Abstract
Background and aim
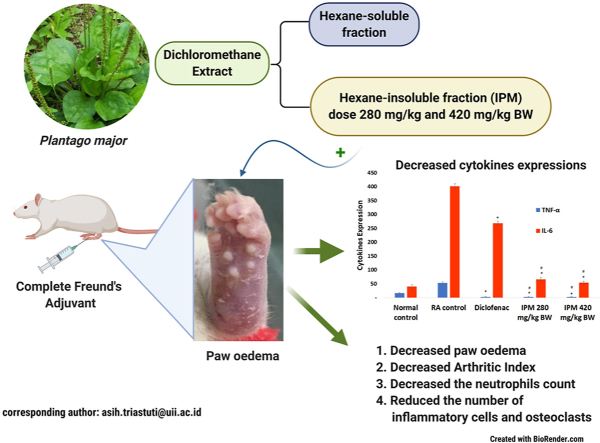
Plantago major has long been used for medical purposes in Indonesia. However, reports on the anti-arthritic activities of P. major are limited.
Experimental procedure
The anti-arthritic properties of an n-hexane-insoluble fraction of dichloromethane extracts of P. major (IPM) were evaluated using Complete Freund's Adjuvant (CFA)-induced arthritis induced in female Wistar rat by CFA. Diclofenac was used as a positive control. The volume of paw oedema, white blood cell count, lymphocytes, neutrophils, expression of TNF-α and Interleukin-6 and the histopathological features of the joint tissues were assessed to characterise IPM activity.
Results
The IPM extract at doses of 280 and 420 mg/kg BW and diclofenac inhibited paw oedema by 15.70 %, 15.94 % and 19.71 % respectively. IPM also reduced the incidence of arthritis and arthritic index. Unlike untreated rats, animals treated with IPM showed a significant decrease in the number of neutrophils and decreased expression of TNF-α and Interleukin-6. Histopathological examination showed a reduction in the number of inflammatory cells and hyperplasia of the synovium after IPM treatment.
Conclusion
This study showed that P. major displays anti-rheumatoid arthritis activity.
Keywords: Arthritis, Histopathology, Cytokines, Paw oedema
Graphical abstract
Highlights
-
•
In Indonesia, P. major has long been used for medical purposes but the reports on the anti-arthritic activities are limited.
-
•
IPM reduced the volume of oedema, the incidence of arthritis, and the arthritic index in rheumatoid arthritis rat model.
-
•
IPM prevents the development of arthritis by decreasing inflammatory cells and the expression of TNF-α and IL-6.
List of abbreviations
- CFA
Complete Freund's Adjuvant
- IPM
n-hexane insoluble fraction of dichloromethane P. major extracts
- TNF-α
Tumor necrosis factor-α
- IL-6
Interleukin 6
- RA
Rheumatoid arthritis
- COX-2
Cyclooxygenase-2
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune, chronic, inflammatory joint condition that causes joint deterioration with discomfort, rigidity, functional loss and systemic comorbidity.1 A characteristic of RA is chronic synovitis triggered by continuous infiltration of immune cells into joints. Effector T cells together with B cells and other innate immune cells form a complex network that promotes the production of pro-inflammatory cytokines. These molecules activate resident fibroblast-like synoviocytes and contribute to cartilage and bone damage. Innate immune cells, such as neutrophils and mast cells, also contribute to synovitis, as do macrophages, which work by releasing pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) and small-molecule inflammatory mediators.2 Cytokines can be divided into two groups: pro- and anti-inflammatory. The balance between these groups is considered an important therapeutic objective for the treatment of RA.
Plantago major is a member of the Plantaginaceae family. P. major is effective as an antioxidant,3 wound healer,4,5 anti-diabetic,6 anti-diarrhoeal,7 anti-inflammatory,8,9 anti-bacterial and anti-viral agent.10,11 Characteristics of P. major reflect various plant components. The species produces a variety of bioactive compounds, including flavonoids, alkaloids, terpenoids, phenolic compounds (caffeic acid derivatives), iridoid glycosides, fatty acids, polysaccharides and vitamins.10,12 These compounds are found in nearly all parts of the plant - seeds, leaves, flowers and roots. Chemical analyses of leaves showed the presence of aucubine, a glycoside, which is reported to be a powerful antitoxin.13,14 P. major leaves also contain 0.07 % oleanolic acid and 0.22 % ursolic acid, two major terpenoids.15, 16, 17 Ursolic acid is a selective inhibitor of cyclooxygenase-2 catalysed prostaglandin biosynthesis.16 This inhibition could explain the anti-inflammatory activity of the plant. Dichloromethane extracts of P. major inhibit leukocyte migration in thioglycollate-induced peritonitis in mice.18 Moreover, ethanol extracts also demonstrate anti-inflammatory activity in mouse ear oedema induced by croton oil.19
Adjuvant arthritis in rats is a commonly used model for RA and predicts the clinical effectiveness of certain medicines for human RA.1,20, 21, 22 This study evaluated the activity of an insoluble hexane fraction of P. major extracts in arthritic female Wistar rats induced with Complete Freund's Adjuvant (CFA).
2. Material and methods
2.1. Drug and chemicals
Complete Freund's Adjuvant (CFA), sodium diclofenac, antibody anti- IL-6, antibody anti- TNF-α and sodium carboxymethylcellulose (Na-CMC) were purchased from Merck. All other chemicals were of the highest purity and analytical grade.
2.2. Preparations of extracts and fractions
P. major was collected from B2P2TOOT (Centre for Research and Development of Medicinal Plants and Traditional Medicines), Tawangmangu, Indonesia. The voucher specimen was deposited at the Laboratory of Pharmaceutical Biology, Department of Pharmacy, Universitas Islam, Indonesia. Powdered plant material (1000 g) was macerated with dichloromethane (3 L) for 24 h at room temperature. After filtration, the residue was re-macerated with dichloromethane. Filtrates were mixed and evaporated under reduced pressure. The dried extract (170 g) was then dissolved in 425 mL n-hexane to produce n-hexane soluble and insoluble fractions. The fractionation was repeated six times before a colourless n-hexane soluble fraction was obtained. This method yielded 77.4 g and 44.1 g of soluble and insoluble n-hexane fractions, respectively. IPM was then used in tests for anti-arthritic activity.
2.3. Animals
Female albino Wistar rats, age 12 weeks, were obtained from the Laboratory of Pharmacology, Department of Pharmacy Universitas Islam Indonesia. Animals were maintained under standard laboratory conditions (12 h light/darkness; at 25 °C ± 3 °C) with standard animal diet and water available ad libitum. All animal experiments were approved by the Medical and Health Research Ethics Committee Faculty of Medicine Universitas Islam Indonesia, with reference number 541/Dek/70-TA/Bag.TA/VIII/2018.
2.4. Experimental design
Rats were acclimatised in the animal house for a week, then randomly divided into five groups of five animals. Group I rats were the normal control; group II animals were administered CFA and served as the RA control group; rats in groups III, IV and V were administered CFA and treated as indicated in Table 1.
Table 1.
Experimental design.
| Group | Treatment |
|---|---|
| Group 1 Group 2 Group 3 Group 4 Group 5 |
Normal Control (NC) RA Control (RAC) CFA + Na diclofenac 5 mg/kg CFA + IPM 280 mg/kg BW CFA + IPM 420 mg/kg BW |
Extracts/Na diclofenac were orally administered daily from day 16–46.
IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major.
RA: Rheumatoid arthritis.
CFA: Complete Freund's Adjuvant.
2.4.1. Dose selection
Acute toxicity studies were not conducted since safety up to a dose of 6200 mg/kg has been reported.23 The two doses 280 and 420 mg/kg BW were selected based on a previous study using thioglycollate-induced leukocyte migration in mice.18
2.4.2. Evaluation of anti-RA
Paw volume of each animal was measured (day 0) to establish a baseline. Arthritis was induced as previously described.24 Briefly, 0.15 mL of CFA was injected into the plantar area of the right hind paw. An equal amount of saline was injected into the left paw at day 0. Volume of oedema was measured every 3 days up to day 47 using a plethysmometer (Ugo Basile, Italy). RA was confirmed by assessing the arthritis index as previously described25 every 3 days for 3 weeks. The clinical severity of arthritis was ranked based on changes in swelling and redness of the toes, footpads and ankles, with a maximum score of 2 per paw. The mean of the cumulative value for all paws was taken as the arthritic index (AI). Rats were considered to have arthritis when AI was at least 1.25 From days 16–46, treated animals received IPM orally with doses of 280 or 420 mg/kg BW. Rats in negative and positive control groups received CMC or diclofenac sodium 5 mg/kg BW, respectively. Animals were sacrificed by administering a high dose of ketamine-xylazine and joint tissue was dissected for histological studies.
2.4.3. Haematological parameters
The blood samples were collected by retro-orbital puncture. Calculation of total leukocytes and differential counts of leukocytes was performed at day 0 and day 47 using a Sysmex KX-21 haematology analyser (Japan).
2.4.4. Haematoxylin -eosin staining
At day 47, all rats were sacrificed after blood sample collection. Hind paws were removed above the knee joint for histological examination. Paws were fixed in 10 % phosphate-buffered formalin, decalcified in 10 % EDTA for 30 days at 4 °C and embedded in paraffin. Serial 5 μm sections were cut and stained with haematoxylin and eosin, then examined under a light microscope to identify areas containing inflammatory cells. Microscopic fields were analysed at a magnification of 400×.
2.4.5. TNF-α and IL-6 staining of the joint tissue
Immunohistochemistry assays used the streptavidin-biotin-peroxidase process. Analyses were carried out in the Pathology Department of the Central General Hospital, Dr. Sardjito, Yogyakarta. Joint tissue was fixed in 10 % buffered formalin for 24 h, followed by 70 % alcohol solution for 24 h, then imbedded in a paraffin block. Four-micron joint tissue sections were placed on poly-lysine coated slides. Sections were deparaffinised, dehydrated in xylol and ethanol and heated in a microwave oven in 0.1 M citrate buffer (pH 6) for about 40 min for antigen recovery. After cooling at room temperature for 20 min, slides were washed with phosphate-buffered saline (PBS), followed by a 15-min blockade of endogenous peroxidase with 3% H2O2. Slides were then incubated with rabbit primary antibodies (anti-TNF-α and anti-IL6) in PBS-BSA overnight at 4 °C following manufacturer instructions. The next day, slides were washed in PBS, the secondary biotinylated rabbit antibody (anti-IgG) in PBS-BSA was added and incubated for 30 min. After washing in PBS, slides were incubated with conjugated streptavidin peroxidase complex (Merck) at room temperature for 15 min, followed by incubating with 3,3 ′Diaminobenzidine-peroxide (DAB) chromophore and counter-stained with Harris haematoxylin. Image Raster and OptiLab Viewer software (USA) quantified histopathological data by counting the number of immunoreactive cells that expressed TNF-α and IL-6.
3. Statistical analysis
The results are expressed as mean ± S.E and analysed using one-way ANOVA followed by Kruskal–Wallis and Mann–Whitney tests. A difference was considered significant at P < 0.05. Expression of TNF-α and IL-6 was measured qualitatively and semi-quantitatively by determining the percentage of positive brown cells under a microscope with a magnification of 400×. Analysis of TNF-α and IL-6 expression used Shapiro-Wilk tests for normality. Differences between experimental groups were tested using one-way ANOVA followed by a Tukey test.
4. Results
4.1. Rat hind paw volume
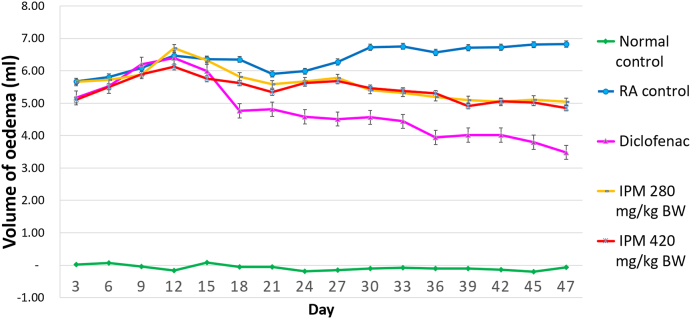
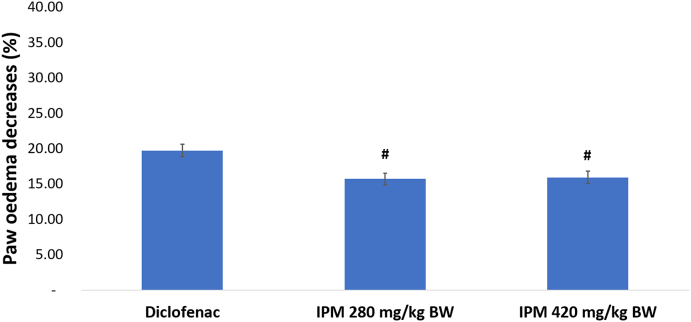
The effect of IPM on CFA-induced arthritis was examined by measuring paw volume. Swelling of CFA-injected hind paws progressed in arthritic rats over time until day 47 (Fig. 1). Rats that received 280 or 420 mg/kg BW of IPM, or sodium diclofenac showed significantly reduced paw oedema compared to RA controls (P < 0.05). IPM extracts at doses of 280 and 420 mg/kg BW and diclofenac inhibit paw oedema by 15.70 %, 15.94 % and 19.71 %, respectively (Fig. 2).
Fig. 1.
Effect of prolonged treatment with IPM on paw volume.
Fig. 2.
The decrease of paw oedema following n-hexane-insoluble fraction of dichloromethane extracts of P. major (IPM) and diclofenac treatment. Values expressed as means ± SE, n = 5, #P < 0.05 with diclofenac. IPM extracts at doses of 280 and 420 mg/kg BW and diclofenac inhibit paw oedema.
Extracts/Na diclofenac were orally administered daily from day 16–46. IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major; RA: Rheumatoid arthritis.
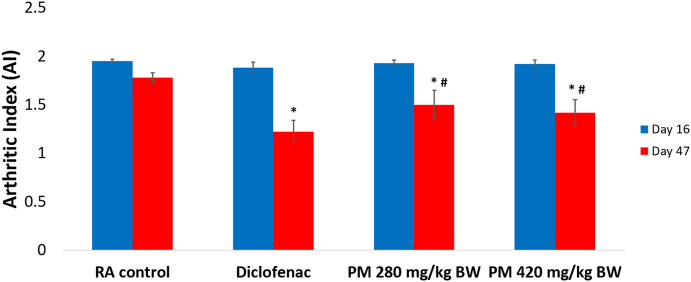
Further, treatment with IPM and diclofenac also inhibited arthritic changes by 20 % (Supplementary Fig. 1). All animals administered CFA started showing signs of clinical inflammation in the right hind paws at day 4 (Supplementary Fig. 2). The severity of arthritis (AI >1) was reduced significantly in IPM and diclofenac-treated rats compared to RA controls from day 16 to day 47 (Fig. 3).
Fig. 3.
Comparison of the Arthritic Index (AI) of treated animals on day 16 and 47. Values expressed as means ± SE, n = 5, ∗P < 0.05, with RA Control, #P < 0.05 with diclofenac. IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major; RA: Rheumatoid arthritis.
4.2. Haematological parameters
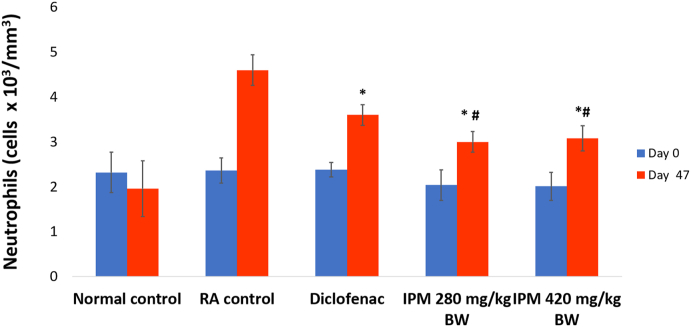
Induction of arthritis resulted in no significant difference in WBC count (cells x 103/mm3) and lymphocytes (Supplementary Figs. 3 and 4). IPM and diclofenac treatments induced a significant decrease in neutrophil counts (Fig. 4).
Fig. 4.
Effect of arthritis on neutrophil counts. Values expressed as means ± SE, n = 5, ∗P < 0.05, with RA Control, #P < 0.05 with diclofenac. IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major; RA: Rheumatoid arthritis.
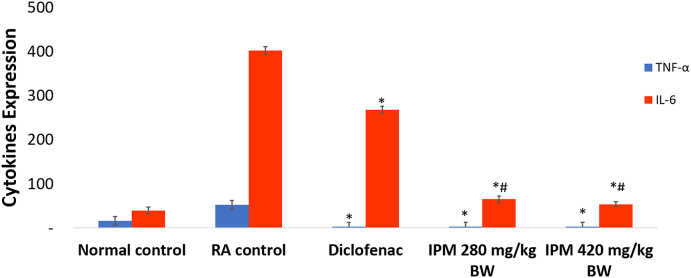
4.3. Expression of TNF-α and IL-6 in joint tissue
The expression of TNF-α and IL-6 significantly increased when arthritis was fully developed (P < 0.05). In arthritic rats, expression of cytokines, TNF-α and IL-6, were 53.00 ± 3.7 and 402.00 ± 8.4, respectively at day 47. Post-treatment with an IPM dose of 420 mg/kg BW reduced expression of both TNF-α and IL-6. The TNF-α reduction were equal to IPM dose of 280 mg/kg BW and diclofenac while the IL-6 reduction was greater than the reduction induced by diclofenac and IPM dose of 280 mg/kg BW, which were equivalent (Fig. 5). Histopathological features of TNF-α and IL-6 expression are depicted in Supplementary Figs. 5 and 6.
Fig. 5.
Effect of IPM on expression of TNF-α and IL-6 in joint tissues. Values expressed as means ± SE, n = 5, ∗P < 0.05 with RA control, #P < 0.05 with diclofenac. IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major; RA: Rheumatoid arthritis.
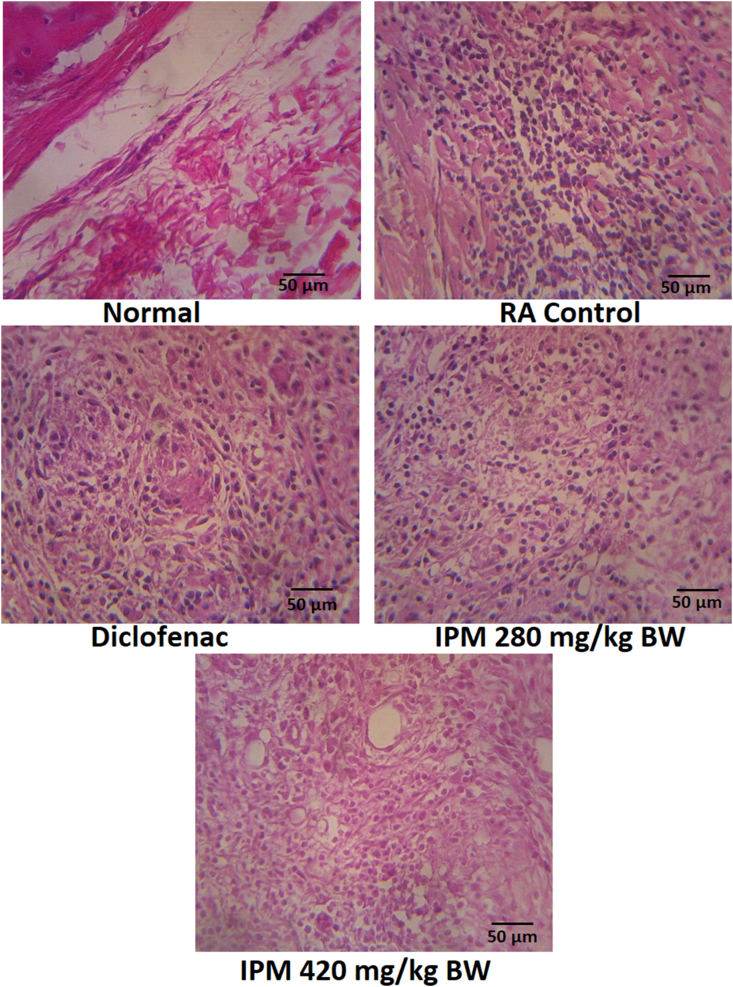
4.4. Histopathological examination
Severity of disease and effects of IPM on joint histology was assessed in photomicrographs of sections stained with H&E (Fig. 6). No inflammation or cartilage erosion was seen in sections of normal rats. In contrast, CFA-treated animals were characterised by extensive inflammation and cellular infiltration resulting in erosion of the articular cartilage.
Fig. 6.
Histopathological features of the joints tissue with a 400 × magnification at five different fields (scale bar: 50 μm). RA animal shows infiltration of inflammatory cells compared to the normal group. Reduction of inflammatory cells is found in group treated with IPM and diclofenac. IPM: n-hexane-insoluble fraction of dichloromethane extracts of P. major; RA: Rheumatoid arthritis.
5. Discussion
Currently, increased interest in the therapeutic potential of medicinal plants is driving new research. These efforts reflect prophylactic or therapeutic efficacy, low toxicity and fewer side effects seen for many plant extracts compared to conventional synthetic drugs. In Indonesia, P. major has long been used for medical purposes, including anti-inflammation, and has been developed in pharmaceutical forms. However, reports on anti-inflammatory and anti-arthritic activity of P. major are limited. We analysed the effects of P. major on CFA-induced arthritic rats. Chlorophyll was removed from the dichloromethane extract of P. major leaves by partitioning with n-hexane to produce an n-hexane-insoluble fraction (IPM). This process was intended to maximise the secondary metabolite content of the extract.
CFA-induced arthritis is a well-established in vivo model used to investigate RA pathogenesis and to identify possible therapeutic targets. This model is similar to human RA in both pathological and serological aspects, including the involvement of paw oedema, arthrodynia, decreased body weight and cartilage degradation.1,21,26 CFA triggers inflammation characterised by redness and swelling in the digits, soles and ankles of examined animals. CFA in joints causes histamine, kinins and prostaglandins to be released into the bloodstream. These chemicals increase blood vessel permeability and blood flow to the inflamed region. This effect causes oedema, heat, erythema and discomfort. Granulocyte migration to sites of inflammation is also facilitated by increased vascular permeability.
In this study, paw swelling, arthritis index, arthritic incidence and haematological changes were used to assess disease progression. Treatment with IPM markedly suppressed the development of arthritis as measured by paw volume (Figs. 1 and 2), arthritic incidence (Supplementary Fig. 1) and arthritic index (Fig. 3).
The IPM reduced the volume of oedema in arthritic rats although activity was less compared to diclofenac. Diclofenac inhibited paw oedema by 19.71 % and the IPM at doses of 280 and 420 mg/kg BW inhibited paw oedema by 15.70 % and 15.94 % respectively. Rats in each group induced with CFA were assessed by arthritic incidence and progression of arthritis severity every four days until day 47, using arthritis index. Arthritic incidence was used to determine whether rats suffer from RA and arthritis index is a scale used to measure the severity of disease. Rats that developed RA were characterised by redness and swelling of toes and changes in the shape of the soles of the feet. Diclofenac and IPM reduced the incidence of RA by 80 % (Supplementary Fig. 1). In addition, IPM reduced AI. The AI of IPM for doses 280 and 420 mg/kg BW were 1.5 ± 0.15 and 1.42 ± 0.13 respectively, significantly different from diclofenac, 1.22 ± 0.12. IPM fractions are thus a source of anti-inflammatory compounds.
IPM treatments did not change WBC or lymphocyte levels but reduced neutrophil counts in arthritic rats. These findings were significantly different from RA control animals and diclofenac-treated rats (P < 0.05) (Fig. 4). Neutrophils are the most abundant leukocytes in inflamed joints, and the importance of these cells is demonstrated for both the initiation and progression of RA humans and murine models. Neutrophils release toxic products, such as reactive oxygen species, that are thought to be partly responsible for associated tissue destruction. Neutrophils are important to articular inflammation and modulation of neutrophil functions and represent a target for pharmacological action in arthritis.27,28 The IPM fraction capable of decreasing neutrophil migration may attenuate inflammatory processes in parallel with our previous findings18,19 and the study by Reina et al.29 The most important compounds in P. major, baicalein and aucubin, are known for antioxidant properties and as free radical scavengers. Baicalein is also known as an anti-inflammatory agent, specifically for its ability to inhibit inflammatory cytokine release in human mast cells.
In this study, the expression of cytokines, TNF-α and IL-6, known as inflammatory mediators of RA, were also analysed.30, 31, 32 TNF-α is the key cytokine for regulating the production of other inflammatory mediators in synovial tissue.33 These cytokines activate synoviocytes and chondrocytes and result in the secretion of matrix metalloproteinase to the synovial fluid. This matrix leads to cartilage and synovial membrane destruction.30,34 An increase in the expression of all these parameters confirms the induction of arthritis. IPM doses of 280 and 420 mg/kg significantly reduced TNF-α levels compared to RA controls but were not significantly different from diclofenac (Fig. 5, Supplementary Fig. 5). Elevated levels of IL-6 in synovial fluid may cause an increased influx of neutrophils to joints.35 IPM doses of 280 and 420 mg/kg significantly reduced IL-6 expression compared to RA control and diclofenac-treated animals (Supplementary Fig. 6). IPM-induced reduction in neutrophil levels (Fig. 4) may correlate with this reduction in IL-6 levels.
Histopathological data showed CFA-induced arthritis reflected in intense subcutaneous changes associated with granulomatous inflammation. IPM doses of 280 and 420 mg/kg BW reduced these features of inflammation in RA rats, including infiltration of inflammatory cells, hyperplasia in the synovium and disruption of cartilage (Fig. 6). Suppression of disease progression suggests that IPM might prevent arthritic progression by reducing joint inflammation reactions and paw tissue destruction.
Our findings are consistent with previous research that reports anti-inflammatory and anti-arthritic activities of P. major in various pre-clinical models. Ursolic acid and oleanolic acid likely contributed to their anti-inflammatory and anti-arthritic activities. Ursolic acid is reported as a selective inhibitor of COX-2 catalysed prostaglandin,16 and targets MMP-activating pathways including c-Jun N-terminal kinase (JNK), NF-κB and mitogen-activated protein kinase/protein kinase B (MAPK/Akt), which limits invasion by immune cells.36 Moreover, ursolic acid induces apoptosis in synovial fibroblasts in RA by increasing expression of SP1 and inducing degradation of Mcl-1.37 It also suppressed leukocyte migration and prostaglandin E2 (PGE2) production in CFA-induced arthritis38 and decreased expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-21 and IL-17) and oxidative stress markers (nitrotyrosine and iNOS) in collagen-induced arthritis (CIA) in mice.39 Oleanolic acid reduced expression and production of inflammatory mediators, such as cytokines and matrix metalloproteinase (MMP)-1/3, in ankle joint tissue and synovial fibroblasts in CIA.40 It also regulates the process of protein synthesis and MMP-3 secretion and affects the proteolytic activity of MMP-3 in tissues of osteoarthritic articular cartilage.41
6. Conclusions
The hexane-insoluble fraction of P. major dichloromethane extract prevents the development of arthritis in an RA rat model by decreasing inflammatory cells and the expression of TNF-α and IL-6. Data from this study support the need for further IPM analysis, including intensive mechanistic studies and documented anti-arthritic effects in other animal models.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jtcme.2021.07.006.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Mossiat C., Laroche D., Prati C., Pozzo T., Demougeot C., Marie C. Association between arthritis score at the onset of the disease and long-term locomotor outcome in adjuvant-induced arthritis in rats. Arthritis Res Ther. 2015;17(1):1–12. doi: 10.1186/s13075-015-0700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z., Bozec A., Ramming A., Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(1):9–17. doi: 10.1038/s41584-018-0109-2. [DOI] [PubMed] [Google Scholar]
- 3.Parhizgar S., Hosseinian S., Hadjzadeh M.A.R., et al. Renoprotective effect of Plantago major against nephrotoxicity and oxidative stress induced by cisplatin. Iran. J. Kidney Dis. 2016;10(4):182–188. doi: 10.1111/aor.12232. [DOI] [PubMed] [Google Scholar]
- 4.Zubair M., Nybom H., Lindholm C., Brandner J.M., Rumpunen K. Promotion of wound healing by Plantago major L. leaf extracts - ex-vivo experiments confirm experiences from traditional medicine. Nat Prod Res. 2016;30(5):622–624. doi: 10.1080/14786419.2015.1034714. [DOI] [PubMed] [Google Scholar]
- 5.Amini M., Kherad M., Mehrabani D., Azarpira N., Panjehshahin M.R., Tanideh N. Effect of Plantago major on burn wound healing in rat. J Appl Anim Res. 2010;37(1):53–56. doi: 10.1080/09712119.2010.9707093. [DOI] [Google Scholar]
- 6.Abdulghani M.A., Hamid I., Al-Naggar R.A., Osman M.T. Potential antidiabetic activity of Plantago major leaves extract in Streptozocin-induced diabetic rats. Res J Pharmaceut Biol Chem Sci. 2014;5(2):896–902. [Google Scholar]
- 7.Nazarizadeh A., Mikaili P., Moloudizargari M., Aghajanshakeri S. Therapeutic uses and pharmacological properties of Plantago major L. And its active constituents antisecretory effect of hydrogen sulfide on gastric acid secretion and the involvement of nitric oxide view project. J. Basic Appl. Sci. Res. 2013;3(9):212–221. https://www.researchgate.net/publication/256494446 [Google Scholar]
- 8.Zubair M., Widén C., Renvert S., Rumpunen K. Water and ethanol extracts of Plantago major leaves show anti-inflammatory activity on oral epithelial cells. J. Tradit. Complement. Med. 2019;9(3):169–171. doi: 10.1016/j.jtcme.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussan F., Mansor A.S., Hassan S.N., Kamaruddin N.T.T.N.E., Budin S.B., Othman F. Anti-inflammatory property of Plantago major leaf extract reduces the inflammatory reaction in experimental acetaminophen-induced liver injury. Evid-Based Complementary Altern. Med. 2015 doi: 10.1155/2015/347861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adom M.B., Taher M., Mutalabisin M.F., et al. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017;96(May):348–360. doi: 10.1016/j.biopha.2017.09.152. [DOI] [PubMed] [Google Scholar]
- 11.Chiang L., Chiang W., Chang M., Lin C. In: Antiviral and Immunomodulatory Effects of Plantago Major and Plantago Asiatica. Am. J. Chin. Med. Cytotoxic Vitro., editor. vol. 31. 2003. pp. 225–234. 2. [DOI] [PubMed] [Google Scholar]
- 12.Handjieva N., Spassov S., Bodurova G., et al. Majoroside, an iridoid glucoside from Plantago major. Phytochemistry. 1991;30(4):1317–1318. doi: 10.1016/S0031-9422(00)95224-5. [DOI] [Google Scholar]
- 13.Kartini K. A.Azminah. Chromatographic fingerprinting and clustering of Plantago major L. from different areas in Indonesia. Asian J Pharmaceut Clin Res. 2012;5(February):191–195. [Google Scholar]
- 14.Shirley K.P., Windsor L.J., Eckert G.J., Gregory R.L. Vitro effects of Plantago major extract, aucubin, and baicalein on Candida albicans biofilm formation, metabolic activity, and cell surface hydrophobicity. J Prosthodont. 2015 doi: 10.1111/jopr.12411. [DOI] [PubMed] [Google Scholar]
- 15.Samuelsen A.B., B Samuelsen A. A review: the traditional uses, chemical constituents and biological activities of Plantago major L. J Ethnopharmacol. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringbom T., Segura L., Noreen Y., Perera P., Bohlin L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J Nat Prod. 1998;61(10):1212–1215. doi: 10.1021/np980088i. [DOI] [PubMed] [Google Scholar]
- 17.Chiang L.C., Ng L.T., Chiang W., Chang M.Y., Lin C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003;69(7):600–604. doi: 10.1055/s-2003-41113. [DOI] [PubMed] [Google Scholar]
- 18.Triastuti A. Aktivitas penghambatan migrasi leukosit ekstrak diklorometana daun sendok (Plantago major) pada mencit yang diinduksi tioglikolat. Eksakta. 2019;19:208–215. doi: 10.20885/eksakta.vol19.iss2.art11. [DOI] [Google Scholar]
- 19.Triastuti A., Indrati O., Hayati F. In: The 5th International Mediterranean Symposium on Medicinal and Aromatic Plants (5MESMAP). Cappadocia. Sekeroglu N., editor. 2019. Development of microemulsion containing Plantago major extracts: formulation and evaluation of topical anti-inflammatory activities; pp. 211–215.http://www.mesmap.com/FileUpload/ks831353/File/mesmap-5_proceeding_book_06062019_yayinlanan.pdf [Google Scholar]
- 20.Wang Y., Lu Y., Wang J., et al. Preparation and analysis of active rat model of rheumatoid arthritis with features of TCM toxic heat-stasis painful obstruction. J Tradit Chin Med Sci. 2015;2(3):166–172. doi: 10.1016/j.jtcms.2016.01.002. [DOI] [Google Scholar]
- 21.Jones G.W., Hill D.G., Sime K., Williams A.S. In vivo models for inflammatory arthritis. Methods Mol Biol. 2018;1725:101–118. doi: 10.1007/978-1-4939-7568-6_9. [DOI] [PubMed] [Google Scholar]
- 22.Damerau A., Gaber T. Modeling rheumatoid arthritis in vitro: from experimental feasibility to physiological proximity. Int J Mol Sci. 2020;21(21):1–25. doi: 10.3390/ijms21217916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avantor . Rutland; 2015. Safety Data Sheet Plantago Major Tincture.https://us.vwr.com/assetsvc/asset/en_US/id/16490607/contents [Google Scholar]
- 24.Newbould B.B. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol. 1963;21(1):127–136. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smit H.F., Kroes B.H., Van Den Berg A.J.J., et al. Immunomodulatory and anti-inflammatory activity of Picrorhiza scrophulariiflora. J Ethnopharmacol. 2000;73(1-2):101–109. doi: 10.1016/S0378-8741(00)00268-3. [DOI] [PubMed] [Google Scholar]
- 26.Eze F.I., Uzor P.F., Ikechukwu P., Obi B.C., Osadebe P.O. In vitro and in vivo models for anti-inflammation: an evaluative review. ITPS. 2019;2(2):3–15. doi: 10.36922/itps.v2i2.775. [DOI] [Google Scholar]
- 27.Rosas E.C., Correa L.B. 2017. Henriques M das G. Neutrophils in Rheumatoid Arthritis: a Target for Discovering New Therapies Based on Natural Products. [DOI] [Google Scholar]
- 28.Chen W., Wang Q., Ke Y., Lin J. Neutrophil function in an inflammatory milieu of rheumatoid arthritis. J Immunol Res. 2018:2018. doi: 10.1155/2018/8549329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reina E., Al-Shibbani N., Allam E., et al. The effects of Plantago major on the activation of the neutrophil respiratory burst. J. Tradit. Complement. Med. 2013;3(4):268–272. doi: 10.4103/2225-4110.119706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grötsch B., Bozec A., Schett G. In vivo models of rheumatoid arthritis. Methods Mol Biol. 2019;1914:269–280. doi: 10.1007/978-1-4939-8997-3_14. [DOI] [PubMed] [Google Scholar]
- 31.Mateen S., Zafar A., Moin S., Khan A.Q., Zubair S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta. 2016;455:161–171. doi: 10.1016/j.cca.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998 Jan 1;391(6662):82–86. doi: 10.1038/34184. PMID: 9422509. [DOI] [PubMed] [Google Scholar]
- 33.Vasanthi P., Nalini G., Rajasekhar G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: a review. J Rheumatol. 2007;10(4):270–274. doi: 10.1111/j.1479-8077.2007.00305.x. [DOI] [Google Scholar]
- 34.Burska A., Boissinot M., Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediat Inflamm. 2014:2014. doi: 10.1155/2014/545493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson G.D., Hauser S.D., McGarity K.L., Bremer M.E., Isakson P.C., Gregory S.A. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97(11):2672–2679. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashyap D., Tuli H.S., Sharma A.K. Ursolic acid (UA): a metabolite with promising therapeutic potential. Life Sci. 2016;146:201–213. doi: 10.1016/j.lfs.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Kim E.Y., Sudini K., Singh A.K., et al. Ursolic acid facilitates apoptosis in rheumatoid arthritis synovial fibroblasts by inducing SP1-mediated Noxa expression and proteasomal degradation of Mcl-1. Faseb J. 2018;32(11):6174–6185. doi: 10.1096/fj.201800425R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang S.-Y., Yoon S.-Y., Roh D.-H., et al. The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J Pharm Pharmacol. 2008;60(10):1347–1354. doi: 10.1211/jpp/60.10.0011. [DOI] [PubMed] [Google Scholar]
- 39.Baek S.Y., Lee J., Lee D.G., et al. Ursolic acid ameliorates autoimmune arthritis via suppression of Th17 and B cell differentiation. Acta Pharmacol Sin. 2014;35(9):1177–1187. doi: 10.1038/aps.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi J.K., Kim S.W., Kim D.S., et al. Oleanolic acid acetate inhibits rheumatoid arthritis by modulating T cell immune responses and matrix-degrading enzymes. Toxicol Appl Pharmacol. 2016;290:1–9. doi: 10.1016/j.taap.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Kang D.G., Lee H.J., Kim K.T., Hwang S.C., Lee C.J., Park J.S. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J Physiol Pharmacol. 2017;21(2):197–204. doi: 10.4196/kjpp.2017.21.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.