Abstract
Spirulina (blue-green algae) contains a wide range of nutrients with medicinal properties which include β-carotene, chromium, and moderate amounts of vitamins B12. This study aims to determine the preventive effect of spirulina against bone fragility linked to type 2 diabetes mellitus. Thirty Sprague-Dawley rats were divided into five groups (n = 6) and diabetes was induced using streptozocin. Rats with a plasma glucose level of 10 mmol/L and above were orally treated for twelve weeks with either a single dose of spirulina, metformin, or a combined dose of spirulina + metformin per day. After the treatment, blood and bones were taken for biochemical analysis, three-dimensional imaging, 3-point biomechanical analysis, histology imaging and gene expression using qPCR. Results showed that diabetes induction and treatment with metformin caused destruction in the trabecular microarchitecture of the femur bone, reduction in serum bone marker and expression of bone formation marker genes in the experimental rats. Spirulina supplementation showed improved trabecular microarchitecture with a denser trabecular network, increased 25-OH vitamin D levels, and lowered the level of phosphate and calcium in the serum. Biomechanical tests revealed increased maximum force, stress strain, young modulus and histology images showed improvement in regular mesh and an increase in osteoblasts and osteocytes. There was an increase in the expression of bone formation marker osteocalcin. The results suggest that spirulina supplementation was more effective at improving bone structural strength and stiffness in diabetic rats compared to metformin. Spirulina may be able to prevent T2DM-related brittle bone, lowering the risk of fracture.
Keywords: Spirulina, Osteoporosis, Diabetes
Graphical abstract
1. Introduction
Diabetes is a metabolic disorder in which the body either produces insufficient amount of insulin to regulate blood glucose levels or the body cells are no longer sensitive towards the produced insulin.1 According to the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the three most common types of diabetes are: Type 1 diabetes mellitus, type 2 diabetes mellitus (T2DM) and gestational diabetes.2 People with diabetes have an increased risk of developing a number of serious health problems. Consistent high blood glucose levels lead to serious complications affecting the heart and blood vessels (cardiovascular diseases), eyes (diabetic retinopathy), kidneys (diabetic nephropathy), nerves (diabetic neuropathy), teeth (periodontal diseases) and limbs.
Besides the above mentioned complications, diabetes mellitus-induced bone fragility is also a serious, yet often neglected complication. Persistently elevated blood glucose levels (hyperglycemia) cause increased non-enzymatic glycation of proteins, lipids and nucleic acids and leads to the formation of advanced glycation end products (AGEs).3 AGEs increase production of reactive carbonyl and oxygen species (ROS) causing oxidative damage that led to bone inflammatory responses. AGEs also cause inhibition of osteoblast proliferation and differentiation, impair the crosslinking of collagen and production of osteocalcin, downregulate bone metabolism, decrease bone density and induce apoptosis of osteoblasts.3
In addition, some oral anti-diabetic drugs have been experimentally and clinically shown to have an association with adverse effects on the skeleton.4 Metformin belongs to the biguanide class of drugs and is frequently prescribed as the first-line therapy for the treatment of T2DM. Metformin acts as an insulin sensitizer to indirectly activate AMP-activated protein kinase (AMPK) pathways in multiple tissues.5 It inhibits hepatic gluconeogenesis, fatty-acid synthesis, and stimulates glucose uptake and fatty-acid oxidation. Although metformin was reported to be able to effectively reduce osteoclast numbers and attenuate alveolar bone resorption by modulating the ratio between the receptor activator of nuclear factor κB-ligand (RANKL) and osteoprotegerin (OPG) (RANKL/OPG ratio),6 there is a study showing that metformin reduces the expression of osteopontin, thus causing a negative effect on bone structure.7 A recent study reported a decrease in bone formation markers in patients using metformin.8 According to Jager et al.,9 long-term metformin treatment increases the risk of vitamin B12 deficiency, which leads to elevated homocysteine levels. In a similar study, Vaes et al.,10 found that vitamin B12 deficiency increased homocysteine and methylmalonic acid (MMA) levels, which stimulated osteoclastogenesis in vitro, implying that vitamin B12 deficiency may indirectly increase osteoclastogenesis through its effect on MMA and homocysteine levels.
Therefore, new compounds with lesser or no side effects are of interest especially in patients with higher risks of developing bone fragility e.g., obese people with diabetes. Malaysia is a biodiversity rich country whose natural products may contain a plant/animal/fungus that contains substances that could prevent the development of bone fragility.
Spirulina, a filamentous cyanobacterium (previously called unicellular blue-green algae) which is rich in minerals and growth factors, might be one of the natural resources that have the potential ability to improve bone metabolism.11 Spirulina is rich in protein (60–70%) and contains a broad range of nutrients that include B-complex vitamins (vitamin B12), Pro-vitamin (β-carotene), vitamin E, minerals (calcium, chromium and magnesium) and gamma-linolenic acid.12 In the United Nations world food conference, spirulina was declared as “the best food for tomorrow”, and, in recent years, it has been gaining popularity as a food supplement. Spirulina was reported in a previous study as a powerful anti-viral, anti–cancer, hypo-cholesterolemic and health improvement agent, thus, it is highly promising as an ideal nutraceutical and pharmaceutical ingredient.13 A study by Simon et al.,14 has shown that spirulina regulates diabetic pathological pathways through its anti-oxidation and free-radical scavenging capability, yet little is known about its protective effect on bone health. Therefore, this study will investigate the protective effect of spirulina on diabetes induced bone fragility and compare its effect with metformin.
2. Materials and methods
2.1. Reagents and chemicals
The reagents and chemicals used and their sources were as follows: chloroform (Mallinckrodt Chemicals, USA); streptozotocin, (STZ, Aldrich, USA); NaCl (Fisher Chemicals, England); citric acid monohydrate (AnApur, Malaysia); trisodium citrate dihydrate (Fisher, UK); metformin and ketamine (Troy Laboratories, Australia); xylazine (Indian Immunologicals, India); spirulina (Alpha Active Industries Sdn Bhd, Malaysia), boric acid and neutral buffered saline (R & M Chemicals, UK); haematoxylin, eosin and paraffin wax (Chem Soln, Malaysia) and xylene (Bendosen Chemicals, Malaysia).
Spirulina used in this experiment was obtained from Alpha Active Industries Sdn Bhd, Malaysia. Analysis provided showed that the spirulina sample used in this experiment contains; crude protein, ash, lipid, carbohydrate, sugar, fiber, chlorophyll, phycocyanin, calcium, chromium, sodium, magnesium, gamma-linolenic acid, γ-linolenic acid, vitamin B12, vitamin D, total carotenoids (β-carotene). This is according to analysis done by Pharmaceutical Industries, Ministry of Industry Myanmar.
2.2. Preparation of animals and treatment
Prior to the experiment pre-test dose – response studies were done by current research group using different doses ranging from 50 to 1000 mg/kg with 300mg/kg giving the best result (Data not shown). This dose was adopted in our previous studies on in vivo and in vitro anti-diabetic and antioxidant activity of spirulina published in 2019.15
Thirty female Sprague–Dawley rats (4 weeks old, weighing approximately 180–200 g) were purchased from Sinar Scientific Sdn. Bhd., Malaysia. The rats were kept in the UCSI University animal holding unit for 2 weeks for acclimatization under a standard temperature (24–28 °C), relative humidity (60–70%) and 12 h:12 h light-dark cycle with free access to water and pellets. This study and procedures were performed in accordance with the national guidelines for the use and care of laboratory animals and were approved by the Universiti Kebangsaan Malaysia Animal Ethics Committee (ethical approval number: UCSI/2016/PATRICK/23-NOV./801-JAN. - 2017-DEC.-2018). The rats were divided into five groups (n = 6). Diabetes was induced in 20 rats (groups II – V) via an intraperitoneal injection of STZ (50 mg/kg) according to the method described by Ekeuku et al.16 and El-Mottaleb et al.17 Seven days after diabetes induction, blood glucose levels were measured, and animals with blood glucose levels exceeding 10 mM were considered diabetic. The rats were treated as follows: normal control (group I); saline-diabetic control (group II); metformin (300 mg/kg) (group III); spirulina (300 mg/kg) (group IV) and metformin (300 mg/kg) + spirulina (300 mg/kg) (group V). Drugs were dissolved in 0.9% NaCl (saline) and orally administered to the animals once daily for 12 weeks following the above experimental grouping. The drug doses were adopted from Devesh et al.12; La Fontaine et al.18; Borst et al.19; Prakash et al.20 and has already been published in previous study by Okechukwu et al.15
2.3. Biochemical and bone assessment test
2.3.1. Treatment of the animals and harvest of bones
After the treatment period, blood was collected, and micro-CT imaging was carried out in vivo. After micro-CT imaging, the rats were anaesthetized using xylazil, zoletil and ketamine (1:2:1) at a dose of 0.2 ml/100 g body weight and blood was obtained intravenously from the tail vein and sent to a commercial pathology laboratory for the respective biochemical (HOMA-IR, HbA1c, albumin and alkaline phosphatase) and serum bone (calcium, phosphate, and 25-OH vitamin D) parameters analysis. After blood collection, the rats were euthanised, and the femurs were harvested within 1 h after euthanization. Fur, skin and muscles from the legs were removed with surgical blades, scissors and tweezers until the whole bone and the hip joint were exposed. The femur was separated from the hip joint and the tibia and rinsed in saline. Whole femurs for gene expression studies were immediately snap frozen in liquid nitrogen and put in a centrifuge tube before storing in −80 °C for further studies.
2.3.2. In vivo micro-computed tomography (micro-CT)
Micro-architectural bone parameters were assessed using an in vivo micro-CT system (Skyscan 1076 scanner, Scanco Medical, Switzerland). The image areas were chosen based on recommendations made in a publication by Effendy et al.21 Before scanning, 30 rats (5 groups, 6 rats per group) were anaesthetized using a cocktail of ketamine, xylazine and zoletil at a ratio of 2:1:1 at a dose of 0.2 ml/100 g body weight. The animals were placed in plastic holders and secured properly, and measurement protocols were created to define parameters such as the source of energy, intensity, and filter of the micro-CT system. The electrical potential and intensity used were 100 kV and 100 μA, respectively. The filter was 1.0-mm Al, and a high resolution of 18 μm was used to obtain the best images of living animals. The trabecular bone parameters were measured at the distal end of the femur. The number of slices was approximately 200, and the region of interest was the metaphyseal area located approximately 1.5 mm below the epiphyseal growth plate and extending 2.0 mm towards the proximal direction. The region, which was set via the reconstructed cross-sections, was rich in blood supply, and it had a high level of bone turnover activity.
2.3.3. Femur three-point bend testing and analysis (biomechanical testing)
The three-point bending tests were conducted on the femurs to estimate the mechanical properties of the cortical bone.22 Thirty femur bones (five groups, six rats per group) were used for this experiment. Each bone was placed on two lower supports (15-mm span) with the anterior aspect facing down and loaded from above at the midpoint between the two supports. The supporting and loading contacts were round with a diameter of 3 mm. An Autograph AGS-X 500 N model (Shimadzu, Japan) test machine was used with a stroke rate of 5 mm/min. Displacement was measured via a linear variable differential transformer mounted to the crosshead. Load deflection data were recorded at 10 Hz using Trapezium X software and analyzed using an attached personal computer. The same basic extrinsic and intrinsic properties were determined as previously indicated except that the analysis for estimating intrinsic properties invoked classical beam theory assumptions in this case.22
2.3.4. Histomorphology of the femur
The histomorphological examination was performed using decalcified femurs. To decalcify bone, the harvested femurs were soaked in 10% neutral buffered formalin for 2 days at 4 °C, after which they were transferred to 10% EDTA pH 7.4 for 30 days at 4 °C. The EDTA solution was changed three times per week to facilitate the decalcification process. The decalcified bones were cut longitudinally into 1-cm fragments, placed in a plastic cassette, and soaked in a beaker containing 10% neutral buffered formalin overnight. After overnight soaking, the fragments were fixed using 10% neutral buffered formalin for 80 min, dehydrated using a graded alcohol series for 6 h and cleared using xylene for 130 min. The fragments were then embedded in paraffin wax for 140 min and sectioned into thin slices using a microtome. The thin slices were collected using microscope slides and passed through xylene, decreasing strengths of alcohol (100–0%) and finally water. The slides were stained with haematoxylin 3G, and eosin (H&E) dehydrated in xylene. The slides were observed under an AxioVert A1 fluorescence inverted microscope at 40x magnification to check for trabecular bone degradation and images were acquired.23 Osteocyte counting was limited to 1.5 mm below the epiphyseal growth plate and extending 2.0 mm towards the proximal direction.
2.4. Gene expression
Total RNA from whole femur bone was extracted using a commercial kit (innuPREP RNA mini kit, Analytik Jena, Germany) according to the manufacturer's instruction. The concentration and quality of RNA was checked using a biophotometer (Eppendorf, Germany), while its integrity was checked using gel electrophoresis to confirm that 18s and 28s rRNA were intact. 10 μL of extracted total RNA was used as a template for cDNA synthesis in a 20 μL volume using a commercial kit (SensifastFast cDNA synthesis kit, Bioline, USA). Real-time PCR was performed using the 5 μL cDNA, with SensiFAST SYBR HI-ROX kit (Bioline, USA) and specific primers (Table 1). The qPCR reaction was carried out in the StepOne real-time PCR system machine (Applied Biosystems, USA) and the amplification program consisted of 1 cycle for 1 min at 95 °C followed by 45 cycles of 94 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s. Two technical replicates and two biological replicates were used, and the relative quantification of gene expression was calculated using the ΔΔCT method. Melt curve and gel analyses were used to verify specific products of appropriate size. Levels of gene expression were shown relative to the expression of reference genes (GAPDH, β-actin and γ-actin). The choice of the reference genes and use of multiple reference genes were based on recommendations made in a publication by Kapila et al.24 Primer sequence was obtained from NCBI database. The primers were purchased from Apical Scientific, Malaysia. Gene names and primer sequences are shown in Table 1. The data were analyzed using StepOne real-time PCR system software, and the changes in target gene expression were calculated using the comparative CT method (Relative expression = 2−ΔΔCT).25, 26, 27
Table 1.
Primers used for qPCR.
| Primers | Forward (5′-3′) | Reverse (3′-5′) |
|---|---|---|
| GAPDH | AGG GCT GCC TTC TCT TGT G | TTG AAC TTG CCG TGG GTA GAG |
| B-actin | GTG GTC AAC GTC ACC TAC TCT AAC | ACC TTC AGG GCA TGG TTC TC |
| G-actin | GGA TCT CTG TGA GCA CCA TGT AG | GGA CTT TCC CAC CCT GTT AGA C |
| OCN | ATC TAT GGC ACC ACC GTT TAG G | CGT CCA TAC TTT CGA GGC AGA G |
| TRAP5b | GAT GAG AAC GGT GTG GGC TAT G | AAA GCG TAG GTA GCC GTT |
2.5. Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences software (SPSS 27.0, Chicago, IL, USA). The analysis was performed using analysis of variance (ANOVA) followed by Tukey's HSD test. All results were expressed as the mean ± SEM. Values were considered statistically significant when ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic control.
3. Results and discussion
3.1. Diabetic study
All tested parameters were found to be within the physiological reference range for the normal/untreated group (control group). There was a constant increase in plasma glucose levels in the diabetic control group until the end of the 12 weeks treatment. The plasma glucose level of the metformin + spirulina treated group was significantly increased at week 4 compared to the diabetic control. By week 12, there was a significant reduction in metformin, spirulina, and metformin + spirulina treated groups compared to the diabetic control (Table 2). There was a constant decrease in weight of the rats in the diabetic control groups until the end of the 12 weeks treatment. At week 8, the spirulina treated group showed a significant increase in weight compared to the diabetic control. By week 12, there was a significant increase in weight of rats in metformin, spirulina, and metformin + spirulina treated groups (Table 2). Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was increased in the diabetic control, but significantly reduced in the metformin, spirulina, and metformin + spirulina treated groups (Table 2).
Table 2.
Diabetic study. Mean blood glucose, body weight and HOMA-IR levels of rats per group.
| Normal Control | Diabetic Control | Metformin | Spirulina | Metformin + Spirulina | ||
|---|---|---|---|---|---|---|
| Mean Blood Glucose Level (mmol/L) | Before induction | 6.33 ± 0.27 | 6.63 ± 0.54 | 6.34 ± 0.35 | 6.19 ± 0.23 | 6.09 ± 0.18 |
| Week 0 | 6.21 ± 0.7 | 27.43 ± 1.18 | 32.75 ± 1.01 | 32.17 ± 0.22 | 33.11 ± 0.99 | |
| Week 4 | 6.24 ± 0.74 | 28.43 ± 1.4 | 29.17 ± 2.48 | 28.42 ± 0.83 | 31.43 ± 1.56∗∗ | |
| Week 8 | 6.57 ± 0.29 | 31.48 ± 1.54 | 25.75 ± 3.82∗∗∗ | 25.48 ± 0.77∗∗∗ | 29.90 ± 1.99 | |
| Week 12 | 6.4 ± 0.34 | 32.32 ± 1.17 | 17.06 ± 2.59∗∗∗∗ | 13.71 ± 1.63∗∗∗∗ | 27.04 ± 0.60∗ | |
| Mean Body Weight (g) | Before induction | 149.11 ± 6.13 | 185 ± 13.52 | 136.16 ± 2.67 | 151.99 ± 1.39 | 152.03 ± 4.57 |
| Week 0 | 167.51 ± 1.75 | 179.22 ± 28.46 | 120.59 ± 10.09 | 135.62 ± 1.79 | 134.97 ± 4.78 | |
| Week 4 | 177.91 ± 0.58 | 173.68 ± 17.78 | 157.28 ± 25.39 | 179.56 ± 16.07 | 165.66 ± 12.58 | |
| Week 8 | 187.62 ± 5.52 | 165.2 ± 2.41 | 168.33 ± 20.49 | 193.51 ± 24.39∗∗ | 170.87 ± 9.84 | |
| Week 12 | 194.89 ± 2.57 | 144.02 ± 7.02 | 178.50 ± 20.04∗ | 206.50 ± 34.35∗∗∗∗ | 180.36 ± 8.46∗ | |
| HOMA-IR | 0.06 ± 0.003 | 0.29 ± 0.01$$$$ | 0.15 ± 0.01∗∗∗∗ | 0.12 ± 0.01∗∗∗∗ | 0.24 ± 0.04∗∗∗∗ | |
Rats were treated over a period of 12 weeks. All data are presented as mean (±) standard error mean (SEM) (n = 6) using StatPlus. The data were statistically analyzed by two-way ANOVA followed by Tukey's HSD test. Values were considered statistically significant when ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic control.
Diabetes mellitus is a clinical condition, characterized by hyperglycemia triggered by an absence or insufficiency of insulin or a resistance to the action of insulin at the cellular level.28 In current study, there was an increase in blood glucose level (hyperglycemia) in the diabetic control group after STZ induction. Hyperglycemia attributed to STZ induction led to progressive insulin resistance of peripheral tissues. The insulin resistance develops when insulin secretion is reduced and the blood glucose level is elevated.29,30 The hyperglycemia observed in current study could be due to reduced insulin production caused by STZ-induction which led to insulin resistance. Insufficient insulin prevents glucose from getting into the cell thus leads to accumulation of glucose in the blood. Glucose is a vital source of cellular energy. In the state of insulin insufficiency, less glucose permeates the body cells, the body utilizes fat and muscle as energy source causing a reduction in overall body weight.31 This explains the observed reduction in weight among diabetic control rats in current experiment. Metformin is a biguanide, which has been reported to improve glycemic control by insulin sensitization. Metformin prevents the liver from making new glucose via gluconeogenesis and stimulates the muscle and fat cells to remove glucose from the blood.32 Metformin also lowers plasma cholesterol and triglyceride levels and does not cause weight gain.33 In this study the effect of metformin was in accordance with the previous study results. The plasma glucose lowering effect observed in the spirulina and metformin + spirulina treated groups could be attributed to chromium that is naturally present in spirulina. The presence of chromium in spirulina makes it a highly beneficial adjuvant therapy.28 Chromium binds to a peptide known as Apo- LMWCr that in turn binds to the insulin receptor thereby enhancing its glucose lowering activity.34 The presence of high fiber content and generation of polypeptides from the digestion of high protein contents in spirulina may also be responsible for the lowering of the plasma glucose level by interfering with the absorption of glucose.35
3.2. Biochemical tests
Glycated hemoglobin (HbA1c) and alkaline phosphatase levels were significantly elevated, while the albumin level was significantly decreased in the diabetic control group compared to the normal group. The metformin treated group showed a significant decrease in HbA1c and alkaline phosphatase. Albumin levels showed no change compared to the diabetic control. Spirulina and metformin + spirulina treated groups showed a significant decrease in HbA1c and alkaline phosphatase levels. There was a significant increase in albumin levels in the spirulina treated group. Although the metformin + spirulina treated group showed an increase in albumin levels, the difference was not significant. The improvement of biochemical parameters in the metformin + spirulina treated group was more prominent compared to the metformin treated group, but spirulina was more effective in stabilizing the biochemical parameters (Table 3).
Table 3.
HbA1c, alkaline phosphatase and albumin serum levels after 12 weeks of treatment.
| HbA1c (%) | Alkaline phosphatase (U/L) | Albumin (g/L) | |
|---|---|---|---|
| Normal Control | 5.2 ± 0.48 | 84.13 ± 9.76 | 43.92 ± 4.27 |
| Diabetic Control | 14.92 ± 1.59$$$$ | 334.63 ± 6.66$$$$ | 26.74 ± 3.07$$$$ |
| Metformin | 12.06 ± 0.49∗∗∗ | 273.82 ± 19.71∗∗∗ | 26.8 ± 0.94 |
| Spirulina | 7.27 ± 0.5∗∗∗∗ | 157.34 ± 19.32∗∗∗∗ | 41.17 ± 3.4∗∗∗∗ |
| Metformin + Spirulina | 10.06 ± 1.1∗∗∗∗ | 210.96 ± 17.73∗∗∗∗ | 30.69 ± 1.8 |
HbA1c, alkaline phosphatase and albumin serum levels for all groups (n = 6) after 12 weeks of treatment. Data are presented as mean ± SEM when ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic control; $$$$p < 0.0001 vs. Normal.
HbA1c elevation as observed in the diabetic control maybe due to its response to oxidative stress generated by STZ induction.36,37 The reduction in plasma glucose level as the result of spirulina and metformin treatment may have contributed to the reduction in HbA1c. Similar finding has been reported by Shashi et al.38 that the reduction of plasma glucose level leads to reduction in plasma HbA1c. ALP, a marker of biliary function and cholestasis which reflects liver synthetic function39 was elevated in the diabetic control. ALP has been associated with diabetes and it has been linked to oxidative stress from reactive lipid peroxidation, peroxisomal beta-oxidation, and recruited inflammatory cells.40 Elevation in ALP in the negative control may be due to oxidative stress generated by STZ induction. This observation is supported by a study by Harris,40 which reports that elevated levels of ALP are due to oxidative stress from reactive LPO. The reduction in ALP by spirulina treatment as observed in the spirulina and metformin + spirulina treated groups may be attributed to the presence of ß-carotene in spirulina, which has been previously reported to possess hepatoprotective activity.35 The antioxidant properties of spirulina may improve the renal function by alleviating the oxidative stress damage to protect the liver and kidney cells.
3.3. Serum bone assessment test
The serum bone assessment test showed a significant decrease in 25-OH vitamin D levels and significant increase in phosphate and calcium levels in the diabetic control group compared to the normal group. The metformin, spirulina, and metformin + spirulina treated groups showed a significant increase in 25-OH vitamin D levels and a significant decrease in phosphate level compared to the diabetic control. There was a significant decrease in calcium levels in the spirulina treated group which was not seen in metformin and metformin + spirulina treated groups (Table 4).
Table 4.
Serum 25-OH vitamin D, calcium, and phosphate levels after 12 weeks of treatment.
| 25-OH vitamin D (mmol/L) | Calcium (mmol/L) | Phosphate (mmol/L) | |
|---|---|---|---|
| Normal Control | 143.33 ± 4.18 | 2.51 ± 0.05 | 2.03 ± 0.06 |
| Diabetic Control | 26.67 ± 5.35$$$$ | 2.7 ± 0.09$$$ | 3.23 ± 0.14$$$$ |
| Metformin | 60.17 ± 7.36∗∗∗ | 2.75 ± 0.05 | 2.73 ± 0.03∗∗∗∗ |
| Spirulina | 143.67 ± 3.72∗∗∗∗ | 2.35 ± 0.07∗∗∗∗ | 2.28 ± 0.02∗∗∗∗ |
| Metformin + Spirulina | 71.5 ± 29.24∗∗∗∗ | 2.65 ± 0.02 | 2.66 ± 0.03∗∗∗∗ |
25-OH vitamin D, calcium, and phosphate levels for all groups (n = 6) after 12 weeks of treatment. Data are presented as mean ± SEM when ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic control; $$$p < 0.001, $$$$p < 0.0001 vs. Normal.
Type 2 diabetes mellitus (T2DM) compromises bone microarchitecture by inducing abnormal bone cell function and matrix structure, increasing osteoblast apoptosis, diminishing osteoblast differentiation, and enhancing osteoclast-mediated bone resorption.41,42 Lou et al.44 reported that 25-OH vitamin D led to increased level of osteogenesis in vivo which signifies increased osteoblastic activity. Increased osteoblastic activity is associated with the movement of calcium and phosphate from blood to bone for the process of bone formation leading to reduced blood calcium and phosphorus levels.52 Increased resorption of bone tissue on the other hand, releases calcium and phosphate from the bone into the blood43 leading to increased serum calcium and phosphate levels. In the current study, the diabetic control group showed decreased 25-OH vitamin D levels suggesting reduced osteogenesis and osteoblastic activity which was evidenced by an increase in the levels of calcium and phosphorus in the blood of diabetic control rats. This suggests an increased osteoclast-mediated resorption caused by STZ-induced T2DM. According to Out et al.,54 metformin supplementation does not lead to 25-OH vitamin D deficiency. This was in line with current study were metformin treated rats showed significant increase in 25-OH vitamin D compared to the diabetic control rats. The increase in 25-OH vitamin D level could be due to glycemic control by the metformin supplementation which led to an improvement in 25-OH vitamin D as reported by Al-Timimi & Ali.45 The increased in 25-OH vitamin D levels lowered the serum phosphate levels following metformin supplementation. However, serum calcium levels were not affected by metformin supplementation. Increased 25-OH vitamin D levels accompanied by normalized calcium and phosphate serum levels were observed in the spirulina and metformin + spirulina treated groups. This may be because of the calcium and phosphate from blood enter bone for the bone formation52 activities, increases of osteogenesis and osteoblactic activities in association of increased 25-OH vitamin D.44
3.4. In vivo micro-CT
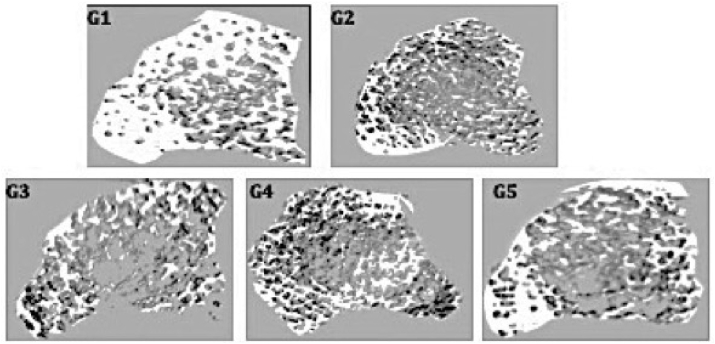
The three-dimensional (3D) images of the distal femur metaphysis revealed that the trabecular network of group G1 was denser than those of G2, G3, G4 and G5. G2 and G3 exhibited higher porosity in the trabecular network compared with the findings in G1. These results are shown in Fig. 1. Trabecular thickness (Tb. Th), trabecular number (Tb. N), trabecular separation (Tb. Sp), bone volume fraction (BV/TV) and BMD were obtained from the in vivo micro-CT scans. These results are shown in Table 5. The data obtained indicated that Tb. Th, BV/TV, and BMD were significantly decreased in diabetic control compared to the normal group. Metformin, spirulina, and metformin + spirulina treated groups showed no significant difference in Tb. Th, Tb. N, Tb. Sp, BV/TV and BMD compared to the diabetic control (Table 5).
Fig. 1.
Three-dimensional micro-CT images of trabecular microarchitecture of distal femur metaphysis after 12 weeks of treatment. G1: Normal control, G2: Diabetic control, G3: metformin-treated group, G4: spirulina-treated group, G5: metformin + spirulina-treated group.
Table 5.
Trabecular thickness (Tb.Th), Trabecular number (Tb.N), Trabecular separation (Tb.Sp), Bone volume (BV/TV) and Bone mineral density (BMD) after 12 weeks of treatment.
| Tb.Th (mm) | Tb.N (1/mm) | BV/TV (%) | Tb.Sp (mm) | BMD (g) | |
|---|---|---|---|---|---|
| Normal Control | 0.22 ± 0.02 | 3.89 ± 0.37 | 85.02 ± 3.22 | 0.09 ± 0.01 | 0.47 ± 0.21 |
| Diabetic Control | 0.12 ± 0.01$$$$ | 3.03 ± 0.88 | 35.65 ± 8.96$$$ | 0.22 ± 0.06 | 0.21 ± 0.06$$$ |
| Metformin | 0.11 ± 0.01 | 3.39 ± 1.75 | 35.99 ± 7.79 | 0.23 ± 0.17 | 0.19 ± 0.05 |
| Spirulina | 0.12 ± 0.03 | 3.39 ± 0.69 | 43.81 ± 26.63 | 0.18 ± 0.04 | 0.30 ± 0.02 |
| Metformin + Spirulina | 0.13 ± 0.02 | 3.42 ± 0.59 | 45.44 ± 7.74 | 0.17 ± 0.02 | 0.19 ± 0.06 |
Mean Trabecular thickness (Tb.Th), Trabecular number (Tb.N), Trabecular separation (Tb.Sp), Bone volume (BV/TV) and Bone mineral density (BMD) for all groups (n = 6) after 12 weeks of treatment. Data are presented as mean ± SEM when $$$p < 0.001, $$$$p < 0.0001 vs. Normal.
Micro-computed tomography provides a three-dimensional view of trabecular and cortical bone micro-architecture at a high resolution. As a result, it is a benchmark technique for evaluating skeletal geometry.55 The BV/TV, Tb.N, Tb.Th, and Tb.Sp parameters are the bare minimum for describing trabecular bone micro-architecture56 which is critical in determining bone quality and strength.57 The amount of calcium and other minerals packed in a segment of bone is determined by bone mineral density (BMD). Results revealed that T2DM due to STZ-induction was accompanied by shortfalls in trabecular bone microarchitecture in the femurs and this was manifested in decreased BV/TV, Tb.N, Tb.Th, BMD and increased Tb.Sp. The 3D images showed increased porosity implying that STZ-induced T2DM reduced trabecular bone quality and strength in rats. A decrease in BMD was also observed, implying that the amount of calcium in the bone has decreased. This matched the findings of serum bone assessments, which revealed higher serum calcium levels, implying that calcium is being lost from bone due to increased resorption, resulting in lower calcium levels in the bone and thus lower BMD. There was no significant change in the metformin treated group, suggesting that these treatments did not improve bone quality and strength after STZ-induction. Although trabecular parameters were not affected by spirulina supplementation, the 3D image showed a regrowth in the trabecular meshwork. This is in line with a study by Gupta et al.53 which reported that spirulina supplementation contributed to the intactness and integrity of the bone surface as well as the bone strength. Spirulina may have assisted remineralization of bones by decreasing resorption pits and stimulating bone formation.53
3.5. 3-Point bend test
The three-point bending test was used to measure the structural properties of bones and characterize the tissue in its intact form. As shown in Table 6, the maximum force, maximum strength, and elastic modulus were significantly reduced in femurs of diabetic control rats as compared to normal rats. The metformin treated group showed no significant difference in maximum force, maximum strength, and elastic modulus. The spirulina and metformin + spirulina treated groups showed a significant increase in maximum force and maximum stress compared to the diabetic control. Young modulus was significantly increased in the spirulina treated group while the metformin + spirulina group showed no significant difference (Table 6).
Table 6.
Maximum force, maximum stress and young modulus after 12 weeks of treatment.
| Maximum force (N) | Maximum stress (GPa) | Young Modulus (GPa) | |
|---|---|---|---|
| Normal Control | 181.71 ± 29.44 | 14.53 ± 2.98 | 2.64 ± 0.71 |
| Diabetic Control | 68.43 ± 3.56$$$$ | 5.17 ± 0.54$$$$ | 1.05 ± 0.23$$$$ |
| Metformin | 86.43 ± 4.36 | 6.59 ± 0.86 | 1.12 ± 0.15 |
| Spirulina | 140.17 ± 5.01∗∗∗∗ | 10.73 ± 1.69∗∗∗ | 1.79 ± 0.20∗ |
| Metformin + Spirulina | 120.24 ± 8.33∗∗∗∗ | 8.68 ± 1.4∗ | 1.49 ± 0.13 |
Mean Maximum force, maximum stress, and young modulus for all groups (n = 6) after 12 weeks of treatment. Data are presented as mean ± SEM where ∗p < 0.05, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic; $$$p < 0.001, vs. Normal.
In the diabetic control, parameters such as ultimate load (maximum force), stiffness (maximum stress), and energy to fracture (Young modulus), which are indicators of structural properties of bones and characterize the tissue in its intact form,46 were lower, implying a reduction in bone structural strength and stiffness.47 This supports the findings of the serum bone assessment, which suggest that STZ-induced T2DM is associated with decreased osteogenesis and osteoblastic activity, which would increase resorption and reduce bone stiffness and strength in diabetic control rats. Metformin supplementation however did not improve the reduction in bone structural strength and stiffness. The maximum force, maximum stress, and young modulus of the spirulina-treated groups, on the other hand, were increased. This backs up the findings from the bone serum analysis, which showed that spirulina increased osteogenesis and osteoblastic activity by raising serum 25-OH vitamin D levels. As a result, serum calcium and phosphorus levels were lowered, and bone strength and stiffness were improved.
3.6. Histomorphology of the femur
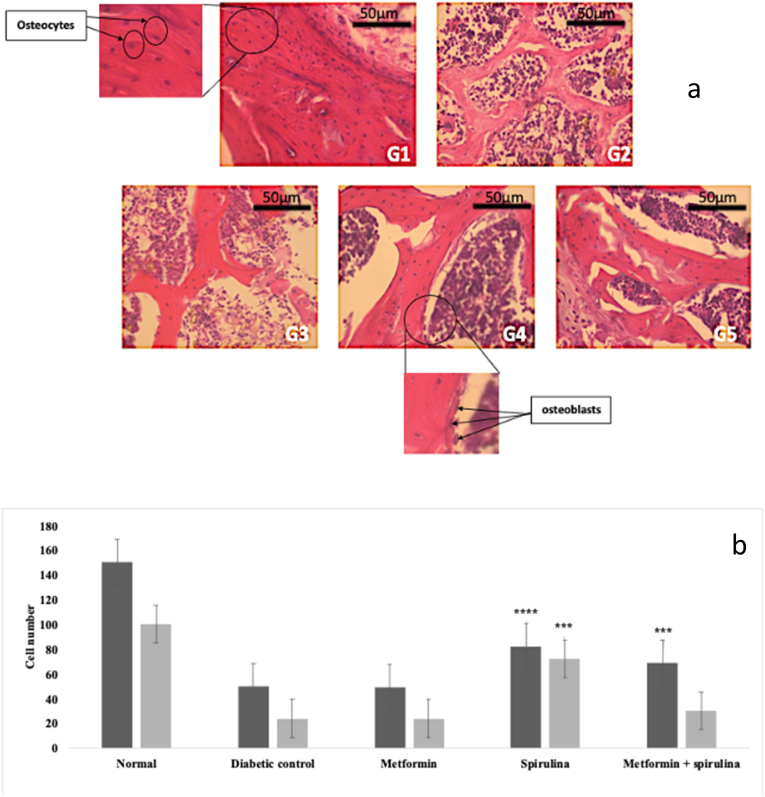
H&E staining revealed a dense and regular meshwork of trabecular bone, a high number of osteocytes and osteoblasts in the femurs of normal non-diabetic rats (G1). By contrast, the trabecular bone in the femurs of rats in the diabetic control group (G2) and metformin treated group (G3) showed reduced osteocyte and osteoblast numbers with loss of trabecular bone. The spirulina (G5) and metformin + spirulina (G5) treated groups were associated with restoration of the structure of the trabecular bone and an increase in osteocyte counts, however, only the spirulina treated group showed a significant increase in osteoblast number (Fig. 2a +b).
Fig. 2.
Histomorphology of femur. a. H & E staining of trabecular bone of femur after 12 weeks of treatment. G1: Normal control, G2: Diabetic control, G3: Metformin-treated group, G4: spirulina-treated group, G5: Metformin + spirulina-treated group (40x magnification). b. Quantitative numbers of osteocytes in G1: Normal control, G2: Diabetic control, G3: Metformin-treated group, G4: spirulina-treated group, G5: Metformin + spirulina-treated group. Data are presented as the mean ± SEM when ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 vs. diabetic control.
The femurs in the diabetic control group were characterized by a reduction in osteocyte and osteoblast numbers. Osteoblasts are bone forming cells and osteocytes are developed osteoblasts implanted within the bone matrix during the growth phase of bone remodeling.48 According to Tatsumi et al.,49 experimental destruction of osteocytes is characterized by a significant increase in bone resorption, decreased bone formation and trabecular bone loss. This was in line with current study which showed decreased osteocyte numbers and thinner trabecular bone in the diabetic control group. Maycas et al.,50 reported a pro-apoptotic effect (promotes apoptosis) of high glucose level on osteocytic cells which suggests that STZ-induced hyperglycemia could have promoted osteocyte apoptosis which led to reduced osteocyte number and trabecular bone loss observed in the diabetic control group. Shanbhogue et al.59 reported that hyperglycemia impacts bone by affecting osteoblast differentiation which in turn reduced the quality of bone matrix implying that hyperglycemia due to STZ induction may be responsible for the decreased osteoblast numbers in the diabetic control group.
Although metformin reduced the high glucose level associated with STZ -induction, reduced osteocyte number was still observed in the metformin treated group. According to Jager et al.,9 and Vaes et al.,10 long-term metformin treatment increases the risk of vitamin B12 deficiency, which may indirectly increase osteoclastogenesis through its effect on MMA and homocysteine levels. Increased homocysteine levels may also lead to osteocyte apoptosis.51 Vitamin B12 has been shown to have a significant effect on osteoblast proliferation in vitro as vitamin B12 stimulation increases alkaline phosphatase activity in osteoblastic cells.60 This may explain the decreased osteocyte and osteoblast numbers in the metformin treated group. Increased osteocyte numbers were observed in the spirulina treated groups. The spirulina treated groups showed increased osteocyte and osteoblast numbers. This could be because of the hypoglycemic effect of spirulina. Reduction in the glucose level may have prevented osteocyte apoptosis and improved osteoblast differentiation thereby increasing osteocyte and osteoblast numbers. Spirulina may also be preventing vitamin B12 deficiency associated with metformin usage. According to Cho et al.,58 spirulina contains a moderate amount of vitamin B12 which may be replenishing its levels when spirulina is used in combination with metformin thereby stimulating osteoblast proliferation and preventing osteocyte apoptosis. Although metformin + spirulina supplementation significantly increased osteocyte number, it did not affect osteoblast number.
3.7. Gene expression
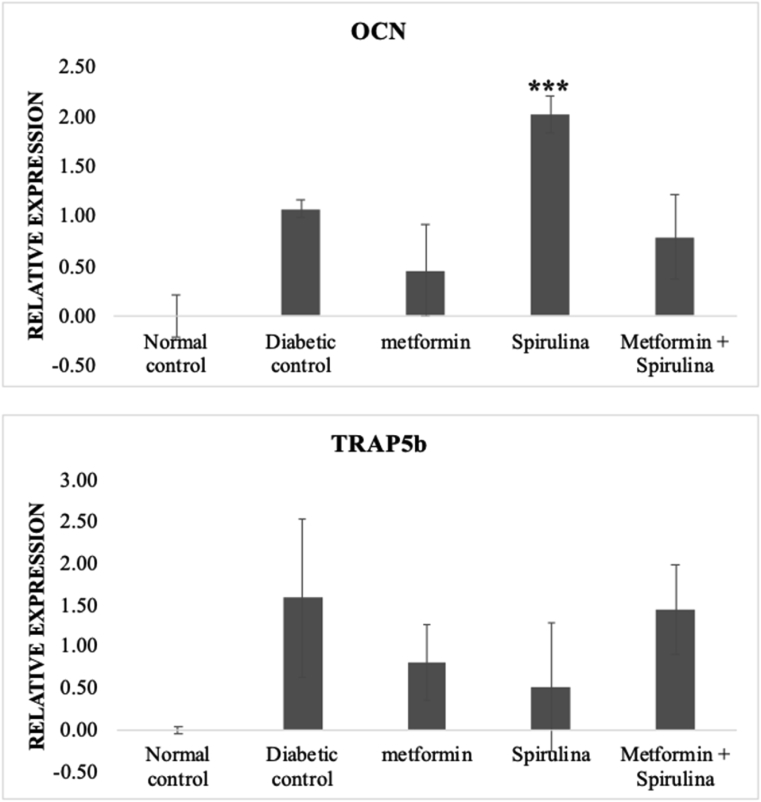
There was no significant change in the expression of OCN and TRAP5b in metformin and metformin + spirulina when compared to diabetic control groups. Spirulina treatment significantly increased the expression of OCN but there was no significant change on the expression of TRAP5b when compared to the diabetic control group. These results are shown in Fig. 3.
Fig. 3.
Relative gene expression of OCN and TRAP5b Gene expression levels were normalized against the geometric mean of three housekeeping genes (GAPDH, β-actin and γ-actin). All data are presented as mean (±) standard error mean (SEM) using StatPlus. The data were statistically analyzed by two-way ANOVA followed by Tukey's HSD test. ∗∗∗p < 0.001.
According to Huang et al.,61 the most specific marker for osteoblastic activity is osteocalcin. OCN expression is suppressed by chronic hyperglycemia62 and blood glucose levels in mice are positively associated with bone marrow-induced osteoblast death and negatively associated with bone OCN expression.63 Reduced expression of OCN was observed in the diabetic control group suggesting that there was a reduction in osteoblastic activity because of hyperglycemia due to STZ-induction. According to Suzuki et al.,64 T2DM patients exhibited higher levels of tartrate-resistant acid phosphatase in their blood, indicating increased osteoclast activity. Similarly, increased TRAP5b expression was observed in the diabetic control group. According to in vitro research, hyperglycaemia predisposes patients to increased osteoclast development.65 suggesting that hyperglycemia due to STZ-induction may be responsible for increased TRAP5b expression in diabetic control group. Metformin and metformin + spirulina treated groups showed no significant change in the expression of OCN and TRAP5b compared to diabetic control. Increased expression of OCN was observed in STZ-induced T2DM rats treated with spirulina only. This finding suggests that spirulina increased osteoblastic activity in diabetic rats, subsequently increases bone formation. This result corresponds to the results obtained from the histology assessment where we observed increased osteoblast numbers in the spirulina treated group. However, spirulina supplementation showed no change in the expression of TRAP5b, suggesting that spirulina supplementation did not affect osteoclast activity.
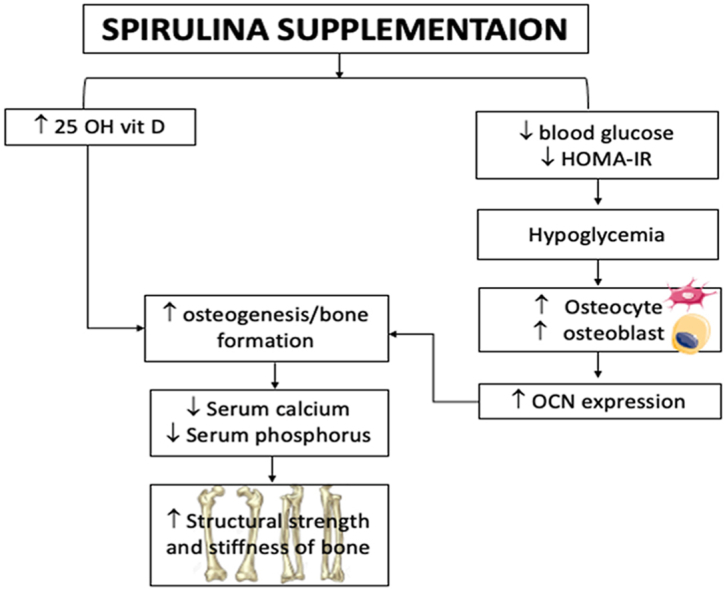
The presence of chromium, phycocyanin, and a high fiber content in spirulina may be responsible for its hypoglycemic effect. Chromium may have reduced blood glucose levels by increasing insulin receptors' glucose-lowering activity, causing GLUT-4 to bring more glucose into the cell. Spirulina's high fiber content and production of polypeptides from protein digestion may have lowered plasma glucose by interfering with glucose absorption. Spirulina contains ß-carotene, which may have contributed to its hepatoprotective properties and protect ß-cells from free radical damage. Spirulina's hypoglycemic properties, as well as its ability to raise serum 25 OH vitamin D, may explain its bone-protective properties, as it promotes bone formation as evidenced by increased osteoblast number and OCN expression. In STZ-induced T2DM rats, this would result in lower serum calcium and phosphate levels, resulting in increased bone structural strength and stiffness.
4. Conclusions
In STZ-induced diabetic rats, spirulina improved bone formation, which resulted in improved bone strength and stiffness. Increased expression of OCN, increased osteocyte/osteoblast numbers, and lower serum calcium and phosphorus levels were all evidence of this. This suggests that spirulina may be able to prevent T2DM-related brittle bone, lowering the risk of fracture.
Funding
This work was funded by The Centre of Excellence for Research, Value, Innovation and Entrepreneurship Research Grant Scheme of UCSI University (UCSI-CERVIE-PISF Proj-In-FAS 039).
Declaration of competing interest
Authors declare no conflict of interest.
Acknowledgments
We acknowledge Alpha Active Industries Sdn. Bhd Malaysia for providing the spirulina.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.07.010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gatineau M., Hancock C., Holman N., et al. Public Health England; Oxford: 2014. Adult Obesity and Type 2 Diabetes. [Google Scholar]
- 2.https://www.niddk.nih.gov/health-information/diabetes/overview/what-is-diabetes
- 3.AL-Khateeb A.R., Md Shaari N., Muid S. Abd, Froemming G.R.A. Critical link between advanced glycation end products, osteoporosis and type 2 diabetes mellitus. Regen Res. 2018;6:1–9. [Google Scholar]
- 4.de Paula F.J.A., Rosen C.J. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq Bras Endocrinol Metabol. 2010;54(2):150–157. doi: 10.1590/S0004-27302010000200010. [DOI] [PubMed] [Google Scholar]
- 5.Adil M., Khan R.A., Kalam A., et al. Effect of anti-diabetic drugs on bone metabolism: evidence from preclinical and clinical studies. Pharmacol Rep. 2017;69(6):1328–1340. doi: 10.1016/j.pharep.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Zhan C., Hu Y., Peng B. Protective effect of metformin on periapical lesions in rats by decreasing the ratio of receptor activator of nuclear factor kappa B ligand/osteoprotegerin. J Endod. 2012;38:943–947. doi: 10.1016/j.joen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Śmieszek A., Basińska K., Chrząstek K., Marycz K. In Vitro and in vivo effects of metformin on osteopontin expression in mice adipose-derived multipotent stromal cells and adipose tissue. J Diabetes Res. 2015;2015:1–16. doi: 10.1155/2015/814896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lecka-Czernik B., Schwartz V.A. Springer international publishing; 2016. Diabetic Bone Disease: Basic and Translational Research and Clinical; pp. 129–130. [Google Scholar]
- 9.de Jager J., Kooy A., Lehert P., et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B12 deficiency: randomized placebo-controlled trial. BMJ. 2010;340:c2181. doi: 10.1136/bmj.c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaes B.L., Lute C., Blom H.J., et al. Vitamin B12 deficiency stimulates osteoclastogenesis via increased homocysteine and methylmalonic acid. Calcif Tissue Int. 2009;84(5):413–422. doi: 10.1007/s00223-009-9244-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumari P., Khanam S., Varma M.C. Study the spirulina as a potential antidiabetic. J Chem Biol Phys Sci. 2013;3:1963–1971. [Google Scholar]
- 12.Devesh C., Mehla K., Nair A., Sehajpal P.K., Gupta S. Spirulina reverses histomorphological changes in diabetic osteoporosis in pioglitazone treated rats. J Diabetes Metabol. 2012;1:1–7. doi: 10.4172/2155-6156.S1-006. [DOI] [Google Scholar]
- 13.Layam A., Reddy C.L.K. Antidiabetic property of spirulina. Diabetol Croat. 2007;35:29–33. [Google Scholar]
- 14.Simon J.P., Baskaran U.L., Shallauddin K.B., Ramalingam G., Evan S.P. Evidence of antidiabetic activity of Spirulina fusiformis against streptozotocin-induced diabetic Wistar albino rats. 3 Biotech. 2018;8:129. doi: 10.1007/s13205-018-1156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okechukwu P.N., Ekeuku S.O., Sharma M., et al. In Vivo and in vitro antidiabetic and antioxidant activity of spirulina. Phcog Mag. 2019;15:17–29. Suppl S1. [Google Scholar]
- 16.Ekeuku S.O., Okechukwu P.N., Gabriel A.A., Teo S.S., Stepfanie N.S., Froemming G.R.A. Plasma glucose lowering activity of Palmatine and its effect on liver, kidney, and antioxidant enzymes parameters in STZ induced diabetic rat model. Curr Bioact Compd. 2015;11(4):256–263. doi: 10.2174/1573407212666151105185802. [DOI] [Google Scholar]
- 17.El-mottaleb N.A.A.B.D., El-aziz E.A.A.B.D., Mostafa D.G. Effect of ginger on bone of streptozotocin induced diabetic rats. Med J Cairo Univ. 2016;84:395–403. [Google Scholar]
- 18.La Fontaine J., Chen C., Hunt N., Jude E., Lavery L. Type 2 diabetes and metformin influence on fracture healing in an experimental rat model. J Foot Ankle Surg. 2016;55:955–960. doi: 10.1053/j.jfas.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Borst S.E., Snellen H.G., Lai H.L. Metformin treatment enhances insulin-stimulated glucose transport in skeletal muscle of Sprague-Dawley rats. Life Sci. 2000;67(2):165–174. doi: 10.1016/S0024-3205(00)00612-3. [DOI] [PubMed] [Google Scholar]
- 20.Prakash R.S., Madhab K.D., Rosalin S. Antihyperglycemic activity of aerial parts of mirabilis jalapa l. In normoglycemic and streptozotocin induced hyperglycemic rats. Int J Drug Dev Res. 2012;4:334–341. [Google Scholar]
- 21.Effendy N.M., Khamis M.F., Soelaiman I.N., Shuid A.N. The effects of Labisia pumila on postmenopausal osteoporotic rat model: dose and time-dependent micro-CT analysis. J X Ray Sci Technol. 2014;22:503–518. doi: 10.3233/XST-140441. [DOI] [PubMed] [Google Scholar]
- 22.Hogan H.A., Ruhmann S.P., Sampson H.W. The mechanical properties of cancellous bone in the proximal tibia of ovariectomized rats. J Bone Miner Res. 2000;15(2):284–292. doi: 10.1359/jbmr.2000.15.2.284. [DOI] [PubMed] [Google Scholar]
- 23.The histology guides. http://www.histology.leeds.ac.uk/what-is-histology/histological_sections.php Available from: Accessed.
- 24.Kapila N., Kishore A., Sodhi M., et al. Identification of Appropriate reference genes for qRT-PCR analysis of heat-stressed mammary epithelial cells in riverine buffaloes (Bubalus bubalis) Biotechnol. 2013:1–9. doi: 10.5402/2013/735053. (735053) (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittgen T.D., Livak K.J. Analysing real-time PCR data by the comparative Ct method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Lu W., Wang W., Wang S., Feng Y., Liu K. Rosiglitazone promotes bone marrow adipogenesis to impair myelopoiesis under stress. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0149543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao X., Huang X., Zhou Z., Lin X. An improvement of the 2^(delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostatist. Bioinf. Biomath. 2013;3:71–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Anjaneyulu M., Chopra K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2004;31(4):244–248. doi: 10.1111/j.1440-1681.2004.03982.x. [DOI] [PubMed] [Google Scholar]
- 29.Nasry M.R., Abo-Youssef A.M., Abd El-Latif H.A. Anti-diabetic activity of the petroleum ether extract of Guar gum in streptozotocin-induced diabetic rats: a comparative study. Beni-Seuf Univ J Appl Sci. 2013;2(1):51–59. doi: 10.1016/j.bjbas.2013.09.008. [DOI] [Google Scholar]
- 30.Ordonez P., Moreno M., Alonso A., Fernández R., Díaz F., González C. Insulin sensitivity in streptozotocin-induced diabetic rats treated with different doses of 17 beta-oestradiol or progesterone. Exp Physiol. 2007;92(1):241–249. doi: 10.1113/expphysiol.2006.035006. [DOI] [PubMed] [Google Scholar]
- 31.Unexplained weight loss. https://www.diabetes.co.uk/symptoms/unexplained-weight-loss.html Available from:
- 32.Stocker D.J., Taylor A.J., Langley R.W., Jezior M.R., Robert A., Vigersky R.A. A randomized trial of the effects of rosiglitazone and metformin on inflammation and subclinical atherosclerosis in patients with type 2 diabetes. Am Heart J. 2007;153(3):445. doi: 10.1016/j.ahj.2006.11.005. .e1-445.e6. [DOI] [PubMed] [Google Scholar]
- 33.Metformin. https://www.diabetesselfmanagement.com/diabetes-resources/definitions/metformin/ Available from:
- 34.Piñero E.J.E., Bermejo B.P., Villar del Fresno A.M. Antioxidant activity of different fractions of spirulina platensis protean extract. Il Farmaco. 2001;56(5-7):497–500. doi: 10.1016/S0014-827X(01)01084-9. [DOI] [PubMed] [Google Scholar]
- 35.Anwer R., Alam A., Khursheed S., S Kashif M., Kabir H., Fatma T. Spirulina: possible pharmacological evaluation for insulin-like protein. J Appl Phycol. 2012;24(6) doi: 10.1007/s10811-012-9924-z. [DOI] [Google Scholar]
- 36.Falcioni G., Cincolà G., Brunori M. Glutathione peroxidase and oxidative hemolysis in trout red blood cells. FEBS (Fed Eur Biochem Soc) Lett. 1987;221(2):355–358. doi: 10.1016/0014-5793(87)80955-9. [DOI] [PubMed] [Google Scholar]
- 37.Giardina B., Messana I., Scatena R., Castagnola M. The multiple functions of hemoglobin. Crit Rev Biochem Mol Biol. 1995;30(3):165–196. doi: 10.3109/10409239509085142. [DOI] [PubMed] [Google Scholar]
- 38.Shashi S.B., Manisha, Raghbendra P. Evaluation of antidiabetic effect of Spirulina on blood of Alloxan Monohydrate induced mice. Bioglobia. 2016;3(2):79–86. [Google Scholar]
- 39.Marsano L.S., Mendez C., Hill D., Barve S., Mcclain C.J. Diagnosis and treatment of alcoholic liver disease and its complications. Alcohol Res Health. 2003;27(3):248–256. [PMC free article] [PubMed] [Google Scholar]
- 40.Harris E.H. Elevated liver function tests in type 2 diabetes. Clin Diabetes. 2005;23(3):115–119. [Google Scholar]
- 41.Sanches C.P., Vianna A.G.D., Barreto F.C. The impact of type 2 diabetes on bone metabolism. Diabetol Metab Syndrome. 2017;9(85):1–7. doi: 10.1186/s13098-017-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vianna A.G.D., Sanches C.P., Barreto F.C. Review article: effects of type 2 diabetes therapies on bone metabolism. Diabetol Metab Syndrome. 2017;9:1–11. doi: 10.1186/s13098-017-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teitelbaum S.L. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 44.Lou Y.R., Toh T.C., Tee Y.H., Yu H. 25-Hydroxyvitamin D3 induces osteogenic differentiation of human mesenchymal stem cells. Sci Rep. 2017;7:42816. doi: 10.1038/srep42816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Timimi D.J., Ali A.F. Serum 25(OH) D in diabetes mellitus type 2: relation to glycaemic control. J Clin Diagn Res. 2013;7(12):2686–2688. doi: 10.7860/JCDR/2013/6712.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oksztulska-Kolanek E., Znorko B., Michalowska M., Pawlak K. The biomechanical testing for the assessment of bone quality in an experimental model of chronic kidney disease. Nephron. 2015;132:51–58. doi: 10.1159/000442714. [DOI] [PubMed] [Google Scholar]
- 47.Ma R., Wang L., Zhao B., et al. Diabetes perturbs bone microarchitecture and bone strength through regulation of Sema3A/IGF-1/β-catenin in rats. Cell Physiol Biochem. 2017;41(1):55–66. doi: 10.1159/000455936. [DOI] [PubMed] [Google Scholar]
- 48.Graham J.M., Ayati B.P., Holstein S.A., Martin J.A. The role of osteocytes in targeted bone remodeling: a mathematical model. PloS One. 2013;8(5) doi: 10.1371/journal.pone.0063884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatsumi S., Ishii K., Amizuka N., et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metabol. 2007;5(6):464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Maycas M., McAndrews K.A., Sato A.Y., et al. PTHrP-Derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J Bone Miner Res. 2017;32(3):486–497. doi: 10.1002/jbmr.3007. [DOI] [PubMed] [Google Scholar]
- 51.Vijayan V., Gupta S. How Homocysteine Modulates the Function of Osteoblasts and Osteocytes, Non-proteinogenic Amino Acids. Nina Filip and Cristina Elena Iancu; IntechOpen: 2018. https://www.intechopen.com/books/non-proteinogenic-amino-acids/how-homocysteine-modulates-the-function-of-osteoblasts-and-osteocytes 10.5772/intechopen.76398. Available from: [Google Scholar]
- 52.Osteoblasts L. Crampton. Osteoclasts, calcium, and bone remodelling. 2013. https://owlcation.com/stem/Osteoblasts-Osteoclasts-Calcium-and-Bone-Remodeling Available from:
- 53.Gupta S., Hrishikeshvan H.J., Sehajpal P.K. Spirulina protects against Rosiglitazone induced osteoporosis in insulin resistance rats. Diabetes Res Clin Pract. 2010;87(1):38–43. doi: 10.1016/j.diabres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Out M., Top W.M.C., Lehert P., Schalkwijk C.A., Stehouwer C.D.A., Kooy A. Long-term treatment with metformin in type 2 diabetes and vitamin D levels: a post-hoc analysis of a randomized placebo-controlled trial. Diabetes Obes Metabol. 2018;20(8):1951–1956. doi: 10.1111/dom.13327. [DOI] [PubMed] [Google Scholar]
- 55.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Müller R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 56.Pirih F., Lu J., Ye F., et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27(2):309–318. doi: 10.1002/jbmr.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen F., Wang Y., Wang H., et al. Flaxseed oil ameliorated high-fat-diet-induced bone loss in rats by promoting osteoblastic function in rat primary osteoblasts. Nutr Metab. 2019;16(1):71. doi: 10.1186/s12986-019-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho J.A., Baek S.Y., Cheong S.H., Kim M.R. Spirulina enhances bone modeling in growing male rats by regulating growth-related hormones. Nutrients. 2020;12(4):1187. doi: 10.3390/nu12041187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shanbhogue V.V., Mitchell D.M., Rosen C.J., Bouxsein M.L. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4(2):159–173. doi: 10.1016/S2213-8587(15)00283-1. [DOI] [PubMed] [Google Scholar]
- 60.Kim G.S., Kim C.H., Park J.Y., Lee K.U., Park C.S. Effects of vitamin B12 on cell proliferation and cellular alkaline phosphatase activity in human bone marrow stromal osteoprogenitor cells and UMR106 osteoblastic cells. Metabolism. 1996;45(12):1443–1446. doi: 10.1016/s0026-0495(96)90171-7. [DOI] [PubMed] [Google Scholar]
- 61.Huang W., Yang S., Shao J., P Li Y. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–3092. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Botolin S., McCabe L.R. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J Cell Biochem. 2006;99:411–424. doi: 10.1002/jcb.20842. [DOI] [PubMed] [Google Scholar]
- 63.Coe L.M., Irwin R., Li D., ner, McCabe L.R. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. J Cell Physiol. 2011;226:477–483. doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki K., Kurose T., Takizawa M., et al. Osteoclastic function is accelerated in male patients with type 2 diabetes mellitus: the preventive role of osteoclastogenesis inhibitory factor/osteoprotegerin (OCIF/OPG) on the decrease of bone mineral density. Diabetes Res Clin Pract. 2005;68(2):117–125. doi: 10.1016/j.diabres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Catalfamo D.L., Britten T.M., Storch D.L., Calderon N.L., Sorenson H.L., Wallet S.M. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013;19(3):303–312. doi: 10.1111/odi.12002. PMID: 24079914; PMCID: PMC3800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.