. faecalis strains of different origins (16 to 37%) but not in non-E. faecalis isolates.
This study has demonstrated the cotransference of vancomycin and erythromycin resistance with bacteriocin production (but not β-hemolysin) in 12 vanA-containing enterococcal isolates, corresponding to nine different PFGE patterns of E. faecalis (nine isolates, six clones) and E. faecium (three isolates, three clones). In all cases, the selection for transconjugants was first performed for vancomycin resistance, and bacteriocin production and other antibiotic resistance determinants were then evaluated in Vanr transconjugants. Notably cotransference does not mean association between bacteriocin production and antibiotic resistance, and the eventual presence of more than one bacteriocin in the transconjugants cannot be ruled out. Nevertheless, the frequent cotransference may have ecological consequences. In 1990, Handwerger et al. (28) described the cotransference of vancomycin resistance and a pheromone response as well as β-hemolytic activity in E. faecium. Cotransference of high-level aminoglycoside, erythromycin, and chloramphenicol resistance and bacteriocin/hemolysin has also been previously reported (30, 39, 40).
Sequence analysis of the bacteriocin RC714 revealed that our bacteriocin belongs to the class II bacteriocins (small, heat-stable, non-lantionine-containing peptides) (20). A bacteriocin similar to RC714 was previously described in an E. faecalis strain from Japan by Tomita et al. (53) and was named bacteriocin 31. Bacteriocin RC714 has a difference of 5 amino acids from the deduced sequence of 42 amino acids of bacteriocin 31 (Fig. 3) and originated from a clinical vanA-containing E. faecium strain. Also, our bacteriocin showed a wide range of activity against different indicator isolates of different genera (5) and species (10) of gram-positive bacteria. On the basis of the N-terminal amino acid sequences, the bacteriocin RC714 could represent a new bacteriocin (enterocin) different from bacteriocin 31.
The use of glycopeptides in humans and animals has been previously associated with the dissemination of Vanr enterococci (45). The present work suggests that other factors should be taken into account. The very frequent association of bacteriocin production and vancomycin resistance in different enterococcal clones isolated from human clinical samples suggests that the production of amensalistic substances may enhance extra-intestinal colonization. In contrast, bacteriocin production was infrequently found among our vancomycin-resistant enterococcal strains from human fecal samples. That may suggest that these nonbacteriocinogenic vancomycin-resistant fecal isolates may remain at low density in the intestinal tract. Whether these observations help explain the discrepancies in the rates of vancomycin resistance among enterococcal isolates from invasive infections versus fecal isolates in Europe and the United States remains to be evaluated. Such evaluation will require a broader sampling of vancomycin-resistant strains of different origins and/or the use of animal models. This work indicates that a complete understanding of the epidemiology of vancomycin resistance in Enterococcus will probably require the simultaneous consideration of different factors involved in the dissemination and selection of particular strains in different environmental compartments.
ACKNOWLEDGMENTS
We are grateful to F. Marco, B. Robledo, J. Castillo, E. Cercenado, M. Lantero, and A. Fleites, as well as to the Spanish Culture Type Collection, for providing us with some of the strains used in this study. We also thank M. Martínez-Bueno for providing us with the plasmid pAM401-61, A. Portillo and C. Martinez for technical assistance, and L. de Rafael, M. Morosini, and T. Coque for critical review of the manuscript.
R. D. C. was supported by a grant from the Diputación General de Aragón of Spain (project P49/97) and from the Sociedad Española de Quimioterapia. This work has been supported in part by a grant from the Fondo de Investigaciones Sanitarias (00/0545) of Spain.
REFERENCES
- 1.Aarestrup F M. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb Drug Resist. 1995;1:255–257. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Americh T, Holo H, Havarstein L S, Hugas M, Garriga M, Nes I F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol. 1996;62:1676–1682. doi: 10.1128/aem.62.5.1676-1682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An F Y, Clewell D B. Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid. 1994;31:215–221. doi: 10.1006/plas.1994.1023. [DOI] [PubMed] [Google Scholar]
- 4.Basinger S F, Jackson R W. Bacteriocin (hemolysin) of Streptococcus zymogenes. J Bacteriol. 1968;96:1895–1902. doi: 10.1128/jb.96.6.1895-1902.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates E M, Jordens J Z, Griffits D T. Farm animals as a putative reservoir for vancomycin resistant enterococcal infections in man. J Antimicrob Chemother. 1994;34:507–516. doi: 10.1093/jac/34.4.507. [DOI] [PubMed] [Google Scholar]
- 6.Bhunia M C, Ray B. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Ind Microbiol. 1987;2:319–322. [Google Scholar]
- 7.Brock T D, Davie J M. Probable identity of a group D hemolysin with a bacteriocine. J Bacteriol. 1963;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butaye P, Devriese L A, Goossens H, Ieven M, Haesebrouck F. Enterococci with acquired vancomycin resistance in pigs and chickens of different age groups. Antimicrob Agents Chemother. 1999;43:365–366. doi: 10.1128/aac.43.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casaus P, Nilsen T, Cintas L M, Nes I F, Hernández P E, Holo H. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology. 1997;143:2287–2294. doi: 10.1099/00221287-143-7-2287. [DOI] [PubMed] [Google Scholar]
- 10.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cintas L M, Casaus P, Håvarstein L S, Hernández P E, Nes I F. Biochemical and genetic characterization of enterocin P, a novel plasmid sec-dependent bacteriocin from Enterococcus faecium P13 with a broad antimicrobial spectrum. Appl Environ Microbiol. 1997;63:4321–4330. doi: 10.1128/aem.63.11.4321-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cintas L M, Casaus P, Holo H, Hernández P E, Nes I F, Havarstein L S. Enterocins L50A and L50B, two novel bacteriocins from Enterococcus faecium L50, are related to staphylococcal hemolysins. J Bacteriol. 1998;180:1988–1994. doi: 10.1128/jb.180.8.1988-1994.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clewell D B, Brown B L. Sex pheromone cAD1 in Streptococcus faecalis: induction of a function related to plasmid transfer. J Bacteriol. 1980;143:1063–1065. doi: 10.1128/jb.143.2.1063-1065.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colmar I, Horaud T. Enterococcus faecalis hemolysin-bacteriocin plasmids belong to the same incompatibility group. Appl Environ Microbiol. 1987;53:567–570. doi: 10.1128/aem.53.3.567-570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coque T M, Patterson J E, Steckelberg J M, Murray B E. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis. 1995;171:1223–1229. doi: 10.1093/infdis/171.5.1223. [DOI] [PubMed] [Google Scholar]
- 16.Costa Y, Galimand M, Leclercq R, Duval J, Courvalin P. Characterization of the chromosomal aac(6′)-Ii gene specific for Enterococcus faecium. Antimicrob Agents Chemother. 1993;37:1896–1903. doi: 10.1128/aac.37.9.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devriese L A, Ieven M, Goossens H, Vandamme P, Pot B, Hommez J, Haesebrouck F. Presence of vancomycin-resistant enterococci in farm and pet animals. Antimicrob Agents Chemother. 1996;40:2285–2287. doi: 10.1128/aac.40.10.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dukta-Malen S, Evers E, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunny G M, Craig R A, Carron R, Clewell D B. Plasmid transfer in Streptococcus faecalis. Production of multiple sex pheromones by recipients. Plasmid. 1979;2:454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 20.Ennahar S, Sashihara T, Sonomoto K, Ishizaki A. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiol Rev. 2000;24:85–106. doi: 10.1111/j.1574-6976.2000.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 21.Facklam R R. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl Microbiol. 1972;23:1131–1139. doi: 10.1128/am.23.6.1131-1139.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floriano B, Ruiz-Barba J L, Jiménez-Díaz R. Purification and genetic characterization of enterocin I from Enterococcus faecium 6T1a, a novel antilisterial plasmid-encoded bacteriocin which does not belong to the pediocin family of bacteriocins. Appl Environ Microbiol. 1998;64:4883–4890. doi: 10.1128/aem.64.12.4883-4890.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz C M A P, Schillinger U, Holzapfel W H. Production and characterization of enterocin 900, a bacteriocin produced by E. faecium BFE900 from black olives. Int J Food Microbiol. 1996;29:255–270. doi: 10.1016/0168-1605(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 24.Gálvez A, Valdivia E, Martínez M, Maqueda M. Effect of peptide AS-48 on Enterococcus faecalis subsp. liquefaciens S-47. Antimicrob Agents Chemother. 1989;33:641–645. doi: 10.1128/aac.33.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold H S, Ünal S, Cercenado E, Thauvin-Eliopoulos C, Eliopoulos G M, Wennersten C B, Moellering R C., Jr A gene conferring resistance to vancomycin but not teicoplanin in isolates of Enterococcus faecalis and Enterococcus faecium demonstrates homology with vanB, vanA, and vanC genes of enterococci. Antimicrob Agents Chemother. 1993;37:1604–1609. doi: 10.1128/aac.37.8.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González C, Langdon G M, Bruix M, Gálvez A, Valdivia E, Maqueda M. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc Natl Acad Sci USA. 2000;97:11221–11226. doi: 10.1073/pnas.210301097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granato P A, Jackson R W. Bicomponent nature of lysin from Streptococcus zymogenes. J Bacteriol. 1969;100:865–868. doi: 10.1128/jb.100.2.865-868.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handwerger S, Pucci M J, Kolokathis A. Vancomycin resistance is encoded on a pheromone response plasmid in Enterococcus faecium 228. Antimicrob Agents Chemother. 1990;34:358–360. doi: 10.1128/aac.34.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ike Y, Hashimoto H, Clewell D B. Hemolysin of Streptococcus faecalis subspecies zymogenes contributes to virulence in mice. Infect Immun. 1984;45:528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ike Y, Hashimoto H, Clewell D B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infections. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob A E, Douglas G J, Hobbs S J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975;121:863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jett B D, Jensen H G, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiménez-Díaz R, Rios-Sanchez R M, Desmazeaud M, Ruiz-Barba J L, Piard J C. Plantaricins S and T, two new bacteriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl Environ Microbiol. 1993;59:1416–1424. doi: 10.1128/aem.59.5.1416-1424.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiménez-Díaz R, Ruiz-Barba J L, Cathcart D P, Holo H, Nes I F, Sletten K H, Warner P J. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl Environ Microbiol. 1995;61:4459–4463. doi: 10.1128/aem.61.12.4459-4463.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joosten H M L J, Rodriguez E, Nuñez M. PCR detection of sequences similar to the AS-48 structural gene in bacteriocin-producing enterococci. Lett Appl Microbiol. 1997;24:40–42. doi: 10.1046/j.1472-765x.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- 37.Klare I, Heier H, Claus H, Rissbrodt R, Witte W. Enterococcus faecium strains with vanA-mediated high-level glycopeptide resistance isolated from animal foodstuffs and fecal samples of human in the community. Microb Drug Resist. 1995;1:265–272. doi: 10.1089/mdr.1995.1.265. [DOI] [PubMed] [Google Scholar]
- 38.Klein G, Pack A, Reuter G. Antibiotic resistance patterns of enterococci and occurrence of vancomycin-resistant enterococci in raw minced beef and pork in Germany. Appl Environ Microbiol. 1998;64:1825–1830. doi: 10.1128/aem.64.5.1825-1830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Libertin C R, Dumitru R, Stein D S. The hemolysin/bacteriocin produced by enterococci is a marker of pathogenicity. Diagn Microbiol Infect Dis. 1992;15:115–120. doi: 10.1016/0732-8893(92)90033-p. [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Kudo M, Takahashi A, Tanimoto K, Ike Y. Evidence of nosocomial infection in Japan caused by high-level gentamicin-resistant Enterococcus faecalis and identification of the pheromone-responsive conjugative plasmid encoding gentamicin resistance. J Clin Microbiol. 1998;36:2460–2464. doi: 10.1128/jcm.36.9.2460-2464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maisnier-Patin S, Forni E, Richard J. Purification, partial characterization and mode of action of enterocin EFS2, an antilisterial bacteriocin produced by a strain of Enterococcus faecalis isolated from a cheese. Int J Food Microbiol. 1996;30:255–270. doi: 10.1016/0168-1605(96)00950-6. [DOI] [PubMed] [Google Scholar]
- 42.Merril C R, Goldman D, Sedman S A, Ebert M H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981;211:1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- 43.Murray B E. The life and times of Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray B E, Singh K V, Heath J D, Sharma B R, Weinstock G M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990;28:2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murray B E. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 46.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility testing on bacteria that grow aerobically. 4th ed. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 47.Parente E, Brienza C, Ricciardi A, Addari G. Growth and bacteriocin production by E. faecium DPC1146 in batch and continuous culture. J Ind Microbiol Biotechnol. 1997;18:62–67. doi: 10.1038/sj.jim.2900368. [DOI] [PubMed] [Google Scholar]
- 48.Riley M A. Molecular mechanisms of bacteriocin evolution. Annu Rev Genet. 1998;32:255–278. doi: 10.1146/annurev.genet.32.1.255. [DOI] [PubMed] [Google Scholar]
- 49.Robredo B, Singh K V, Baquero F, Murray B E, Torres C. Vancomycin-resistant enterococci isolated from animals and food. Int J Food Microbiol. 2000;54:197–204. doi: 10.1016/s0168-1605(99)00195-6. [DOI] [PubMed] [Google Scholar]
- 50.Tanimoto K, Florence Y, Clewell D B. Characterization of the traC determinant of the E. faecalis hemolysin-bacteriocin plasmid pAD1: binding to sex pheromone. J Bacteriol. 1993;175:5260–5264. doi: 10.1128/jb.175.16.5260-5264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomich P K, An F Y, Damle S P, Clewell D B. Plasmid-related transmissibility and multiple drug resistance in Streptococcus faecalis subsp. zymogenes strain DS16. Antimicrob Agents Chemother. 1979;15:828–830. doi: 10.1128/aac.15.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomita H, Fujimoto S, Tanimoto K, Ike Y. Cloning and genetic organization of the bacteriocin 31 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pY117. J Bacteriol. 1996;178:3585–3593. doi: 10.1128/jb.178.12.3585-3593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torres C, Reguera J A, SanMartín M J, Pérez-Díaz J C, Baquero F. vanA-mediated vancomycin-resistant Enterococcus spp. isolates from sewage. J Antimicrob Chemother. 1994;33:553–561. doi: 10.1093/jac/33.3.553. [DOI] [PubMed] [Google Scholar]
- 55.Vandamme P, Vercauteren E, Lammens C, Pensart N, Ieven M, Pot B, Leclercq R, Goossens H. Survey of enterococcal susceptibility patterns in Belgium. J Clin Microbiol. 1996;34:2572–2576. doi: 10.1128/jcm.34.10.2572-2576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Incidence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]
- 57.Wegener H C, Madsen M, Nielsen N, Aarestrup F M. Isolation of vancomycin resistant Enterococcus faecium from food. Int J Food Microbiol. 1997;35:57–66. doi: 10.1016/s0168-1605(96)01221-4. [DOI] [PubMed] [Google Scholar]
- 58.Witte W, Klare I. Glycopeptide resistant Enterococcus faecium outside hospitals: a commentary. Microb Drug Resist. 1995;3:259–263. doi: 10.1089/mdr.1995.1.259. [DOI] [PubMed] [Google Scholar]
- 59.Woodford N, Morrison D, Johnson A P, Briant V, George R C, Cookson B. Application of DNA probes for rRNA and vanA genes to investigation of a nosocomial cluster of vancomycin-resistant enterococci. J Clin Microbiol. 1993;31:653–658. doi: 10.1128/jcm.31.3.653-658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yagi Y, Kessler R E, Shaw J H, Lopatin D E, An F, Clewell D B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983;129:1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]