Abstract
Skin thickness and strength differ between male and female chickens. This study aimed to clarify the effects of estradiol on the expression of estrogen receptors and collagen mRNA in chicken skin. Estradiol was administered to male chicks for 3 weeks, then cryosections of skin collected from the cervical, thoracic, dorsal, and pelvic limb regions were stained with hematoxylin and eosin, and dermal thickness was measured. Estrogen receptor and collagen mRNA expression was assessed using real-time RT-PCR, and collagen contents were determined. Estradiol did not alter dermal thickness or the collagen content of the skin from any tested region. Among the estrogen receptors, significantly more ESR1 mRNA was expressed in the thoracic skin of chicks administered with estradiol compared with vehicle (control), and in the thoracic skin compared with skin from other regions within each group. Estradiol did not affect ESR2, GPER, and COL1A1 mRNA expression. These results suggested that estradiol stimulates ESR1 expression in thoracic skin, but does not affect collagen synthesis in skin from any other region of male chicks.
Keywords: chicken, collagen, estradiol, estrogen receptor, skin
Introduction
Chicken meat is a major livestock product that is consumed worldwide as well as beef and pork. However, chicken meat is usually supplied to the market accompanied by the skin, unlike beef and pork. The skin covers the entire animal and it is one of the largest organs. Chicken skin is edible and considered valuable as a food commodity. The skin also protects against environmental damage. Consequently, damage to the skin such as tears during rearing or processing affects underlying skeletal muscles, which reduces meat quality (Salim et al., 2012).
The thickness of skin differs among regions of the body between male and female chickens. The skin is thicker in the back than in the thigh of all chickens (Salim et al., 2012; Ji et al., 2021), and in male, compared with female chickens (Salim et al., 2012). The skin is also stronger in male, than in female chickens (Bilgili et al., 1993). Skin strength is similar between 3-week-old male and female chicks. However, the skin starts to become stronger with overall growth in 5-week-old male, but not female chicks (Christensen et al., 1994). Therefore, sex hormones might be associated with these differences in the thickness and strength of chicken skin.
Skin comprises the epidermis, dermis, and subcutis, among which, the dermis has abundant collagen fibers, and more collagen content in male, than in female chickens (Salim et al., 2012). Adult female chickens have less hydroxyproline, a constituent of collagen, in the skin than males (Bruce and Anastassiadis, 1977). Therefore, skin strength and the dermal collagen content are apparently associated. Estradiol decreases collagen content in the skin of male rats (Smith and Allison, 1966) and guinea pigs (Henneman, 1968). Our previous study of chicken liver showed that estradiol inhibits collagen synthesis in hepatic stellate cells (Nishimura et al., 2017). Although the inhibitory mechanism of collagen synthesis in chicken liver has not yet been determined, estradiol might regulate the mechanism of collagen synthesis in chicken skin.
Vertebrates have estrogen receptors α (ERα or ER1) and β (ERβ or ER2), and estrogen-responsive G protein-coupled receptor (GPER or GPR30) (Eyster, 2016). Both ERα and ERβ are ligand-activated transcription factors that share a delayed onset and persistent response to genomic action. However, GPER, which is located in the cell membrane, mediates the rapid and non-genomic actions of estrogen (Eyster, 2016). The expression profiles of these receptors and how they relate to estrogen in collagen synthesis in chicken skin remain unknown.
The present study aimed to define the role of estrogen in collagen synthesis in the chicken dermis and to obtain basic understanding that will help to increase the added value of the skin. Thus, we investigated the effects of estradiol on estrogen receptor expression and collagen synthesis in chicken skin.
Materials and Methods
Ethics Statement
All experiments were proceeded according to the Guidelines for the Proper Conduct of Animal Experiments published by the Science Council of Japan. All experimental protocols were approved by the Ethics Committee of Kyushu University (approval no. A20-028-0).
Chickens
Rhode Island Red (Gallus gallus domesticus) hens and male chicks obtained as fertilized eggs (National Livestock Breeding Center, Okazaki Station, Okazaki, Japan) were hatched and reared in 2×1.5-m pens with wood-chip or saw-dust floors at the Poultry Breeding and Experiment Facility, Faculty of Agriculture, Kyushu University (Fukuoka, Japan). Hens and chicks were fed with a commercial feed mixture, Power layer 17Y and Power chick ZK (JA Kitakyushu Kumiai Shiryo, Fukuoka, Japan) and water ad libitum. At the end of the experimental period, the birds were intravenously injected with sodium pentobarbital, then exsanguinated via the carotid artery under deep anesthesia. Skin samples were rapidly dissected for experimentation.
Measurement of Serum Estradiol in Hens
Blood samples from seven euthanized laying hens were collected into tubes from the carotid artery. The samples were clotted on crushed ice for 30-60 min, then serum was separated by centrifugation at 1,200×g for 15 min, and stored at −20°C. Serum estradiol concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kits (Cayman Chemical Company, Ann Arbor, MI, USA) as described by the manufacturer, then absorbance as measured at 405 nm using a plate reader (Multiskan FC; Thermo Fisher Scientific Inc., Waltham, MA, USA). A standard curve was calculated using ImageJ software (https://imagej.nih.gov/ij/).
Hormone Administration
The amount of hormone administered was calculated from the serum estradiol values in hens as follows.
Beta estradiol (Sigma-Aldrich Co., St. Louis, MO, USA) was dissolved in ethanol to a concentration of 1mg/mL, then diluted 1:50,000 with physiological saline (working dilution). Five-week-old male chicks were weighed (and again in every trial), then intraperitoneally injected with either β-estradiol (800 pg/mL of blood; 80 ng/kg body weight [BW]) or vehicle (physiological saline control) daily for 21 days.
Histology
Tissue specimens from male chicks administered with vehicle or β-estradiol were dissected from the cervical, thoracic, and dorsal regions, and from the lateral side of the pelvic limb after plucking the feathers. Cervical skin was obtained from the fifth region of the craniodorsal part of the neck. Specimens were mounted in Tissue-Tek O.C.T. compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan), frozen in liquid nitrogen and stored at −20°C. Frozen specimens were cut into 8212 µm sections using a cryostat (CM1850; Leica Microsystems GmbH, Wetzlar, Germany), fixed in acetone for 5 min at 4°C, air-dried, then conventionally stained with hematoxylin and eosin (HE). The sections were visualized and images were obtained using an Eclipse 80i microscope (Nikon Corp., Tokyo, Japan). Three randomly-selected, 100-µm-wide dermal areas in images of each section were measured using NIS-Elements D version 3.22.14 software (Nikon Corp.). Dermal thickness in each section was calculated by dividing the area by the width.
Real-time RT-PCR
We investigated the mRNA expression of the chicken ERα (ESR1), ERβ (ESR2), GPER, and alpha-1 type I collagen (COL1A1) genes in skin excised from the cervical, thoracic, and dorsal regions, as well as the lateral side of the pelvic limbs of male chicks administered with β-estradiol or vehicle (n=4 each). Total RNA was isolated using Isogen II (Nippon Gene, Tokyo, Japan) as described by the manufacturer. Genomic DNA was removed using DNase I (Nippon Gene), as described by the manufacturer. Real-time RT-PCR proceeded in duplicate using One Step TB Green PrimeScrip RT-PCR Kit II (Takara Bio Inc., Kusatsu, Japan) as described by the manufacturer and an Mx3000P QPCR system (Agilent Technologies, Inc., Santa Clara, CA, USA) under the following cycling condi tions: 42°C for 5 min, 95°C for 10 s, 40 cycles of 95°C for 5 s, and 60°C for 34 s, 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. Table 1 shows the primers that we designed according to information downloaded from a nucleotide database (US National Center for Biotechnology Information [NCBI], Bethesda, MD, USA). The results were normalized to that of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene.
Table 1. Primers used for real-time reverse transcriptional polymerase chain reaction.
| Target gene | Accession No. | Primer forward | Primer reverse | Product (bp) |
|---|---|---|---|---|
| COL1A1 | XM_025144131.1 | GAGCGACGGCTTCCAGTTTGAG | GTGTCGTGGTCCATGTAGGC | 153 |
| ESR1 | NM_205183.2 | CATCATCCTGCTCAATTCTGGTGTG | GCGTCCAGCATCTCCAGTAAG | 208 |
| ESR2 | NM_204794.2 | AGTGGGAATGATGAAATGTGGCTC | CATGGAGGCCTCGGTGAATG | 241 |
| GPER | NM_001162405.1 | GGAAAAATGGAATTAAGGTGGAAGG | TTGCAGATGGGAGCACTTTC | 202 |
| GAPDH | NM_204305.1 | ACTGTCAAGGCTGAGAACGG | ACCTGCATCTGCCCATTTGA | 99 |
Total Collagen Assay
Collagen content was measured in 50-100 mg of skin excised from the locations described above using total collagen assay kits (Quick-Zyme Biosciences B.V., Leiden, The Netherlands) as described by the manufacturer. Briefly, samples were incubated with 1 mL of HCl (6 mol/L) at 95°C for 20 h, cooled to room temperature, then centrifuged at 13,000×g for 10 min. The absorbance of the supernatants was measured in 96-well plates at a wavelength of 570 nm using a Multiskan FC (Thermo Fisher Scientific Inc.).
Statistical Analyses
Data were statistically analyzed by two-way analyses of variance, and Tukey-Kramer tests of significance using R (The R Project for Statistical Computing, Vienna, Austria). Data are shown as means±standard error of the mean (SEM). Irregular data were excluded using the Smirnoff-Grubbs rejection test.
Results
As hens have less collagen in the skin than in cocks (Bruce and Anastassiadis, 1977) and the sex difference in collagen content in the skin is not significant until 7 weeks of age (Pines et al., 1996), the dose of β-estradiol was decided based on the circulating level in hens. The mean β-estradiol level in hens was 807±243.8 pg/mL (n=7). The dose of β-estradiol given to male chicks with a putative blood volume of ∼10% of the BW (Sturkie, 1954) was 800 pg/mL of blood (80 ng/kg BW).
Histology
Figure 1 shows photomicrographs of histological sections of control male chick skin stained with HE. The skin surface was rugged (Fig. 1). An intricate outline was obvious in the cervical region, and the surface of the thoracic skin was relatively flat compared with the other regions. The epidermis in all regions was thin and comprised an eosinophilic Stratum corneum, a Stratum intermedium with only a few cell layers, and the Stratum basale. The dermis was distinguished from the Stratum superficiale and Stratum profundum. The dermis was not uniformly thick in all skin regions. Cervical skin had well-developed muscle layers beneath the dermis, which differed from other regions (Fig. 1a). These histological features did not significantly differ between the control (Fig. 1) and estradiol groups (data not shown).
Fig. 1.
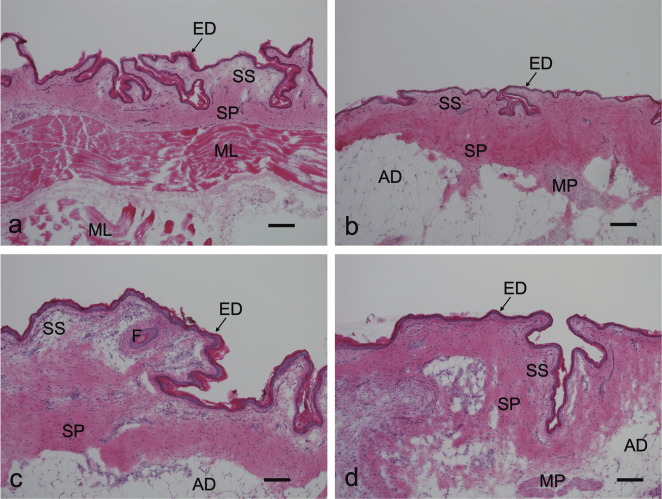
Representative images of skin from various regions of control male chicks. Sections were stained with hematoxylin and eosin; a, cervical region; b, thoracic region; c, dorsal region; d, lateral side of pelvic limb. AD, adipose tissue; ED, epidermis; F, Folliculus; ML, muscle layer; MP, Mm. pennarum; SP, Stratum profundum of the dermis; SS, Stratum superficiale of the dermis. Bars indicate 100 µm.
Table 2 shows that the thickness of the dermis did not significantly differ in any of the tested regions between the estradiol and control chicks, or between these regions within each group. However, light microscopy findings indicated that the dermis of the dorsal skin was thicker than that in any other regions.
Table 2. Dermal thickness (µm) in male chicks administered with estradiol (E2) or vehicle (control).
| Cervical | Thoracic | Dorsal | Lateral side of leg | |
|---|---|---|---|---|
| E2 | 127±25.8 | 205±32.4 | 170±28.0 | 181±28.1 |
| Control | 148±15.7 | 200±21.3 | 227±22.5 | 195±13.9 |
Means±SEM (n=5 per group).
Real-time RT-PCR Analyses
Figure 2 shows ER expression in skin from the various regions. Messenger RNA levels of ESR1 were significantly higher in the thoracic region of chicks administered with estradiol, than vehicle (p<0.05) and significantly higher than in any other regions within the estradiol group (p<0.001). The mRNA expression of ESR1 was significantly higher in the thoracic region than in the pelvic limb of control chicks (p<0.05), whereas that of ESR2 and GPER mRNA did not significantly differ in any region between the groups or within each group.
Fig. 2.
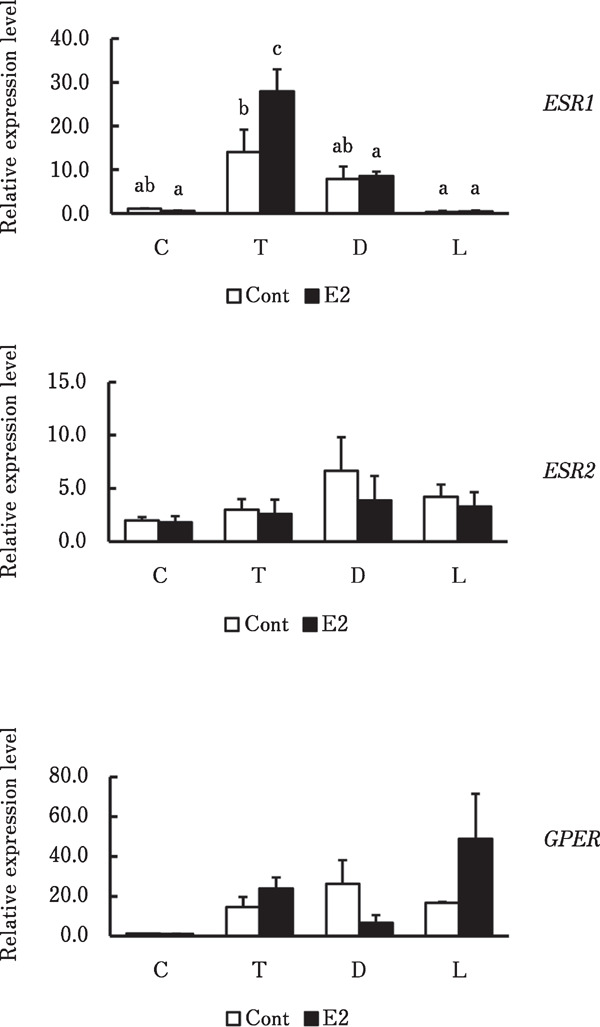
Relative ESR1, ESR2 and GPER mRNA expression in skin from various regions of male chicks. Cervical (C), thoracic (T), dorsal (D) regions and lateral side of pelvic limb (L) in skin from male chicks administered with vehicle (Cont) or estradiol (E2). a, b, c Significant differences in means (p<0.05). Data are shown as means±SEM (n=3–4).
The real-time RT-PCR results showed that COL1A1 mRNA expression in any region did not differ between the groups (Fig. 3). However, COL1A1 mRNA levels were significantly higher in the lateral side of the pelvic limb than in other regions in the control and estradiol groups (p<0.05 and p<0.001, respectively).
Fig. 3.
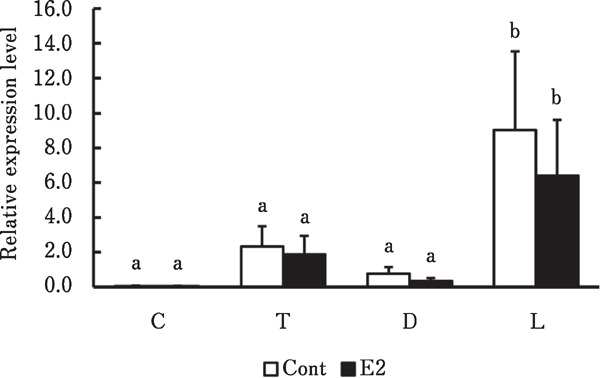
Relative COL1A1 mRNA expression levels in skin from various regions of male chicks. Cervical (C), thoracic (T), dorsal (D) regions and lateral side of pelvic limb (L) in skin from male chicks administered with vehicle (Cont) or estradiol (E2). a, b Significant differences in means (p<0.01). Data are shown as means±SEM (n=3–4 per group).
Collagen Content
Figure 4 shows that the collagen content in the skin of each body region did not significantly differ between the groups. However, collagen was significantly more abundant in the pelvic limb than in the other regions in the two groups (p<0.001 for both).
Fig. 4.
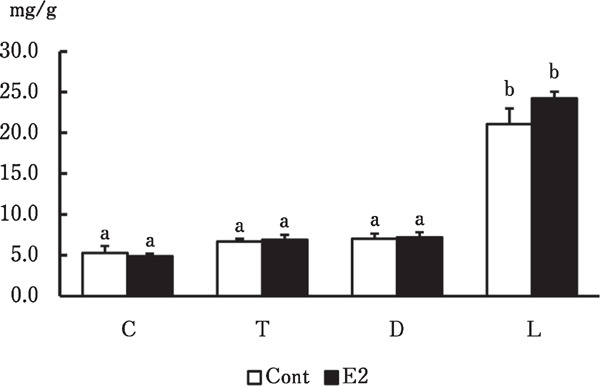
Collagen contents in skin from various regions of male chicks. Cervical (C), thoracic (T), dorsal (D) regions and lateral side of pelvic limb (L) in skin from male chicks administered with vehicle (Cont) or estradiol (E2). a, b Significant differences in means (p<0.001). Data are shown as means±SEM (n=4 per group).
Discussion
The collagen content in skin and the effects of collagen on human skin aging have been extensively studied (Calleja-Agius et al., 2007). Skin collagen and estrogen closely correlate in postmenopausal women (Brincat et al., 2005; Hall and Phillips, 2005). However, collagen in skin has mostly been investigated in humans and rodents. Only a few studies have examined this topic in domestic animals despite the economic importance of leather and food products prepared from skin (Maiorano et al., 1995; Pines et al., 1996; Fang et al., 2012; Erickson et al., 2013). The collagen concentration in skin is greater in normal rams than in castrated or zeranol-implanted rams (Maiorano et al., 1995). Furthermore, the ratio (%) of soluble collagen in the skin is higher in rams and zeranol-implanted rams than in wethers, and insoluble collagen concentrations are lowest in the skin of zeranol-implanted rams. Because zeranol is a non-steroidal estrogen agonist, estrogen might decrease collagen concentrations in sheep skin. The collagen content in the skin of chicks increases with age and is higher in males than in females, but this difference is not significant until 7 weeks of age (Pines et al., 1996). Our previous findings suggested that estradiol inhibits collagen synthesis in the livers of growing chickens (Nishimura et al., 2017). Therefore, we speculated that estradiol also inhibits collagen synthesis in chicken skin.
Ovariectomized female rats have reduced epidermal growth, and the subcutaneous administration of low and high concentrations of estrogen respectively induce and suppress proliferation (Dunaif and Finerty, 1950). In contrast, estrogen administered to ovariectomized female mice for 4 days does not change epidermal or dermal thickness in skin from four regions of the body (Coppola and O'Connell, 1989). Estrogen prevents collagen loss and increases skin thickness in post-menopausal women (Archer, 2012), whereas estradiol administered to ovariectomized rats for 36 weeks does not change dermal thickness (Berger et al., 2010). The collagen content is increased in chick thoracic skin, but collagen type I gene expression decreases with growth, although it is higher in male, than in female chicks (Pines et al., 1996). The findings of light microscopy did not reveal any notable differences in the epidermis and dermis of male chicks given β-estradiol for 3 weeks compared with control male chicks. Although the skin is thicker in male, than in female chickens (Salim et al., 2012), we showed that estradiol did not change the collagen content in chick skin. These findings suggested that the thickness and collagen content of male chick skin are not affected by estradiol. Further studies are needed to clarify sex differences in the thickness of the chicken skin.
Both ERα and ERβ are localized in the human epidermis (Pelletier and Ren, 2004), and ERα does not change, whereas ERβ gradually decreases with age (Inoue et al., 2011). Estrone injected into mice induces an increase in epidermal mitosis and thickness (Bullough, 1947). However, the skin is thicker and contains more collagen in ERα-deficient, than WT mice, whereas an ERβ deficiency leads to a decrease in thickness and collagen content (Markiewicz et al., 2013). We found significantly more ESR1 mRNA expression in the thoracic, than in any other tested region in both groups, and that estradiol significantly increased these levels. However, ESR2 and GPER mRNA expression did not significantly differ in any tested regions between the groups. Although COL1A1 mRNA expression was more abundant in the pelvic limb than in the other regions, the relative amount of ESR1 mRNA was the lowest in this area. These results suggested that a high collagen content and more abundant COL1A1 mRNA expression in the pelvic limb skin are not associated with estradiol stimulation. In contrast, thoracic skin was the most sensitive to estradiol stimulation, because ESR1 expression increased in the four regions investigated herein.
Considering the effect of estrogen on the thoracic skin, many birds develop a brood patch (ventral apterium) during the breeding season, and this can be induced by exogenous estradiol (Steel and Hinde, 1964). Dermal thickness is increased by connective tissue hyperplasia during patch formation (Jones, 1971). The present study found that estradiol did not affect collagen synthesis or dermal thickness, but increased ESR1 mRNA expression in thoracic skin. Furthermore, ESR1 mRNA expression was more abundant in the thoracic skin than in the other regions tested, even in control chicks. Although we used male chicks in the present study and did not observe incubation behavior in Rhode Island Red hens reared in pens, increased ESR1 mRNA expression in thoracic skin might have been associated with brood patch formation.
In conclusion, collagen synthesis in chicken skin is most active in the pelvic limb, but estradiol does not affect dermal thickness and collagen synthesis in male chick skin. Estradiol stimulates ESR1 mRNA expression in thoracic skin, which might be related to brood patch formation. It is probable that other factors will affect the sex difference in the collagen content in chicken skin. Further studies need to clarify it.
Acknowledgments
We thank Ellen Knapp, PhD, from Edanz (https://jp.edanz.com/ac) for editing the draft of this manuscript.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Archer DF. Postmenopausal skin and estrogen. Gynecological Endocrinology, 28(S2): 2-6. 2012. [DOI] [PubMed] [Google Scholar]
- Berger L, El-Alfy M, Martel C and Labrie F. Effect of long-term topical application of dehydroepiandrosterone (DHEA) and oral estrogens on morphology, cell proliferation, procollagen A1 and androgen receptor levels in rat skin. Hormone Molecular Biology and Clinical Investigation, 2: 267-275. 2010. [DOI] [PubMed] [Google Scholar]
- Bilgili SF, Eckman MK and Bushong RD. Broiler Skin Strength: Influence of age, sex, and feathering rate. Journal of Applied Poultry Research, 2: 135-141. 1993. [Google Scholar]
- Brincat MP, Baron YM and Galea R. Estrogens and the skin. Climacteric, 8: 110-123. 2005. [DOI] [PubMed] [Google Scholar]
- Bruce KR and Anastassiadis PA. Connective tissue constituents of the fowl. Effects of exogenous estrogen. Poultry Science, 56: 1073-1085. 1977. [DOI] [PubMed] [Google Scholar]
- Bullough HF. Epidermal thickness following oestrone injections in the mouse. Nature, 159: 101-102. 1947. [DOI] [PubMed] [Google Scholar]
- Calleja-Agius J, Muscat-Baron Y and Brincat MP. Skin ageing. Menopause International, 13: 60-64. 2007. [DOI] [PubMed] [Google Scholar]
- Christensen KD, Zimmermann NG, Wyatt CL, Goodman TN, Buhr RJ and Twining P. Dietary and environmental factors affecting skin strength in broiler chickens. Poultry Science, 73: 224-235. 1994. [DOI] [PubMed] [Google Scholar]
- Coppola DM and O'Connell RJ. Sexual skin in rodents: an across body region, gender, and species analysis. Biology of Reproduction, 41: 543-550. 1989. [DOI] [PubMed] [Google Scholar]
- Dunaif CB and Finerty JC. The effects of estrogen administration upon epidermal proliferation. Journal of Investigative Dermatology, 15: 363-371. 1950. [DOI] [PubMed] [Google Scholar]
- Erickson B, Fang M, Wallace JM, Orr BG, Les CM and Banaszak Holl MM. Nanoscale structure of type I collagen fibrils: quantitative measurement of D-spacing. Biotechnology Journal, 8: 117-126. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster KM. (ed.) Estrogen Receptors: Methods and Protocols, Methods in Molecular Biology, 1366: 1-10. 2016. [DOI] [PubMed] [Google Scholar]
- Fang M, Liroff KG, Turner AS, Les CM, Orr BG and Holl MM. Estrogen depletion results in nanoscale morphology changes in dermal collagen. Journal of Investigative Dermatology, 132: 1791-1797. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G and Phillips TJ. Estrogen and skin: The effects of estrogen, menopause, and hormone replacement therapy on the skin. Journal of the American Academy of Dermatology, 53: 555-568. 2005. [DOI] [PubMed] [Google Scholar]
- Henneman DH. Effect of estrogen on in vivo and in vitro collagen biosynthesis and maturation in old and young female guinea pigs. Endocrinology, 83: 678-690. 1968. [DOI] [PubMed] [Google Scholar]
- Inoue T, Miki Y, Abe K, Hatori M, Hosaka M, Kariya Y, Kakuo S, Fujimura T, Hachiya A, Aiba S and Sasano H. The role of estrogen-metabolizing enzymes and estrogen receptors in human epidermis. Molecular and Cellular Endocrinology, 344: 35-40. 2011. [DOI] [PubMed] [Google Scholar]
- Ji GG, Zhang M, Liu YF, Shan YJ, Tu YJ, Ju XJ, Zou JM, Shu JT, Wu JF and Xie JF. A gene co-expression network analysis of the candidate genes and molecular pathways associated with feather follicle traits of chicken skin. Journal of Animal Breeding and Genetics, 138: 122-134. 2021. [DOI] [PubMed] [Google Scholar]
- Jones RE. The incubation patch of birds. Biological Reviews, 46: 315-339. 1971. [Google Scholar]
- Maiorano G, McCormick RJ, Field RA and Snowder GD. Collagen characteristics of skin, fell, and epimysium from rams, wethers, and zeranol-implanted ram lambs. Journal of Animal Science, 73: 393-398. 1995. [DOI] [PubMed] [Google Scholar]
- Markiewicz M, Znoyko S, Stawski L, Ghatnekar A, Gilkeson G and Trojanowska M. A role for estrogen receptor-α and estrogen receptor-β in collagen biosynthesis in mouse skin. Journal of Investigative Dermatology, 133: 120-127. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Teshima A, Kawabata F and Tabata S. Estradiol inhibits hepatic stellate cell area and collagen synthesis in the chicken liver. Animal Science Journal, 88: 1827-1834. 2017. [DOI] [PubMed] [Google Scholar]
- Pelletier G and Ren L. Localization of sex steroid receptors in human skin. Histology and Histopathology, 19: 629-636. 2004. [DOI] [PubMed] [Google Scholar]
- Pines M, Schickler M, Hurwitz S and Yamauchi M. Developmental changes in skin collagen biosynthesis pathway in posthatch male and female chickens. Poultry Science, 75: 484-490. 1996. [DOI] [PubMed] [Google Scholar]
- Salim HM, Lee HR, Jo C, Lee SK and Lee BD. Effect of dietary zinc proteinate supplementation on growth performance, and skin and meat quality of male and female broiler chicks. British Poultry Science, 53: 116-124. 2012. [DOI] [PubMed] [Google Scholar]
- Smith QT and Allison DJ. Changes of collagen content in skin, femur and uterus of 17-beta-estradiol benzoate-treated rats. Endocrinology, 79: 486-492. 1966. [DOI] [PubMed] [Google Scholar]
- Steel E and Hinde RA. Effect of Exogenous OEstrogen on Brood Patch Development of Intact and Ovariectomized Canaries. Nature, 202: 718-719. 1964. [DOI] [PubMed] [Google Scholar]
- Sturkie PD. Avian Physiology. Comstock publishing Associates. Ithaca. New York. 1954. [Google Scholar]


