Abstract
The mucosa of the intestine and oviduct of hens are susceptible to pathogens. Pathogenic infections in the mucosal tissues of laying hens lead to worsened health of the host animal, decreased egg production, and bacterial contamination of eggs. Therefore, better understanding of the mechanisms underlying mucosal barrier function is needed to prevent infection by pathogens. In addition, pathogen infection in the mucosal tissue generally causes mucosal inflammation. Recently, it has been shown that inflammation in the oviduct and intestinal tissue caused by disruption of the mucosal barrier function, can affect egg production. Therefore, it is vitla to understand the relationship between mucosal barrier function and egg production to improve poultry egg production. This paper reviews the studies on (1) oviductal mucosal immune function and egg production, (2) intestinal inflammation and egg production, and (3) improvement of mucosal immune function by probiotics. The findings introduced in this review will contribute to the understanding of the mucosal barrier function of the intestine and oviduct and improve poultry egg production in laying hens.
Keywords: egg production, inflammation, intestine, mucosal barrier, oviduct
1. Introduction
Pathogenic infections of the intestinal tract and oviduct negatively impact the health of host animals and contaminated eggs cause food poisoning in humans. Ameliorating infectious disease prevention is vital to maintain chicken health and to produce safe eggs. Chickens have a mucosal barrier in their digestive and reproductive tracts, because the mucosal tissue, as an “outside of the inside” tissue, is exposed to external bacteria and viruses. The mucosal barrier consists of four parts: the biological barrier formed by microbiota, chemical barrier formed by mucus including antimicrobial peptides and antibodies, mechanical barrier formed by tight junctions between mucosal epithelial cells, and immune barrier consisting of immune-competent cells in the lamina propria (Wang et al., 2016). These barriers work together to prevent pathogenic infections in mucosal tissues. An understanding of the control mechanisms and enhancement of mucosal barrier function should help prevent infection in chickens.
With the expansion of bans on growth-promoting antibiotics in poultry feed, new problems, such as disruption of the intestinal environment due to intestinal infection (Huyghebaert et al., 2011; Ducatelle et al., 2018). Non-caged house systems, such as deep litter, free range, and organic systems, are major breeding systems that improve poultry welfare in terms of behavioral freedom in the EU (Sokołowicz et al., 2020). However, non-caged house systems also bring another problem in the form of an increased risk of pathogenic infections such as coccidiosis, rubella, E. coli, pasteurellosis, histomoniasis, and ascariasis in comparison to cage systems (Relic et al., 2019; Bari et al., 2020). Therefore, protecting chickens from these pathogens by ameliorating the mucosal barrier function of the intestine and oviduct is becoming increasingly important.
In addition, it has been suggested that the oviductal mucosal immune reaction caused by antigen stimulation affects egg production. Intestinal inflammation caused by disruption of mucosal barrier function may also strongly affect egg production in laying hens. Understanding the mechanism of the relationship between mucosal barrier function and egg production is important for the improvement of egg production. This review introduces (1) the regulation of oviductal mucosal immune function and egg production, (2) the effects of intestinal inflammation on egg production, and (3) enhancement of intestinal mucosal barrier function by probiotic treatment.
2. Oviductal mucosal immune function and egg production
2-1. Regulation of Mucosal Immune Function in the Oviduct
The hen oviduct is susceptible to infections by bacterial and viral pathogenic microorganisms. When these organisms (such as Salmonella, Escherichia coli, and Mycoplasma) infect the oviduct, it leads to functional disorders of egg formation and concomitant production of contaminated eggs (Feberwee et al., 2009; Gantois et al., 2009; Neubauer et al., 2009; Ozaki and Murase, 2009). Contamination of eggs increases the mortality of newly hatched chicks and causes foodborne diseases in humans. For example, Salmonella Enteritidis is phagocytized by intestinal macrophages, which may then translocate to the ovary and oviduct through the bloodstream (De Buck et al., 2004). These pathogens colonizing the cloaca may invade the oviduct through the vagina. Therefore, enhancing the mucosal barrier function of the hen oviduct is needed to prevent pathogenic infections and maintain hygienic egg production.
Oviduct tissue has unique characteristics; namely, the development and regression of the oviduct tissue are regulated by gonadal steroids. Zheng et al. (1998) reported that estrogen stimulates the growth and differentiation of oviductal mucosal cells, as well as the influx of immunocompetent cells such as T and B cells. The report suggests that the mucosal immune function of the oviduct may be affected by gonadal steroids, similar to oviductal tissue. Similarly, the oviduct is more susceptible to bacterial infection in the molting phase, which is the non-laying phase with lower gonadal steroid concentration, compared with normal laying conditions (Holt, 1993; Gantois et al., 2009). In addition, eggs laid by post-molting hens were more heavily contaminated than those of pre-molting hens (Golden et al., 2008).
Lipopolysaccharides (LPS) are components of the gram-negative bacterial cell wall, and it is recognized by toll-like receptor (TLR) 4, which triggers an immune response. The recruitment of CD8+ T cells by LPS stimulation in the hen oviduct was decreased during the molting phase, and this may be a reason why the susceptibility to infections is higher during the molting phase (Nii et al., 2011). We also reported that the cellular immune response to the infectious bronchitis (IB) viral antigen stimulation was decreased with lower TLR7 gene expression during the molting phase in the oviductal mucosa, but the responsiveness was recovered by estrogen stimulation (Nii et al., 2015). Similarly, mRNA expression levels of Claudin-1, -3, -5 and protein expression of Claudin-1 were significantly decreased in molting hens, but these expressions were recovered by estrogen stimulation in the oviduct of laying hens (Ariyadi et al., 2013). The gene and protein expression of avian β-defensin (AvBD)-11, a component of the chemical barrier, was enhanced by estrogen stimulation in the hen oviduct (Lim, et al., 2013). In addition, AvBD expression was decreased in the mucosal epithelium of the regressed oviduct of molting hens (Yoshimura et al., 2006). Therefore, estrogen plays an important role in enhancing the chemical, physical, and immunological barriers in the oviductal mucosa of laying hens.
The frequency of antigen exposure is factor that affects the mucosal immune function of the oviduct. The density of T cells was higher in the vagina than in the other parts of the laying hen oviduct (Withanage et al., 1997; Yoshimura et al., 1997). The vagina opens to the cloaca, and this segment is exposed to antigens colonizing the cloaca. Therefore, it is expected that exposure to proper antigens may enhance the immune function of the oviduct. In fact, LPS stimulation conducted five times increased the numbers of CD4+, CD8+, and TCRγδ+ T cells in the mucosal tissue of the vagina of laying hens, but they did not change after a single LPS stimulation (Nii et al., 2013). This suggests that proper antigen stimulation may be effective for the accumulation of the T cell pool in the oviductal mucosa, and these cells may contribute to protection from antigen infection.
Thus, proper antigen stimulation results in a T cell subset pool, which may contribute to the prequiescence of host immunity in the oviductal mucosa, and estrogen stimulation may be responsible for enhancing oviductal mucosal barrier function. To increase the production of high-quality eggs by enhancing the mucosal barrier function in the oviduct, the establishment of the following methods in the oviduct may be considered: (1) stimulation with the effective antigen and (2) reinforcement of estrogen efficacy by increasing estrogen levels or receptor expression.
2-2. Immune Function of the Oviductal Mucosa and Egg Pro duction
The IB virus infects bronchial tubes and causes respiratory disease in chickens. This virus also infects the epithelial surface of the oviduct through blood circulation from bronchitis to the kidney and translocation from the kidney to the cloaca, reaching the oviduct (Dolz et al., 2012). Infection by the IB virus leads to disordered eggshell formation and a decline in egg production (Feberwee and Landman, 2010). Thus, how IB virus infection causes egg production disorders is an important question. It has been reported that IB virus infection negatively affects eggshell formation by disturbing the gene expression of collagen type I in the isthmus and CaBP-D28K (calbindin) is associated with increased proinflammatory cytokine gene expression in the uterus (Nii et al., 2014). In addition, both IL-1β and IL-6 stimulation significantly decreased CaBP-D28K protein density in cultured uterine tissue compared to that in the control group (Nii et al., 2018). These reports suggest that proinflammatory cytokines produced by viral infection lead to eggshell malformation through a decrease in CaBP-D28K protein expression.
H9N2 avian influenza virus infection increased proin flammatory cytokine expression in the uterus as well as disturbed eggshell formation through a decrease in CaBP-D28K (Wang et al., 2015; Qi et al., 2016). This report supports the investigation of the mechanism of eggshell malformation caused by viral infection. Therefore, bacterial or viral infection in the oviduct may cause disorder of eggshell formation through an increase in proinflammatory cytokines and a decrease in CaBP-D28K protein density. Preventing pathogenic infections, namely pathological mucosal inflammation, in the hen oviduct is important for healthy egg production in laying hens.
Collectively, cytokine production and attraction of lymphocytes through the interaction with TLRs with their ligands are important to prevent infections by pathogenic bacteria and viruses in the hen oviduct (Fig. 1). Estrogen is likely necessary for enhancing the immune reaction in the oviduct. Recent studies have also established that eggshell malformation caused by pathological microbe infection is likely led by the production of IL-1β and IL-6 induced by microbial infection. This knowledge is expected to be useful for the development of technology for preventive hygiene in the oviduct and production of eggs.
Fig. 1.
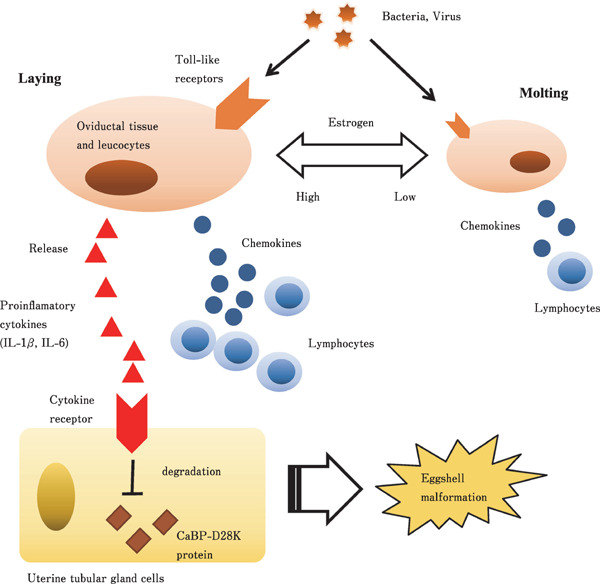
Representative diagram describing the regulatory mechanism of the recruitment of cytotoxic cells in hen oviduct, and eggshell malformation caused by infection. Cytokines are released and cytotoxic cells are recruited through the interaction between Tool-like receptors and its ligands by pathogenic bacteria and virus infection in hen oviductal mucosa. These immunoresponse is weakened during molting phase, but estrogen is likely to enhance this response. IL-1β and IL-6 produced by pathological microbe infection lead eggshell malformation through the degradation of calbindin protein. It is likely the cytotoxic cells and cytotoxic factors led by infection also affects eggshell malformation.
3. Intestinal inflammation and egg production
3-1. DSS-induced Acute Heavy Intestinal Inflammation and Egg Production in Laying Hens
The chicken gut mucosa is susceptible to pathogen infection in feed or water. Pathogens such as bacteria, parasites, and viral infection of intestinal tissue lead to decreased growth performance and egg production in chickens. In particular, Eimeria, one of the principal intestinal infectious pathogens causing coccidiosis, leads to a decrease in feed intake, growth, and egg production of layer and broiler breeder hens (Lensing et al., 2012; Ritzi et al., 2014). This pathogenic infection increases proinflammatory cytokine production (IL-1β and IL-6) and the frequency of histological inflammatory events in the intestinal mucosa (Hong et al., 2006; Belote et al., 2018; Fasina and Lillehoj, 2019). These reports suggest that intestinal inflammation reduces egg production in laying hens.
Dextran sulfate sodium (DSS), an inducer of intestinal inflammation, is commonly used as a model animal for colitis, inflammatory bowel disease, and colitis-associated cancer (Saleh and Trinchieri, 2011; Heijmans et al., 2014). DSS directly damages epithelial cells in the intestinal mucosa, and the intestinal microbiome enters the lamina propria and causes inflammation by immunocompetent cells (Saleh and Trinchieri, 2011). The effects of DSS administration on the intestinal mucosa of chickens was verified by Menconi et al. (2015), who showed that 0.75% DSS in drinking water for 8 d caused depression, anemia, watery and bloody diarrhea, decreased body weight (BW), and decreased villus and epithelial cell height of the duodenum and ileum in young broiler chicks. Kuttappan et al. (2015) examined the optimal DSS administration method for chicks. They administered two doses of oral gavage of 0.45 g DSS/bird to cause gut leakage in 3- and 4-day-old broiler chicks, because the method showed less mortality and more consistent results compared with the method using 1.25% DSS in drinking water. It was reported that DSS treatment in broiler and layer chicks caused histological damage of the intestine, including shortening and loss of villi and crypts, as well as intestinal inflammation (Simon et al., 2016). These reports confirm that DSS administration in broiler and layer chicks causes several symptoms such as intestinal inflammation, similar to those of rodents. Recently, the effects of intestinal inflammation caused by DSS treatment on egg production in laying hens have also been reported (Nii et al., 2020a). The report showed that oral administration of DSS (0.9 g/kg BW DSS for 5 d) caused severe acute intestinal inflammation, including histological disintegration of the cecal mucosa in laying hens (Fig. 2b), which may reduce egg production by disrupting egg-yolk precursor production in association with liver inflammation caused by the influx of LPS from the intestine.
Fig. 2.
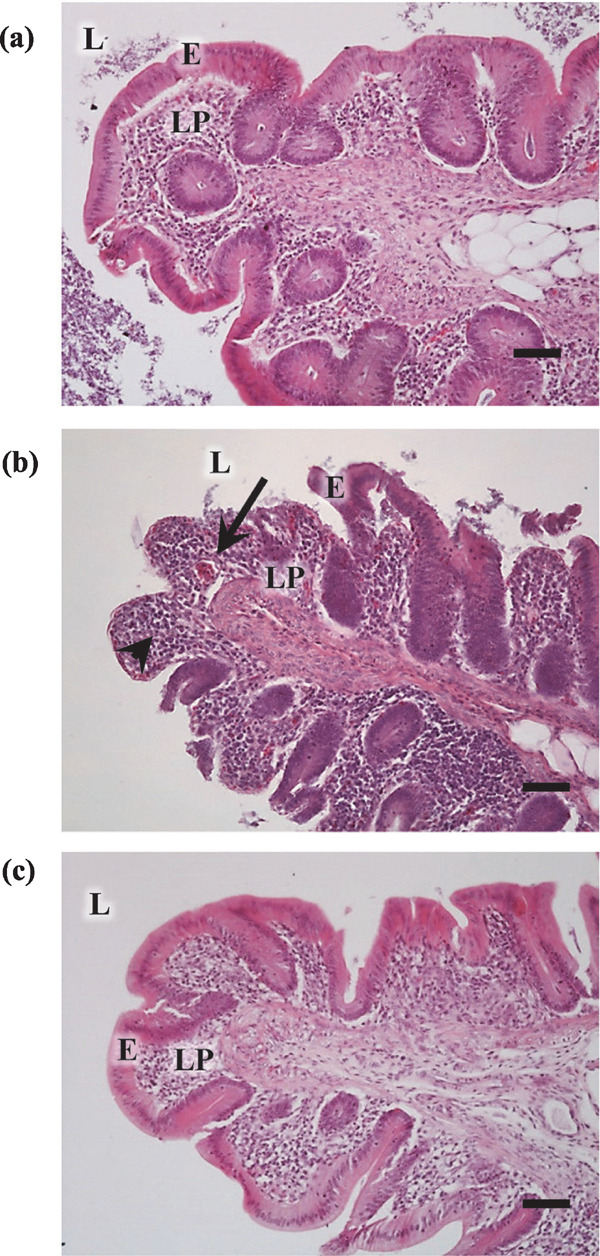
Micrographs showing the histology of the cecum in hens orally administered 0.9 g/kg BW of dextran sulphate sodium for 5 days (High-DSS group), 0.225 g/kg BW of DSS for 28 days (Low-DSS group) or water (control group). The cecum of hens in the control (A), High-DSS (B) and Low-DSS (C) groups. HE staining. Leukocytes are distributed in the lamina propria in the cecum of hens in the DSS group (arrows). The luminal epithelial cells of the cecum in the DSS group had disintegrated or were lost (arrow head). E=mucosal epithelium, L=lumen, LP= lamina propria. Scale bars=50 µm. (Nii et al., 2020a, b).
3-2. Slight Intestinal Inflammation and Egg Production in Laying Hens
Minor disruption of the intestinal environment without severe symptoms is related to intestinal barrier dysfunction, intestinal inflammation, and dysbiosis during the rearing period and it has become a common problem since the ban on growth-promoting antibiotics in poultry feed (Huyghebaert et al., 2011; Ducatelle et al., 2018). Therefore, an experimental model for long, minor disruptions of the intestinal environment are highly important to simulate real farm conditions. Oral administration of 0.225 g/kg BW DSS for 28 d led to a slight disruption of the intestinal environment; namely, shorter villi and a decrease in cecal microbiome diversity without any histological damage or symptoms (Fig. 2c) (Nii et al., 2020b). Slight disruption of the intestinal environment decreased egg-laying ratio and egg yolk size (Fig. 3), and this low egg productivity was likely caused by dysfunction in the egg-yolk precursor uptake in the ovarian follicle in association with an increase in circulating LPS without disrupting liver function. However, the mechanism by which egg yolk size is decreased by disruption of the intestinal environment is still unknown.
Fig. 3.
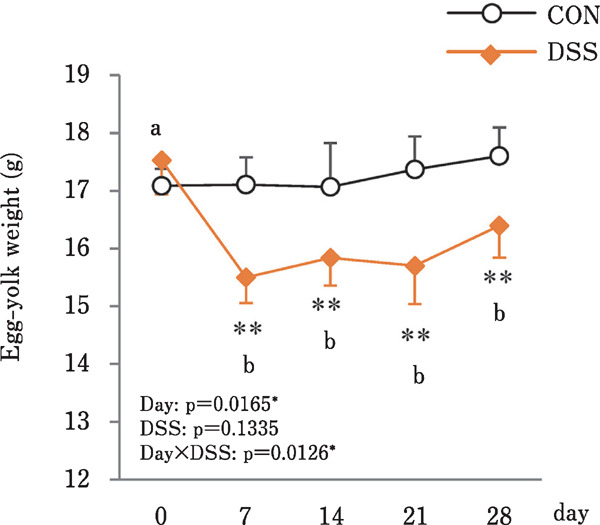
Effects of the oral administration of 0.225 g/kg BW of dextran sulphate sodium (DSS) on the changes in the egg-yolk weight in hens administered DSS (DSS group) or water (CON group). Open bars represent the control group, and orange filled bars represent the DSS group. Values are the mean±SEM (CON and DSS, n=8 and 7). Asterisks (**) indicate significant differences be tween the CON and DSS groups (P<0.01). Lower case let ters indicate significant differences between the time points (P<0.05). (Nii et al., 2020b).
Disruption of the intestinal environment causes histological damage and shortening of villi, which absorb nutrients (Nii et al., 2020a, b). Therefore, disruption of the intestinal environment is suspected to decrease the function of nutrient absorption in chickens and may affect poultry productivity, including the size of the yolk. Restricting feed ing to 55%–80% of the general feed intake for 5 d decreased whole egg weight on day 5 (Nii et al., 2020a). However, an increase in the energy concentration of the diet does not affect the weight of egg yolk (Pérez-Bonilla et al., 2012). Thus, decreased nutrient absorption caused by a disrupted intestinal environment may contribute to the decreased egg yolk size, but a change in energy intake does not always seem to affect the egg yolk weight.
Elkin et al. (2012) reviewed the characteristics of the “restricted ovulatory” (R/O) chicken strain, which shows smaller ovarian follicles and higher circulating cholesterol (CHO) and triglycerides (TG) compared with wild-type chickens. Ho et al. (1974) reported that high concentrations of cholesterol in R/O hens was not caused by overproduction of cholesterol, but was mainly caused by the failure to remove cholesterol from blood circulation by incorporating it into egg yolks. In addition, the R/O hens lack a 95-kDa membrane protein known as LR8, which is a lipoprotein receptor for uptake of VLDLy, an egg yolk precursor, into oocytes (Nimpf et al., 1989). Thus, the lack of LR8 in ovarian follicles may be a primary factor underlying disrupted follicular growth and increased CHO and TG concentrations in the plasma. In our previous study, plasma TG and total CHO were greatly increased in both high and low concentrations of orally administered DSS hens, which showed lower egg productivity (Nii et al., 2020a, b). However, the gene expression of LR8 in granulosa cells of F1 follicles was higher in the DSS group than in the control group (Nii et al., 2020b). The reason for this contradictory result is unknown, but the increase in LR8 gene expression may be caused by feedback from slow-growing follicles. Therefore, a slight disruption of the intestinal environment may decrease egg production and egg yolk size by disrupting VLDLy receptors such as LR8.
Endocrine changes, such as changes in stress or gonadal hormones caused by disruption of the intestinal environment, may also lead to a decrease in egg yolk size. It has been reported that DSS-mediated intestinal inflammation increases plasma corticosterone levels in mice (Hassan et al., 2014). Corticosterone stimulation by implantation of corticosterone pellets decreased plasma estrogen levels in White Leghorn hens (Henriksen et al., 2011). However, R/O hens exhibited higher circulating FSH levels caused by a lack of negative feedback inhibition from lower inhibin secretion (Ocón-Grove, et al., 2007). In addition, slight disruption of the intestinal environment caused by DSS stimulation increased the mRNA expression of FSH in the pituitary gland of laying hens (Nii et al., 2020b). Therefore, disordered endocrine hormone production caused by the disrupted intestinal environment may cause the decreased egg yolk size.
Taken together, DSS is a useful chemical that causes severe or mild artificial intestinal inflammation in laying hens. Severe intestinal inflammation causes disruption of liver function and ovarian growth function by circulating endo-toxins from intestinal bacteria, resulting in the cessation of egg production. In contrast, slight disruption of the intestinal environment may cause dysfunction of egg yolk precursor uptake into ovarian follicles, but not suppression of liver function. Thus, disorders of the intestinal environment, including inflammation, may first lead to the cessation or delay of ovarian growth, the yolk precursor remaining in the blood, and finally, downregulation of yolk precursor production in the liver by negative feedback. However, the factors that disrupt ovarian growth are still unknown.
4. Improvement of mucosal immune function by probiotics
Disruption of the intestinal environment causes intestinal inflammation through disordered intestinal mucosal barrier function, resulting in decreased in egg production in chickens. Thus, maintaining optimal conditions in the intestinal environment and enhancing the mucosal barrier function are likely important for egg production.
Probiotics are live bacteria that exert health benefits in host animals, and they have been well studied for their potential to improve poultry health and production. Treatment with Lactobacillus salivarius and L. reuteri, two lactic acid bacteria, increases the ratio of villus heights/crypt depth (V/C), which is an indicator of intestinal health in the duodenum of broiler chickens (Awad et al., 2010). Bacillus and Entero-coccus also increase the V/C ratio in the small intestine of chicks (Li et al., 2018; Huang et al., 2019). Our previous study showed that oral administration of 1×108 cfu of L. reuteri increased villus height in the ileum of broiler chicks (Nii et al., 2020c, d). Therefore, probiotics are expected to improve intestinal health by increasing the V/C ratio. Tight junctions, paracellular structures formed by several molecules, such as claudins, junctional adhesion molecules (JAMs), and zonula occludens (ZOs), are respon sible for the physical barrier function of the epithelium against pathogen invasion into mucosal tissue (Awad et al., 2017). Treatment with L. plantarum decreases gut permeability by enhancing the expression levels of ZO1 and Claudin5 in the intestine of Salmonella-infected chickens (Wang et al., 2018). In our study, L. reuteri treatment increased the gene expression of JAM2 in the digestive tract of broiler chicks (Nii et al., 2020c). L. reuteri also increased the expressions of Claudin1, Claudin5, JAM2, and ZO2, and decreased cecal permeability in the cecum of Salmonella Typhimurium heat-killed bacteria stimulated broiler chicks (Nii et al., 2020d). In addition, L. reuteri and C. butyricum treatment enhanced the expression of TLRs, AvBDs, and pro- and anti-inflammatory cytokine genes (Terada et al., 2020). These results suggest that pro-biotics enhance intestinal mucosal barrier function by improving tight junctions and innate immune function in the intestine.
Davis and Anderson (2002) reported that probiotic mixtures, including those containing L. acidophilus, L. casei, E. faecium, and Bifidobacterium thermophilum, improve micro-flora and egg production in laying hens. Bacillus licheniformis powder (2×1010 cfu/g) increased villus height in the jejunum of White Leghorn hens and improved egg quality, including shell thickness (Lei et al., 2013). It has been reported that the Bacillus family plays a role in improving the microbiome and morphology of the intestine following egg production (Popov et al., 2021). However, DSS-induced disruption of the intestinal environment resulted in a decrease in the V/C ratio and tight junction-related gene expression in the lower parts of the intestine, which was associated with decreased egg production (Nii et al., 2020b). These results suggest that decreased mucosal barrier function is probably related to egg production in laying hens. Thus, probiotics are expected to ameliorate egg production in laying hens by improving the intestinal environment, including mucosal barrier function. The mechanism of how probiotics affect egg production in laying hens are not well understood. We believe our DSS experimental model providing stress in the intestine is useful for studies to determine the mechanisms of how probiotics affect egg production in laying hens.
Recently, it has been reported that the same genera of microbiota, including Lactobacillus, exists in both the cloaca and oviduct, and it has been suggested that some probiotic bacteria can translocate from the intestine to the oviduct (Lee et al., 2019; Shterzer et al., 2020). In humans, intravaginal administration as suppositories or topical application as a probiotic gel improves the mucosal immune response of the reproductive organ (Bustamante et al., 2020). Therefore, probiotics should enhance the mucosal barrier function of the oviductal mucosa, similar to the effects of probiotics in the intestines of chickens. Further research is needed to determine the effects of probiotics on the mucosal barrier function of the oviduct of chickens.
5. Conclusion
Pathogenic infections in both the intestine and oviduct lead to dysfunction of egg production in laying hens through inflammation caused by mucosal immune reactions (Fig. 4). Intestinal inflammation causes an influx of LPS to the liver, disrupting liver function following disordered follicular growth in the ovary (Fig. 4a). Intestinal inflammation may directly disrupt follicular growth through LPS translocation. Oviductal inflammation causes an increase in proinflammatory cytokine production, leading to disrupted eggshell formation through decreased CaBP-D28K protein density (Fig. 4b). However, probiotic treatment may prevent pathogenic infection by enhancing the mucosal barrier function of the intestine, leading to improvement of liver function and ovarian follicular growth (Fig. 4c). Estrogen and proper antigens, such as probiotic stimulation, may also prevent infections by ameliorating the mucosal barrier function of the oviduct, leading to improved eggshell formation (Fig. 4d). Enhancing mucosal barrier function in the intestine and oviduct should improve egg production in laying hens. However, the mechanism underlying the decrease in egg yolk size caused by the disruption of the intestinal environment is still unknown. Further studies are necessary to address this problem.
Fig. 4.
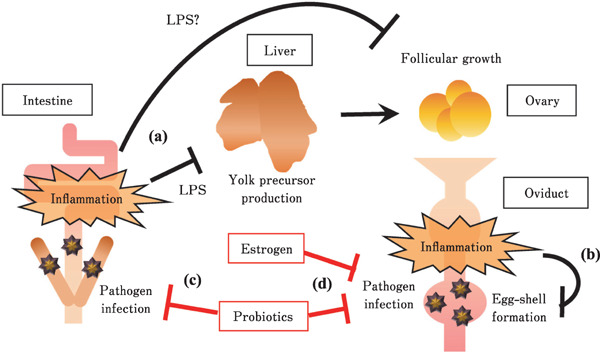
Representative diagram describing the effects of inflammation condition and enhancement of mucosal barrier function in the intestine and oviduct on egg production of laying hens. (a) Intestinal inflammation caused by pathogen infection leads disruption of liver function through influx of lipopolysaccharide (LPS), and disorder of follicular growth. LPS probably directly disrupts the function of follicular growth in the ovary. (b) Inflammation in oviduct causes disorder of egg-shell formation. These inflammation in both intestine and oviduct leads disorder of egg production. (c) Probiotics enhances mucosal barrier function in the intestine, it may be following prevention of liver and ovarian functional disorder caused by disruption of intestinal mucosal barrier function. (d) Estrogen and proper antigen stimulation such as probiotics stimulation enhance mucosal barrier function in the oviduct. The enhancement of barrier function in both intestine and ovary may contribute to improvement of egg production.
In addition, the DSS experimental model introduced in this review is expected to be a new method for studies on the mechanisms of the relationship between the intestinal environment and poultry production, as well as to examine the effects of feed additives in laying hens. The findings introduced in this review will contribute to the understanding of the mucosal barrier function of the intestine and oviduct, and improve poultry egg production in laying hens.
Acknowledgments
The author, Takahiro Nii, was awarded the 2021 Young Scientist Prize by the Japan Poultry Science Association for “Studies on the relationship between mucosal immune function of the intestine and oviduct and egg production in laying hens.” I thank Dr. Yukinori Yoshimura, Hiroshima University, for his critical reading of the manuscript. This work was supported by a Grant-in-Aid for JSPS Fellows (No. 13J02100) to T.N., Grant-in-Aid for Research Activity Start-up (No. 17H06892) to T.N. and Grant-in-Aid for Early Career Scientists (No. 18K14569) to T.N. from the Japan Society for Promotion of Science (JSPS).
Author Contributions
Takahiro Nii was responsible for all elements of this review.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ariyadi B, Isobe N and Yoshimura Y. Expression of tight junction molecule “claudins” in the lower oviductal segments and their changes with egg-laying phase and gonadal steroid stimulation in hens. Theriogenology, 79: 211-218. 2013. [DOI] [PubMed] [Google Scholar]
- Awad WA, Ghareeb K and Bohm J. Effect of addition of a probiotic micro-organism to broiler diet on intestinal mucosal architecture and electrophysiological parameters. Journal of Animal Physiology and Animal Nutrition, 94: 486-494. 2010. [DOI] [PubMed] [Google Scholar]
- Awad WA, Hess C and Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins, 9: 60. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari MS, Laurenson YCSM, Cohen-Barnhouse AM, Walkden-Brown SW and Campbell DLM. Effects of outdoor ranging on external and internal health parameters for hens from different rearing enrichments. PeerJ, 8: e8720-e8720. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote BL, Tujimoto-Silva A, Hummelgen PH, Sanches AWD, Wammes JCS, Hayashi RM and Santin E. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poultry Science, 97: 2287-2294. 2018. [DOI] [PubMed] [Google Scholar]
- Bustamante M, Oomah BD, Oliveira WP, Burgos-Díaz C, Rubilar M and Shene C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiologica, 65: 245-264. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GS and Anderson KE. The effects of feeding the direct-fed microbial, primalac, on growth parameters and egg production in single comb white leghorn hens. Poultry Science, 81: 755-759. 2002. [DOI] [PubMed] [Google Scholar]
- De Buck J, Van Immerseel F, Haesebrouck F, and Ducatelle R. Colonization of the chicken reproductive tract and egg contamination by Salmonella. Journal of Applied Microbiology, 97: 233-245. 2004. [DOI] [PubMed] [Google Scholar]
- Dolz R, Vergara-Alert J, Pérez M, Pujols J and Majó N. New insights on infectious bronchitis virus pathogenesis: characterization of Italy 02 serotype in chicks and adult hens. Veterinary Microbiology, 156: 256-264. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F and Van Immerseel F. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Veterinary Research, 49: 43. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin RG, Bauer R and Schneider WJ. The restricted ovulator chicken strain: an oviparous vertebrate model of reproductive dysfunction caused by a gene defect affecting an oocyte-specific receptor. Animal Reproduction Science, 136: 1-13. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasina YO and Lillehoj HS. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poultry Science, 98: 188-198. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feberwee A, de Wit JJ and Landman WJ. Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies. Avian Pathology, 38: 77-85. 2009. [DOI] [PubMed] [Google Scholar]
- Feberwee A and Landman WJM. Induction of eggshell apex abnormalities in broiler breeder hens. Avian Pathology, 39: 133-137. 2010. [DOI] [PubMed] [Google Scholar]
- Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey TJ and Van Immerseel F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiology Reviews, 33: 718-738. 2009. [DOI] [PubMed] [Google Scholar]
- Golden NJ, Marks HH, Coleman ME, Schroeder CM, Bauer NE and Schlosser WD. Review of induced molting by feed removal and contamination of eggs with Salmonella enterica serovar Enteritidis. Veterinary Microbiology, 131: 215-228. 2008. [DOI] [PubMed] [Google Scholar]
- Hassan AM, Jain P, Reichmann F, Mayerhofer R, Farzi A, Schuligoi R and Holzer P. Repeated predictable stress causes resilience against colitis-induced behavioral changes in mice. Frontiers in Behavioral Neuroscience, 8: 386-386. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans J, Wielenga MC, Rosekrans SL, van Lidth de Jeude JF, Roelofs J, Groothuis P, Ederveen A, de Jonge-Muller ES, Biemond I, Hardwick JC, D'Haens G, Hommes DW, Muncan V and van den Brink GR. Oestrogens promote tumorigenesis in a mouse model for colitis-associated cancer. Gut, 63: 310-316. 2014. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Groothuis TG and Rettenbacher S. Elevated plasma corticosterone decreases yolk testosterone and progesterone in chickens: linking maternal stress and hormone-mediated maternal effects. PLoS One, 6: e23824. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KJ, Lawrence WD, Lewis LA, Liu LB and Taylor CB. Hereditary hyperlipidemia in nonlaying chickens. Archives of Pathology, 98: 161-172. 1974. [PubMed] [Google Scholar]
- Holt PS. Effect of induced molting on the susceptibility of White Leghorn hens to a Salmonella enteritidis infection. Avian Diseases, 37: 412-417. 1993. [PubMed] [Google Scholar]
- Hong YH, Lillehoj HS, Lee SH, Dalloul RA and Lillehoj EP. Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Veterinary Immunology and Immunopathology, 114: 209-223. 2006. [DOI] [PubMed] [Google Scholar]
- Huang L, Luo L, Zhang Y, Wang Z and Xia Z. Effects of the Dietary Probiotic, Enterococcus faecium NCIMB11181, on the Intestinal Barrier and System Immune Status in Escherichia coli O78-Challenged Broiler Chickens. Probiotics and Antimicrobial Proteins. 11: 946-956. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert G, Ducatelle R and Immerseel FV. An update on alternatives to antimicrobial growth promoters for broilers. The Veterinary Journal, 187: 182-188. 2011. [DOI] [PubMed] [Google Scholar]
- Kuttappan VA, Berghman LR, Vicuna EA, Latorre JD, Menconi A, Wolchok JD, Wolfenden AD, Faulkner OB, Tellez GI, Hargis BM and Bielke LR. Poultry enteric inflammation model with dextran sodium sulfate mediated chemical induction and feed restriction in broilers. Poultry Science, 94: 1220-1226. 2015. [DOI] [PubMed] [Google Scholar]
- Lee S, La TM, Lee HJ, Choi IS, Song CS, Park SY, Lee JB and Lee SW. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Scientific Reports, 9: 6838. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Li YL, Yu DY, Rajput IR and Li WF. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poultry Science, 92: 2389-2395. 2013. [DOI] [PubMed] [Google Scholar]
- Lensing M, van der Klis JD, Yoon I and Moore DT. Efficacy of Saccharomyces cerevisiae fermentation product on intestinal health and productivity of coccidian-challenged laying hens. Poultry Science, 91: 1590-1597. 2012. [DOI] [PubMed] [Google Scholar]
- Li CL, Wang J, Zhang HJ, Wu SG, Hui QR, Yang CB, Fang RJ and Qi GH. Intestinal morphologic and microbiota responses to dietary Bacillus spp. in a broiler chicken model. Frontiers in Physiology, 9: 1968. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Jeong W, Kim J, Yoshimura Y, Bazer FW, Han JY and Song G. Expression and regulation of beta-defensin 11 in the oviduct in response to estrogen and in ovarian tumors of chickens. Molecular and Cellular Endocrinology, 366: 1-8. 2013. [DOI] [PubMed] [Google Scholar]
- Menconi A, Hernandez-Velasco X, Vicuna EA, Kuttappan VA, Faulkner OB, Tellez G, Hargis BM and Bielke LR. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poultry Science, 94: 906-911. 2015. [DOI] [PubMed] [Google Scholar]
- Neubauer C, De Souza-Pilz M, Bojesen AM, Bisgaard M and Hess M. Tissue distribution of haemolytic Gallibacterium anatis isolates in laying birds with reproductive disorders. Avian Pathology, 38: 1-7. 2009. [DOI] [PubMed] [Google Scholar]
- Nii T, Sonoda Y, Isobe N and Yoshimura Y. Effects of lipo-polysaccharide on the expression of proinflammatory cytokines and chemokines and the subsequent recruitment of immuno-competent cells in the oviduct of laying and molting hens. Poultry Science, 90: 2332-2341. 2011. [DOI] [PubMed] [Google Scholar]
- Nii T, Isobe N and Yoshimura Y. Effects of repeated lipopolysaccharide stimulation on the development of antigen-presenting cells and T cells pool in hen vagina. Journal of Poultry Science, 50: 83-89. 2013. [Google Scholar]
- Nii T, Isobe N and Yoshimura Y. Effects of avian infectious bronchitis virus antigen on eggshell formation and immunoreaction in hen oviduct. Theriogenology, 81: 1129-1138. 2014. [DOI] [PubMed] [Google Scholar]
- Nii T, Isobe N and Yoshimura Y. The effect of estrogen on the early cytotoxic response to IB virus infection in hen oviduct. Veterinary Immunology and Immunopathology, 164: 56-66. 2015. [DOI] [PubMed] [Google Scholar]
- Nii T, Isobe N and Yoshimura Y. Effects of interleukin-1β and -6 on the expression of ion transporters for mineralization of eggshell in cultured uterine mucosal tissue of hen. Journal of Poultry Science, 55: 142-149. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Bungo T, Isobe N and Yoshimura Y. Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poultry Science, 99: 1663-1677. 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Bungo T, Isobe N and Yoshimura Y. Slight disruption in intestinal environment by dextran sodium sulfate reduces egg yolk size through disfunction of ovarian follicle growth. Frontiers in Physiology, 11: 607369. 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Jirapat J, Isobe N and Yoshimura Y. Effects of oral administration of Lactobacillus reuteri on mucosal barrier function in the digestive tract of broiler chicks. Journal of Poultry Science, 57: 67-76. 2020c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T, Kakuya H, Isobe N and Yoshimura Y. Lactobacillus reuteri enhances the mucosal barrier function against heat-killed Salmonella Typhimurium in the intestine of broiler chicks. Journal of Poultry Science, 57: 148-159. 2020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimpf J, Radosavljevic MJ and Schneider WJ. Oocytes from the mutant restricted ovulator hen lack receptor for very low density lipoprotein. Journal of Biological Chemistry, 264: 1393-1398. 1989. [PubMed] [Google Scholar]
- Ocón-Grove OM, Maddineni S, Hendricks GL, Elkin RG, Proudman JA and Ramachandran R. Pituitary progesterone receptor expression and plasma gonadotrophin concentrations in the reproductively dysfunctional mutant restricted ovulator chicken. Domestic Animal Endocrinology, 32: 201-215. 2007. [DOI] [PubMed] [Google Scholar]
- Ozaki H and Murase T. Multiple routes of entry for Escherichia coli causing colibacillosis in commercial layer chickens. Journal of Veterinary Medical Science, 71: 1685-1689. 2009. [DOI] [PubMed] [Google Scholar]
- Pérez-Bonilla A, Novoa S, García J, Mohiti-Asli M, Frikha M and Mateos GG. Effects of energy concentration of the diet on productive performance and egg quality of brown egg-laying hens differing in initial body weight. Poultry Science, 91: 3156-3166. 2012. [DOI] [PubMed] [Google Scholar]
- Popov IV, Algburi A, Prazdnova EV, Mazanko MS, Elisashvili V, Bren AB, Chistyakov VA, Tkacheva EV, Trukhachev VI, Donnik IM, Ivanov YA, Rudoy D, Ermakov AM, Weeks RM and Chikindas ML. A review of the effects and production of spore-forming probiotics for poultry. Animals, 11: 1941. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Tan D, Wu C, Tang C, Li T, Han X, Wang J, Liu C, Li R and Wang J. Deterioration of eggshell quality in laying hens experimentally infected with H9N2 avian influenza virus. Veterinary Research, 47: 35. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relic R, Sossidou E, Dedousi A, Peric L, Bozickovic I and Dukic-Stojcic M. Behavioral and health problems of poultry related to rearing systems. Ankara Universitesi Veteriner Fakultesi Dergisi, 66: 423-428. 2019. [Google Scholar]
- Ritzi MM, Abdelrahman W, Mohnl M and Dalloul RA. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poultry Science, 93: 2772-2778. 2014. [DOI] [PubMed] [Google Scholar]
- Saleh M and Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nature Reviews Immunology, 11: 9-20. 2011. [DOI] [PubMed] [Google Scholar]
- Shterzer N, Rothschild N, Sbehat Y, Stern E, Nazarov A and Mills E. Large overlap between the intestinal and reproductive tract microbiomes of chickens. Frontiers in Microbiology, 11: 1508-1508. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon K, Arts JA, de Vries Reilingh G, Kemp B and Lammers A. Effects of early life dextran sulfate sodium administration on pathology and immune response in broilers and layers. Poultry Science, 95: 1529-1542. 2016. [DOI] [PubMed] [Google Scholar]
- Sokołowicz Z, Dykiel M, Topczewska J, Krawczyk J and Augustyńska-Prejsnar A. The effect of the type of non-caged housing system, genotype and age on the behaviour of laying hens. Animals, 10: 2450. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T, Nii T, Isobe N and Yoshimura Y. Effects of probiotics Lactobacillus reuteri and Clostridium butyricum on the expression of toll-like receptors, pro- and anti-inflammatory cytokines, and antimicrobial peptides in broiler chick intestine. The Journal of Poultry Science, 57: 310-318. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tang C, Wang Q, Li R, Chen Z, Han X and Xu X. Apoptosis induction and release of inflammatory cytokines in the oviduct of egg-laying hens experimentally infected with H9N2 avian influenza virus. Veterinary Microbiology, 177: 302-314. 2015. [DOI] [PubMed] [Google Scholar]
- Wang K, Wu L, Dou C, Guan X, Wu H and Liu H. Research advance in intestinal mucosal barrier and pathogenesis of Crohn's disease. Gastroenterology Research and Practice, 2016: 9686238. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li L, Lv Y, Chen Q, Feng J and Zhao X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Scientific Reports, 8: 2229. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withanage GS, Baba E, Sasai K, Fukata T, Kuwamura M, Miyamoto T and Arakawa A. Localization and enumeration of T and B lymphocytes in the reproductive tract of laying hens. Poultry Science, 76: 671-676. 1997. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Okamoto T and Tamura T. Localisation of MHC class II, lymphocytes and immunoglobulins in the oviduct of laying and moulting hens. British Poultry Science, 38: 590-596. 1997. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Ohashi H, Subedi K, Nishibori M and Isobe N. Effects of age, egg-laying activity, and Salmonella-inoculation on the expressions of gallinacin mRNA in the vagina of the hen oviduct. Journal of Reproduction and Development, 52: 211-218. 2006. [DOI] [PubMed] [Google Scholar]
- Zheng WM, Yoshimura Y and Tamura T. Effects of age and gonadal steroids on the localization of antigen-presenting cells, and T and B cells in the chicken oviduct. Journal of Reproduction and Fertility, 114: 45-54. 1998. [DOI] [PubMed] [Google Scholar]


