Key Teaching Points.
-
•
Leadless pacemaker (PM) implantation (Micra; Medtronic, Minneapolis, MN) through the mechanical tricuspid valve (TV) can be done.
-
•
The size of the implanted mechanical TV should be considered first. The diameter of the Micra device is 6.7 mm, and the delivery catheter is 23F (about 7.7 mm).
-
•
Although our procedure was successful, more studies and discussions are needed to determine whether leadless PM could be a reasonable alternative option for patients with the mechanical TV requiring PM.
Introduction
The leadless pacemaker (LPM) has become an effective alternative to the traditional single-chamber ventricular transvenous pacemaker (PM) in selected patient populations.1 The LPM does not require a subcutaneous pocket or use of a transvenous lead, potentially mitigating many of the short- and long-term risks inherent to transvenous PMs, including infections, lead fractures, and venous occlusions.2
In patients who have a prosthetic tricuspid valve (TV), 5 options can be considered for the PM ventricular lead implantation: implant epicardial leads; implant a standard right ventricular transvenous lead; implant a para-Hisian lead; implant a coronary sinus (CS) lead for left ventricular (LV) pacing only; or implant an LPM.3
However, only 2 options are available in patients with a mechanical TV: implantation of an epicardial lead or implantation of a CS lead for LV pacing. Further, currently, implanting an LPM through a mechanical TV is not yet considered as an alternative option. Therefore, we report the experience of an LPM implantation in an Ebstein anomaly patient with a mechanical TV as a last resort.
Case report
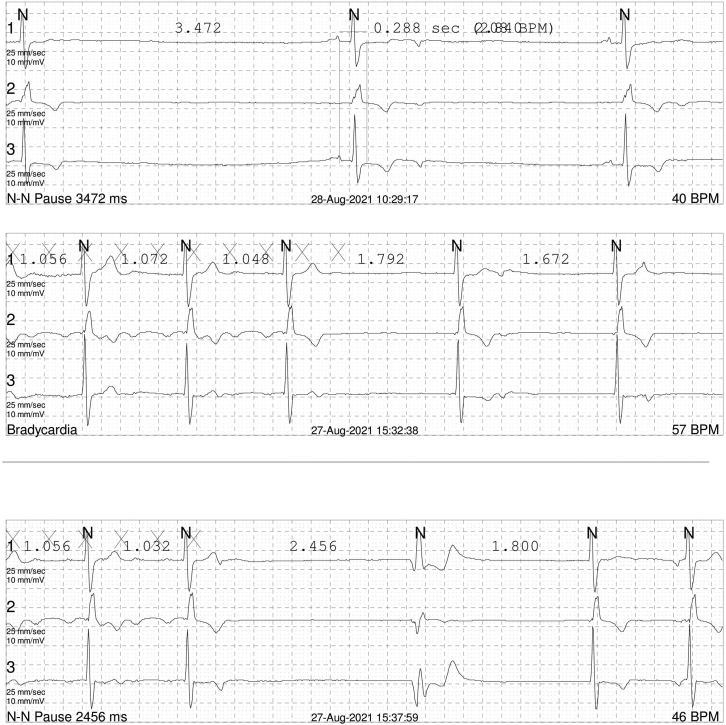
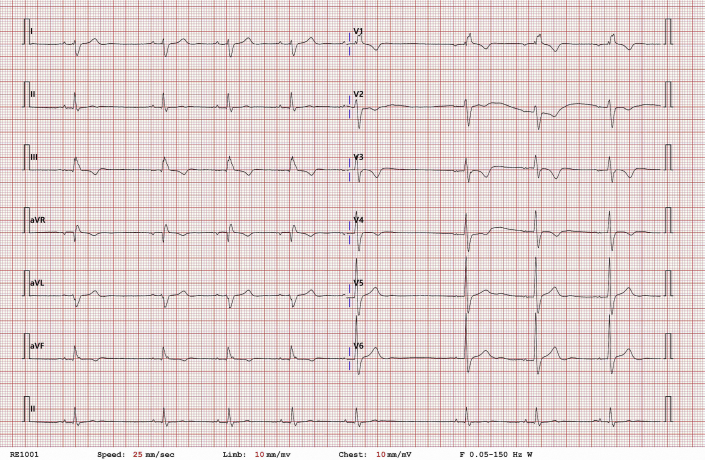
A 34-year-old man diagnosed with an Ebstein anomaly at age 5 and underwent a mechanical TV replacement surgery (TVR) at age 7 was referred to a cardiac implantable electronic device clinic for recurrent dizziness and near syncope. He was diagnosed with paroxysmal atrial flutter and tachycardia-bradycardia syndrome 6 years prior, and, at that time, a mechanical TV thrombosis and valve malfunction were also noted. Hence, he had a redo TVR with a mechanical valve and epicardial PM implantation performed simultaneously. After the operation, the epicardial PM system became infected. Despite continuous antibiotic treatment, 6 minor debridement surgeries, and 2 advancement flap surgeries, the infection was not controlled. He had no choice but to remove the total epicardial PM system (third open heart surgery). Fortunately, he was in a stable state without the PM for several years, but dizziness recently occurred again, and the Holter monitoring documented paroxysmal atrial flutter followed by a 3.5-second-long atrial pause accompanied with symptoms (Supplemental Figure 1).
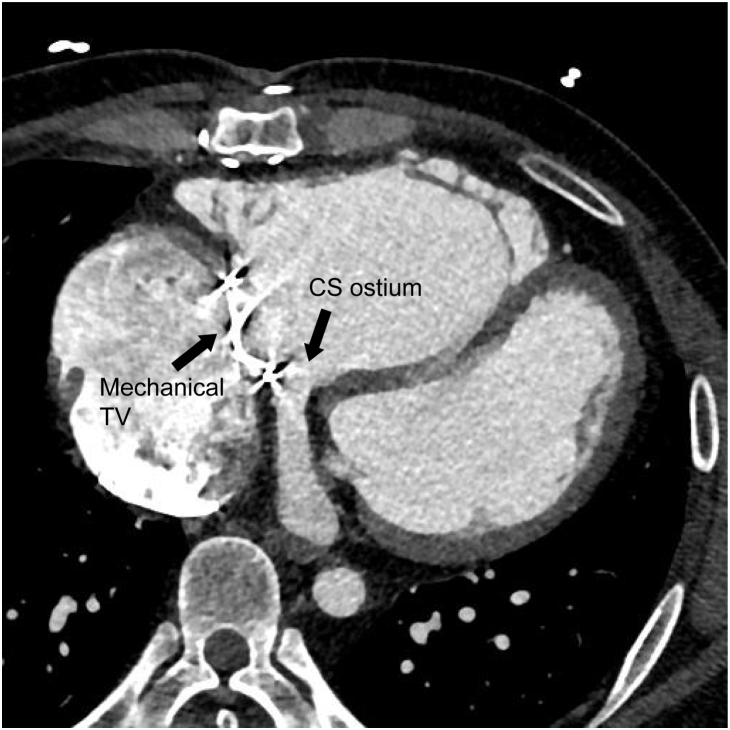
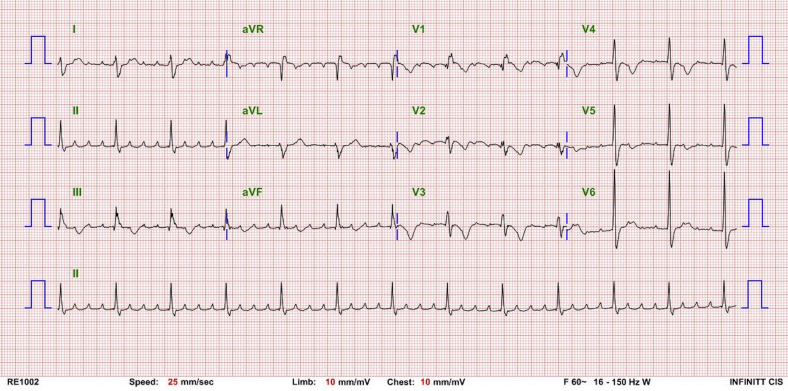
One of the 2 options, including implanting a CS lead or redo epicardial PM, was considered. However, a preoperative computed tomography scan showed that the CS ostium was below the mechanical valve (Figure 1). Therefore, only 2 alternative options were available: an AAI-type transvenous PM or redo epicardial PM. An electrophysiologic (EP) study was performed to evaluate the atrioventricular (AV) conduction and status of the right atrial scar. Right atrial pacing was possible but 2:1 AV block occurred at an atrial pacing cycle length of 700 ms, suggesting a poor AV node function. The patient was awake during the EP study, and considering his age and the circumstances of the EP lab, we assumed that a hypervagotonia would not affect the AV node function during the EP study. Detailed EP study around the His bundle area was impossible owing to his distorted anatomy (CS ostium under mechanical TV). Further, his baseline ECG exhibited complete right bundle branch block (Supplemental Figure 2) and he had a redo TVR, which could not exclude the possibility of any damage to the AV node during the surgery. In addition, his average heart rate was around 60 beats/min and maximal heart rate around 100 beats/min during atrial tachycardia without any AV nodal blocking agent on the Holter monitoring and 12-lead ECG (Supplemental Figures 3 and 4). For the above reasons, we concluded that implanting only an AAI-type PM was not the safest PM option for this patient. Thus, a redo epicardial PM implantation was recommended. It would be the fourth open heart surgery for the patient. However, the thoracic surgeon was worried about difficulty in finding a portion of the ventricle with an acceptable pacing threshold owing to the prior surgery. We had an in-depth discussion with the patient about the possible options and, finally, we decided to try a Micra (Micra Transcatheter Pacing System; Medtronic, Minneapolis, MN) implantation; however, it was currently not recommended.
Figure 1.
Computed tomography image of the patient’s heart showed the coronary sinus ostium under the mechanical tricuspid valve. CS = coronary sinus; TV = tricuspid valve.
Before the procedure, we simulated whether the Micra device could freely move in and out through the mechanical valve (Supplemental Video 1, using a dummy model of a St. Jude Mechanical Heart Valve 27 mm and Micra device). In this simulation, the Micra device could freely pass through the fully opened leaflet of the mechanical heart valve. The patient’s TV valve was a St. Jude Mechanical Heart Valve 31 mm model, and the diameter of the Micra device was 6.7 mm. Therefore, we assured that the implantation of the Micra through a mechanical TV would be possible.
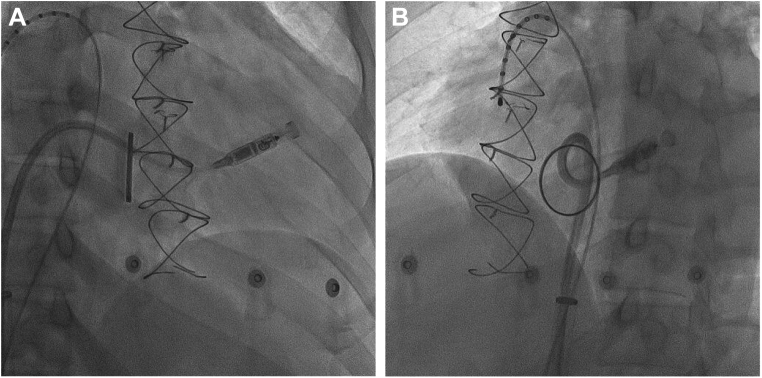
Then the procedure was performed. After puncturing of the right femoral vein with an 8F short sheath, a sequential dilation of the entry site was done until the 27F Micra introducer sheath was inserted. After introduction of the delivery catheter with the Micra device into the right atrium, the mechanical valve was crossed with the Micra delivery sheath (Supplemental Video 2A and 2B). Some resistance was felt because the distal end of the delivery catheter, which contained the Micra device, was slightly bigger than the other parts of the delivery catheter. This resistance was overcome by gently pushing the catheter. Since the diameter of the main body of the delivery catheter was much smaller than that of the fully opened valve leaflet, no resistance was felt during subsequent manipulation of the delivery catheter. The typical apical septal location was not suitable to apply enough tip pressure because the right ventricle was markedly enlarged, and there was a limitation of movement of the delivery system owing to the mechanical valve. After several attempts, we were able to successfully deploy the Micra device in the right ventricular outflow tract (Figure 2).
Figure 2.
Fluoroscopy image of the final position and reverse curve of the Micra delivery catheter. A: Right anterior oblique (20-degree) fluoroscopy image. B: Left anterior oblique (35-degree) fluoroscopy image.
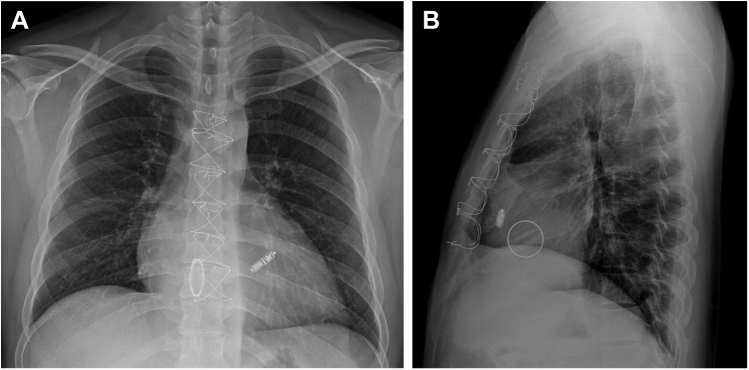
The pull and hold test revealed that 3 tines were engaged, and the electrical measurements were within the recommended values: R wave: >20 V; impedance: 1010 ohms; threshold: 0.5 V @ 0.24 ms. The electrical parameters were stable after the pull and hold tests; we cut and removed the entire tether. The withdrawal of the delivery catheter was relatively easy, and no resistance was felt during withdrawal of the delivery system (Supplemental Video 3). Subsequent fluoroscopy and echocardiography did not show any abnormal movement of the mechanical valve. The total procedural and fluoroscopy times were 60 minutes and 16.5 minutes, respectively. The periprocedural anticoagulation strategy was as follows: The day before the procedure, the patient’s international normalized ratio (INR) was 2.63. The nighttime warfarin dosing for the day before the procedure was skipped and the INR of the procedure day was 2.42. Although he was in the therapeutic range of the INR, we administered a bolus of 3000 international units of unfractionated heparin for the complexity of the procedure. A heparinized saline drip through the introducer was also maintained during the procedure. Hemostasis was successfully achieved with a figure-of-8-suture and there were no complications during the hospitalization. The patient was discharged after a week. Now he visits our hospital regularly, without any further symptoms. Figure 3 shows postprocedure chest radiography image.
Figure 3.
Chest radiography after the procedure. A: Posteroanterior view. B: Lateral view.
Discussion
TV surgery carries a significant risk of conduction disorders requiring an implantable electronic device.3 The implantation rate of PMs has tended to decrease over the decades, but rates as high as 27% have recently been described after a TVR.4 However, implanting cardiac electronic devices in patients who had a TV surgery is a challenging procedure. Especially, as described above, there are only 2 options to implant a ventricular lead of a PM in patients with a mechanical TV: (1) LV-only pacing through the CS like cerclage pacing,5 or (2) epicardial PM. And considering the degenerative nature of conduction system disorders, implanting an only AAI-type PM is not a reliable solution for this type of patient.
In this report, the mechanical TV was unintentionally placed over the CS ostium; therefore there was no option other than an epicardial PM implantation. Since the patient had already undergone 3 open heart surgeries, it was expected that finding epicardial sites with an acceptable pacing threshold would be difficult. Furthermore, his AV conduction was not good for atrial pacing only. The patient was a young 34-year-old man who strongly wanted an alternative method other than the fourth open-heart surgery. So, we decided to try to implant the LPM through the mechanical TV as a last resort.
The following points should be considered when deciding whether to perform the procedure as in this case. Above all, the size of the implanted mechanical TV is most important. The diameter of the Micra device is 6.7 mm, and the delivery catheter is 23F (about 7.7 mm). Hence, the diameter of 1 side of the fully opened mechanical TV should be greater than 23F. After passing through the mechanical TV, the delivery catheter was not difficult to manipulate owing to the main body of the delivery catheter being smaller than the Micra device. Since the TV is firmly fixed, it is not necessary to focus on a typical apical septal location to apply adequate tip pressure and a good reverse curve of the delivery catheter. Needless to say, preprocedural imaging for the planning and guidance of the procedure is mandatory. Especially, a thoughtful discussion is required about the trajectory of the device/delivery catheter through imaging. The right internal jugular vein has been demonstrated as a safe alternative for LPM implantations in adults and pediatric patients6,7; a superior approach may be appropriate depending on the case to avoid any significant catheter tip deflection after going across the mechanical TV. After deployment of the device and performance of the pull and hold test, the recapture cone should remain within the right ventricle so that the tether does not get caught on the mechanical TV. Despite the lower catheter profile of the delivery catheter without a Micra device, there is at least a theoretical concern of an oblique withdrawal across the valve resulting in catheter entrapment along the edge of the coaptation line, where a pincer entrapment is possible. To avoid this situation, the use of adenosine may be considered prior to the catheter withdrawal to temporarily suspend the valvular motion. This has been conventionally taught as a technique to mitigate catheter entanglement risk if a mechanical mitral valve is inadvertently crossed during a left atrial ablation procedure. Finally, in order to minimize any damage to the mechanical TV, it is recommended to shorten the procedure time as much as possible.
Conclusion
In this case, we performed an implantation of an LPM through a mechanical TV as a last resort. Although our procedure was successful, more studies and discussion are needed to determine whether the LPM could be a reasonable alternative option for patients with mechanical TVs requiring a PM.
Acknowledgments
We thank Ms Jae-Hee Kim from Medtronic Korea for her assistance and Mr John Martin for his linguistic assistance.
Footnotes
Funding Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The authors have no conflicts to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2022.01.010.
Appendix. Supplementary data
Preprocedural simulation video using a dummy model of a St. Jude Mechanical Heart Valve 27mm and Medtronic Micra device (diameter 6.7mm). In this simulation, the Micra device could freely pass through the fully opened leaflet of the mechanical heart valve.
AFailed attempt to cross the mechanical tricuspid valve with Micra delivery catheter. The distal end of the delivery catheter, which contained the Micra device, was slightly bigger than the other parts of the delivery catheter. Hence, some resistance was felt during the attempt to cross the mechanical tricuspid valve, but the resistance was overcome by gently pushing the catheter.2
2BSuccessful advancement of the Micra delivery catheter through right ventricle across the mechanical tricuspid valve.3
Withdrawal of the Micra delivery catheter after Micra device deployment and removal of the tether. It was relatively easy, and we felt no resistance during the withdrawal of the delivery catheter.4
Supplemental Figure 1.
Representative electrocardiography in Holter monitoring of the patient. The image showed paroxysmal atrial flutter followed by a 3.5-second-long atrial pause during daytime.
Supplemental Figure 2.
Baseline 12-lead electrocardiography of the patient. It showed complete right bundle branch block.
Supplemental Figure 3.
Summarized results of the patient Holter monitoring. The average heart rate was around 60 bpm and maximal heart rate around 100bpm during atrial tachycardia. The patient was 36-year-old, and any atrioventricular nodal blocking or antiarrhythmic agents were not administered.
Supplemental Figure 4.
The 12-lead electrocardiography of the patient during atrial tachycardia. It showed 4:1 atrioventricular conduction. Any atrioventricular nodal blocking or antiarrhythmic agents were also not administered.
References
- 1.Reynolds D., Duray G.Z., Omar R., et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 2.Pagan E., Gabriels J., Khodak A., et al. Safety of leadless pacemaker implantation in the very elderly. Heart Rhythm. 2020;17:2023–2028. doi: 10.1016/j.hrthm.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Martins R.P., Galand V., Leclercq C., Daubert J.C. Cardiac electronic implantable devices after tricuspid valve surgery. Heart Rhythm. 2018;15:1081–1088. doi: 10.1016/j.hrthm.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Mar P.L., Angus C.R., Kabra R., et al. Perioperative predictors of permanent pacing and long-term dependence following tricuspid valve surgery: a multicentre analysis. Europace. 2017;19:1988–1993. doi: 10.1093/europace/euw391. [DOI] [PubMed] [Google Scholar]
- 5.Cho M.S., Chon M.K., Choi J.H., et al. Cerclage parahisian septal pacing through the septal perforator branch of the great cardiac vein: bedside-to-bench development of a novel technique and lead. Heart Rhythm. 2019;16:1834–1840. doi: 10.1016/j.hrthm.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolek M.J., Crossley G.H., Ellis C.R. Implantation of a MICRA leadless pacemaker via right internal jugular vein. JACC Clin Electrophysiol. 2018;4:420–421. doi: 10.1016/j.jacep.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Surti A.K., Ambrose M., Cortez D. First description of a successful leadless pacemaker implantation via the left internal jugular vein (in a 20 kg patient) J Electrocardiol. 2020;60:1–2. doi: 10.1016/j.jelectrocard.2020.02.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preprocedural simulation video using a dummy model of a St. Jude Mechanical Heart Valve 27mm and Medtronic Micra device (diameter 6.7mm). In this simulation, the Micra device could freely pass through the fully opened leaflet of the mechanical heart valve.
AFailed attempt to cross the mechanical tricuspid valve with Micra delivery catheter. The distal end of the delivery catheter, which contained the Micra device, was slightly bigger than the other parts of the delivery catheter. Hence, some resistance was felt during the attempt to cross the mechanical tricuspid valve, but the resistance was overcome by gently pushing the catheter.2
2BSuccessful advancement of the Micra delivery catheter through right ventricle across the mechanical tricuspid valve.3
Withdrawal of the Micra delivery catheter after Micra device deployment and removal of the tether. It was relatively easy, and we felt no resistance during the withdrawal of the delivery catheter.4