Abstract
Cefiderocol is a novel synthetic siderophore-conjugated antibiotic that hijacks the bacterial iron transport systems facilitating drug entry into cells, achieving high periplasmic concentrations. This systematic review analyzed the currently available literature on cefiderocol. It summarized in vitro susceptibility data, in vivo antimicrobial activity, pharmacokinetics/pharmacodynamics (PK/PD), clinical efficacy, safety and resistance mechanisms of cefiderocol. Cefiderocol has potent in vitro and in vivo activity against multidrug-resistant (MDR) Gram-negative bacteria, including carbapenem-resistant isolates. But New Delhi Metallo-β-lactamase (NDM)- positive isolates showed significantly higher MICs than other carbapenemase-producing Enterobacterales, with a susceptible rate of 83.4% for cefiderocol. Cefiderocol is well-tolerated, and the PK/PD target values can be achieved using a standard dose regimen or adjusted doses according to renal function. Clinical trials demonstrated that cefiderocol was non-inferiority to the comparator drugs in treating complicated urinary tract infection and nosocomial pneumonia. Case reports and series showed that cefiderocol was a promising therapeutic agent in carbapenem-resistant infections. However, resistant isolates and reduced susceptibility during treatment to cefiderocol have already been reported. In conclusion, cefiderocol is a promising powerful weapon for treating MDR recalcitrant infections.
Keywords: cefiderocol, multidrug resistant, carbapenem-resistant, gram-negative bacteria, systematic review
Introduction
The spread of multi-drug resistant (MDR) bacteria is a great threat to public health. In 2017, the World Health Organisation (WHO) designated the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) as “priority status”, for which new antibiotics are urgently needed (De Oliveira et al., 2020; Tacconelli et al., 2017). Carbapenem-resistant gram-negative bacteria, including carbapenem-resistant Enterobacteriaceae, carbapenem-resistant P. aeruginosa, and carbapenem-resistant A. baumannii, are considered superbugs in healthcare settings. They are associated with resistance to nearly all classes of antibiotics commonly used in clinical settings. Due to the limited therapeutic options, polymyxins, a class of cationic peptide drugs abandoned in the last century due to high nephrotoxicity, are currently used to treat recalcitrant infections caused by carbapenem-resistant Gram-negative bacteria (Li et al., 2006). However, polymyxins are associated with unsatisfactory clinical outcomes and a high mortality rate among critically ill patients.
A few antibiotics being churned out of the drug discovery and development pipeline give hope of curbing antibiotic resistance. All bacteria, especially Gram-negative bacteria, need iron as an enzyme cofactor to catalyze redox reactions involved in various fundamental cellular processes. (Kramer et al., 2020). Taking advantage of this unique feature, cefiderocol, a novel synthetic siderophore-conjugated antibiotic has been developed, which can hijack the bacterial iron transport systems to facilitate the drug to enter cells, thereby achieving high periplasmic concentrations (Page, 2019). In addition, cefiderocol has a high affinity for penicillin binding proteins 3 (PBP3). The C-7 side chain in cefiderocol improves the transport across the bacterial outer membrane and can resist the hydrolysis by several β-lactamases (Aoki et al., 2018). Further, cefiderocol shows high in vitro potency against pathogenic carbapenem-resistant Gram-negative bacteria, with the minimum inhibitory concentration (MIC) lower than 4 mg/L for most Enterobacterales, P. aeruginosa and A. baumannii isolates (Yamano, 2019). It is approved by the Food and Drug Administration (FDA) to treat nosocomial pneumonia and complicated urinary tract infections (cUTIs).
Although cefiderocol is a promising antimicrobial agent against MDR Gram-negative bacteria, its efficacy in treating infections caused by carbapenem-resistant pathogens is uncertain (Simner and Patel, 2020). Furthermore, emergence of resistance has already been reported. Therefore, it is important to have a deep understanding of this novel siderophore-cephalosporin to promote rational use and thus reduce the emergence of resistance. This systematic review analyzes currently available literature evaluating the role of cefiderocol in treating MDR Gram-negative bacterial infections.
Materials and Methods
Search Strategy and Study Eligibility
This systematic review was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards (Page et al., 2021). We systematically searched PUBMED, EMBASE and Cochrane Library databases from inception to 12 January 2022. The search terms included “cefiderocol”, “S-6492660” and “Fetroja”. Further, we reviewed the conference proceedings of the International Symposium on Antimicrobial Agents and Resistance (ISARR), Infectious Diseases Society of America (IDSA), and European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) from the year 2015–2021 to reduce publication bias. Finally, we manually searched the reference lists of the included studies and systematic reviews to select relevant articles. This study was registered in the International Prospective Register of Systematic Reviews (Registration number: CRD42021286832).
Studies were considered eligible for inclusion if they reported on in vitro or in vivo antimicrobial activity, pharmacokinetics (PK) and pharmacodynamics (PD), clinical use and resistance of cefiderocol. Studies published in languages other than English or having duplicated data were excluded. The literature search and the study selection were carried out by two independent reviewers (WC and YD). Any disagreements were resolved by a third reviewer, and a final consensus was reached among all authors.
Data Extraction and Quality Assessment
The following data were extracted by two independent reviewers: authors, publication year, details of the experimental methods or study design, number of tested strains, animals or patients, main characteristics of the tested strains or the study population, and the outcome measures. Cochrane risk of bias tool was used to assess the risk of bias of the included clinical trials.
End-points
The primary end-point for in vitro studies on antimicrobial activity was the MICs and the susceptibility rate. The primary end-point in the animal studies was the in vivo efficacy. For the PK/PD studies, the primary end-point was the PK/PD targets. Finally, the primary end-points in clinical studies and trials were the clinical response and all-cause mortality.
Quantitative Data Synthesis
Quantitative data were analyzed using Stata 14.0 (Stata Corporation, College Station, TX). Risk ratio (RR) and 95% confidence intervals (CI) were used as the effect measures of outcomes for meta-analysis of clinical trials. Statistical heterogeneity among studies was assessed with the I 2 index (I 2 > 50% was considered substantial heterogeneity). The random-effect model was used when the heterogeneity was significant; in all other cases, the fixed effect model was used.
Results and Discussion
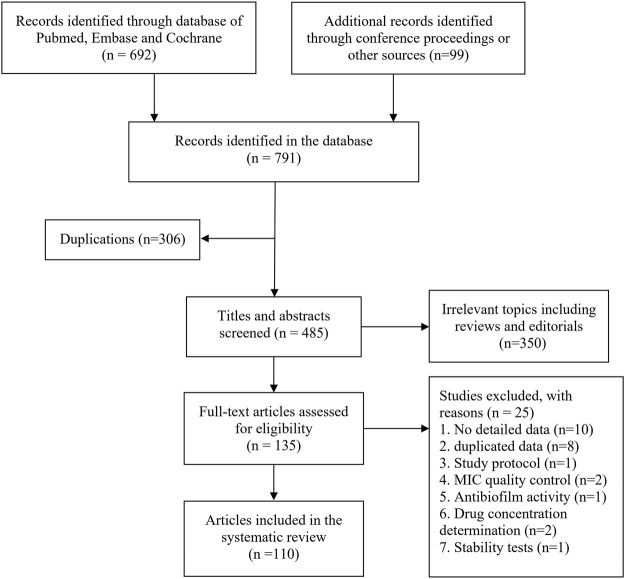
The literature search of databases yielded 692 citations. In addition, 99 conference proceedings on cefiderocol were included. Irrelevant studies were excluded after reviewing the full text. Finally, a total of 110 citations were included in this systematic review. Figure 1 shows a flow diagram of the literature search.
FIGURE 1.
Flow diagram of literature search.
In vitro Antimicrobial Activities
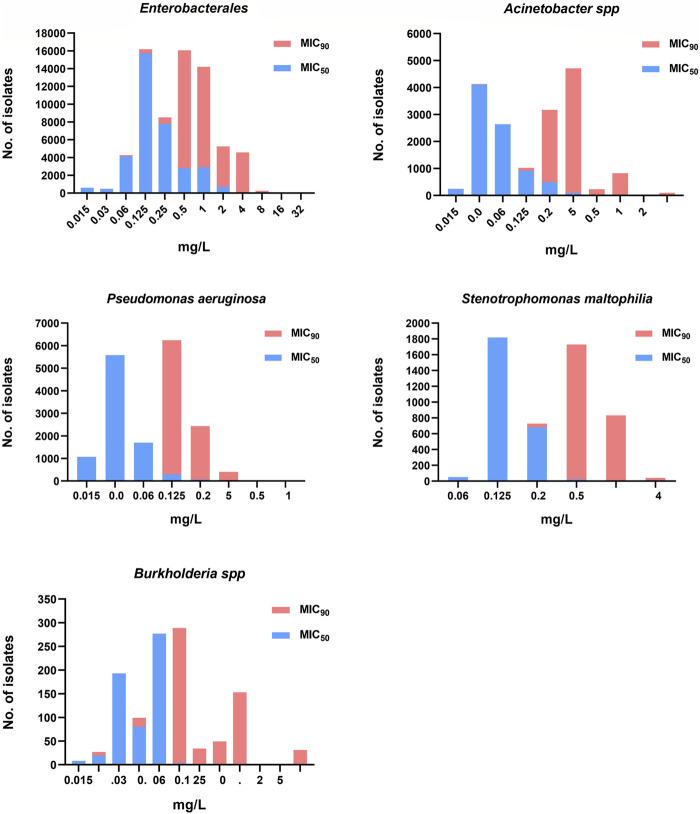
Thirty-eight studies reporting on the in vitro activity of cefiderocol against Gram-negative bacteria were included (Ito et al., 2015; Ito et al., 2016; Kohira et al., 2015; Falagas et al., 2017; Dobias et al., 2017; Hackel et al., 2017; Kanazawa et al., 2017; Yamano et al., 2017; Hackel et al., 2018; Ito et al., 2018a; Jacobs et al., 2018; Karlowsky et al., 2019; Kazmierczak et al., 2019; Hsueh et al., 2019; Iregui et al., 2019; Albano et al., 2020; Biagi et al., 2020; Delgado-Valverde et al., 2020; Golden et al., 2020; Johnston et al., 2020; Kresken et al., 2020; Longshaw et al., 2020; Morris et al., 2020; Mushtaq et al., 2020; Rolston et al., 2020; Trebosc et al., 2020; Abdul-Mutakabbir et al., 2021; Bhagwat et al., 2021; Bianco et al., 2021; Burnard et al., 2021; Cercenado et al., 2021; Gant et al., 2021; Candel et al., 2022; Johnston et al., 2021; Lee et al., 2021; Liu et al., 2021; Stracquadanio et al., 2021; Zalacain et al., 2021). A total of 53,416 isolates, including 34,805 Enterobacterales, 8297 P. aeruginosa, 7249 Acinetobacter spp, 2508 Stenotrophomonas maltophilia and 549 Burkholderia spp, mainly collected from North America, Europe and East Asia excluding Chinese mainland, were tested for cefiderocol susceptibility. The distribution of MIC50 and MIC90 for the significant pathogens is shown in Figure 2. Most studies reported that the MIC90 of cefiderocol for Enterobacterales ranged between 0.5 and 4 mg/L. Two studies including 393 isolates reported a MIC90>4 mg/L for Enterobacterales, indicating that cefiderocol had a susceptibility rate lower than 90% (Albano et al., 2020; Morris et al., 2020). The MIC90 of cefiderocol in Acinetobacter spp was similar to that of Enterobacterales. However, six studies including 920 isolates, reported a MIC90 higher than 4 mg/L (Ito et al., 2015; Hackel et al., 2018; Hsueh et al., 2019; Albano et al., 2020; Morris et al., 2020; Trebosc et al., 2020). The MIC90 for P. aeruginosa, S. maltophilia and Burkholderia spp was lower than Enterobacterales and Acinetobacter spp, suggesting a higher sensitivity to cefiderocol.
FIGURE 2.
The cefiderocol MIC50 and MIC90 distribution of Enterobacterales, Acinetobacter spp, Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia spp.
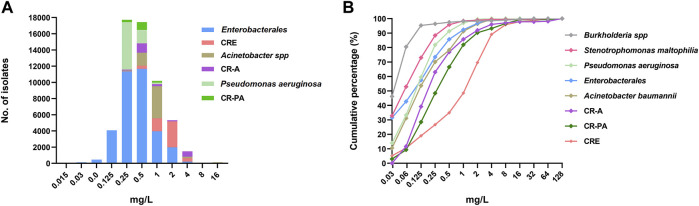
Further, the MIC90 distribution for carbapenem-resistant isolates was compared with that of the “putative carbapenem-susceptible” isolates (data obtained from studies that did not report on susceptibility to carbapenems). As show in Figure 3A, the MIC90 for carbapenem-resistant isolates, especially the Enterobacterales and Acinetobacter spp, was higher than that for the ‘putative carbapenem-susceptible’ isolates. The MIC90 for carbapenem-resistant Enterobacterales (CRE) was higher than that of carbapenem-resistant Acinetobacter spp and carbapenem-resistant P. aeruginosa. The specific MIC values for 9305 isolates were obtained from 13 studies. The cumulative MIC distribution curves for cefiderocol also showed that the MICs for carbapenem-resistant isolates were higher than for the ‘putative carbapenem-susceptible’ isolates (Figure 3B).
FIGURE 3.
The cefiderocol MIC profile of Enterobacterales, Acinetobacter spp and Pseudomonas aeruginosa with or without carbapenem-resistance. (A), the MIC90 distribution of the three Gram-negative bacteria; (B), the cumulative curves of MICs of 9305 isolates (n = 13). CRE, carbapenem-resistant Enterobacterales; CR-A, carbapenem-resistant Acinetobacter spp; CR-PA, carbapenem-resistant P. aeruginosa.
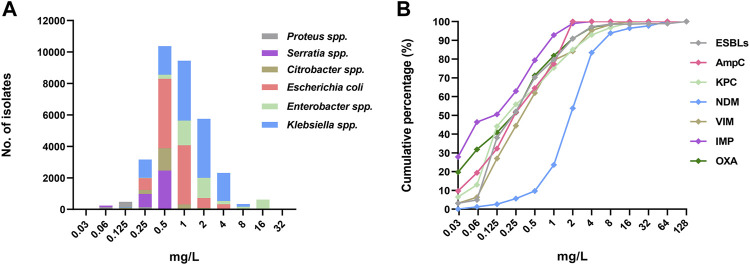
The MIC90 distribution for different Enterobacterales species is shown in Figure 4A. The MIC90 for Enterobacter spp and Klebsiella spp were higher than for others species. Further, the distribution of MICs for Enterobacterales (1264 isolates) harboring different β-lactamase genes were obtained from 15 studies and analyzed. As shown in Figure 4B, the MICs for New Delhi metallo- β-lactamase (NDM) positive isolates were significantly higher than those harboring other β-lactamase genes, with a susceptibility rate of 83.4% for cefiderocol.
FIGURE 4.
The cefiderocol MIC profile of different species of Enterobacterales, (A), the MIC90 distribution; (B), the cumulative curves of MICs of 1264 isolates (n = 15) harboring different β-lactamase.
In addition, four studies investigate the synergistic in vitro activity of cefiderocol combined with other antimicrobial agents against Gram-negative bacteria (Tsuji et al., 2016; Yamano et al., 2020a; Biagi et al., 2020; Abdul-Mutakabbir et al., 2021). Abdul-Mutakabbir, et al. assessed the combination of β-lactamase inhibitors (BLIs) on reversing cefiderocol resistance for MDR A. baumannii (the MICs for cefiderocol were 16–32 mg/L) (Abdul-Mutakabbir et al., 2021). They found that the addition of BLIs resulted in lower MIC values. Avibactam exerted the strongest effects with 4–64 folds reduction in the MIC values (0.5–8 mg/L) (Abdul-Mutakabbir et al., 2021). Another study by Yamano et al. reported that cefiderocol combined with avibactam or sulbactam showed synergistic activity against cefiderocol-resistant PER-producing A. baumannii (Yamano et al., 2020a). Besides, the two studies reported synergistic activity in combination therapy of cefiderocol-meropenem, cefiderocol-amikacin, cefiderocol-tigecycline, cefiderocol-minocycline and cefiderocol-ampicillin-sulbactam, even though the isolates showed resistance to both cefiderocol and meropenem/amikacin (Yamano et al., 2020a; Abdul-Mutakabbir et al., 2021). Biagi, et al. used time-kill assays to show the synergy when cefiderocol combined with levofloxacin, minocycline, polymyxin B, or TMP-SMZ against S. maltophilia were 44.4% (4/9), 66.7% (6/9), 55.5% (5/9), and 66.7% (6/9), respectively (Biagi et al., 2020). Using the checkboard method, Tsuji, et al. showed that cefiderocol combined with meropenem, ciprofloxacin, and amikacin showed synergistic effects against A. baumannii, P. aeruginosa and K. pneumoniae (Tsuji et al., 2016).
The Clinical and Laboratory Standards Institute recommends the use of iron-depleted cation-adjusted Mueller–Hinton broth (ID-CAMHB) for the determination of cefiderocol MICs (Clinical and Laboratory S, 2020). Among the 40 in vitro studies, 6 did not report the concrete methodologies used for determination of the MICs of cefiderocol (Ito et al., 2015; Ito et al., 2018a; Rolston et al., 2020; Trebosc et al., 2020; Abdul-Mutakabbir et al., 2021; Bhagwat et al., 2021), and the others used iron-depleted broth medium in the MIC testing. There may be potential some bias in the pooled results of susceptibility tests.
PK/PD and Animal Studies
Twenty-five studies investigated the characteristics of PK and/or PD of cefiderocol (Katsube et al., 2016; Katsube et al., 2017; Matsumoto et al., 2017; Monogue et al., 2017; Ghazi et al., 2018a; Ghazi et al., 2018b; Katsube et al., 2018; Kawaguchi et al., 2018; Saisho et al., 2018; Katsube et al., 2019a; Kidd et al., 2019a; Katsube et al., 2019b; Kidd et al., 2019b; Chen et al., 2019; Miyazaki et al., 2019; Nakamura et al., 2019; Stainton et al., 2019; Matsumoto et al., 2020; Ota et al., 2020; Gill et al., 2021; Katsube et al., 2021; Kawaguchi et al., 2021; Kobic et al., 2021; König et al., 2021; Nakamura et al., 2021). A phase I study including healthy Japanese and Caucasian volunteers showed exhibit linear PK at doses of up to 2,000 mg, with low to moderate interindividual variability (Saisho et al., 2018). Cefiderocol was mainly eliminated unchanged in urine (Miyazaki et al., 2019), with metabolism contributing to less than 10% elimination (Saisho et al., 2018). Since cefiderocol is primarily eliminated through the renal route, renal impairment alters area under the plasma concentration-time curve (AUC), total drug clearance from plasma (CL) and terminal half-life (t1/2), without significantly affecting the maximum plasma concentration (Cmax) (Katsube et al., 2017; Kawaguchi et al., 2018; Kobic et al., 2021; König et al., 2021). Kawaguchi, et al. evaluated the PK of cefiderocol in patients with pneumonia, bloodstream infection/sepsis, or complicated urinary tract infection, finding that no other factors, including infection sites and mechanical ventilation, were statistically significant covariates in the population PK analysis (Kawaguchi et al., 2021). The intrapulmonary PK of cefiderocol was further evaluated in healthy adult subjects (n = 20) and mechanically ventilated patients with pneumonia (n = 7) (Katsube et al., 2019a; Katsube et al., 2021). In the healthy subjects, the geometric mean concentrations of cefiderocol in epithelial lining fluid (ELF) were 13.8, 6.7, 2.8 and 1.4 mg/L at 1, 2, 4 and 6 h from infusion initiation, respectively. The ratios of ELF concentration to total plasma concentration over 6 h ranged from 0.093 to 0.12 (Katsube et al., 2019a). In the mechanically ventilated patients with pneumonia, the ELF concentration was 7.63 mg/L at the end of infusion and 10.40 mg/L at 2 h after the end of infusion. The ratios of ELF concentration to total plasma concentration ranged from 0.09 to 0.42 at the end of infusion and 0.44–0.82 at 2 h after the end of the infusion (Katsube et al., 2021). These results suggest that cefiderocol can penetrate into the ELF.
Kidd et al. established neutropenic murine thigh infection models with iron overload and deficiency (Kidd et al., 2019a). They showed that the plasma concentrations of cefiderocol were similar in the iron overload models and the control group (Kidd et al., 2019a). However, the plasma concentrations in the iron-depleted mice were lower than that in the control group, indicating that in vivo iron deficiency might alter the PK of cefiderocol (Kidd et al., 2019a). Moreover, Katsube, et al. showed that administration of cefiderocol did not significantly affect OAT1, OAT3, OCT1, OCT2, and MATE2-K drug transporters, suggesting no clinically significant drug-drug interaction potential via the transporters (Katsube et al., 2018).
Animal studies demonstrated that cefiderocol exhibited time-dependent PD similar to other β-lactam antibiotics (Ghazi et al., 2018a; Nakamura et al., 2019). Considering that the bactericidal activity of β-lactam antibiotics can be enhanced by prolonging the infusion time, the recommended standard dose regimen for cefiderocol is 2g q8h with a 3-h infusion (Fetroja (Cefiderocol), 2021). An in vitro PK/PD study showed that the standard dose could completely kill meropenem-resistant gram-negative isolates showing cefiderocol MICs of 0.5–4 g/ml within 24 h (Matsumoto et al., 2020). Nine animal studies using neutropenic murine thigh models or respiratory tract infection models mimicking humanized exposures (2g q8h with a 3-h infusion) showed a >1 log10 reduction in bacterial colony forming units (CFU) of most Gram-negative bacteria with MICs ≤4 g/ml, but not for the isolates with MICs ≥ 8 mg/L (Supplementary Table S1) (Matsumoto et al., 2017; Monogue et al., 2017; Ghazi et al., 2018b; Kidd et al., 2019b; Chen et al., 2019; Stainton et al., 2019; Ota et al., 2020; Gill et al., 2021; Nakamura et al., 2021).
Monte-Carlo simulations based on population PK models in accounting for protein binding of 57.8% showed the standard dose yielded >90% probability of target attainment (PTA) for 75% T f>MIC for an MIC ≤4 g/ml for adults or pediatric patients with normal renal function (Katsube et al., 2016; Katsube et al., 2019b). The dose of cefiderocol should be adjusted according to the renal function and whether patients are on hemodialysis or continuous renal replacement therapy. Another Monte-Carlo simulation study found >90% PTA for 100% T f>MIC for an MIC ≤4 g/ml in different infections and renal function groups could be achieved, except for bloodstream infection/sepsis patients with normal renal function (85%) (Kawaguchi et al., 2021).
Clinical Trials
By far, the clinical efficacy of cefiderocol has been investigated in three randomized controlled trials (RCTs), including one phase II trial (APEKS-cUTI) and two phase III trials (APEKS-NP and CREDIBLE-CR) (Portsmouth et al., 2018; Bassetti et al., 2021; Wunderink et al., 2021). The baseline demographics and pathogen distribution of the study populations are shown in Supplementary Table S2. Further, the risk of bias of the three RCTs is shown in Supplementary Figure S1.
The APEKS-cUTI trial compared the efficacy of cefiderocol versus imipenem/cilastatin in the treatment of complicated urinary tract infections (cUTIs) (Portsmouth et al., 2018). The primary endpoint included both clinical and microbiological outcomes at test of cure (7 days after treatment cessation). A total of 371 patients [cefiderocol (n = 252); imipenem/cilastatin (n = 119)] with qualifying Gram-negative uropathogen (≥1 × 10⁵ CFU/mL) were included in the primary efficacy analysis. The most common pathogens in both groups were Escherichia coli and K. pneumoniae. The primary efficacy endpoint was achieved by 72.6% (183/252) patients in the cefiderocol group and 54.6% (65/119) patients in the control group with an adjusted treatment difference of 18.6% (95% CI: 8.2–28.9, p = 0.0004). These results suggested that cefiderocol was non-inferior to imipenem/cilastatin for cUTIs.
The APEKS-NP trial evaluated the efficacy of cefiderocol versus meropenem with high-dose, extended-infusion (2g q8h with a 3-h infusion) for nosocomial pneumonia (hospital-acquired pneumonia, ventilator-associated pneumonia, or health-care-associated pneumonia) caused by gram-negative bacteria (Wunderink et al., 2021). A total of 292 patients were included in the modified intention-to-treat population, with 145 in the cefiderocol group and 147 in the meropenem group. The most common pathogens were K. pneumoniae followed by P. aeruginosa and A. baumannii. There were no significant differences in the primary endpoint (all-cause mortality at day 14) observed between two groups (12.4% in cefiderocol versus 11.6% in the meropenem group, the adjusted difference was 0.8%, 95% CI: 6.6–8.2%).
The CREDIBLE-CR trial evaluated the efficacy of cefiderocol versus the best available therapies (mainly colistin-based regimens) in adults with severe infections caused by carbapenem-resistant Gram-negative bacteria. This study enrolled 150 patients with nosocomial pneumonia (n = 67, 44.6%), bloodstream infection/sepsis (n = 47, 31.3%) or cUTIs (n = 36, 24.0%) (Bassetti et al., 2021). The most common pathogens were carbapenem-resistant Acinetobacter spp (n = 56), K. pneumoniae (n = 39) and P. aeruginosa (n = 22), with cefiderocol MIC90 of 1 g/ml, 4 mg/ml, and 2 mg/ml, respectively. The clinical cure rate for nosocomial pneumonia or bloodstream infection/sepsis and the microbiological eradication rate in cUTIs were not significantly different between the two groups. However, the mortality rate in the cefiderocol group [33.7% (34/101)] was higher than that of the control group [18.3% (9/49)]. Most deaths due to treatment failure in the cefiderocol group occurred in patients with infection due to Acinetobacter spp (13/16). Only one death (1/4) due to Acinetobacter spp infections was reported in the control group. In patients with infections due to other bacteria, no differences in mortality rates were noticed between the two groups. The efficacy of cefiderocol for treating MDR Acinetobacter spp infections deserves further clinical investigation.
A recent meta-analysis pooled the results of the three studies, and found no significant difference between cefiderocol and the comparators in terms of clinical response, microbiological response, all-cause mortality and adverse events (Hsueh et al., 2021). The most common reported adverse events were nausea, diarrhea, rash, elevated aminotransferase levels, and hypokalemia. Besides, a phase I study conducted in healthy persons showed that therapeutic doses of cefiderocol had no apparent effect on the QT interval.
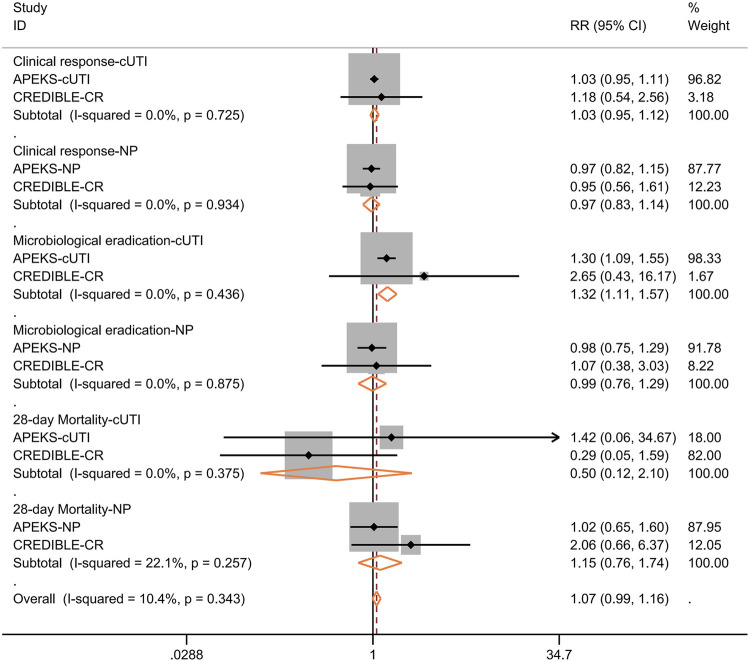
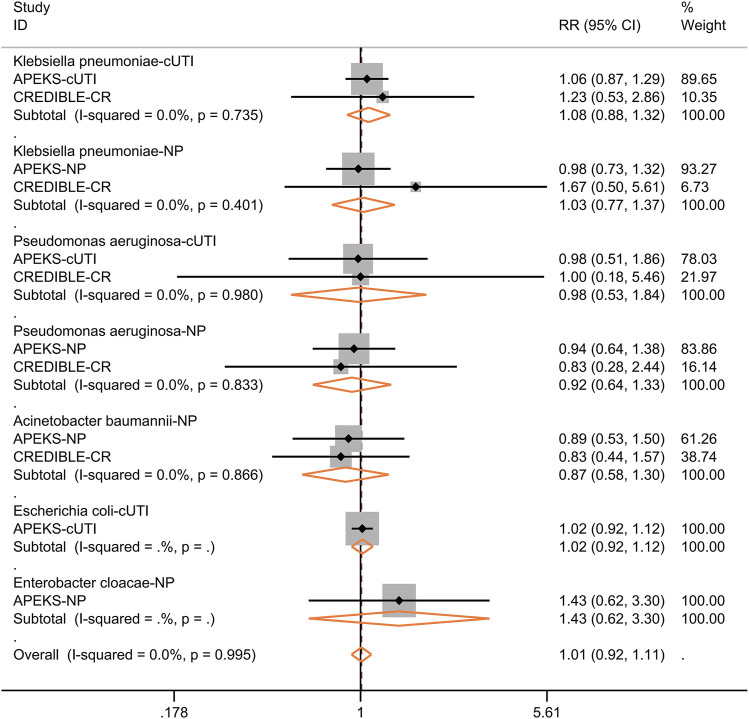
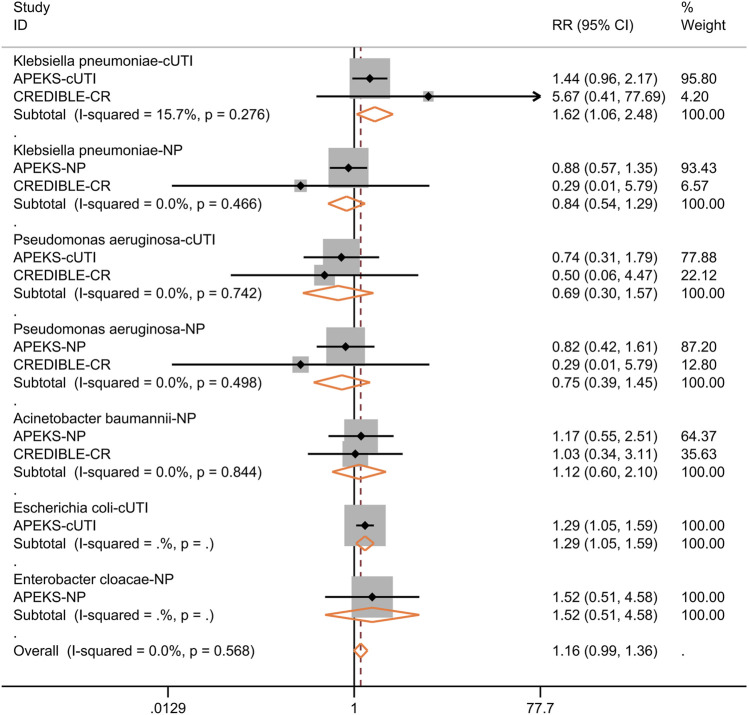
We further performed subgroup analysis for the efficacy of cefiderocol in treating nosocomial pneumonia or cUTI. As shown in Figure 5, the clinical response at the time of test of cure, microbiological response, 28-days all-cause mortality were not significantly different between cefiderocol and comparators. The subgroup analysis for different pathogens showed the clinical response was similar in the two groups (Figure 6). In the subgroup analysis for microbiological eradication of different pathogens (Figure 7), the cefiderocol group had higher microbiological eradication when treating cUTI caused by K. pneumoniae (RR = 1.6, 95% CI: 1.1–2.5, I 2 = 15.7%) or E. coli (RR = 1.3, 95% CI: 1.1–1.6).
FIGURE 5.
Forest plot for the pooled analysis of clinical response at test of cure, microbiological response and 28-days all-cause mortality between cefiderocol and comparators for the treatment of complicated urinary tract infection (cUTI) or nosocomial pneumonia (NP).
FIGURE 6.
Forest plot for the pooled analysis of clinical response at test of cure between cefiderocol and comparators for the treatment of infections caused by specific pathogens. Complicated urinary tract infection, cUTI; Nosocomial pneumonia, NP.
FIGURE 7.
Forest plot for the pooled analysis of microbiological eradication at test of cure between cefiderocol and comparators for the treatment of infections caused by specific pathogens. Complicated urinary tract infection, cUTI; Nosocomial pneumonia, NP.
Case Reports and Case Series
We identified 30 case reports and case series including 78 patients who had recalcitrant infections caused by MDR Gram-negative bacteria, and treated with salvage treatment or compassionate use of cefiderocol in real-world settings (Edgeworth et al., 2019; Stevens and Clancy, 2019; Trecarichi et al., 2019; Alamarat et al., 2020; Contreras et al., 2020; Dagher et al., 2020; Kufel et al., 2020; Lampejo et al., 2020; Oliva et al., 2020; Siméon et al., 2020; Zingg et al., 2020; Bavaro et al., 2021a; Bavaro et al., 2021b; Bleibtreu et al., 2021; Bodro et al., 2021; Borghesi et al., 2021; Carney et al., 2021; Chavda et al., 2021; Cipko et al., 2021; Falcone et al., 2021; Fratoni et al., 2021; Grande Perez et al., 2021; Grasa et al., 2021; Klein et al., 2021; König et al., 2021; Mabayoje et al., 2021; Martinez et al., 2021; Mc Gann et al., 2021; Warner et al., 2021; Zaidan et al., 2021). The detailed characteristics of these cases are summarized in Supplementary Table S3. Most patients were adult (74/78), and the most common reason for hospitalization were COVID-19, trauma and bone fracture, organ transplantation and cystic fibrosis, et al. The patients mostly had bloodstream infections (n = 26), lower respiratory tract infections (n = 24), including ventilator-associated pneumonia (n = 8), wound infections (n = 6), osteomyelitis (n = 5), and intra-abdominal infections (n = 4), caused mostly by A. baumannii (n = 33), P. aeruginosa (n = 25), K. pneumoniae (n = 12) and Achromobacter spp (n = 10). Eleven patients had polymicrobial infections.
Twenty-three studies including 47 patients, reported on therapy regimens given before using cefiderocol. Among them, 42 patients received colistin (polymyxin E) or polymyxin B-based therapies. Four patients received colistin monotherapy, and the other patients received polymyxin combination therapies. Tigeycline, meropenem and fosfomycin were the most common antibiotics used in combination therapy. The most frequent reasons for switching to cefiderocol based regimen was treatment failure (n = 36), and/or polymyxin-associated toxicity (n = 13) ([enal toxicity (n = 7), neurotoxicity (n = 4)]. Among the 73 patients with detailed cefiderocol-based regimens, 30 received cefiderocol monotherapy, and the others received combination therapy (mainly combined with polymyxins, tigecycline, fosfomycin, meropenem or ceftazidime-avibatam). The total clinical response, microbiological eradication and mortality rates were 73.1% (57/78), 74.3% (57/77), 24.4% (19/78), respectively. Cefiderocol associated adverse events were reported in six patients, including leukopenia (n = 2), thrombocytopenia (n = 2), acute kidney injury (n = 2). The clinical response of cefiderocol for treating cefiderocol-susceptible A. baumannii, Enterobacterales and P. aeruginosa were 85.2% (23/27), 100% (8/8) and 81.3% (13/16), respectively. These data supported the role of cefiderocol in treating MDR Gram-negative bacteria infections. Nevertheless, it should be noted that the pooled analysis results of case reports and case series were better than those of the CREDIBLE-CR trial, due to possible selection bias and/or publication bias.
Resistant Mechanisms
Overall, the worldwide resistant rate (MIC > 8 mg/L) of MDR gram-negative bacteria for cefiderocol is quite low. However, clinical resistance has been reported. In the APEKS-NP and CREDIBLE-CR studies, a ≥4-fold MIC increase during the treatment was found in 4.4% (7/159) and 11.3% (12/106) isolates, respectively (Bassetti et al., 2021; Wunderink et al., 2021). Klein, et al. reported the development of high resistance within 21 days of cefiderocol therapy in a patient with intra-abdominal and bloodstream infections caused by carbapenemase-producing Enterobacter cloacae (Klein et al., 2021). In addition, Choby, et al. reported widespread cefiderocol heteroresistance in carbapenem-resistant A. baumannii (59%), Klebsiella spp (30%), and S. maltophilia (48%) (Choby et al., 2021). Though in vitro heteroresistance of bacteria has not been clinically validated to be predictive of clinical or microbiological outcomes in vivo, the presence of resistant subpopulation in heteroresistant isolates may be selected and predominates, ultimately resulting in cefiderocol resistance.
Various mechanisms are associated with reduced susceptibility to cefiderocol. Firstly, several studies showed that cefiderocol-resistant isolates often harbored genes encoding NDM, PER and VEB β-lactamases, suggesting that these β-lactamases may contribute to cefiderocol resistance (Ito et al., 2019; Yamano et al., 2020b; Kohira et al., 2020; Poirel et al., 2021). The addition of avibactam could significantly decrease the MICs of non-susceptible A. baumannii isolates (Abdul-Mutakabbir et al., 2021), suggesting the involvement of β-lactamases in resistance. Secondly, structural changes in AmpC and KPC β-lactamases could confer reduced susceptibility to the cefiderocol, ceftazidime-avibactam and other cephalosporins (Shields et al., 2020; Hobson et al., 2021; Simner et al., 2021). Thirdly, reduced expression or mutation of genes involving iron transport pathways, especially the siderophore receptor genes (pirA, cirA, et al.) are associated with cefiderocol resistance (Ito et al., 2018b; Yamano et al., 2020b; Yamano et al., 2020c; Malik et al., 2020; Klein et al., 2021; Streling et al., 2021). Lastly, two studies found that mutations in the target gene PBP-3 might contribute to cefiderocol resistance (Malik et al., 2020; Takemura et al., 2020).
Conclusion
Cefiderocol shows extensive in vitro and in vivo activities against MDR Gram-negative bacteria, including carbapenem-resistant isolates. It is well tolerated and the PK/PD target can be achieved in most patients by using standard dosage (2g q8h) or adjusting doses according to the renal function. Clinical trials and case reports/series show that cefiderocol is a promising therapeutic option for carbapenem-resistant recalcitrant infections. Since resistant isolates have already been reported, cefiderocol should be used judiciously to prevent widespread resistance. More clinical data is still needed to testify its efficacy.
Author Contributions
NW and WC raised the research question and objectives of this systematic review. WC, YD, and WY searched the literature, screened titles and abstracts, and performed data extraction and analyses. NW, WC, and YD drafted the manuscript. NW and WY reviewed manuscript drafts. All authors approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81903672), Peking University People’s Hospital Research and Development Funds (RS2020-04), and China International Medical Foundation (Z-2018-35-2003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.896971/full#supplementary-material
References
- Abdul-Mutakabbir J. C., Nguyen L., Maassen P. T., Stamper K. C., Kebriaei R., Kaye K. S., et al. (2021). In Vitro antibacterial Activity of Cefiderocol against Multidrug-Resistant Acinetobacter Baumannii. Antimicrob. Agents Chemother. 65, e0264620. 10.1128/AAC.02646-20 PubMed Abstract | 10.1128/AAC.02646-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamarat Z. I., Babic J., Tran T. T., Wootton S. H., Dinh A. Q., Miller W. R., et al. (2020). Long-Term Compassionate Use of Cefiderocol to Treat Chronic Osteomyelitis Caused by Extensively Drug-Resistant Pseudomonas aeruginosa and Extended-Spectrum-β-Lactamase-Producing Klebsiella pneumoniae in a Pediatric Patient. Antimicrob. Agents Chemother. 64, e01872–19. 10.1128/AAC.01872-19 PubMed Abstract | 10.1128/AAC.01872-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano M., Karau M. J., Schuetz A. N., Patel R. (2020). Comparison of agar Dilution to Broth Microdilution for Testing In Vitro Activity of Cefiderocol against Gram-Negative Bacilli. J. Clin. Microbiol. 59, e00966–20. 10.1128/JCM.00966-20 PubMed Abstract | 10.1128/JCM.00966-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Yoshizawa H., Yamawaki K., Yokoo K., Sato J., Hisakawa S., et al. (2018). Cefiderocol (S-649266), A New Siderophore Cephalosporin Exhibiting Potent Activities against Pseudomonas aeruginosa and Other Gram-Negative Pathogens Including Multi-Drug Resistant Bacteria: Structure Activity Relationship. Eur. J. Med. Chem. 155, 847–868. 10.1016/j.ejmech.2018.06.014 PubMed Abstract | 10.1016/j.ejmech.2018.06.014 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., et al. (2021). Efficacy and Safety of Cefiderocol or Best Available Therapy for the Treatment of Serious Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria (CREDIBLE-CR): a Randomised, Open-Label, Multicentre, Pathogen-Focused, Descriptive, Phase 3 Trial. Lancet Infect. Dis. 21, 226–240. 10.1016/S1473-3099(20)30796-9 PubMed Abstract | 10.1016/S1473-3099(20)30796-9 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bavaro D. F., Belati A., Diella L., Stufano M., Romanelli F., Scalone L., et al. (2021). Cefiderocol-based Combination Therapy for "Difficult-To-Treat" Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics (Basel) 10, 652. 10.3390/antibiotics10060652 PubMed Abstract | 10.3390/antibiotics10060652 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaro D. F., Romanelli F., Stolfa S., Belati A., Diella L., Ronga L., et al. (2021). Recurrent Neurosurgical Site Infection by Extensively Drug-Resistant P. aeruginosa Treated with Cefiderocol: a Case Report and Literature Review. Infect. Dis. (Lond) 53, 206–211. 10.1080/23744235.2020.1856921 PubMed Abstract | 10.1080/23744235.2020.1856921 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bhagwat S. S., Legakis N. J., Skalidis T., Loannidis A., Goumenopoulos C., Joshi P. R., et al. (2021). In Vitro activity of Cefepime/zidebactam (WCK 5222) against Recent Gram-Negative Isolates Collected from High Resistance Settings of Greek Hospitals. Diagn. Microbiol. Infect. Dis. 100, 115327. 10.1016/j.diagmicrobio.2021.115327 PubMed Abstract | 10.1016/j.diagmicrobio.2021.115327 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Biagi M., Vialichka A., Jurkovic M., Wu T., Shajee A., Lee M., et al. (2020). Activity of Cefiderocol Alone and in Combination with Levofloxacin, Minocycline, Polymyxin B, or Trimethoprim-Sulfamethoxazole against Multidrug-Resistant Stenotrophomonas Maltophilia. Antimicrob. Agents Chemother. 64, e00559–20. 10.1128/AAC.00559-20 PubMed Abstract | 10.1128/AAC.00559-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco G., Boattini M., Comini S., Iannaccone M., Bondi A., Cavallo R., et al. (2021). In Vitro activity of Cefiderocol against Ceftazidime-Avibactam Susceptible and Resistant KPC-Producing Enterobacterales: Cross-Resistance and Synergistic Effects. Eur. J. Clin. Microbiol. Infect. Dis. 41, 63–70. [Online ahead of print]. 10.1007/s10096-021-04341-z PubMed Abstract | 10.1007/s10096-021-04341-z | Google Scholar [DOI] [PubMed] [Google Scholar]
- Bleibtreu A., Dortet L., Bonnin R. A., Wyplosz B., Sacleux S. C., Mihaila L., et al. (2021). Susceptibility Testing Is Key for the success of Cefiderocol Treatment: A Retrospective Cohort Study. Microorganisms 9, 282. 10.3390/microorganisms9020282 PubMed Abstract | 10.3390/microorganisms9020282 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodro M., Hernández-Meneses M., Ambrosioni J., Linares L., Moreno A., Sandoval E., et al. (2021). Salvage Treatment with Cefiderocol Regimens in Two Intravascular Foreign Body Infections by MDR Gram-Negative Pathogens, Involving Non-removable Devices. Infect. Dis. Ther. 10, 575–581. 10.1007/s40121-020-00385-4 PubMed Abstract | 10.1007/s40121-020-00385-4 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesi L., Viaggi V., Franzetti M., Montoli M., Mauri C., Moioli G., et al. (2021). Successful Prolonged Cefiderocol Treatment of a Chronic Left Pleural Empyema Caused by Pseudomonas aeruginosa in a Patient Affected by COVID-19: a Case Report. J. Glob. Antimicrob. Resist. 27, 157–159. 10.1016/j.jgar.2021.09.005 PubMed Abstract | 10.1016/j.jgar.2021.09.005 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnard D., Robertson G., Henderson A., Falconer C., Bauer M. J., Cottrell K., et al. (2021). Burkholderia Pseudomallei Clinical Isolates Are Highly Susceptible In Vitro to Cefiderocol, a Siderophore Cephalosporin. Antimicrob. Agents Chemother. 65, e00685–20. 10.1128/AAC.00685-20 PubMed Abstract | 10.1128/AAC.00685-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candel F. J., Santerre Henriksen A., Longshaw C., Yamano Y., Oliver A. (2022). In Vitro activity of the Novel Siderophore Cephalosporin, Cefiderocol, in Gram-Negative Pathogens in Europe by Site of Infection. Clin. Microbiol. Infect. 28 (21), e1–447. 10.1016/j.cmi.2021.07.018 10.1016/j.cmi.2021.07.018 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Carney B. W., Rizzo J. A., Alderete J. F., Cindass R., Markelz A. E., Cancio L. C. (2021). Carbapenem-resistant Enterobacterales Infection after Massive Blast Injury: Use of Cefiderocol Based Combination Therapy. Mil. Med. 186, 1241–1245. 10.1093/milmed/usab350 PubMed Abstract | 10.1093/milmed/usab350 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Cercenado E., Cardenoso L., Penin R., Longshaw C., Henriksen A. S., Pascual A. (2021). In Vitro activity of Cefiderocol and Comparators against Isolates of Gram-Negative Bacterial Pathogens from a Range of Infection Sources: SIDERO-WT-2014-2018 S-tudies in Spain. J. Glob. Antimicrob. Resist. 26, 292–300. 10.1016/j.jgar.2021.06.011 PubMed Abstract | 10.1016/j.jgar.2021.06.011 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Chavda A., Gilchrist M., Samarasinghe D. (2021). Education: A Compassionate Use of Cefiderocol to Treat Osteomyelitis Caused by an XDR Pseudomonas aeruginosa . JAC Antimicrob. Resist. 3 (Suppl. 1), i18–i20. Erratum in: JAC Antimicrob Resist. 2021 Aug 28;3(3):dlab109. Erratum in: JAC Antimicrob Resist. 2021;3:dlab110. 10.1093/jacamr/dlab054 PubMed Abstract | 10.1093/jacamr/dlab054 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. H., Kidd J. M., Abdelraouf K., Nicolau D. P. (2019). Comparative In Vivo Antibacterial Activity of Human-Simulated Exposures of Cefiderocol and Ceftazidime against Stenotrophomonas Maltophilia in the Murine Thigh Model. Antimicrob. Agents Chemother. 63, e01558–19. 10.1128/AAC.01558-19 10.1128/AAC.01558-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choby J. E., Ozturk T., Satola S. W., Jacob J. T., Weiss D. S. (2021). Widespread Cefiderocol Heteroresistance in Carbapenem-Resistant Gram-Negative Pathogens. Lancet Infect. Dis. 21, 597–598. 10.1016/S1473-3099(21)00194-8 10.1016/S1473-3099(21)00194-8 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipko K., Kizny Gordon A., Adhikari S., Konecny P. (2021). Cefiderocol Treatment of Pseudomonas aeruginosa and Extensively Drug-Resistant Acinetobacter Baumannii Retained Spinal Hardware Infection Causing Reversible Acute Interstitial Nephritis: Recto: Cefiderocol Causing Acute Interstitial Nephritis. Int. J. Infect. Dis. 109, 108–111. 10.1016/j.ijid.2021.06.035 PubMed Abstract | 10.1016/j.ijid.2021.06.035 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) (2020). M100 Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Wayne: Clinical and Laboratory Standards Institute. Google Scholar [Google Scholar]
- Contreras D. A., Fitzwater S. P., Nanayakkara D. D., Schaenman J., Aldrovandi G. M., Garner O. B., et al. (2020). Coinfections of Two Strains of NDM-1- and OXA-232-Coproducing klebsiella Pneumoniae in a Kidney Transplant Patient. Antimicrob. Agents Chemother. 64, e00948–19. 10.1128/AAC.00948-19 PubMed Abstract | 10.1128/AAC.00948-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher M., Ruffin F., Marshall S., Taracila M., Bonomo R. A., Reilly R., et al. (2020). Case Report: Successful rescue Therapy of Extensively Drug-Resistant Acinetobacter Baumannii Osteomyelitis with Cefiderocol. Open Forum Infect. Dis. 7, ofaa150. 10.1093/ofid/ofaa150 PubMed Abstract | 10.1093/ofid/ofaa150 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira D. M. P., Forde B. M., Kidd T. J., Harris P. N. A., Schembri M. A., Beatson S. A., et al. (2020). Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 33, e00181–19. 10.1128/CMR.00181-19 PubMed Abstract | 10.1128/CMR.00181-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Valverde M., Conejo M. D. C., Serrano L., Fernández-Cuenca F., Pascual Á. (2020). Activity of Cefiderocol against High-Risk Clones of Multidrug-Resistant Enterobacterales, Acinetobacter Baumannii, Pseudomonas aeruginosa and Stenotrophomonas Maltophilia. J. Antimicrob. Chemother. 75, 1840–1849. 10.1093/jac/dkaa117 PubMed Abstract | 10.1093/jac/dkaa117 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobias J., Dénervaud-Tendon V., Poirel L., Nordmann P. (2017). Activity of the Novel Siderophore Cephalosporin Cefiderocol against Multidrug-Resistant Gram-Negative Pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 36, 2319–2327. 10.1007/s10096-017-3063-z PubMed Abstract | 10.1007/s10096-017-3063-z | Google Scholar [DOI] [PubMed] [Google Scholar]
- Edgeworth J. D., Merante D., Patel S., Young C., Jones P., Vithlani S., et al. (2019). Compassionate Use of Cefiderocol as Adjunctive Treatment of Native Aortic Valve Endocarditis Due to Extremely Drug-Resistant Pseudomonas aeruginosa . Clin. Infect. Dis. 68, 1932–1934. 10.1093/cid/ciy963 PubMed Abstract | 10.1093/cid/ciy963 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas M. E., Skalidis T., Vardakas K. Z., Legakis N. J. Hellenic Cefiderocol Study Group (2017). Activity of Cefiderocol (S-649266) against Carbapenem-Resistant Gram-Negative Bacteria Collected from Inpatients in Greek Hospitals. J. Antimicrob. Chemother. 72, 1704–1708. 10.1093/jac/dkx049 PubMed Abstract | 10.1093/jac/dkx049 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Falcone M., Tiseo G., Nicastro M., Leonildi A., Vecchione A., Casella C., et al. (2021). Cefiderocol as rescue Therapy for Acinetobacter Baumannii and Other Carbapenem-Resistant Gram-Negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 72, 2021–2024. 10.1093/cid/ciaa1410 PubMed Abstract | 10.1093/cid/ciaa1410 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Fetroja (Cefiderocol) (2021). Fetroja (Cefiderocol for Injection) Drug. Osaka, Japan: Shionogi & Co., Ltd. [Package Insert]Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209445s000lbl.pdf . Google Scholar [Google Scholar]
- Fratoni A. J., Kuti J. L., Nicolau D. P. (2021). Optimised Cefiderocol Exposures in a Successfully Treated Critically Ill Patient with Polymicrobial Stenotrophomonas Maltophilia Bacteraemia and Pneumonia Receiving Continuous Venovenous Haemodiafiltration. Int. J. Antimicrob. Agents 58, 106395. 10.1016/j.ijantimicag.2021.106395 PubMed Abstract | 10.1016/j.ijantimicag.2021.106395 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Gant V., Hussain A., Bain M., Longshaw C., Henriksen A. S. (2021). In Vitro activity of Cefiderocol and Comparators against Gram-Negative Bacterial Isolates from a Series of Surveillance Studies in England: 2014-2018. J. Glob. Antimicrob. Resist. 27, 1–11. 10.1016/j.jgar.2021.07.014 PubMed Abstract | 10.1016/j.jgar.2021.07.014 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ghazi I. M., Monogue M. L., Tsuji M., Nicolau D. P. (2018). Humanized Exposures of Cefiderocol, a Siderophore Cephalosporin, Display Sustained In Vivo Activity against Siderophore-Resistant Pseudomonas aeruginosa . Pharmacology 101, 278–284. 10.1159/000487441 PubMed Abstract | 10.1159/000487441 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi I. M., Monogue M. L., Tsuji M., Nicolau D. P. (2018). Pharmacodynamics of Cefiderocol, a Novel Siderophore Cephalosporin, in a Pseudomonas aeruginosa Neutropenic Murine Thigh Model. Int. J. Antimicrob. Agents 51, 206–212. 10.1016/j.ijantimicag.2017.10.008 PubMed Abstract | 10.1016/j.ijantimicag.2017.10.008 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Gill C. M., Abdelraouf K., Oota M., Nakamura R., Kuroiwa M., Gahara Y., et al. (2021). Discrepancy in Sustained Efficacy and Resistance Emergence under Human-Simulated Exposure of Cefiderocol against Stenotrophomonas Maltophilia between In Vitro Chemostat and In Vivo Murine Infection Models. J. Antimicrob. Chemother. 76, 2615–2621. 10.1093/jac/dkab221 PubMed Abstract | 10.1093/jac/dkab221 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Golden A. R., Adam H. J., Baxter M., Walkty A., Lagacé-Wiens P., Karlowsky J. A., et al. (2020). In Vitro activity of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated from Patients in canadian Intensive Care Units. Diagn. Microbiol. Infect. Dis. 97, 115012. 10.1016/j.diagmicrobio.2020.115012 PubMed Abstract | 10.1016/j.diagmicrobio.2020.115012 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Grande Perez C., Maillart E., Miendje Deyi V. Y., Huang T. D., Kamgang P., Dernier Y., et al. (2021). Compassionate Use of Cefiderocol in a Pancreatic Abscess and Emergence of Resistance. Infect. Dis. Now 51, 399–401. 10.1016/j.medmal.2020.10.022 PubMed Abstract | 10.1016/j.medmal.2020.10.022 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Grasa C. D., Gómez-Gil M. R., San Román Pacheco S., Del Rosal T., Moreno F., Gerig N., et al. (2021). Compassionate Use of Cefiderocol for VIM Metallo-β-Lactamase-Producing Pseudomonas aeruginosa Infection in a Toddler with Burkitt Lymphoma. J. Glob. Antimicrob. Resist. 26, 91–92. 10.1016/j.jgar.2021.04.025 PubMed Abstract | 10.1016/j.jgar.2021.04.025 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hackel M. A., Tsuji M., Yamano Y., Echols R., Karlowsky J. A., Sahm D. F. (2017). In Vitro activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Antimicrob. Agents Chemother. 61, e00093–17. 10.1128/AAC.00093-17 PubMed Abstract | 10.1128/AAC.00093-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel M. A., Tsuji M., Yamano Y., Echols R., Karlowsky J. A., Sahm D. F. (2018). In Vitro activity of the Siderophore Cephalosporin, Cefiderocol, against Carbapenem-Nonsusceptible and Multidrug-Resistant Isolates of Gram-Negative Bacilli Collected Worldwide in 2014 to 2016. Antimicrob. Agents Chemother. 62, e01968–17. 10.1128/AAC.01968-17 PubMed Abstract | 10.1128/AAC.01968-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson C. A., Cointe A., Jacquier H., Choudhury A., Magnan M., Courroux C., et al. (2021). Cross-resistance to Cefiderocol and Ceftazidime-Avibactam in KPC β-lactamase Mutants and the Inoculum Effect. Clin. Microbiol. Infect. 27, 1172. e7-e10. 10.1016/j.cmi.2021.04.016 PubMed Abstract | 10.1016/j.cmi.2021.04.016 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hsueh S. C., Chao C. M., Wang C. Y., Lai C. C., Chen C. H. (2021). Clinical Efficacy and Safety of Cefiderocol in the Treatment of Acute Bacterial Infections: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Glob. Antimicrob. Resist. 24, 376–382. 10.1016/j.jgar.2021.02.004 PubMed Abstract | 10.1016/j.jgar.2021.02.004 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Hsueh S. C., Lee Y. J., Huang Y. T., Liao C. H., Tsuji M., Hsueh P. R. (2019). In Vitro activities of Cefiderocol, Ceftolozane/tazobactam, Ceftazidime/avibactam and Other Comparative Drugs against Imipenem-Resistant Pseudomonas aeruginosa and Acinetobacter Baumannii, and Stenotrophomonas Maltophilia, All Associated with Bloodstream Infections in Taiwan. J. Antimicrob. Chemother. 74, 380–386. 10.1093/jac/dky425 PubMed Abstract | 10.1093/jac/dky425 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Iregui A., Khan Z., Landman D., Quale J. M. (2019). In Vitro activity of Cefiderocol against Gram-Negative Clinical Isolates from New York City. Open Forum Infect. Dis. 6, S324. 10.1093/ofid/ofz360.78910.1093/ofid/ofz360.796 10.1093/ofid/ofz360.78910.1093/ofid/ofz360.796 | Google Scholar [DOI] [Google Scholar]
- Ito A., Hackel M., Sahm D., Tsuji M., Tamano Y. (2019). “Characterization of Isolates Showing High MICs to Cefiderocol from Global Surveillance Study SIDERO-CR-2014/2016,” in Proceedings of the 29th European Congress of Clinical Microbiology and Infectious Diseases (Amsterdam: ECCMID; ). [abstract P1857]. Google Scholar [Google Scholar]
- Ito A., Kohira N., Bouchillon S. K., West J., Rittenhouse S., Sader H. S., et al. (2016). In Vitro antimicrobial Activity of S-649266, a Catechol-Substituted Siderophore Cephalosporin, when Tested against Non-fermenting Gram-Negative Bacteria. J. Antimicrob. Chemother. 71, 670–677. 10.1093/jac/dkv402 PubMed Abstract | 10.1093/jac/dkv402 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ito A., Kohira N., Nakamura R., Tsuji M., Kreiswirth Y. Y., Yamano Y. (2015). “S-649266, a Novel Siderophore Cephalosporin: In Vitro Activity against Gram-Negative Bacteria Including Carbapenem Resistant Strains,” in Proceedings of the 25th European Congress of Clinical Microbiology and Infectious Diseases (Copenhagen: ECCMID; ). [abstract P0252]. Google Scholar [Google Scholar]
- Ito A., Nishikawa T., Ishii R., Kuroiwa M., Ishioka Y., Kurihara N., et al. (2018). 696. Mechanism of Cefiderocol High MIC Mutants Obtained in Non-clinical FoR Studies. Open Forum Infect. Dis. 5, S251. 10.1093/ofid/ofy210.703 10.1093/ofid/ofy210.703 | Google Scholar [DOI] [Google Scholar]
- Ito A., Ota M., Nakamura R., Tsuji M., Sato T., Yamano Y., et al. (2018). 1366. In Vitro and In Vivo Activity of Cefiderocol against Stenotrophomonas Maltophilia Clinical Isolates. Open Forum Infect. Dis. 5, S418. 10.1093/ofid/ofy210.1197 10.1093/ofid/ofy210.1197 | Google Scholar [DOI] [Google Scholar]
- Jacobs M. R., Abdelhamed A. M., Good C. E., Rhoads D. D., Hujer K. M., Hujer A. M., et al. (2018). ARGONAUT-I: Activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, Including Carbapenem-Resistant Nonfermenters and Enterobacteriaceae with Defined Extended-Spectrum β-Lactamases and Carbapenemases. Antimicrob. Agents Chemother. 63, e01801–18. 10.1128/AAC.01801-18 PubMed Abstract | 10.1128/AAC.01801-18 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. D., Thuras P., Porter S. B., Anacker M., VonBank B., Snippes Vagnone P., et al. (2020). Activity of Cefiderocol, Ceftazidime-Avibactam, and Eravacycline against Carbapenem-Resistant Escherichia coli Isolates from the United States and International Sites in Relation to Clonal Background, Resistance Genes, Coresistance, and Region. Antimicrob. Agents Chemother. 64, e00797–20. 10.1128/AAC.00797-20 PubMed Abstract | 10.1128/AAC.00797-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B. D., Thuras P., Porter S. B., Clabots C., Johnsona J. R. (2021). Activity of Cefiderocol, Ceftazidime-Avibactam, and Eravacycline against Extended-Spectrum Cephalosporin-Resistant Escherichia coli Clinical Isolates (2012-20017) in Relation to Phylogenetic Background, Sequence Type 131 Subclones, blaCTX-M Genotype, and Coresistance. Diagn. Microbiol. Infect. Dis. 100, 115314. 10.1016/j.diagmicrobio.2021.115314 PubMed Abstract | 10.1016/j.diagmicrobio.2021.115314 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kanazawa S., Sato T., Kohira N., Ito-Horiyama T., Tsuji M., Yamano Y. (2017). Susceptibility of Imipenem-Susceptible but Meropenem-Resistant blaIMP-6-Carrying Enterobacteriaceae to Various Antibacterials, Including the Siderophore Cephalosporin Cefiderocol. Antimicrob. Agents Chemother. 61, e00576–17. 10.1128/AAC.00576-17 PubMed Abstract | 10.1128/AAC.00576-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlowsky J. A., Hackel M. A., Tsuji M., Yamano Y., Echols R., Sahm D. F. (2019). In Vitro activity of Cefiderocol, a Siderophore Cephalosporin, against Gram-Negative Bacilli Isolated by Clinical Laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int. J. Antimicrob. Agents 53, 456–466. 10.1016/j.ijantimicag.2018.11.007 PubMed Abstract | 10.1016/j.ijantimicag.2018.11.007 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Katsube T., Echols R., Arjona Ferreira J. C., Krenz H. K., Berg J. K., Galloway C. (2017). Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects with Renal Impairment. J. Clin. Pharmacol. 57, 584–591. 10.1002/jcph.841 PubMed Abstract | 10.1002/jcph.841 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube T., Echols R., Wajima T. (2019). Prediction of Cefiderocol Pharmacokinetics and Probability of Target Attainment in Pediatric Subjects for Proposing Dose Regimens. Open Forum Infect. Dis. 6, S330–S331. 10.1093/ofid/ofz360.807 10.1093/ofid/ofz360.807 | Google Scholar [DOI] [Google Scholar]
- Katsube T., Miyazaki S., Narukawa Y., Hernandez-Illas M., Wajima T. (2018). Drug-drug Interaction of Cefiderocol, a Siderophore Cephalosporin, via Human Drug Transporters. Eur. J. Clin. Pharmacol. 74, 931–938. 10.1007/s00228-018-2458-9 PubMed Abstract | 10.1007/s00228-018-2458-9 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Katsube T., Nicolau D. P., Rodvold K. A., Wunderink R. G., Echols R., Matsunaga Y., et al. (2021). Intrapulmonary Pharmacokinetic Profile of Cefiderocol in Mechanically Ventilated Patients with Pneumonia. J. Antimicrob. Chemother. 76, 2902–2905. 10.1093/jac/dkab280 PubMed Abstract | 10.1093/jac/dkab280 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube T., Saisho Y., Shimada J., Furuie H. (2019). Intrapulmonary Pharmacokinetics of Cefiderocol, a Novel Siderophore Cephalosporin, in Healthy Adult Subjects. J. Antimicrob. Chemother. 74, 1971–1974. 10.1093/jac/dkz123 PubMed Abstract | 10.1093/jac/dkz123 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube T., Wajima T., Ishibashi T., Arjona Ferreira J. C., Echols R. (2016). Pharmacokinetic/pharmacodynamic Modeling and Simulation of Cefiderocol, a Parenteral Siderophore Cephalosporin, for Dose Adjustment Based on Renal Function. Antimicrob. Agents Chemother. 61, e01381–16. 10.1128/AAC.01381-16 PubMed Abstract | 10.1128/AAC.01381-16 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi N., Katsube T., Echols R., Wajima T. (2018). Population Pharmacokinetic Analysis of Cefiderocol, a Parenteral Siderophore Cephalosporin, in Healthy Subjects, Subjects with Various Degrees of Renal Function, and Patients with Complicated Urinary Tract Infection or Acute Uncomplicated Pyelonephritis. Antimicrob. Agents Chemother. 62, e01391–17. 10.1128/AAC.01391-17 PubMed Abstract | 10.1128/AAC.01391-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi N., Katsube T., Echols R., Wajima T. (2021). Population Pharmacokinetic and Pharmacokinetic/pharmacodynamic Analyses of Cefiderocol, a Parenteral Siderophore Cephalosporin, in Patients with Pneumonia, Bloodstream Infection/sepsis, or Complicated Urinary Tract Infection. Antimicrob. Agents Chemother. 65, e01437–20. 10.1128/AAC.01437-20 PubMed Abstract | 10.1128/AAC.01437-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak K. M., Tsuji M., Wise M. G., Hackel M., Yamano Y., Echols R., et al. (2019). In Vitro activity of Cefiderocol, a Siderophore Cephalosporin, against a Recent Collection of Clinically Relevant Carbapenem-Non-Susceptible Gram-Negative Bacilli, Including Serine Carbapenemase- and Metallo-β-Lactamase-Producing Isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 53, 177–184. 10.1016/j.ijantimicag.2018.10.007 PubMed Abstract | 10.1016/j.ijantimicag.2018.10.007 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kidd J. M., Abdelraouf K., Nicolau D. P. (2019). Development of Neutropenic Murine Models of Iron Overload and Depletion to Study the Efficacy of Siderophore-Antibiotic Conjugates. Antimicrob. Agents Chemother. 64, e01961–19. 10.1128/AAC.01961-19 PubMed Abstract | 10.1128/AAC.01961-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd J. M., Abdelraouf K., Nicolau D. P. (2019). Efficacy of Humanized Cefiderocol Exposure Is Unaltered by Host Iron Overload in the Thigh Infection Model. Antimicrob. Agents Chemother. 64, e01767–19. 10.1128/AAC.01767-19 PubMed Abstract | 10.1128/AAC.01767-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S., Boutin S., Kocer K., Fiedler M. O., Störzinger D., Weigand M. A., et al. (2021). Rapid Development of Cefiderocol Resistance in Carbapenem-Resistant Enterobacter cloacae during Therapy Is Associated with Heterogeneous Mutations in the Catecholate Siderophore Receptor Cira. Clin. Infect. Dis. 74, 905–908. 10.1093/cid/ciab511 10.1093/cid/ciab511 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobic E., Gill C. M., Mochon A. B., Nicolasora N. P., Nicolau D. P. (2021). Cefiderocol Pharmacokinetics in a Patient Receiving Continuous Venovenous Hemodiafiltration. Open Forum Infect. Dis. 8, ofab252. 10.1093/ofid/ofab252 PubMed Abstract | 10.1093/ofid/ofab252 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohira N., Hackel M. A., Ishioka Y., Kuroiwa M., Sahm D. F., Sato T., et al. (2020). Reduced Susceptibility Mechanism to Cefiderocol, a Siderophore Cephalosporin, Among Clinical Isolates from a Global Surveillance Programme (SIDERO-WT-2014). J. Glob. Antimicrob. Resist. 22, 738–741. 10.1016/j.jgar.2020.07.009 PubMed Abstract | 10.1016/j.jgar.2020.07.009 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kohira N., West J., Ito A., Ito-Horiyama T., Nakamura R., Sato T., et al. (2015). In Vitro Antimicrobial Activity of a Siderophore Cephalosporin, S-649266, against Enterobacteriaceae Clinical Isolates, Including Carbapenem-Resistant Strains. Antimicrob. Agents Chemother. 60, 729–734. 10.1128/AAC.01695-15 PubMed Abstract | 10.1128/AAC.01695-15 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- König C., Both A., Rohde H., Kluge S., Frey O. R., Röhr A. C., et al. (2021). Cefiderocol in Critically Ill Patients with Multi-Drug Resistant Pathogens: Real-Life Data on Pharmacokinetics and Microbiological Surveillance. Antibiotics (Basel) 10, 649. 10.3390/antibiotics10060649 PubMed Abstract | 10.3390/antibiotics10060649 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J., Özkaya Ö., Kümmerli R. (2020). Bacterial Siderophores in Community and Host Interactions. Nat. Rev. Microbiol. 18, 152–163. 10.1038/s41579-019-0284-4 PubMed Abstract | 10.1038/s41579-019-0284-4 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresken M., Korte-Berwanger M., Gatermann S. G., Pfeifer Y., Pfennigwerth N., Seifert H., et al. (2020). In Vitro activity of Cefiderocol against Aerobic Gram-Negative Bacterial Pathogens from Germany. Int. J. Antimicrob. Agents 56, 106128. 10.1016/j.ijantimicag.2020.106128 PubMed Abstract | 10.1016/j.ijantimicag.2020.106128 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Kufel W. D., Steele J. M., Riddell S. W., Jones Z., Shakeraneh P., Endy T. P. (2020). Cefiderocol for Treatment of an Empyema Due to Extensively Drug-Resistant Pseudomonas aeruginosa: Clinical Observations and Susceptibility Testing Considerations. IDCases 21, e00863. 10.1016/j.idcr.2020.e00863 PubMed Abstract | 10.1016/j.idcr.2020.e00863 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampejo T., Cherian B. P., Tan M. G. M., Wareham D. W. (2020). Cefiderocol in the Treatment of Systemic Carbapenemase-Producing Multidrug-Resistant Klebsiella pneumoniae Infection. J. Glob. Antimicrob. Resist. 23, 338–339. 10.1016/j.jgar.2020.10.008 PubMed Abstract | 10.1016/j.jgar.2020.10.008 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Lee Y. L., Ko W. C., Lee W. S., Lu P. L., Chen Y. H., Cheng S. H., et al. (2021). In-vitro Activity of Cefiderocol, Cefepime/zidebactam, Cefepime/enmetazobactam, Omadacycline, Eravacycline and Other Comparative Agents against Carbapenem-Nonsusceptible Enterobacterales: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2017-2020. Int. J. Antimicrob. Agents 58, 106377. 10.1016/j.ijantimicag.2021.106377 PubMed Abstract | 10.1016/j.ijantimicag.2021.106377 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Li J., Nation R. L., Turnidge J. D., Milne R. W., Coulthard K., Rayner C. R., et al. (2006). Colistin: the Re-emerging Antibiotic for Multidrug-Resistant Gram-Negative Bacterial Infections. Lancet Infect. Dis. 6, 589–601. 10.1016/S1473-3099(06)70580-1 PubMed Abstract | 10.1016/S1473-3099(06)70580-1 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Liu P.-Y., Ko W.-C., Lee W.-S., Lu P.-L., Chen Y.-H., Cheng S.-H., et al. (2021). In Vitro activity of Cefiderocol, Cefepime/enmetazobactam, Cefepime/zidebactam, Eravacycline, Omadacycline, and Other Comparative Agents against Carbapenem-Non-Susceptible Pseudomonas aeruginosa and Acinetobacter Baumannii Isolates Associated from Bloodstream Infection in Taiwan between 2018-2020. J. Microbiol. Immunol. Infect. S1684-1182 (21), 00186–00189. 10.1016/j.jmii.2021.08.012 10.1016/j.jmii.2021.08.012 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Longshaw C., Manissero D., Tsuji M., Echols R., Yamano Y. (2020). In Vitro activity of the Siderophore Cephalosporin, Cefiderocol, against Molecularly Characterized, Carbapenem-Non-Susceptible Gram-Negative Bacteria from Europe. JAC Antimicrob. Resist. 2, dlaa060. 10.1093/jacamr/dlaa060 PubMed Abstract | 10.1093/jacamr/dlaa060 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabayoje D. A., NicFhogartaigh C., Cherian B. P., Tan M. G. M., Wareham D. W. (2021). Compassionate Use of Cefiderocol for Carbapenem-Resistant Acinetobacter Baumannii Prosthetic Joint Infection. JAC Antimicrob. Resist. 3, i21–4. Erratum in: JAC Antimicrob Resist. 2021 Aug 28;3(3):dlab109. Erratum in: JAC Antimicrob Resist. 2021 Aug 28;3(3):dlab110. 10.1093/jacamr/dlab055 PubMed Abstract | 10.1093/jacamr/dlab055 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S., Kaminski M., Landman D., Quale J. (2020). Cefiderocol Resistance in Acinetobacter Baumannii: Roles of β-Lactamases, Siderophore Receptors, and Penicillin Binding Protein 3. Antimicrob. Agents Chemother. 64, e01221–20. 10.1128/AAC.01221-20 PubMed Abstract | 10.1128/AAC.01221-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. E., Attarha B., Lung J. (2021). Investigational Cefiderocol Use in Treatment of Multi-Drug Resistant Achromobacter Spp. J. Invest. Med. 68, 693. 10.1136/jim-2020-SRM.632 10.1136/jim-2020-SRM.632 | Google Scholar [DOI] [Google Scholar]
- Matsumoto S., Kanazawa S., Sato T., Yamano Y. (2020). Activities of Cefiderocol with Simulated Human Plasma Concentrations against Carbapenem-Resistant Gram-Negative Bacilli in an In Vitro Chemostat Model. Antimicrob. Agents Chemother. 64, e01128–20. 10.1128/AAC.01128-20 PubMed Abstract | 10.1128/AAC.01128-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Singley C. M., Hoover J., Nakamura R., Echols R., Rittenhouse S., et al. (2017). Efficacy of Cefiderocol against Carbapenem-Resistant Gram-Negative Bacilli in Immunocompetent-Rat Respiratory Tract Infection Models Recreating Human Plasma Pharmacokinetics. Antimicrob. Agents Chemother. 61, e00700–17. 10.1128/AAC.00700-17 PubMed Abstract | 10.1128/AAC.00700-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Gann P., Geringer M. R., Hall L. R., Lebreton F., Markelz E., Kwak Y. I., et al. (2021). Pan-drug Resistant Providencia Rettgeri Contributing to a Fatal Case of COVID-19. J. Med. Microbiol. 70, 001406. 10.1099/jmm.0.001406 10.1099/jmm.0.001406 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Katsube T., Shen H., Tomek C., Narukawa Y. (2019). Metabolism, Excretion, and Pharmacokinetics of [14 C]-cefiderocol (S-649266), a Siderophore Cephalosporin, in Healthy Subjects Following Intravenous Administration. J. Clin. Pharmacol. 59, 958–967. 10.1002/jcph.1386 PubMed Abstract | 10.1002/jcph.1386 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monogue M. L., Tsuji M., Yamano Y., Echols R., Nicolau D. P. (2017). Efficacy of Humanized Exposures of Cefiderocol (S-649266) against a Diverse Population of Gram-Negative Bacteria in a Murine Thigh Infection Model. Antimicrob. Agents Chemother. 61, e01022–17. 10.1128/AAC.01022-17 PubMed Abstract | 10.1128/AAC.01022-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. P., Bergman Y., Tekle T., Fissel J., Tamma P. D., Simner P. J. (2020). Cefiderocol Antimicrobial Susceptibility Testing against Multidrug-Resistant Gram-Negative Bacilli: a Comparison of Disk Diffusion to Broth Microdilution. J. Clin. Microbiol. 59, e01649–20. 10.1128/JCM.01649-20 PubMed Abstract | 10.1128/JCM.01649-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq S., Sadouki Z., Vickers A., Livermore D. M., Woodford N. (2020). In Vitro Activity of Cefiderocol, a Siderophore Cephalosporin, against Multidrug-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 64, e01582–20. 10.1128/AAC.01582-20 PubMed Abstract | 10.1128/AAC.01582-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R., Ito-Horiyama T., Takemura M., Toba S., Matsumoto S., Ikehara T., et al. (2019). In Vivo pharmacodynamic Study of Cefiderocol, a Novel Parenteral Siderophore Cephalosporin, in Murine Thigh and Lung Infection Models. Antimicrob. Agents Chemother. 63, e02031–18. 10.1128/AAC.02031-18 PubMed Abstract | 10.1128/AAC.02031-18 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R., Oota M., Matsumoto S., Sato T., Yamano Y. (2021). In Vitro activity and In Vivo Efficacy of Cefiderocol against Stenotrophomonas Maltophilia. Antimicrob. Agents Chemother. 65, e01436–20. 10.1128/AAC.01436-20 PubMed Abstract | 10.1128/AAC.01436-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A., Ceccarelli G., De Angelis M., Sacco F., Miele M. C., Mastroianni C. M., et al. (2020). Cefiderocol for Compassionate Use in the Treatment of Complicated Infections Caused by Extensively and Pan-Resistant Acinetobacter Baumannii. J. Glob. Antimicrob. Resist. 23, 292–296. 10.1016/j.jgar.2020.09.019 PubMed Abstract | 10.1016/j.jgar.2020.09.019 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Ota K., Kaku N., Uno N., Sakamoto K., Kosai K., Hasegawa H., et al. (2020). 1273. Efficacy of Cefiderocol against Carbapenem-Resistant A. Baumannii and P. aeruginosa in Ventilator-Associated Pneumonia Mouse Model. Open Forum Infect. Dis. 7, S653. 10.1093/ofid/ofaa439.1457 10.1093/ofid/ofaa439.1457 | Google Scholar [DOI] [Google Scholar]
- Page M. G. P. (2019). The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 69 (Suppl. 7), S529–S37. 10.1093/cid/ciz825 PubMed Abstract | 10.1093/cid/ciz825 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. BMJ 372, n71. 10.1136/bmj.n71 PubMed Abstract | 10.1136/bmj.n71 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L., Sadek M., Nordmann P. (2021). Contribution of PER-type and NDM-type β-Lactamases to Cefiderocol Resistance in Acinetobacter Baumannii. Antimicrob. Agents Chemother. 65, e0087721. 10.1128/AAC.00877-21 PubMed Abstract | 10.1128/AAC.00877-21 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portsmouth S., van Veenhuyzen D., Echols R., Machida M., Ferreira J. C. A., Ariyasu M., et al. (2018). Cefiderocol versus Imipenem-Cilastatin for the Treatment of Complicated Urinary Tract Infections Caused by Gram-Negative Uropathogens: a Phase 2, Randomised, Double-Blind, Non-inferiority Trial. Lancet Infect. Dis. 18, 1319–1328. 10.1016/S1473-3099(18)30554-1 PubMed Abstract | 10.1016/S1473-3099(18)30554-1 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Rolston K. V. I., Gerges B., Shelburne S., Aitken S. L., Raad I., Prince R. A. (2020). Activity of Cefiderocol and Comparators against Isolates from Cancer Patients. Antimicrob. Agents Chemother. 64, e01955–19. 10.1128/AAC.01955-19 PubMed Abstract | 10.1128/AAC.01955-19 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saisho Y., Katsube T., White S., Fukase H., Shimada J. (2018). Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob. Agents Chemother. 62, e02163–17. 10.1128/AAC.02163-17 PubMed Abstract | 10.1128/AAC.02163-17 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R. K., Iovleva A., Kline E. G., Kawai A., McElheny C. L., Doi Y. (2020). Clinical Evolution of AmpC-Mediated Ceftazidime-Avibactam and Cefiderocol Resistance in Enterobacter cloacae Complex Following Exposure to Cefepime. Clin. Infect. Dis. 71, 2713–2716. 10.1093/cid/ciaa355 PubMed Abstract | 10.1093/cid/ciaa355 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siméon S., Dortet L., Bouchand F., Roux A. L., Bonnin R. A., Duran C., et al. (2020). Compassionate Use of Cefiderocol to Treat a Case of Prosthetic Joint Infection Due to Extensively Drug-Resistant Enterobacter Hormaechei. Microorganisms 8, 1236. 10.3390/microorganisms8081236 10.3390/microorganisms8081236 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simner P. J., Beisken S., Bergman Y., Posch A. E., Cosgrove S. E., Tamma P. D. (2021). Cefiderocol Activity against Clinical pseudomonas Aeruginosa Isolates Exhibiting Ceftolozane-Tazobactam Resistance. Open Forum Infect. Dis. 8, ofab311. 10.1093/ofid/ofab311 PubMed Abstract | 10.1093/ofid/ofab311 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simner P. J., Patel R. (2020). Cefiderocol Antimicrobial Susceptibility Testing Considerations: The Achilles Heel of the Trojan Horse? J. Clin. Microbiol. 59, e00951–20. 10.1128/JCM.00951-20 PubMed Abstract | 10.1128/JCM.00951-20 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainton S. M., Monogue M. L., Tsuji M., Yamano Y., Echols R., Nicolau D. P. (2019). Efficacy of Humanized Cefiderocol Exposures over 72 hours against a Diverse Group of Gram-Negative Isolates in the Neutropenic Murine Thigh Infection Model. Antimicrob. Agents Chemother. 63, e01040–18. 10.1128/AAC.01040-18 PubMed Abstract | 10.1128/AAC.01040-18 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. W., Clancy M. (2019). Compassionate Use of Cefiderocol in the Treatment of an Intraabdominal Infection Due to Multidrug-Resistant Pseudomonas aeruginosa: A Case Report. Pharmacotherapy 39, 1113–1118. 10.1002/phar.2334 PubMed Abstract | 10.1002/phar.2334 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Stracquadanio S., Torti E., Longshaw C., Henriksen A. S., Stefani S. (2021). In Vitro activity of Cefiderocol and Comparators against Isolates of Gram-Negative Pathogens from a Range of Infection Sources: SIDERO-WT-2014-2018 Studies in Italy. J. Glob. Antimicrob. Resist. 25, 390–398. 10.1016/j.jgar.2021.04.019 PubMed Abstract | 10.1016/j.jgar.2021.04.019 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Streling A. P., Al Obaidi M. M., Lainhart W. D., Zangeneh T., Khan A., Dinh A. Q., et al. (2021). Evolution of Cefiderocol Non-susceptibility in Pseudomonas aeruginosa in a Patient without Previous Exposure to the Antibiotic. Clin. Infect. Dis. 73, e4472–e4474. 10.1093/cid/ciaa1909 PubMed Abstract | 10.1093/cid/ciaa1909 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M., Yamano Y., Matsunaga Y., Ariyasu M., Echols R., Den Nagata T. (2020). 1266. Characterization of Shifts in Minimum Inhibitory Concentrations during Treatment with Cefiderocol or Comparators in the Phase 3 CREDIBLE-CR and APEKS-NP Studies. Open Forum Infect. Dis. 7, S649–S650. 10.1093/ofid/ofaa439.1450 10.1093/ofid/ofaa439.1450 | Google Scholar [DOI] [Google Scholar]
- Trebosc V., Schellhorn B., Schill J., Lucchini V., Bühler J., Bourotte M., et al. (2020). In Vitro activity of Rifabutin against 293 Contemporary Carbapenem-Resistant Acinetobacter Baumannii Clinical Isolates and Characterization of Rifabutin Mode of Action and Resistance Mechanisms. J. Antimicrob. Chemother. 75, 3552–3562. 10.1093/jac/dkaa370 PubMed Abstract | 10.1093/jac/dkaa370 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecarichi E. M., Quirino A., Scaglione V., Longhini F., Garofalo E., Bruni A., et al. (2019). Successful Treatment with Cefiderocol for Compassionate Use in a Critically Ill Patient with XDR Acinetobacter Baumannii and KPC-Producing Klebsiella pneumoniae: a Case Report. J. Antimicrob. Chemother. 74, 3399–3401. 10.1093/jac/dkz318 PubMed Abstract | 10.1093/jac/dkz318 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Tsuji M., Kohira N., Nakamura R., Sato T., Yamano Y. (2016). “S-649266, a Novel Siderophore Cephalosporin: In Vitro Combination Effect of S-649266 and Other Antibiotics against Gram-Negative Bacteria,” in Proceedings of the 26th European Congress of Clinical Microbiology and Infectious Diseases (Amsterdam: ECCMID; ). [abstract P1312]. Google Scholar [Google Scholar]
- Warner N. C., Bartelt L. A., Lachiewicz A. M., Tompkins K. M., Miller M. B., Alby K., et al. (2021). Cefiderocol for the Treatment of Adult and Pediatric Patients with Cystic Fibrosis and Achromobacter Xylosoxidans Infections. Clin. Infect. Dis. 73, e1754–7. 10.1093/cid/ciaa1847 PubMed Abstract | 10.1093/cid/ciaa1847 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Magrini N. (2017). Global Priority List of Antibiotic Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available at: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (Accessed September 2, 2021). Google Scholar [Google Scholar]
- Wunderink R. G., Matsunaga Y., Ariyasu M., Clevenbergh P., Echols R., Kaye K. S., et al. (2021). Cefiderocol versus High-Dose, Extended-Infusion Meropenem for the Treatment of Gram-Negative Nosocomial Pneumonia (APEKS-NP): a Randomised, Double-Blind, Phase 3, Non-inferiority Trial. Lancet Infect. Dis. 21, 213–225. 10.1016/S1473-3099(20)30731-3 PubMed Abstract | 10.1016/S1473-3099(20)30731-3 | Google Scholar [DOI] [PubMed] [Google Scholar]
- Yamano Y. (2019). In Vitro activity of Cefiderocol against a Broad Range of Clinically Important Gram-Negative Bacteria. Clin. Infect. Dis. 69 (Suppl. 7), S544–S51. 10.1093/cid/ciz827 PubMed Abstract | 10.1093/cid/ciz827 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y., Tsuji M., Hackel M., Echols R., Sahm D. (2017). “ In Vitro activity of Cefiderocol against Gram-Negative Clinical Isolates Collected from Asia and South Pacific in 2014-2016 (SIDERO-CR Study),” in Proceedings of the 30th International Congress of Chemotherapy and Infection (Taipei: ICC2017; ). [abstract OS7-2]. Google Scholar [Google Scholar]
- Yamano Y., Nakamura R., Takemura M., Echols R. (2020). 1455. Potential Mechanisms of Cefiderocol MIC Increase in Enterobacterales in In Vitro Resistance Acquisition Studies. Open Forum Infect. Dis. 7, S730. 10.1093/ofid/ofaa439.1636 10.1093/ofid/ofaa439.1636 | Google Scholar [DOI] [Google Scholar]
- Yamano Y., Takemura M., Anan N., Nakamura R., Echols R. (2020). 1626. Synergistic Effect of Cefiderocol with Other Antibiotics against PER-Producing Acinetobacter Baumannii Isolates from the Multinational SIDERO-WT Studies. Open Forum Infect. Dis. 7, S805. SUPPL 1. 10.1093/ofid/ofaa439.1806 10.1093/ofid/ofaa439.1806 | Google Scholar [DOI] [Google Scholar]
- Yamano Y., Takemura M., Kazmierczak K., Wise M. G. G., SahmHackel D. F., Echols R., et al. (2020). 1452. Molecular Profile of β-Lactamase Genes and Siderophore-dependent Iron Transporter Genes of Cefiderocol High MIC Isolates from SIDERO-WT Studies. Open Forum Infect. Dis. 7, S728–S729. 10.1093/ofid/ofaa439.1633 10.1093/ofid/ofaa439.1633 | Google Scholar [DOI] [Google Scholar]
- Zaidan N., Hornak J. P., Reynoso D. (2021). Extensively Drug-Resistant Acinetobacter Baumannii Nosocomial Pneumonia Successfully Treated with a Novel Antibiotic Combination. Antimicrob. Agents Chemother. 65, e0092421. 10.1128/AAC.00924-21 PubMed Abstract | 10.1128/AAC.00924-21 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalacain M., Lozano C., Llanos A., Sprynski N., Valmont T., De Piano C., et al. (2021). Novel Specific Metallo-β-Lactamase Inhibitor ANT2681 Restores Meropenem Activity to Clinically Effective Levels against NDM-Positive Enterobacterales. Antimicrob. Agents Chemother. 65, e00203–21. 10.1128/AAC.00203-21 PubMed Abstract | 10.1128/AAC.00203-21 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg S., Nicoletti G. J., Kuster S., Junker M., Widmer A., Egli A., et al. (2020). Cefiderocol for Extensively Drug-Resistant Gram-Negative Bacterial Infections: Real-World Experience from a Case Series and Review of the Literature. Open Forum Infect. Dis. 7, ofaa185. 10.1093/ofid/ofaa185 PubMed Abstract | 10.1093/ofid/ofaa185 | Google Scholar [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.