Abstract
Background and aim
The present study evaluates the antidiabetic effects of aqueous (CPAQ) and methanolic (CPME) extract of Costus pictus D. Don singly and/or in combination with metformin in alloxan-induced diabetic rats.
Experimental procedure
CPAQ and CPME (400 mg/kg dose), metformin (120 mg/kg) and two different combinations of plant extracts and metformin (200 + 60 mg/kg and 400 mg/kg + 60 mg/kg) were orally given to alloxan-induced diabetic rats for 21 days. At 0, 7, 14, and 21 days, body weight and blood glucose levels were measured.
Results and conclusion
After 21 days of treatment, biochemical profiling and histopathology analysis were carried out. CPAQ and CPME, when administrated separately, could decrease blood glucose levels (P ≤ 0.05). CPME showed more promising results (P ≤ 0.05) compared to the diabetic control group. Extracts co-administrated with metformin showed dose-dependent significant recovery of hypoglycemic activity of metformin. Fasting blood glucose levels, body weight, protein, and lipid profile of the treatment group were compared to the diabetic and normal control groups. Animal groups co-administered with CPME and metformin showed more significant effects on the recovery of tissue damages. The synergistic effect of plant extracts with metformin has positive effects on all the parameters and enhanced the efficiency and reduction of blood glucose levels.
Keywords: Costaceae, Hypoglycaemia, Synergistic, Insulin, Histopathology
Graphical abstract
Highlights
-
•
Costus pictus D. Don. Leaves used traditionally in mulching also treats diabetes.
-
•
Methanolic extract of the plant has potent antihyperglycaemic activity compared to aqueous extract.
-
•
Simultaneous oral administration of the C. pictus methanolic extract with metformin positively affected the blood-glucose-lowering activity.
-
•
There was a synergistic effect between standard drugs, Metformin and CPAQ and CPME.
-
•
C. pictus and metformin have a great influence on the recovery of damaged tissues of the experimental animals.
List of abbreviations
- CP
Costus pictus
- CPAQ
Costus pictus aqueous extract
- CPME
Costus pictus methanolic extract
- °C
Degree Celsius
- ml
Millilitre
- mg
Milligram
- μl
Microlitre
- μg
Microgram
- g
Gram
- h
Hour
- min
Minute
- mM
Millimole
- μM
Micromolar
- M
Molar
- nm
Nanometer
1. Introduction
Diabetes mellitus being the most widespread endocrine, metabolic disease arises by insulin deficiency in its secretion or resistance.1 It is also a general term for metabolism disturbances that results in chronic hyperglycaemia.2
Available antidiabetic drugs have an adverse effect and many severe complications in the human body, lack efficacy, and rarely reach the mark for symptomatic relief. So, novel remedies are still a necessity that has curative effects. The primary needs for diabetes management are oral hypoglycemic agents and insulin as routine doses.
Metformin is a biguanide medication that primarily reduces hepatic glucose synthesis and boosts peripheral insulin sensitivity.3,4 Vitamin B12 deficiency is proportional to the duration of treatment and dosage of metformin.5 However, in research examining the occurrence of lactic acidosis in patients receiving long-term metformin therapy, serum vitamin B12 levels were monitored annually, and vitamin B12 supplements were administered if deficiencies were discovered.6 Compared to other antihyperglycaemic medications, there is no evidence from prospective comparative trials or observational cohort studies that metformin is associated with an increased risk of lactic acidosis or elevated lactate levels.7 Patients with renal impairment, advanced liver disease, alcoholism, and heart failure, on the other hand, are more susceptible to develop metformin-induced lactic acidosis.8 As previously reported, four weeks after metformin (500 mg twice daily) therapy was initiated in obese type 2 diabetic patients, there were indications of possible metformin-associated liver damage and an increased level of the enzyme alanine aminotransferase.9 To overcome the risk posed by the high dosage of metformin, people are now returning to nature to cure and prevent diabetes. Many plants have been identified as antidiabetic agents and help manage diabetes.10, 11, 12
Costus pictus D. Don, commonly known as Spiral flag or Insulin plant, is a medicinally important plant that belongs to the family Costaceae.13 This plant is grown as an ornamental plant, mainly in South India. The leaves of this plant are widely used by the tribal people of Kolli hills of Tamilnadu as ethnomedicine to treat diabetes.14 Following its introduction into India from the Americas, C. pictus has acquired prominence in studies of its medicinal potential.15
The efficacy of combinatorial and single herbal formulations has been reported,16,17 but the report on the combinatorial effects of medicinal herbs with known diabetic drugs is limited. The possibility of therapeutic correlation between medicinal plants and the conventional antidiabetic agents may be additive, synergistic, or antagonistic. Exploration of medicinal plants for their antidiabetic activities has been intensively done, but the research for herb-drug interactions is insufficient.18 Moreover, some of the patients prefer to use conventional drugs concurrently with herbal medicine.19,20 Interactions between herbs and drugs may increase or decrease the pharmacological or toxicological effects of either component.21 Therefore, it is imperative to promote credible research on herbal medicine's safety and possible interaction with synthetic drugs. There is a necessity to do a systemic investigation for possible interaction and safety of herb-drug interactions.
This study aimed to evaluate the plausible effects of single and co-administration of the aqueous and methanolic extracts of Costus pictus leaves and metformin being the first line of diabetic treatment on blood glucose, lipid, protein, and liver functions of alloxan-induced diabetic rats.
2. Material and methods
2.1. Plant collection and sample preparation
The fresh Costus pictus D. Don leaves were collected from Green Park Nursery and Farm, Doldha, Gujarat State, India, in August 2014. The collected plant was identified and authenticated by a botanist, Dr. Minoo Parabia, at the Department of Bioscience, Veer Narmad South Gujarat University (VNSGU), Surat. The voucher specimen (ABN/1) was deposited at VNSGU and C.G.Bhakta Institute of Biotechnology, Maliba Campus, Uka Tarsadia University, Bardoli, Gujarat. Leaves of Costus pictus were air-dried for three (3) weeks and then blended in an electric grinder to get fine powder and stored in an air-tight container. Fresh and mature leaves were used for the study.
2.2. Extraction procedure
The powdered samples were extracted with distilled water and absolute methanol using a Soxhlet apparatus for 6–8 hs at a temperature not more than their respective boiling points. The extracts were filtered using Whatman No.1 filter paper and then concentrated under vacuum at 40 °C concentrated using a rotary evaporator (Model: Hei-Vap Value G3). The extracts were concentrated to dryness by placing on a water bath at 40 °C. The dried extracts were kept in air-tight containers before further studies.
2.3. Chemicals and reagents
Alloxan was purchased from Sigma Chemicals Company, and metformin was a gift from Sun Pharmaceutical Industries Ltd. Alloxan and metformin were freshly dissolved in 0.9% saline for intraperitoneal (IP) and oral administration. Glucose estimation kit, lipid, protein, and liver profiling kits were procured from Span Diagnostic Limited, Surat, India. Glucometer with compatible and authentic strips (ONETOUCH® Select Simple™, India) was used to measure blood glucose levels.
2.4. In vivo antidiabetic study
2.4.1. Animals
Adult Albino Rats of Wistar strain (1.5 months/250-300 gm) were used for the study. All the animals were fed with a regular diet and water ad libitum. Before commencing the experiment, the rats were acclimatized for seven (7) days under standard environmental conditions (temperature 25 ± 2 °C; RH 50 ± 5%; 12 hrs light/dark cycle). The research adheres strictly and conforms to the principle of Laboratory Animal Care (NIH publication No. 85-23). Institutional Animal Ethics Committee approved the research protocols (Reg. No. MPC/IAEC/15/2016), and the CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) recommendations were strictly adhered to for animal care.
2.4.2. Oral glucose tolerance test
After overnight fasting, rats were divided into eight groups of five animals each (n = 5). Group A received normal saline 10 ml/kg body weight and was kept as normal control. Group B was given metformin (120 mg/kg body weight/10 ml). Groups C to H received different doses of an aqueous and methanolic extract with or without metformin combination/body weight/10 ml. Thirty minutes later; glucose solution (2 g/kg body weight/10 ml) was administered to each animal. All the treatments were given orally. Blood samples were collected from the tail vein at – 30 min (fasting (0); before any treatment) and 15 (Baseline), 30, 60, 90, and 120 min after glucose administration. The blood glucose level of the individual animals was measured with a Glucometer using compatible and authentic strips (ONETOUCH® Select Simple™, India).
2.4.3. Induction and assessment of diabetes
Diabetes was induced by injecting freshly prepared 150 mg/kg alloxan monohydrate intraperitoneally to overnight fasted albino rats of Wistar strain. Hyperglycemia was confirmed five days after induction by drawing blood from the tail vein of the experimental animals. The animals showing Random Blood Glucose (RBG) level ≥ 250 mg/dL were selected for further study. The animals were maintained in a diabetic state for 21 days.
2.4.4. Experimental design
All the rats were randomly divided into nine groups, with six animals in each group (n = 6).
Normal: Non Induced rats administered normal saline for comparative assessment.
Diabetic: Alloxan-induced rats administered saline solution to serve as a diabetic non-treated group.
MET: Alloxan-induced rats administered 120 mg/kg b.wt of metformin used as reference.
CPAQ400: Alloxan-induced rats administered 400 mg/kg b.wt of aqueous C. pictus.
CPME400: Alloxan-induced rats administered 400 mg/kg b.wt of Methanolic C. pictus.
CPAQ200+MET: Alloxan-induced rats administered 200 mg/kg b.wt (low dose) of Aqueous C. pictus and 60 mg/kg b.wt metformin.
CPME200+MET: Alloxan-induced rats administered 200 mg/kg b.wt (low dose) of Methanolic C. pictus and 60 mg/kg b.wt metformin.
CPAQ400+MET: Alloxan-induced rats administered 400 mg/kg b.wt (High dose) of Aqueous C. pictus and 60 mg/kg b.wt metformin.
CPME400+MET: Alloxan-induced rats administered 400 mg/kg b.wt (high dose) of Methanolic C. pictus and 60 mg/kg b.wt metformin.
The extracts and standard drugs were administered for 21 days. All the animal's body weight was measured and recorded every week.
2.4.5. Blood glucose and biochemical parameters estimations
Blood was withdrawn from the overnight fasted rats on the initial (0), 7th, 14th, and 21st days of the study, and blood glucose and insulin level were measured. At the end of the experimental period, on the 21st day, blood was collected from each overnight fasted rat by cardiac puncture under deep ether anaesthesia. The serum was separated from the blood and analyzed for protein profiling; Total protein, Urea, Total albumin, Globulin, Creatinine, alkaline phosphatase, alanine transaminase (SGPT), aspartate transaminase (SGOT), and lipid profiling; Cholesterol, Total triglycerides, HDL, LDL, VLDL, Phospholipids using commercially available kits (Span Diagnostic Ltd.).
2.4.6. Histopathology
Vital organs such as kidneys, liver, and pancreas were isolated, weighed, and fixed in 10% formalin for histopathological studies. Tissue samples of the organs were processed for paraffin embedding, and serial sections were made for staining with hematoxylin and eosin dyes to determine the degree of damage of the various treatments to the organs.
2.5. GC-MS analysis of Costus pictus
The GC-MS analysis was carried out using a Clarus 500 PerkinElmer (Auto system XL) Gas Chromatograph equipped and coupled to a mass detector Turbo mass gold - PerkinElmer Turbomass 5.1 spectrometer with an Elite - 1 (100% Dimethylpolysiloxane), 30 m × 0.25 mm ID × 1 μm capillary column. The instrument was set to an initial temperature of 70 °C and maintained at this temperature for 3 min. At the end of this period, the oven temperature was increased to 300 °C, increasing 10 °C/min, and maintained for 9 min. Injection port temperature was ensured at 250 °C and Helium flow rate at 1.5 ml/min. The ionization voltage was 70 eV. The samples were injected in split mode as 10:1. Mass spectral scan range was set at 40–700 (m/z). The ion source temperature was maintained at 230 °C, and the interface temperature was at 240 °C. The MS start time was 3 min, and the end time was 35 min, with the solvent cut time of 3 min. The identification of components was accomplished by comparing retention time, fragmentation pattern, and mass spectra on a NIST Ver. 11 MS data library and comparing the spectrum obtained through GC-MS, compounds present in the plant samples were identified. The relative percentage of each extract constituent was expressed as a percentage with peak area normalization.
2.6. Statistical analysis
The results are expressed as the mean (n = 5)±Standard Error of Mean (SEM). The means were separated using Duncan Multiple Range Test (DMRT). Values with P ≤ 0.05 between groups were considered significant. The data were analyzed with the help of IBM SPSS Statistics 20 and GraphPad Prism 8.0.1 (GraphPad Software Inc., USA).
3. Results
3.1. Oral glucose tolerance test
In the Oral glucose tolerance test (OGTT), there was a significant reduction in the blood glucose of the experimental animals treated with the standard drug, metformin, and graded concentrations of methanolic and aqueous extracts of C. pictus (Fig. 1). Elevation of glucose was observed 15 min after the 2 g/kg b.wt. glucose load and the induction of significant reduction in the blood glucose was observed at 30 min after the glucose load. It is noteworthy that there was no significant difference between the induced reduction of blood glucose among standard metformin (MET), CPAQ400, CPME200+MET, and CPAQ400+MET, 30 min after the glucose load.
Fig. 1.
Effect of Oral administration of Costus pictus methanolic and aqueous leaf extracts and their co-administration with metformin on glycaemia (mg/dl) after raising oral administration of glucose (2 g/kg b.wt), ∗Values are mean of five replicates ± Standard Error of Mean (SEM)
The same superscript down the column denotes no significant difference (P ≤ 0.05).
Normal = Normal Control; MET = Metformin-treated group; = Group treated with 120 mg/kg b.wt Metformin; CPAQ400 = Group treated wit 400 mg/kg b.wt of aqueous C. pictus; CPME400 = Group treated with 400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Group treated with 200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin; CPME200+MET = Group treated with 200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin; CPAQ400+MET = Group treated with 400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Group treated with 400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin.
3.2. Effect of the administration metformin and C. pictus extracts on alloxan-induced diabetic rats
3.2.1. Effect of on fasting blood glucose (FBG) and insulin levels of diabetic rats
At the end of the experiment, significant fasting blood glucose reduction was observed in the treatment group and positive (metformin) control (Table 1). There was no fasting blood glucose reduction in the diabetic untreated group. The highest %FBG reduction (18.07 ± 3.60) was obtained in the group treated with metformin, followed by the CPME400+MET group (16.32 ± 3.00). The lowest %FBG reduction, however, was observed in CPME200+MET (5.89 ± 2.41). The diabetic control group has shown a significant increase in blood glucose levels throughout the study period when compared with the normal control group (P ≤ 0.05). However, the extract-treated and the standard drug (MET) treated groups had a significant decrease in the blood glucose levels when compared with diabetic control (P ≤ 0.05), which was determined on the 7th, 14th, and 21st day of the experiment (Table 1). The effect of glucose level reduction was more pronounced in CPME400+MET followed by the CPAQ400+MET group as compared to the standard group (MET). Metformin alone and the extract–metformin co-administration caused a steady and significant reduction in glycaemia after 21 days of treatment.
Table 1.
Glucose lowering effect of Costus pictus methanolic and aqueous leaf extracts and their co-administration with metformin in alloxan-induced diabetic rats.
| Group | (Blood glucose level (mg/dl) |
%FBG Reduction | |||
|---|---|---|---|---|---|
| 0 day | 7 days | 14 days | 21 days | ||
| Normal | 88.60 ± 6.24a∗ | 94.00 ± 5.62a | 92.00 ± 4.90a | 87.20 ± 5.51a | – |
| Diabetic | 346.60 ± 10.68b | 368.60 ± 14.19b | 420.60 ± 21.86b | 457.20 ± 27.19b | NR |
| MET | 301.80 ± 14.93b | 266.20 ± 7.55c | 253.80 ± 7.65c | 245.40 ± 6.27c | 18.07 ± 3.60a |
| CPAQ400 | 291.60 ± 16.98b | 280.80 ± 13.39c | 273.80 ± 10.61c | 262.80 ± 10.69c | 9.40 ± 2.37abc |
| CPME400 | 294.60 ± 14.58b | 275.80 ± 12.68c | 266.20 ± 10.91c | 258.60 ± 8.12c | 11.76 ± 2.61abc |
| CPAQ200+MET | 304.60 ± 24.31b | 293.80 ± 21.94c | 291.80 ± 17.23c | 282.20 ± 19.79c | 6.88 ± 2.00bc |
| CPME200+MET | 305.00 ± 20.75b | 294.20 ± 17.49c | 286.60 ± 15.05c | 285.20 ± 14.21c | 5.89 ± 2.41c |
| CPAQ400+MET | 314.60 ± 22.31b | 286.80 ± 23.35c | 273.00 ± 19.86c | 269.00 ± 20.18c | 14.07 ± 4.16abc |
| CPME400+MET | 323.40 ± 20.75b | 291.80 ± 13.87c | 276.20 ± 12.40c | 268.20 ± 9.58c | 16.32 ± 3.00ab |
The Same superscript down the column denotes no significant difference (P ≤ 0.05).
Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin).
∗Values are mean of five replicates ± Standard Error of Mean (SEM).
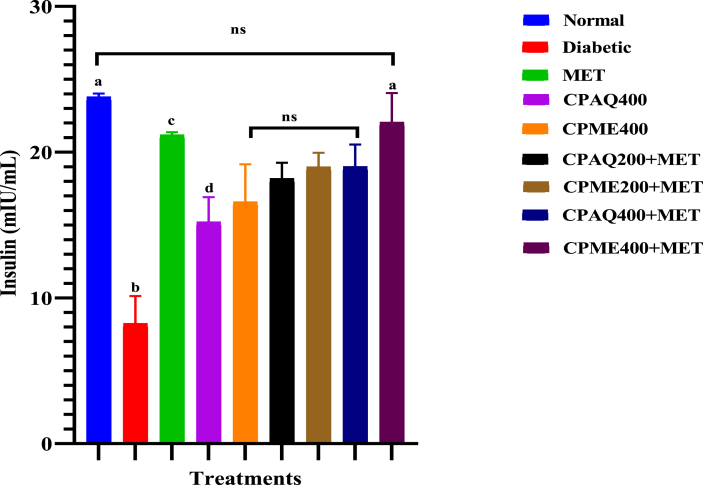
Serum insulin levels in the diabetic group were decreased significantly, and in other treatment groups, the serum insulin levels were increased significantly (P ≤ 0.05) compared to the normal group (Fig. 2). There was no significant difference in the serum insulin level or normal group and group treated with CPME400+MET (P ≤ 0.05). The group treated with metformin also had a considerable increase in the insulin level compared with the rest of the treatment groups.
Fig. 2.
Serum Insulin levels in experimental animals after 21 days of treatment.
Data are means of five replicates ± Standard error of the mean (SEM); ns = No significant difference; different superscripts denote significant different (P ≤ 0.05). Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin).
3.2.2. Effect of extracts administration on body weights
Alloxan-induced diabetic rats showed a significant reduction in body weight. However, the administration of C. pictus and metformin extracts significantly increased the body weight within 21 days (Fig. 3A). The diabetic group had a significant percentage weight loss of about 30%, whereas the treatment groups had no more than 15% weight loss compared to the normal control group with no weight loss (Fig. 3B). Also, other weight observations were presented in Fig. 3C, D.
Fig. 3.
Determination of average weight of the experimental animals (A); Percentage weight loss of the diabetic treated and non-treated groups after 21 days of treatment (B); Average weight of rat organs (C); and the rat organ to body weight ratio (D) after treatment with standard drugs (Metformin) and Costus pictus extracts in comparison with the normal group.
Data are means of five replicates ± Standard error of the mean (SEM); ns = No significant difference; different superscripts denote significant different (P ≤ 0.05). Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); NWL: No weight loss.
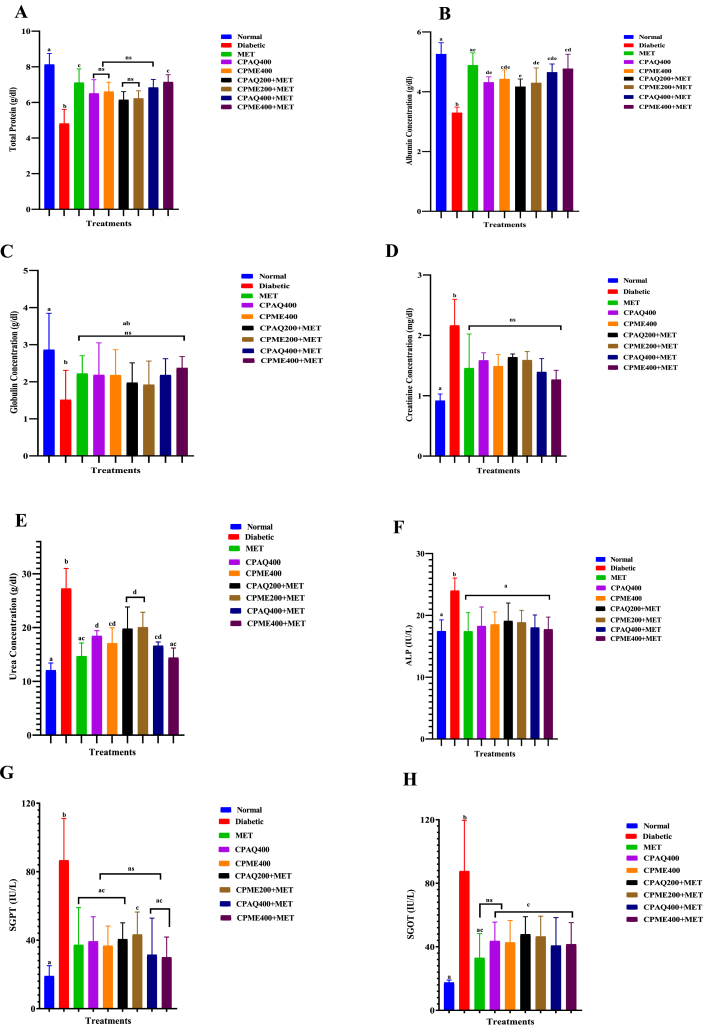
3.2.3. Effect of extracts administration on serum protein and lipid profiles
The effect of oral administration of the aqueous and ethanolic extracts as well as their co-administration with metformin on liver profiling of normal and alloxan-diabetic rats is illustrated in Fig. 4(A-H). Hepatoprotective enzyme levels of AST, ALT, and ALP were significantly increased (P ≤ 0.05) in the plasma of diabetic rats. Oral administration of plant extracts for 21 days lowered the activity of AST, ALT, and ALP to normal levels in the plasma of these treated diabetic rats. The CPME400+MET and CPME200+MET (P ≤ 0.05) were most effective for liver profiling, followed by CPAQ200+MET and CPAQ400+MET (P ≤ 0.05) when compared to the diabetic control group.
Fig. 4.
Average serum protein profile. Total Protein (A); Total Albumin Concentration (B); Globulin Concentration (C); Creatinine Concentration (D); Concentration of Urea (E); Alkaline Phosphatase-ALP (F); Serum Glutamic Pyruvic Transaminase (SGPT) also known as Alanine aminotransferase (G); and Serum Glutamic Oxaloacetate Transaminase (SGOT) also known as Aspartate aminotransferase (H) concentrations after 21 days of treatment with various formulations of Costus pictus.
Data are means of five replicates ± Standard error of the mean (SEM); ns = No significant difference; different superscripts denote significant different (P ≤ 0.05). Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin).
The lipid profile in control and treated experimental rats are presented in Table 2. In Alloxan-induced diabetic rats, a significant (P ≤ 0.05) increase in total cholesterol, triglycerides, LDL, and significant (P ≤ 0.05) decrease in HDL cholesterol in serum compared to normal control were observed. The treatments with CPME400+MET and CPME200+MET significantly (P ≤ 0.05) decreased total cholesterol, triglycerides, LDL, and significantly (P ≤ 0.05) increase HDL cholesterol. This indicates that these leaf extracts have a favourable effect on the lipid metabolism of diabetic rats.
Table 2.
Serum lipid profile of experimental animals 21 days after treatment with Costus pictus methanolic and aqueous leaf extracts and their co-administration with metformin.
| GROUPS | TC∗ (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL-C (mg/dL) | VLDL-C (mg/dL) |
|---|---|---|---|---|---|
| Normal | 107.78 ± 6.17a∗∗ | 94.22 ± 8.38a | 45.18 ± 1.57a | 43.75 ± 7.19a | 18.85 ± 1.68a |
| Diabetic | 221.67 ± 24.76b | 202.67 ± 24.18b | 24.40 ± 2.76b | 156.73 ± 20.39b | 40.53 ± 4.84b |
| MET | 129.31 ± 13.29a | 124.38 ± 16.97a | 24.47 ± 1.35b | 79.96 ± 12.01a | 24.88 ± 3.39a |
| CPAQ400 | 138.20 ± 16.60a | 135.44 ± 18.92a | 33.05 ± 1.90c | 78.06 ± 13.04a | 27.09 ± 3.78a |
| CPME400 | 135.83 ± 6.45a | 133.50 ± 12.48a | 34.61 ± 1.45c | 74.52 ± 4.51a | 26.70 ± 2.50a |
| CPAQ200+MET | 142.78 ± 8.51a | 140.67 ± 13.90a | 31.42 ± 3.58c | 83.22 ± 8.94a | 28.13 ± 2.78a |
| CPME200+MET | 140.14 ± 8.90a | 138.84 ± 12.95a | 32.77 ± 2.64c | 79.61 ± 4.37a | 27.77 ± 2.59a |
| CPAQ400+MET | 132.78 ± 17.30a | 130.09 ± 11.49a | 36.10 ± 1.75c | 70.66 ± 16.18a | 26.02 ± 2.30a |
| CPME400+MET | 130.83 ± 10.74a | 126.44 ± 13.75a | 37.30 ± 1.31c | 68.24 ± 11.00a | 25.29 ± 2.75a |
The Same superscript down the column denotes no significant difference (P ≤ 0.05).
Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin).
∗TC- Total Cholesterol; TG- Total Glyceraldehides; HDL- High-Density Lipoproteins; LDL- Low-Density Lipoprotein; VLDL- Very Low-Density Lipoproteins.
∗∗Values are mean of five replicates ± Standard Error of Mean (SEM).
3.2.4. Effect on haematological profile
The haematological evaluation revealed a significant reduction in the haemoglobin and red blood cells and elevated white blood cells of the diabetic untreated group compared to the normal and treated groups (Table 3).
Table 3.
Effect of CPAQ and CPME and their co-administration with metformin on haematological profile of alloxan-induced diabetic rats.
| GROUPS | Hb (g/dL) | PCV (%) | RBC x 1012 per liter | MCV (fL) | MCH (Pg) | Hematocrit | MCHC (g/dL) | Platelets x109/L | WBC x 109/L | Lymphocytes (%) | Neutrophils (%) | Eosinophils (%) | Monocytes (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | 12.49 ± 0.34a∗ | 38.40 ± 2.07a | 6.75 ± 0.11a | 55.35 ± 2.11ab | 18.50 ± 0.47a | 37.45 ± 1.82a | 33.56 ± 1.23a | 884.91 ± 10.03a∗ | 9.61 ± 0.46a | 46.85 ± 1.56a | 34.42 ± 1.30a | 2.09 ± 0.03a | 4.17 ± .0.11a |
| Diabetic | 9.95 ± 0.27b | 29.67 ± 1.51b | 4.46 ± 0.21b | 67.66 ± 6.07b | 22.51 ± 1.14b | 29.67 ± 1.51b | 33.91 ± 1.98a | 613.24 ± 29.35b | 26.92 ± 2.80b | 57.93 ± 2.30b | 26.97 ± 1.48b | 1.19 ± 0.21b | 5.85 ± .0.26b |
| MET | 10.96 ± 0.49cd | 33.64 ± 2.11abc | 5.91 ± 0.30ac | 57.04 ± 2.78ab | 18.67 ± 1.12ab | 33.64 ± 2.11abc | 33.11 ± 2.61a | 601.77 ± 19.60b | 17.15 ± 0.72c | 52.79 ± 2.58ab | 28.97 ± 1.15bc | 1.82 ± 0.22ac | 3.70 ± .0.20ac |
| CPAQ400 | 11.32 ± 0.16cdef | 31.82 ± 1.60bc | 5.84 ± 0.33ac | 55.08 ± 3.82ab | 19.60 ± 0.96ab | 31.82 ± 1.60bc | 36.01 ± 2.33a | 640.60 ± 35.09b | 15.57 ± 1.68cd | 50.73 ± 3.79ab | 28.48 ± 1.41bc | 1.55 ± 0.18bc | 3.49 ± .0.23ac |
| CPME400 | 11.89 ± 0.30adef | 32.97 ± 0.66bc | 5.90 ± 0.37ac | 56.52 ± 2.83ab | 20.43 ± 1.28ab | 32.97 ± 0.66bc | 36.19 ± 1.55a | 647.76 ± 31.85b | 14.23 ± 1.85acd | 53.33 ± 2.74ab | 29.91 ± 0.54bc | 1.62 ± 0.16abc | 3.50 ± .0.24ac |
| CPAQ200+MET | 10.80 ± 0.37bc | 30.23 ± 2.42b | 5.66 ± 0.18c | 53.57 ± 4.44a | 19.14 ± 0.79ab | 30.22 ± 2.42b | 36.77 ± 3.52a | 629.14 ± 20.55b | 17.79 ± 1.86c | 52.01 ± 3.34ab | 26.91 ± 1.81b | 1.35 ± 0.19bc | 3.20 ± .0.25c |
| CPME200+MET | 11.08 ± 0.26cde | 31.43 ± 1.14bc | 5.78 ± 0.30ac | 55.02 ± 3.61ab | 19.29 ± 0.67ab | 31.43 ± 1.14bc | 35.42 ± 1.49a | 635.77 ± 8.40b | 16.49 ± 2.18c | 53.47 ± 2.72ab | 27.51 ± 1.80b | 1.50 ± 0.16bc | 3.33 ± .0.27c |
| CPAQ400+MET | 12.01 ± 0.26aef | 33.53 ± 1.15abc | 6.17 ± 0.56ac | 56.16 ± 5.35ab | 20.32 ± 2.40ab | 33.53 ± 1.15abc | 36.09 ± 1.90a | 647.97 ± 7.96b | 12.86 ± 1.23acd | 48.96 ± 3.39ab | 30.44 ± 1.10abc | 1.71 ± 0.04ac | 3.72 ± .0.37ac |
| CPME400+MET | 12.25 ± 0.14af | 35.53 ± 0.42ac | 6.27 ± 0.21ac | 56.95 ± 2.03ab | 19.63 ± 0.65ab | 35.53 ± 0.42ac | 34.48 ± 0.35a | 659.53 ± 9.74b | 10.82 ± 0.65ad | 47.01 ± 2.64a | 32.15 ± 1.76ac | 1.80 ± 0.09ac | 3.90 ± .0.20ac |
The Same superscript down the column denotes no significant difference (P ≤ 0.05).
Normal = Normal Control; Diabetic = Diabetic Control; MET = Diabetic +120 mg/kg b.wt Metformin; CPAQ400 = Diabetic+400 mg/kg b.wt of aqueous C. pictus; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin).
∗Values are mean of five replicates ± Standard Error of Mean (SEM).
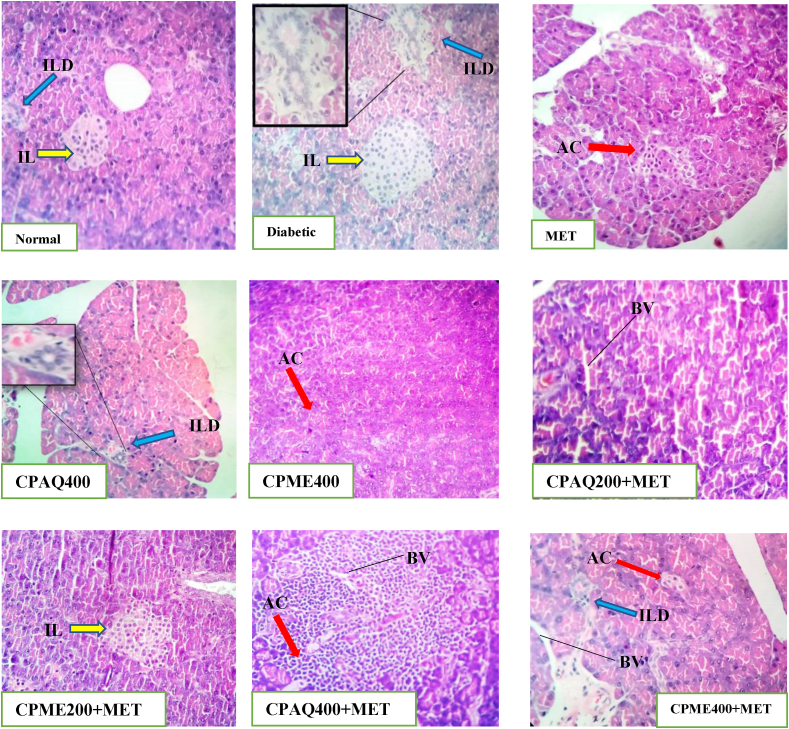
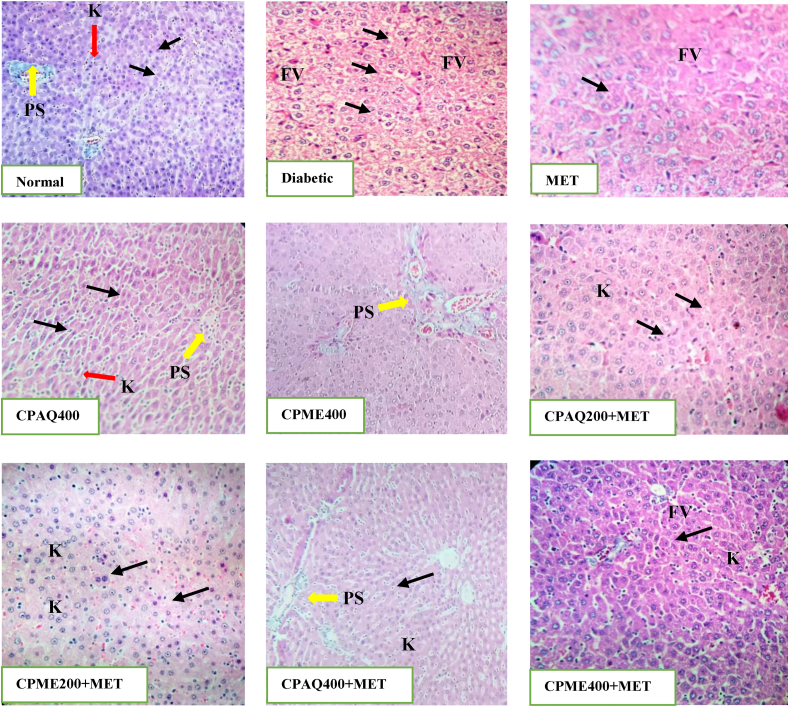
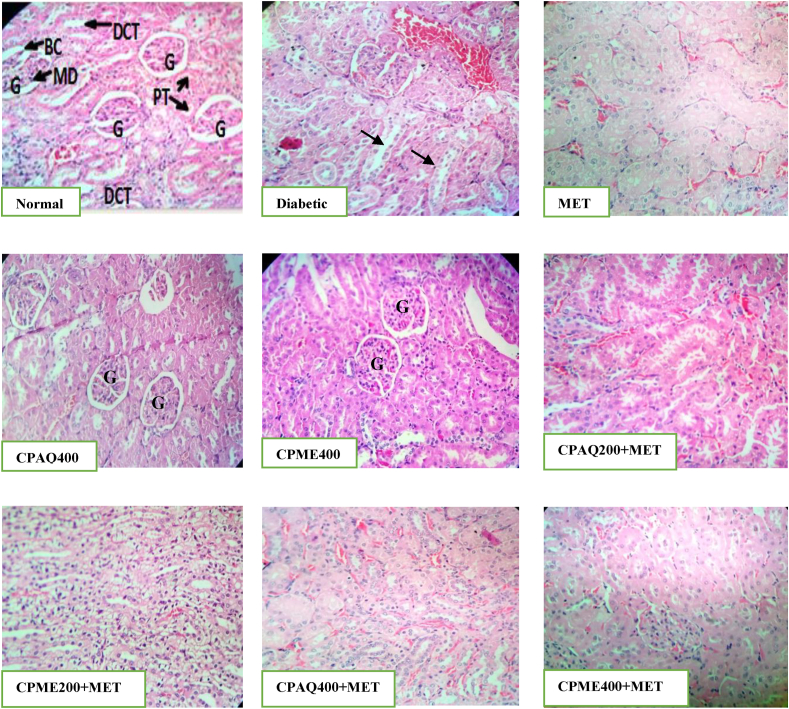
3.2.5. Histopathology of pancreas, liver, and kidney
Histopathological study of the pancreas, liver, and kidney was evaluated (Fig. 5, Fig. 6, Fig. 7). In diabetic control animals (Diabetic), the pancreas section showed degenerative and hyperplasia of islets cells. Islets were damaged, shrunken in size, and infiltration of lymphocytes was observed. While islets of extracts-treated rats, when compared to normal rats, showed mild hyperplasia of islet cells. In diabetic rats, there was a severe fatty change, mild periportal inflammation, fibrosis, and necrosis in liver histology. The liver of diabetic rats treated with the plant extract exhibited minor lipid changes, periportal inflammation, fibrosis, and necrosis. The kidney of diabetic control showed severe tubular epithelial atrophy and moderate congestion of capillaries. The plant extract-treated section showed mild to normal histology.
Fig. 5.
Histopathology of the pancreas.
Normal: Pancreas of the Normal animal showing normal histology of the pancreas tissues; Diabetic: Pancreas of the Diabetic non-treated animal showing destroyed, diminished size and shape of β-cells and damaged acinar cells with an abnormal structure of islet of Langerhans (yellow arrow), and disorganized interlobular duct (blue arrow); MET: Pancreas showing mild hyperplasia of the islet cells (blue arrow), normal acinar cell observed (blue arrow); CPAQ400: Pancrease showing an improved histopathological architecture and reorganized interlobular duct (blue arrow); CPME400: Pancreas of the CPME400-treated animal showing normal histopathology indicating ameliorative effects; CPAQ200+MET: Pancreas showing no defined pathological lesions in histoarchitecture of islets of Langerhans and acinar cells; CPME200+MET: Pancreas showing no defined pathological lesions in histoarchitecture of islets of Langerhans and acinar cells; CPAQ400+MET: Pancreas showing no pathological alteration of the β-cells of islets of Langerhans and normal appearance of acinar cells; CPME400+MET: Pancreas showing significant improvement in pancreatic morphological changes.
ILD: Interlobular Duct; IL: Islet of Langarhans; AC: Acini cells; BV: Blood Vessels; CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin) Magnification × 40; Scale Bar = 10 μm.
Fig. 6.
Histopathology of the liver.
Normal: The liver of Normal group showing normal appearance of hepatocytes, portal space (PS), sinusoids (Black arrows) and Kupffer cells (K); Diabetic: Liver showing progressive worsening of sinusoidal enlargement (Black arrows) and liver fatty degeneration; MET: Liver of MET treated animals showing the onset of sinusoidal enlargement (Black arrows), small amount of fatty vacuoles (FV) were observed; CPAQ400: Normal histology of liver hepatocytes observed indicating ameliorative effect; CPME400: Normal histology of the liver hepatocytes was observed; CPAQ200+MET: Mild sinusoidal enlargement and few fatty acid vacuoles as a results of metformin presence; CPME200+MET: Mild sinusoidal enlargement and few fatty acid vacuoles; CPAQ400+MET: Showing normal histology of liver tissues but with a slight amount of fatty acid vacuole cells; CPME400+MET: showing normal histology of liver tissues but with a slight amount of fatty acid vacuole cells.
CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin) Magnification × 40; Scale Bar = 10 μm.
Fig. 7.
Histopathology of kidney.
Normal: The kidney of Normal group showed no pathological changes appearance of the kidney, normal glomeruli, and tubules, Bowman's capsule (BC), glomerulus (G), proximal tubule (PT), distal convoluted tubular (DCT), Macula Densa cell (MD); Diabetic: kidney showing shrinkage of tubular and inflammation was observed; MET: Kidney showing mild shrinkage of tubules; CPAQ400 & CPME400: Kidney showing normal glomerulus, normal basement membrane, and capillaries, without any inflammatory cells; CPAQ200+MET & CPME200+MET: Kidney showing normal histology with mild shrinkage of tubules; CPAQ400+MET & CPME400+MET: Kidney showing normal histology of kidney tissues with mild shrinkage of tubular.
CPME400 = Diabetic+400 mg/kg b.wt of methanolic C. pictus; CPAQ200+MET = Diabetic+(200 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME200+MET = Diabetic +(200 mg/kg b.wt of CPME+60 mg/kg b.wt metformin); CPAQ400+MET = Diabetic+(400 mg/kg b.wt of CPAQ+60 mg/kg b.wt metformin); CPME400+MET = Diabetic+(400 mg/kg b.wt of CPME+60 mg/kg b.wt metformin) Magnification × 40; Scale Bar = 10 μm.
3.3. GC-MS analysis of methanolic extract of C. pictus
The GC-MS analysis of the methanolic extract revealed ten major peaks (Fig. 8, Suppl. 1). The compounds identified are shown in Table 4 (Suppl. 1). The noticeable compounds were identified like steroids, tocopherol, and palmitic acid, which have many reported biological activities. Stigmastan-6,22-dien, 3,5-dedihydro- occupied the most prominent peak area (66%), followed by dl-alpha.-tocopherol (15.33%) and beta.-sitosterol acetate (13.81%). Other prominent compounds identified to be present are phytol, n-hexadecanoic acid, Di-n-octyl phthalate, 2h-1-benzopyran-6-ol, 3,4-dihydro-2,8-dimethyl-2-(4,8,12-trimethyltridec, 1-Octadecyne, and 5,9-Undecadien-2-one, 6,10-dimethyl-, (z)-.
4. Discussion
Vast numbers of plants have been identified as ethnomedicine for antidiabetic activity, but scientific proofs need to be established through pharmacological evaluations. This study showed that both aqueous and methanolic extracts have antidiabetic activity, but methanolic extract has significant activity.
The oral glucose tolerance test is the gold standard for diagnosing diabetes mellitus. It determines the rate at which glucose is cleared from the body, a function of insulin production and sensitivity, and glucose uptake by peripheral cells.22 The oral glucose tolerance test in the normal rats revealed a significant relationship between the drug dosage and time. Hence, CPAQ and CPME at various graded concentrations with or without metformin kept the blood glucose level at bay even after the glucose load.
Alloxan is a urea derivative that causes selective necrosis of the pancreatic islet β-cells. It induced “chemical diabetes” in albino Wistar rats by damaging the insulin-secreting pancreatic cell, resulting in a decrease in endogenous insulin release.23 Metformin is an antihyperglycemic agent that improves glucose tolerance in type 2 diabetes by lowering basal and postprandial plasma glucose levels. It also stimulates tissue uptake of glucose and reduces insulin sensitivity.24 The methanolic and aqueous extracts of Costus pictus have a similar effect in reducing blood glucose levels, and these effects are more pronounced than metformin.
The increase in body weight in the various treated groups has proved that C. pictus has a beneficiary effect on body weight. The collapse of muscle tissue proteins and adipocytes is increased during the glycogenolysis cycle, and catabolism of fats and proteins directly leads to the loss in body weight in diabetic condition.25 A key symptom of the diabetic condition is frequent urination. Hence, most of the energy is utilized for this urination activity, so converting glycogen to glucose contributes to additional bodyweight loss.26
Alloxan-induced diabetic rats showed a significant increase in blood glucose levels. Alloxan massively reduces insulin secretion by destructing the beta cells of islets of Langerhan's and cause hyperglycemia,27 which may be due to insulin deficiency or insulin resistance state in diabetic rats caused by alloxan treatment. With the lower concentration of insulin, glucose uptake is reduced by peripheral adipose tissues and skeletal muscles.28,29 So, the path of glucose conversion to fat in adipose tissues and glycogen in skeletal muscles is blocked, which results in a large amount of glucose deposition in the blood. With these cellular changes, gluconeogenesis and glycogenolysis are enhanced by glucagon in the liver,30 which leads to higher glucose concentration. When the animals were treated with plant extracts and metformin, diabetic rats showed decreased blood glucose levels, which indicated the reversal of insulin resistance or increased insulin secretion, perhaps by rejuvenating damaged pancreatic β-cells in alloxan-induced diabetic rats.
The fact that diabetic rats were treated individually with the extracts (CPAQ400 and CPME400) and a standard drug (MET), as well as their co-administration, resulted in a significant decrease in glucose level demonstrates that the plant extract may stimulate glucose utilisation by peripheral tissues or hepatic tissues through factors such as activation of glycogen synthesis or release of compounds with insulinogenic properties.31 The hypoglycemic effect of the co-administrative treatments suggests that their antidiabetic activities are additive, indicating that the extract and metformin may be acting through the same mechanism.
In an earlier study,32 some significant observations were made in which diabetic conditions deplete the serum proteins, enhance the deamination, and increase the transportation rate of amino acids. This finally led to the deposition of a large quantity of free ammonia and increased urea levels in the blood and urine. Increased serum urea levels and creatinine in diabetes may be due to the destruction of renal cells by excess glucose flow or by higher deposition of glycosylated proteins in tissues.
In the present study, CPAQ400+MET and CPME400+MET raised the albumin level to be normal, indicating that the extracts have a curative effect on liver and kidney dysfunctions. In diabetic conditions, reduced protein and albumin levels were observed, probably due to proteinuria, albuminuria, or increased protein catabolism, a clinical marker of a diabetic condition. Massive liver necrosis, weak liver function, insulin resistance, and destruction of oxidative phosphorylation of glycogen can cause a lower level of albumin.33
The increased level of liver enzymes in serum can damage the structural integrity of the liver,34 which is considered a sensitive marker of liver injury. These expressions are exhibited in diabetic animals due to altered gluconeogenesis and ketogenesis and/or the onset of hepatic diseases.35
Insulin plays a significant role in lipid metabolism as a potent inhibitor of lipolysis. Lipid breakdown concentrates on the free fatty acids in the liver and kidney. Hypertriglycaemia and hypercholesterolemia are the most common anomalies that arise due to excess amounts of lipid. The immobilisation of free acids from peripheral fats deposits a higher concentration of lipids in the liver and kidney.36 In diabetic conditions, as insulin concentration decreases, lipoprotein lipase activity, which is insulin sensitive, is blocked and can cause hypertriglyceridemia.25
The histopathology of various organs suggested that alloxan-induced diabetic rats presented altered histopathology. These effects can be linked to the chronic organ damage which occurs in human diabetic conditions. The histological studies showed that C. pictus extracts and metformin greatly influence the recovery of damaged tissues, significant for the methanolic extracts treated groups when used separately.
GC-MS analysis provides a detailed analysis of plant samples. The technique provides a detailed chromatographic profile of the sample and consequently measures the relative or absolute amounts of the compounds.37 Phytol and tocopherol have been reported to be antidiabetic and antioxidant agents.38,39 These phytochemicals act as an antidiabetic as they decrease lipid peroxidation, protein glycosylation, and insulin resistance in diabetic rats.40 The antidiabetic activity of the extract might be connected with the presence of Phytol and Tocopherol in the C. pictus extracts.
5. Conclusion
The findings of this present study specify that methanolic extract of the plant has potent antihyperglycaemic activity compared to aqueous extract. The concurrent oral administration of the C. pictus methanolic extract with metformin had a positive effect on the blood-glucose-lowering activity and the reduction in the blood glucose level more effectively than the single applications of both. This study concluded that the combined effect of a high dosage of methanolic plant extract and a low dose of metformin proved to be more effective, additive, and safe because of the most negligible side effects. Hence, the combined effect of methanolic extract of C. pictus and metformin can manage diabetes mellitus effectively.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.08.007.
Contributor Information
Ami Naik, Email: ami.naik@utu.ac.in, naik.ami3@gmail.com.
Sherif Babatunde Adeyemi, Email: adeyemi.sb@unilorin.edu.ng.
Bhavin Vyas, Email: bhavin.vyas@utu.ac.in.
Ramar Krishnamurthy, Email: krishnamurthy@utu.ac.in.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Kerner W., Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(7):384–386. doi: 10.1055/s-0034-1366278. [DOI] [PubMed] [Google Scholar]
- 3.Bailey C.J. Biguanides and NIDDM. Diabetes Care. 1992;15(6):755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M., Nurjhan N., Perriello G., Dailey G., Gerich J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 5.Ting R.Z.-W., Szeto C.C., Chan M.H.-M., Ma K.K., Chow K.M. Risk factors of vitamin B12 deficiency in patients receiving metformin. Arch Intern Med. 2006;166(18):1975–1979. doi: 10.1001/archinte.166.18.1975. [DOI] [PubMed] [Google Scholar]
- 6.Stang M., Wysowski D.K., Butler-Jones D. Incidence of lactic acidosis in metformin users. Diabetes Care. 1999;22(6):925–927. doi: 10.2337/diacare.22.6.925. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter S.R., Greyber E., Pasternak G.A., Salpeter E.E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010:1–233. doi: 10.1002/14651858.CD002967.pub4. [DOI] [PubMed] [Google Scholar]
- 8.Bailey C.J., Turner R.C. Metformin. N. England J. Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 9.Hashmi T. 2011. Probable hepatotoxicity associated with the use of metformin in type 2 diabetes. Case Reports. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabu M., Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81(2):155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 11.Reyes B., Bautista N., Tanquilut N., et al. Antidiabetic potentials of Momordica charantia and Andrographis paniculata and their effects on estrous cyclicity of alloxan-induced diabetic rats. J Ethnopharmacol. 2006;105(1):196–200. doi: 10.1016/j.jep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Hong L., Xun M., Wutong W. Anti-diabetic effect of an α-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J Pharm Pharmacol. 2007;59(4):575–582. doi: 10.1211/jpp.59.4.0013. [DOI] [PubMed] [Google Scholar]
- 13.Ami N., Ramar K., Janardan P. Comparative physicochemical and phytochemical evaluation for insulin plant-Costus pictus D. Don accessions. Int J Appl Agric Res. 2017;3(2):420–426. [Google Scholar]
- 14.Elavarasi S., Saravanan K. Ethnobotanical study of plants used to treat diabetes by tribal people of Kolli Hills, Namakkal District, Tamilnadu, Southern India. Int J Pharm Tech Res. 2012;4(1):404–411. [Google Scholar]
- 15.Hegde P.K., Rao H.A., Rao P.N. A review on Insulin plant (Costus igneus Nak) Phcog Rev. 2014;8(15):67. doi: 10.4103/0973-7847.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojiako O.A., Chikezie P.C., Ogbuji A.C. Blood glucose level and lipid profile of alloxan-induced hyperglycemic rats treated with single and combinatorial herbal formulations. J Trad Compl Med. 2016;6(2):184–192. doi: 10.1016/j.jtcme.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visavadiya N.P., Narasimhacharya A. Ameliorative effects of herbal combinations in hyperlipidemia. Oxid Med Cell Longev. 2011 doi: 10.1155/2011/160408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopalakrishna R.N., Bannimath G., Huded S.P. Herb-drug interaction: effect of poly-herbal formulation on glibenclamide therapy in patients with type-2 diabetes mellitus. Pharmaceut Methods. 2017;8(1):62–70. [Google Scholar]
- 20.Agbabiaka T., Wider B., Watson L.K., Goodman C. Concurrent use of prescription drugs and herbal medicinal products in older adults: a systematic review protocol. Syst Rev. 2016;5(1):65. doi: 10.1186/s13643-016-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355(9198):134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo R.A., Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011;108(3):3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Rohilla A., Ali S. Alloxan induced diabetes: mechanisms and effects. Int J Res Pharmaceut Biomed Sci. 2012;3(2):819–823. [Google Scholar]
- 24.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirwaikar A., Rajendran K., Kumar C.D., Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin–nicotinamide type 2 diabetic rats. J Ethnopharmacol. 2004;91(1):171–175. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Whitton P.D., Hems D.A. Glycogen synthesis in the perfused liver of streptozotocin-diabetic rats. Biochem J. 1975;150(2):153–165. doi: 10.1042/bj1500153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkotby D., Hassan A.K., Emad R., Bahgat I. Histological changes in islets of Langerhans of pancreas in alloxan-induced diabetic rats following Egyptian honey bee venom treatments. Int J Pure Appl Zool. 2018;6(1):1–6. [Google Scholar]
- 28.Baron A.D. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metabol. 1994;267(2):E187–E202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 29.Pitre M., Nadeau A., Bachelard H. Insulin sensitivity and hemodynamic responses to insulin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Endocrinol Metabol. 1996;271(4):E658–E668. doi: 10.1152/ajpendo.1996.271.4.E658. [DOI] [PubMed] [Google Scholar]
- 30.Ramnanan C., Edgerton D., Kraft G., Cherrington A. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metabol. 2011;13:118–125. doi: 10.1111/j.1463-1326.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porchezhian E., Ansari S., Shreedharan N. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia. 2000;71(5):522–526. doi: 10.1016/s0367-326x(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 32.Gupta R., Mathur M., Bajaj V.K., et al. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4(2):164–171. doi: 10.1111/j.1753-0407.2011.00173.x. [DOI] [PubMed] [Google Scholar]
- 33.Krishnasamy G., Muthusamy K., Chellappan D.R., Subbiah N. Antidiabetic, antihyperlipidaemic, and antioxidant activity of Syzygium densiflorum fruits in streptozotocin and nicotinamide-induced diabetic rats. Pharmaceut Biol. 2016;54(9):1716–1726. doi: 10.3109/13880209.2015.1125932. [DOI] [PubMed] [Google Scholar]
- 34.Al-Malki A.L., El Rabey H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. BioMed Res Int. 2015:1–13. doi: 10.1155/2015/381040. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piero M., Nzaro G., Njagi J. Diabetes mellitus-a devastating metabolic disorder. Asian J Biomed Pharmaceut Sci. 2014;4(40):1–7. [Google Scholar]
- 36.Chandran R., Parimelazhagan T., Shanmugam S., Thankarajan S. Antidiabetic activity of Syzygium calophyllifolium in Streptozotocin-Nicotinamide induced Type-2 diabetic rats. Biomed Pharmacother. 2016;82:547–554. doi: 10.1016/j.biopha.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Halket J.M., Waterman D., Przyborowska A.M., Patel R.K., Fraser P.D., Bramley P.M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. J Exp Bot. 2005;56(410):219–243. doi: 10.1093/jxb/eri069. [DOI] [PubMed] [Google Scholar]
- 38.Islam M.T., de Alencar M.V.O.B., da Conceição Machado K., et al. Phytol in a pharma-medico-stance. Chem Biol Interact. 2015;240:60–73. doi: 10.1016/j.cbi.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Sajid M., Khan M.R., Ismail H., et al. Antidiabetic and antioxidant potential of Alnus nitida leaves in alloxan induced diabetic rats. J Ethnopharmacol. 2020;251:112544. doi: 10.1016/j.jep.2020.112544. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes A.A.H., Novelli E.L.B., Okoshi K., et al. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed Pharmacother. 2010;64(3):214–219. doi: 10.1016/j.biopha.2009.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.