Abstract
Purpose
To report 12-month efficacy outcomes of 360° canaloplasty and 180° trabeculotomy using the OMNI surgical system in combination with phacoemulsification in patients with mild-moderate open-angle glaucoma (OAG) and visually significant cataract.
Setting
Fifteen multi-subspecialty ophthalmology practices and surgery centers located in 14 US states.
Design
Prospective, multicenter, IRB approved study of patients treated with canaloplasty (360°) and trabeculotomy (180°). Eligible patients had cataract and mild-moderate OAG with intraocular pressure (IOP) ≤33 mmHg on 1 to 4 hypotensive medications. Unmedicated post-washout mean diurnal IOP (DIOP) ≥21 and ≤36 mmHg.
Methods
Medication washout preoperatively and prior to month 12 DIOP. Effectiveness outcomes were IOP and IOP lowering medication use. Safety outcomes included adverse events and secondary surgical interventions (SSIs). Evaluations at 1, 3, 6, and 12 months.
Results
A total of 149 subjects underwent surgery and 120 were included in the final effectiveness analysis. Mean (standard deviation) unmedicated diurnal IOP was reduced from 23.8 (3.1) mmHg at baseline to 15.6 (4.0) at month 12 (−35%) and medications (before washout) were reduced from 1.8 (0.9) at baseline to 0.4 (0.9) at month 12 (−80%). At month 12, 84.2% of eyes achieved IOP reductions >20% from baseline, 80% of eyes were medication-free, and 76% of eyes achieved IOP between 6–18 mmHg inclusive. Adverse events were uncommon. Most were mild and self-limited including transient hyphema (9 of 149; 6%) and transient IOP elevations (3 of 149; 2.0%). No eyes required SSIs or experienced loss of VA that was attributable to the device or procedure.
Conclusion
Canaloplasty and trabeculotomy performed with the OMNI surgical system at the time of phacoemulsification significantly reduces unmedicated mean diurnal IOP and medication use 12 months postoperatively, with an excellent safety profile. This procedure should be considered for eyes with mild-moderate OAG to reduce IOP, medication burden, or both.
Keywords: viscodilation, MIGS, open-angle glaucoma, glaucoma surgery, canaloplasty, trabeculotomy, OMNI
Introduction
In the past decade, the role of minimally invasive glaucoma surgeries (MIGS) has expanded greatly. Developed to expand the indications for glaucoma surgery by offering safer procedures than traditional trabeculectomy and tube-shunts for patients with more modest therapeutic goals,1 MIGS procedures have eclipsed traditional incisional glaucoma surgeries among Medicare beneficiaries in the US: from 2008 to 2016, the number of traditional surgeries decreased by 12% to ~33,000 while the number of MIGS procedures increased by 426% to ~58,000.2 MIGS adoption and utilization has continued to expand to the present.3
Most MIGS procedures lower intraocular pressure (IOP) by targeting one source of aqueous humor outflow resistance (eg, the trabecular meshwork [TM] or Schlemm’s canal [SC]) or by creating novel aqueous egress pathways (eg, to the suprachoroidal or subconjunctival spaces).4–7 The OMNI surgical system (Sight Sciences) was developed to target 3 distinct sources of elevated IOP: the TM, SC, and the distal collector channels.8–11 The OMNI system consists of a single-use, disposable handpiece with a cannula tip that delivers a microcatheter through which ophthalmic viscosurgical device (OVD) is delivered to perform canaloplasty to viscodilate SC and the collector channels and with which trabeculotomy is performed. The handpiece incorporates an advancement wheel to deploy and retract the microcatheter and a port through which an OVD reservoir can be loaded.12 In published studies, canaloplasty and trabeculotomy performed with the OMNI system produced mean IOP reductions of ~30-40% and medication reductions of ~45-75%.13–15
GEMINI was a prospective, multicenter, interventional, single-arm, clinical study of patients with mild-moderate OAG undergoing 360° canaloplasty followed by 180° trabeculotomy with the OMNI surgical system at the time of phacoemulsification. An interim analysis of the GEMINI data set at 6 months revealed mean IOP reduction of 38%, medication reduction of 67%, with 100% of eyes having achieved IOP reduction ≥20% from baseline and 78% being medication-free.16 In this report, we present the final 12-month analysis of the GEMINI study.
Methods
This was a prospective, multicenter, single-arm, interventional clinical study. A central IRB (Aspire, Western Copernicus Group) reviewed and approved the study protocol before study commencement. Subjects were enrolled between February 2019 and February 2020, and all subjects provided written informed consent to participate. The study was registered at clinicaltrials.gov (NCT03861169) and adhered to the tenets of the Declaration of Helsinki.
The study eligibility criteria, visits, and procedures were modeled after recent pivotal randomized trials conducted to evaluate outcomes of MIGS procedures17,18 and has been reported previously.16 Briefly, the study enrolled adults aged 22 years or older with visually significant cataract and mild to moderate open-angle glaucoma (including pigmentary and pseudoexfoliation glaucomas) according to ICD-10 guidelines and with visual field mean deviation −12 dB or better. Medicated IOP <33 mmHg using a stable regimen of 1–4 topical IOP lowering medications for at least 2 months prior to screening and unmedicated mean diurnal IOP (after washout) between 21–36 mmHg inclusive and at least 3 mmHg higher than screening IOP were required for eligibility. Key exclusion criteria included anterior chamber angle less than Shaffer grade 3 in all four quadrants by gonioscopy, the presence of advanced glaucoma or any form of glaucoma other than OAG, laser trabeculoplasty within 3 months of enrollment, or any prior glaucoma surgery (trabeculectomy, tube-shunt, or any MIGS procedure). The protocol was amended to exclude medication-naïve subjects after 8 subjects on no medications at baseline were enrolled and treated, and the sample size was increased to achieve enough power necessary to test the hypothesis after exclusion of these 8 subjects.
Following a comprehensive medical history and examination, subjects meeting screening criteria underwent washout of IOP lowering medications (5 days for carbonic anhydrase inhibitors, 14 days for alpha adrenergic agonists, 28 days for all others) followed by diurnal IOP assessment at 9AM, 12PM, and 4PM. BCVA was assessed using the ETDRS chart and manifest refraction (at all time points except Day 1). IOP was assessed by Goldmann tonometry using an operator/reader system in which an experienced operator took the measurement and a separate reader recorded the measurement. At each time point, 2 measurements were taken, and the mean recorded. If the two readings differed by more than 2 mmHg, a third measurement was taken and the median recorded. Subjects meeting all eligibility criteria underwent surgery within 7 days.
Eyes with uneventful standard phacoemulsification and intraocular lens implantation (IOL) underwent the 360° canaloplasty followed by 180° trabeculotomy procedure (eyes with intraoperative surgical complications related to the cataract/IOL procedure were exited without undergoing the glaucoma procedure). The procedure has been described previously.16 Briefly, the OMNI handpiece tip was inserted through the cataract surgery incision into the anterior chamber filled with viscoelastic under intraoperative gonioscopy and positioned at the nasal angle. The trabecular meshwork (TM) was incised using the instrument’s tip and its flexible microcatheter was then advanced through 180° of SC. As it was then slowly retracted, a fixed volume (5.5 µL per 180°) of OVD was automatically dispensed to dilate SC and the collector channels in a controlled standardized fashion. These steps were repeated along the remaining 180° of SC through the same TM entry point in the opposite direction to complete the canaloplasty. The microcatheter was then re-advanced into SC and withdrawn slowly to unroof 180° of SC. All eyes received a standard postoperative regimen of steroid, nonsteroidal anti-inflammatory drug, and antibiotic.
Subjects were seen 1 day, 1 week, and 1, 3, 6, and 12 months postoperatively, with eyes using topical IOP lowering medications at month 12 undergoing a washout (as described above) and returning post-washout. Postoperative assessments included best-corrected visual acuity (BCVA), IOP, slit-lamp examination of the anterior segment, gonioscopy (beginning at Week 1), and adverse event reporting (identified by patient report, solicitation by study staff, and examination). At month 12, IOP was measured at 9AM, 12PM, and 4PM and unmedicated mean diurnal IOP calculated.
The first primary efficacy endpoint for this study was the reduction in unmedicated diurnal IOP from baseline to month 12 postoperatively. The control (cataract surgery only) arms of two recent pivotal MIGS trials showed an approximate 6.5 mmHg reduction in IOP at 12 months17,18 therefore a 7.5 mmHg mean reduction in IOP at 12 months for the present study was selected as the minimum reduction required to demonstrate meaningful effectiveness beyond what might be provided by cataract surgery alone. The sample size required to provide 80% power to detect a >7.5 mmHg reduction in unmedicated diurnal IOP using a one-sample t-test with a one-sided significance level of 0.025 was 116 eyes. The second primary efficacy endpoint was the reduction in the number of IOP lowering medications from screening to month 12. Medication reduction at 12 months seen in the control arms of the same two pivotal MIGS trials was approximately 1.0.17,18 Based on a one-sample t-test with a one-sided significance level of 0.025, a sample size of 101 eyes provided 80% power to detect a mean reduction of >1.0 medication. The first secondary efficacy endpoint was the proportion of eyes with >20% reduction in unmedicated IOP from baseline to month 12. Based on the binomial distribution with a one-sided significance level of 0.025, a sample size of 105 eyes provided 80% power to detect a >73% proportion of eyes achieving this endpoint. The second secondary efficacy endpoint was the proportion of eyes with unmedicated diurnal IOP between 6–18 mmHg inclusive at month 12. Based on the binomial distribution with a one-sided significance level of 0.025, a sample size of 91 eyes provided 80% power to detect a >55% proportion of eyes achieving this endpoint. Based on these individual outcome power analyses, and allowing for an ~10% attrition rate, the overall study’s target enrollment was set at 130 eyes. Safety analysis consisted of tabulating the nature and incidence of adverse events. Means are reported with their standard deviations.
Results
Overall, 149 subjects were enrolled and comprised the Safety dataset. Eight subjects enrolled under the original protocol were treatment naive (0 medications at screening) and were thus excluded from analysis following amendment of the protocol requiring at least one medication preoperatively for eligibility. Thirteen subjects were enrolled but were excluded from the final effectiveness analysis due to protocol deviations related to eligibility criteria including one with a cataract surgery complication (capsular tear) and 8 subjects were lost to follow-up. A total of 120 subjects were included in the final effectiveness analysis (per protocol population). Demographic and glaucoma status data are given in Table 1. Subjects had a mean (standard deviation) age of 68.3 (8.5) years, most were female (60%) and White (82%), and most had POAG (93%) that was mild to moderate in severity (mean visual field mean deviation −3.7 [3.6] dB; mean pattern standard deviation 3.7 [2.6] dB).
Table 1.
Demographic and Baseline Glaucoma Status Data for the Study Sample (N=149)
| Parameter | Value (N=149) |
|---|---|
| Age (yr), mean (SD) | 68.3 (8.5) |
| Gender, n (%) | |
| Female | 89 (60) |
| Male | 60 (40) |
| Race/Ethnicity, n (%) | |
| White | 122 (82) |
| Other | 27 (18) |
| Study Eye, n (%) | |
| Right | 74 (50) |
| Left | 75 (50) |
| Glaucoma Diagnosis n, (%) | |
| Primary open-angle | 139 (93) |
| Pseudoexfoliation | 9 (6) |
| Pigmentary | 1 (0.7) |
| Visual Field Mean Deviation (dB), mean (SD) | −3.7 (3.6) |
| Visual Field Pattern Standard Deviation (dB), mean (SD) | 3.7 (2.6) |
Abbreviations: dB, decibels; SD, standard deviation.
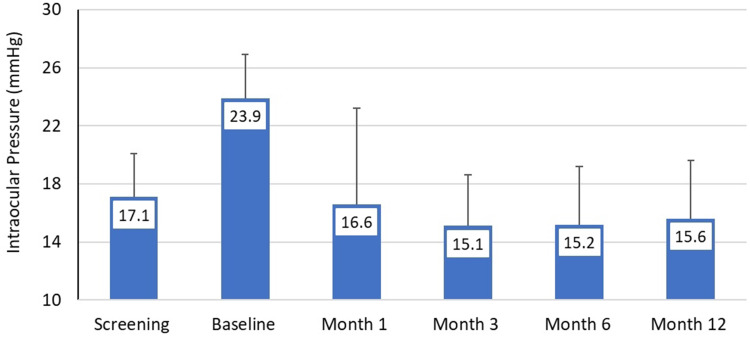
Mean medicated IOP at screening (n=120) was 17.1 (3.0) mmHg and rose to 23.9 (3.0) mmHg at baseline (after washout). Mean IOP at months 1, 3, 6, and 12 (Table 2 and Figure 1) ranged from 15.1–16.6 mmHg (p<0.00001 at each time point). At month 12, after washout of any eyes using IOP lowering medications, mean unmedicated DIOP was 15.6 (4.0), representing an 8.2 (4.1) mmHg reduction from unmedicated baseline DIOP (p<0.00001), and 84.2% of eyes had a ≥20% IOP reduction from baseline. The proportion of eyes on no medications across visits ranged from 67–80% (80% at month 12), and among the eyes on no medications at each time point, mean IOP ranged from 14.3–15.6 mmHg, representing reductions of 7.7–9.0 mmHg and relative reductions of 34–39%. Amongst the eyes on no medications, the proportion of eyes with IOP reductions of ≥20% from baseline ranged from 84–94% across time points (92% at month 12); the proportion meeting both of these criteria and with IOP between 6–18 mmHg inclusive ranged from 75–88% (87% at month 12). Considering only the POAG eyes the results were essentially identical to the overall results while mean IOP reduction for the small subset of secondary OAG eyes (n=7) was somewhat less (24.9 at baseline and 19.1 mmHg at Month 12) (Table 2).
Table 2.
Intraocular Pressure (IOP) Outcomes at Each Postoperative Study Time Point in per Protocol Population (n=120)
| Month 1 | Month 3 | Month 6 | Month 12 | |
|---|---|---|---|---|
| Mean (SD) IOP, mmHg | ||||
| ALL (n=120) | 16.6 (6.6) | 15.1 (3.5) | 15.2 (4.0) | 15.6 (4.0) |
| POAG only (n=113) | 16.6 (6.8) | 15.0 (3.5) | 15.1 (3.9) | 15.4 (3.8) |
| PXF and PG only (n=7) | 18.1 (3.9) | 17.0 (2.2) | 18.0 (3.6) | 19.1 (6.9) |
| Eyes on 0 meds, n (%) | ||||
| ALL (n=120) | 87 (73) | 95 (79) | 96 (80) | 96 (80) |
| POAG only (n=113) | 82 (73) | 90 (81) | 90 (80) | 90 (80) |
| PXF and PG only (n=7) | 5 (71) | 5 (71) | 6 (86) | 6 (86) |
| Eyes with decrease in Diurnal IOP ≥20% at Month 12, n (%) | ||||
| ALL (n=120) | NA | NA | NA | 104 (87) |
| POAG only (n=113) | 100 (89) | |||
| PXF and PG only (n=7) | 4 (57) | |||
| Eyes on 0 meds with decrease in IOP ≥20%, n (%) | 67 (84) | 83 (92) | 87 (94) | 90 (92) |
| Eyes on 0 meds with decrease in IOP ≥20% and IOP ≥6 ≤ 18 mmHg, n (%) | 60 (75) | 79 (88) | 82 (88) | 85 (87) |
| Mean (SD) unmedicated IOP, mmHg* | 15.6 (5.2) | 14.5 (2.9) | 14.3 (3.1) | 14.6 (3.1)** |
| Mean (SD) decrease in unmedicated IOP from Baseline* | 7.7 (4.7) | 8.7 (3.2) | 9.0 (3.3) | 8.2 (4.1)** |
| Mean (SD) decrease in unmedicated IOP (%) from Baseline* | 34 (19) | 37 (12) | 39 (12) | 34 (16)** |
Notes: *Among eyes on no medications at each time point **Unmedicated IOP is measured as mean DIOP.
Abbreviations: mmHg, millimeters of mercury; SD, standard deviation; POAG, primary open-angle glaucoma; PXF, pseudoexfoliation; PG, pigmentary glaucoma.
Figure 1.
Mean intraocular pressure at each study time point. Error bars represent standard deviation in per protocol population (n=120).
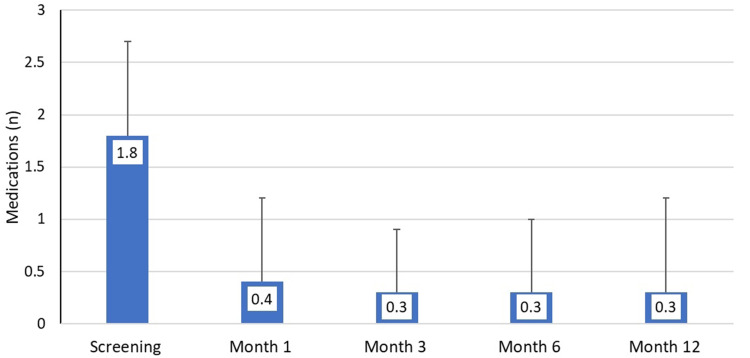
The mean number of medications used per eye at screening was 1.8 (0.9) and at months 1, 3, 6, and 12 and ranged from 0.3–0.4 medications per eye (p<0.00001 at each time point), representing mean medication reductions of 1.4–1.5 per eye and relative reductions of 78–83% (Table 3 and Figure 2). At month 12, before washout, mean medication use was 0.3 (0.9) per eye, a reduction of 1.4 (1.1) medications (p<0.00001) and a 78% relative reduction. All the eyes (100%) on no medications at 3, 6 and 12 months and 96% eyes at 1 month had no increase in IOP from unmedicated baseline. The proportion of eyes that had no increase from screening in the number of medications used ranged from 95–99% (97% at month 12).
Table 3.
Medication Outcomes at Each Postoperative Study Time Point in per Protocol Population (n=120)
| Month 1 | Month 3 | Month 6 | Month 12* | |
|---|---|---|---|---|
| Mean (SD) number of medications | 0.4 (0.8) | 0.3 (0.6) | 0.3 (0.7) | 0.3 (0.9) |
| Mean (SD) reduction in medications | 1.4 (1.2) | 1.5 (0.9) | 1.5 (1.0) | 1.4 (1.1) |
| Mean percent reduction in medications, % | 78 | 83 | 83 | 78 |
| Eyes achieving medication freedom | 80 (67) | 90 (75) | 93 (78) | 98 (82) |
| Of the eyes on 0 meds, eyes with no increase in IOP from Baseline, n (%) | 77 (96) | 90 (100) | 93 (100) | 98 (100) |
| Eyes with no increase in medications, n (%) | 114 (95) | 119 (99) | 119 (99) | 116 (97) |
Note: *Before mandatory medication washout.
Abbreviations: IOP, intraocular pressure; SD, standard deviation.
Figure 2.
Mean medication use at each study visit. Month 12 data are before mandatory medication washout (per protocol dataset, n=120). Error bars represent standard deviation.
One or more ocular hypotensive medications were reintroduced for 23 subjects (safety dataset) prior to the Month 12 visit. Pre- and post washout IOP at Month 12 was available for 22 of these. These included one (n = 16), two (n = 5), and three (n = 1) medications. These were predominantly prostaglandin analogues (PGA) with one medication (12 of 16), or a PGA with another class of medication(s) for two or three medications (5 of 6). Mean (SD) medicated IOP was 16.9 (3.4) mmHg and 21.0 (4.9) mmHg following washout, an average increase of 4.1 mmHg. Broken out by number of medications this was 16.8 (3.6) and 19.8 (4.0) mmHg with a rise of 3.0 (2.9) mmHg for one medication, and 18.6 (4.5) and 21.7 (6.3) mmHg with a rise of 5.8 (4.0) mmHg for two medications. The single patient on three medications went from 17.0 to 30.0 mmHg following washout, an increase of 13 mmHg. For comparison, there was an average increase of 6.5 (2.8) mmHg for all subjects that underwent washout pre-surgically (safety dataset) including 6.0 (2.5) mmHg (1 medication, n = 71), 5.9 (2.0) mmHg (2 medications, n = 36), 8.1 (3.4) mmHg (3 medications, n = 30), and 8.9 (3.9) mmHg (4 medications, n = 4).
Intraoperative, perioperative, and postoperative safety outcomes from the safety population (all enrolled and treated subjects; n=149) are summarized in Table 4. As is common with angle-based procedures, layered hyphema was the most common adverse event, occurring in 9 eyes (6%), that resolved without further intervention or sequelae within four weeks of occurrence. Other AEs included IOP increased 10 mmHg or more above baseline at or after the Month 1 visit (n=3, 2.0%), anterior uveitis (n=2, 1.3%), peripheral anterior synechiae (n=2, 1.3%), clinically significant cystoid macular edema (n=1, 0.7%) and blepharitis (n=3, 2.0%). It is noteworthy that no AE for recurrent hyphema and no secondary surgical interventions were reported. Loss of visual acuity was reported in 2 eyes (10 letters and 13 letters compared to baseline), both secondary to posterior capsular opacity. None of the AE were considered serious by the Investigators.
Table 4.
The Nature and Incidence of Adverse Events
| Adverse Event | N (%) | Serious | Device Related | Status |
|---|---|---|---|---|
| Layered hyphema ≥ 1mm | 9 (6%) | No | Yes | Recovered spontaneously without sequel |
| Intraocular pressure increase ≥ 10 mmHg above baseline at ≥ 1 month | 3 (2.0%) | No | Yes | Recovered without sequel with hypotensive medication added |
| Blepharitis | 2 (1.3%) | No | No | Recovered spontaneously without sequel |
| Clinically significant cystoid macular edema | 1 (0.7%) | No | No | Recovered without sequel after topical steroid |
| Internal Hordeolum | 1 (0.7%) | No | No | Recovered without sequel after topical treatment |
| Keratoconjunctivitis sicca | 1 (0.7%) | No | No | Recovered without sequel after topical treatment |
| Vitreous Hemorrhage | 1 (0.7%) | No | No | Recovered spontaneously without sequel |
| Loss of 2 lines or more BCVA (10 or more ETDRS letters) at or after 3 months postoperative | 2 (1.3%) | No | No | VA loss due to PCO; ongoing |
Discussion
The final results of the GEMINI study demonstrate persistence of the previously reported 6-month findings16 that 360° canaloplasty and 180° trabeculotomy performed with the OMNI surgical system at the time of phacoemulsification significantly reduces both IOP and the need for IOP lowering medications for at least 12 months postoperatively. Unmedicated mean diurnal IOP was reduced by 34%, 84% of eyes achieved ≥20% IOP reductions from baseline, mean medication use was reduced by 78%, and 80% were medication-free at month 12. No new safety findings were observed since the previously-reported interim analysis.16
These results are consistent with 6-month results of the GEMINI study reported in the interim analysis16 and with prior studies of the OMNI surgical system. A prospective study of 360° canaloplasty and 360° trabeculotomy alone or combined with phacoemulsification in 17 eyes with mild-moderate OAG reported 12-month mean IOP reduction of 36% (39% in standalone surgery, 34% in combined surgery) and mean medication reduction of 75% (73% and 76%, respectively).13 In the 12-month retrospective multicenter ROMEO study, 81 eyes (24 with baseline IOP > 18 mmHg and 57 with IOP ≤ 18 mmHg) underwent either 180° or 360° canaloplasty and trabeculotomy combined with phacoemulsification; mean IOP reductions at month 12 were 31% in high baseline IOP eyes and 4% in low baseline IOP eyes, while mean medication reductions were 45% and 44%, respectively.15 In ROMEO, 89% of all eyes achieved 12-month IOP between 6–18 mmHg inclusive (88% and 90%, respectively).15 A single-site retrospective study evaluating 41 eyes undergoing 180° canaloplasty and trabeculotomy combined with phacoemulsification reported mean IOP reductions of 5.6 mmHg (and 9.6 mmHg in eyes with baseline IOP > 22 mmHg).19
Trabecular bypass implants circumvent proximal resistance residing in the juxtacanalicular TM and inner wall of Schlemm’s canal (iStent) or, as with the Hydrus device, also scaffold a quadrant of Schlemm’s canal partially addressing distal resistance (atrophied or collapsed Schlemm’s canal and blocked collector channel ostia).20 The OMNI surgical system targets these three potential sources of resistance circumferentially.21,22 Trabeculotomy addresses the increased aqueous outflow resistance within the TM,8,9 while canaloplasty addresses contributions to outflow resistance by both Schlemm’s canal10 and the distal collector channels.11 This comprehensive approach is a key advantage as it is not currently possible in the clinical setting to assess pre-operatively the main site of abnormal resistance for a specific eye. The relative contributions of canaloplasty and trabeculotomy to the outcomes produced with the combined OMNI procedure have not been evaluated in any study to date. Canaloplasty using the VISCO360 system (a precursor to the OMNI system) or the OMNI for canaloplasty only in both standalone and combined with cataract surgery procedures has been reported to lower mean IOP by 36% and medications by 32% at 18 months post-surgery.23 Recognizing that eyes typically undergo MIGS procedures either to reduce IOP (in eyes with high baseline IOP) or medications (in eyes with low baseline IOP), two studies analyzed VISCO360 outcomes by baseline IOP: In eyes with high IOP, IOP reductions of 22–41% were seen, while in eyes with low IOP, medication reductions of 45–89% were seen.24,25 Another canaloplasty procedure—ab interno canaloplasty, or ABiC—has been shown to reduce mean IOP by 25–40% and mean medication use by 61–97% in various studies.26–29 Similarly, trabeculotomy procedures (TRAB360 [an OMNI precursor] and gonioscopy-assisted transluminal trabeculotomy [GATT]) have been reported in various studies to reduce mean IOP by 28–44% and medication use by 28–82% as standalone procedures,30–37 and GATT lowers mean IOP by 29–67% and medications by 45–93% when combined with phacoemulsification.30,35,38,39
It is noteworthy that the mean IOP reduction in the present study closely matches that achieved by the Hydrus microstent at 12 months in its pivotal trial17 (8.2 mmHg vs 8.5 mmHg) despite starting from a washed out baseline nearly 2 mmHg lower (23.8 mmHg vs 25.5 mmHg). Had the mean baseline IOP in the present study been greater it is likely that mean reduction would also have been greater.
The protocol for this study specified 360° canaloplasty but only 180° trabeculotomy as a means of minimizing potentially confounding differences in the extent of trabeculotomy between participating study sites. It is unknown if a potential dose-response relationship might have produced greater IOP and medication reductions with a 360° trabeculotomy. In a study cited above, 360° canaloplasty and trabeculotomy produced mean IOP and medication reductions of 34% and 76%, respectively, when combined with phacoemulsification.13 These values are consistent with the findings of the current study—34% and 80%, respectively—suggesting that maximal surgical benefit may be obtained with 180° trabeculotomy, although such a conclusion is limited by the many caveats of cross-study comparisons (including differences in study protocols, patients, techniques, etc.).
Intraocular pressure increase following preoperative washout for subjects from the COMPASS and HORIZON studies showed an average increase in IOP post-washout of 5.7, 6.9, 8.8, and 9.5 mmHg for 1, 2, 3, or 4 medications, respectively.40 Results from the smaller cohort in the present study are generally consistent with these results; 6.0, 5.9, 8.1, and 8.9 mmHg underscoring the overall similarity of the subject population from the present study with these larger trials. Interestingly, the subset of subjects that underwent a terminal washout at Month 12 because medications had been added during the follow-up period showed similar (2 medications) or smaller (1 medication) increases in IOP following the 12-month washout, but unmedicated IOP that was lower than the corresponding presurgical IOP (21.0 vs 25.6 mmHg) suggesting that the surgical procedure provided IOP lowering effectiveness even for those subjects.
This study is strengthened by its mirroring procedurally and in terms of study population of recent pivotal MIGS trials. Features such as medication washout at baseline and month 12 to assess its primary endpoint—reduction in unmedicated diurnal IOP—and subgroup analysis of unmedicated eyes at other time points set this study apart from many or even most other MIGS studies. The lack of a control group is a study limitation that precludes quantifying the contribution of elements from the phacoemulsification and OMNI procedures (as would be possible with a phacoemulsification-only arm) or comparison of OMNI to other MIGS procedures (as would be possible with an active comparator arm). Phacoemulsification alone is known to lower IOP and the need for IOP lowering medications in glaucomatous eyes.41 We have attempted to mitigate this limitation by designing the statistical analysis plan around the relevant outcomes of phacoemulsification-only groups (which have been remarkably consistent albeit greater than what has been typically reported in other publications) in recent pivotal MIGS trials,17,18 specifically powering the study to detect an effect size in excess of that expected by phacoemulsification alone in eyes with mild-moderate OAG. The decision to design this study without a cataract surgery only control group was not made lightly. First, the availability of published data for large cohorts of similarly defined patient populations undergoing phacoemulsification only allowed reasonable estimation of the cataract surgery only treatment effect. Second, in the early 2010ʹs when the pivotal studies for iStent, Hydrus, and CyPass were initiated, the treatment landscape for mild to moderate glaucoma in the United States was very different. MIGS was a new modality with limited availability and options.1 Standard treatment was generally medical (drops) or laser (SLT). In contrast, by 2019 when the present study was designed there were several MIGS options available to patients and surgeons with increasing adoption.3 In this evolving environment, execution of a trial where many patients would be randomized to a “no treatment” (aside from cataract surgery) control would have faced increased unwillingness of patients to participate and would arguably be less ethically sound given the many new treatment options available for mild to moderate glaucoma.
In summary, 360° canaloplasty and 180° trabeculotomy performed with the OMNI surgical system at the time of phacoemulsification significantly reduces unmedicated mean diurnal IOP by 34% and medication use by 78% 12 months postoperatively, with an excellent safety profile and no serious adverse events. Importantly, these outcomes were achieved with an implant-free procedure. This procedure should be considered among the options available for eyes with mild-moderate OAG and visually significant cataract in which MIGS is planned at the time of phacoemulsification to lower IOP, reduce the medication burden, or both.
Acknowledgments
Statistical analysis was provided by Yi-Jing Duh, PhD. Tony Realini, MD, MPH (Hypotony Holdings, LLC) created the draft manuscript. Jaime Dickerson, Jr., PhD provided editorial assistance.
The GEMINI Study Group: Mark Gallardo, Steven D Vold, Inder Paul Singh, Alex Cohen, Michael Greenwood, Sebastian Heersink, Arkadiy Yadgarov, Lorne Schlecht, Mark Pyfer, Anil Vedula, Anita Campbell, Brandon Baartman, Steven R Sarkisian Jr, Russell Swan, Brian Flowers, Yi-Jing Duh, Thomas W Samuelson, Kavita Dhamdhere.
Data Sharing Statement
The authors do not intend to share participant level data. Other queries or requests should be directed to the corresponding author (KD).
Disclosure
MJG, SRS, SDV, IPS are consultants and speakers for Sight Sciences. SRS has an equity interest in Sight Sciences and reports grants and/or personal fees from Alcon, Aerie, Bausch & Lomb, Elios, IStar, Allysta Pharmaceuticals, Allergan/AbbVie, BVI, Glaukos, ICare, Katena, MST, Santen, grants/personal fees with equity from Ocular Science, Ocular Therapeutix and Sight Sciences, during the conduct of the study. SDV also reports personal fees from Alcon, Allergan, Bausch & Lomb, Carl Zeiss Meditec, Glaukos, Iridex, iStar Medical, Ivantis, Sight Sciences, Volk Optical, Santen, and Alphaeon, outside the submitted work; In addition, SDV has a patent Gonio lens system (8851676) issued; fasteners/deployment systems for ophthalmic tissue closure (13/434,562, 13/709,375, 14/046,488) pending. MFP reports research support from Sight Sciences, during the conduct of the study and is speakers bureau for Omeros, outside the submitted work. AC reports study fees for participation as principal investigator from Sight Sciences, during the conduct of the study. BF reports grants and/or personal fees from Alcon, Glaukos, Sight Sciences, grants, New World Medical, iStar Medical, Santen, and NiCox, during the conduct of the study. KD is an employee of Sight Sciences, Inc. The authors report no other conflicts of interest in this work.
References
- 1.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23:96–104. doi: 10.1097/ICU.0b013e32834ff1e7 [DOI] [PubMed] [Google Scholar]
- 2.Rathi S, Andrews CA, Greenfield DS, Stein JD. Trends in glaucoma surgeries performed by glaucoma subspecialists versus nonsubspecialists on Medicare beneficiaries from 2008 through 2016. Ophthalmology. 2021;128:30–38. doi: 10.1016/j.ophtha.2020.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SA, Mitchell W, Hall N, et al. Trends and usage patterns of minimally invasive glaucoma surgery in the United States: IRIS® Registry Analysis 2013–2018. Ophthalmol Glaucoma. 2021;4(6):558–568. PMID: 33831643. doi: 10.1016/j.ogla.2021.03.012 [DOI] [PubMed] [Google Scholar]
- 4.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12:e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH.S135316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konopińska J, Lewczuk K, Jabłońska J, Mariak Z, Rękas M. Microinvasive glaucoma surgery: a review of Schlemm’s canal-based procedures. Clin Ophthalmol. 2021;15:1109–1118. doi: 10.2147/OPTH.S293702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- 9.Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-H [DOI] [PubMed] [Google Scholar]
- 10.Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62:101–109. doi: 10.1006/exer.1996.0012 [DOI] [PubMed] [Google Scholar]
- 11.Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickerson JE, Dhamdhere K. Combined circumferential canaloplasty and trabeculotomy ab interno with the OMNI surgical system. Front Ophthalmol. 2021;1:106–115. [Google Scholar]
- 13.Grabska-Liberek I, Duda P, Rogowska M, et al. 12-month interim results of a prospective study of patients with mild to moderate open-angle glaucoma undergoing combined viscodilation of Schlemm’s canal and collector channels and 360 degrees trabeculotomy as a standalone procedure or combined with cataract surgery. Eur J Ophthalmol. 2021;24:1120672121998234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klabe K, Kaymak H. Standalone trabeculotomy and viscodilation of Schlemm’s canal and collector channels in open-angle glaucoma using the OMNI surgical system. Clin Ophthalmol. 2021;15:3121–3129. doi: 10.2147/OPTH.S325394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47:907–915. doi: 10.1097/j.jcrs.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 16.Gallardo MJ, Sarkisian Jr SR Jr., Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification in open-angle glaucoma: interim results from the GEMINI study. Clin Ophthalmol. 2021;15:481–489. doi: 10.2147/OPTH.S296740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126:29–37. doi: 10.1016/j.ophtha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 18.Samuelson TW, Sarkisian SR Jr., Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126:811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 19.Brown RH, Tsegaw S, Dhamdhere K, Lynch MG. Viscodilation of Schlemm canal and trabeculotomy combined with cataract surgery for reducing intraocular pressure in open-angle glaucoma. J Cataract Refract Surg. 2020;46:644–645. doi: 10.1097/j.jcrs.0000000000000107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camras LJ, Yuan F, Fan S, et al. A novel Schlemm’s canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci. 2012;53:6115–6121. doi: 10.1167/iovs.12-9570 [DOI] [PubMed] [Google Scholar]
- 21.Dickerson JE, Brown RH. Circumferential canal surgery: a brief history. Curr Opin Ophthalmol. 2020;31:139–146. doi: 10.1097/ICU.0000000000000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khaimi MA. Canaloplasty: a minimally invasive and maximally effective glaucoma treatment. J Ophthalmol. 2015;2015. doi: 10.1155/2015/485065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes T, Traynor M. Clinical results of ab interno canaloplasty in patients with open-angle glaucoma. Clin Ophthalmol. 2020;14:3641–3650. doi: 10.2147/OPTH.S275087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ondrejka S, Korber N. 360 degrees ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–1246. doi: 10.2147/OPTH.S203917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tracer N, Dickerson JE Jr., Radcliffe NM. Circumferential viscodilation ab interno combined with phacoemulsification for treatment of open-angle glaucoma: 12-month outcomes. Clin Ophthalmol. 2020;14:1357–1364. doi: 10.2147/OPTH.S252965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillmann K, Aref A, Niegowski LJ, Baumgartner JM. Combined ab interno viscocanaloplasty (ABiC) in open-angle glaucoma: 12-month outcomes. Int Ophthalmol. 2021;41:3295–3301. doi: 10.1007/s10792-021-01891-1 [DOI] [PubMed] [Google Scholar]
- 27.Kazerounian S, Zimbelmann M, Lortscher M, et al. Canaloplasty ab interno (AbiC) - 2-year-results of a novel minimally invasive glaucoma surgery (MIGS) technique. Klin Monbl Augenheilkd. 2020;238:1113–1119. doi: 10.1055/a-1250-8431 [DOI] [PubMed] [Google Scholar]
- 28.Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs ab-externo canaloplasty with tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi: 10.2147/OPTH.S178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;12:2149–2155. doi: 10.2147/OPTH.S177597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121:855–861. doi: 10.1016/j.ophtha.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Grover DS, Godfrey DG, Smith O, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26:41–45. doi: 10.1097/IJG.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 32.Rahmatnejad K, Pruzan NL, Amanullah S, et al. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26:1137–1143. doi: 10.1097/IJG.0000000000000802 [DOI] [PubMed] [Google Scholar]
- 33.Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27:393–401. doi: 10.1097/IJG.0000000000000956 [DOI] [PubMed] [Google Scholar]
- 34.Aktas Z, Ozmen MC, Atalay HT, Ucgul AY. Evaluation of episcleral venous fluid wave during gonioscopy assisted transluminal trabeculotomy in patients with advanced glaucoma. Eye. 2019;33:668–673. doi: 10.1038/s41433-018-0254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olgun A, Aktas Z, Ucgul AY. XEN gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol. 2020;40:1085–1093. doi: 10.1007/s10792-019-01271-w [DOI] [PubMed] [Google Scholar]
- 36.Sarkisian S, Allan EJ, Ding K, et al. New way for ab interno trabeculotomy: initial results. American Society of Cataract and Refractive Surgery Annual Meeting. San Diego, CA: 2015. [Google Scholar]
- 37.Sarkisian SR, Mathews B, Ding K, et al. 360° ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161–168. doi: 10.2147/OPTH.S189260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baykara M, Poroy C, Erseven C. Surgical outcomes of combined gonioscopy-assisted transluminal trabeculotomy and cataract surgery. Indian J Ophthalmol. 2019;67:505–508. doi: 10.4103/ijo.IJO_1007_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirabayashi MT, Lee D, King JT, et al. Comparison of surgical outcomes of 360 degrees circumferential trabeculotomy versus sectoral excisional goniotomy with the Kahook Dual Blade at 6 months. Clin Ophthalmol. 2019;13:2017–2024. doi: 10.2147/OPTH.S208468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson TV, Jampel HD. Intraocular pressure following prerandomization glaucoma medication washout in the HORIZON and COMPASS trials. Am J Ophthalmol. 2020;216:110–120. doi: 10.1016/j.ajo.2020.04.008 [DOI] [PubMed] [Google Scholar]
- 41.Armstrong JJ, Wasiuta T, Kiatos E, et al. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26:511–522. [DOI] [PubMed] [Google Scholar]