Abstract
The standard treatment of human leishmaniases involves the use of pentavalent antimony [Sb(V)] compounds, including meglumine antimoniate. The mode of action of these compounds has not been fully elucidated. The possibility that Sb(III) is involved has been suggested; however, the biomolecule that may induce the conversion of Sb(V) to Sb(III) has not yet been identified. In the present study, we investigated both the ability of reduced glutathione (GSH) to promote the reduction of Sb(V) into Sb(III) in meglumine antimoniate and the effects of pH and temperature on this transformation. GSH did promote the reduction of Sb(V) into Sb(III) in a dose-dependent manner. When GSH and meglumine antimoniate were incubated together at a GSH/Sb molar ratio superior or equal to 5:1, all antimony was encountered in the reduced form, indicating a stoichiometry of 5:1 between GSH and Sb(V) in the reaction. The reaction between Sb(V) and GSH was favored at an acidic pH (pH 5) and an elevated temperature (37°C), conditions found within the phagolysosome, in which Leishmania resides. For instance, about 30% of the Sb(V) (concentration, 2mM) was converted to Sb(III) following incubation for 3 days with 10 mM GSH at pH 5 and 37°C. Our data support the hypothesis that Sb(V) would be converted by GSH, or a related thiol compound, to more toxic Sb(III) in the phagolysosome of macrophages.
The leishmaniases are a group of diseases produced by invasion of the reticuloendothelial system of a vertebrate host by a parasite of the genus Leishmania. This parasite is found as a motile promastigote in the sandfly; it transforms into an amastigote when engulfed by host macrophages and resides in the acidic environment of secondary lysosomes (1). These diseases are a significant cause of morbidity and mortality in several countries of the world. The treatment of choice for all forms of leishmaniasis depends on pentavalent antimony [Sb(V)]-containing drugs such as meglumine antimoniate (Glucantime) and sodium stibogluconate (Pentostam). Despite the clinical use of these antileishmanial agents for over half a century, their mechanism of action and the basis for their selective toxicity remain unknown. The hypothesis that Sb(V) acts as a prodrug that is converted to the more toxic trivalent antimony [Sb(III)] at or near the site of action was first suggested by Goodwin and Page (9), after they observed that a host organism can reduce Sb(V) into Sb(III). Recently, hydride generation-atomic absorption spectrometry analysis of serum and urine from patients treated with meglumine antimoniate revealed that 15 to 25% of serum antimony and 50% of urine antimony were trivalent (3, 15). This hypothesis was further supported by the observations that Sb(III) is more toxic than Sb(V) against both parasite stages of different Leishmania species (13, 19, 21) and that mutants of Leishmania infantum amastigotes selected for resistance to Sb(III) were cross-resistant to Sb(V) inside monocytes (22).
Recently, however, Ephros et al. (6) showed that axenically grown amastigotes were highly sensitive to Sb(V) and suggested that Sb(V) is directly and specifically toxic to amastigotes. Until now, the biomolecule that promotes the reduction of Sb(V) into Sb(III) and the location where this reaction occurs have not been identified. Reduced glutathione (GSH) is a likely candidate as a reducing agent for Sb(V). First, GSH is the most prevalent cellular thiol (12), present within the cytosol at high (2 to 10 mM) concentrations. Secondly, GSH was previously found to be oxidized in the presence of arsenate [As(V)] (20). This reaction may also occur with Sb(V), instead of As(V), since antimony lies directly below arsenic in the periodic table.
In the present study, we investigated the ability of GSH to promote the reduction of Sb(V) into Sb(III) in meglumine antimoniate, as well as the effects of pH and temperature on this transformation. The results obtained led us to propose a model for the mechanism of action of antimonials and the basis for their selective toxicity.
MATERIALS AND METHODS
Materials.
GSH (Sigma Chemical Co., St. Louis, Mo.) was used as supplied and stored at 4°C. N-methyl glucamine, bromopyrogallol red (BPR), and potassium antimony tartrate were obtained from Aldrich Chemical Co. (Milwaukee, Wis). Commercial preparations of meglumine antimoniate (Glucantime, also known as RP 2168) were obtained from Rhône-Poulenc SA (Paris, France) in powder form and from Rhodia Farma LTDA (São Paulo, Brazil) as a solution in ampoule. All other reagents were of at least reagent grade. Double-distilled, deionized water was used throughout the experiments.
Drug preparation.
Meglumine antimoniate was synthesized as previously described (4) from equimolar amounts of N-methyl glucamine and oxyhydrated pentavalent antimony. The resulting product contained approximately 30% antimony by weight, as determined by atomic absorption spectroscopy. This product was used throughout the experiments.
Study of reduction of Sb(V) in the presence of GSH.
To assess the effect of the GSH/Sb(V) ratio on the production of Sb(III), different tubes were prepared with aqueous solutions of meglumine antimoniate and GSH at different molar ratios. The Sb(V) concentration was kept at 10 mM, and the GSH concentration varied from 0 to 100 mM. The pH was adjusted to 3.5 when necessary. All solutions were deoxygenated by bubbling with argon, and the tubes were flushed with argon before being closed to protect GSH from air oxidation. Samples were kept at 25°C and analyzed for Sb(III) content 7 days later.
To assess the effect of the pH level on the production of Sb(III), different solutions containing meglumine antimoniate (Sb concentration, 10 mM) and 50 mM GSH were prepared, with the pH level varying from 2 to 8. The pH was adjusted by adding small aliquots of either potassium hydroxide- or hydrogen chloride-concentrated solutions. Samples were kept under an argon atmosphere at 25°C and analyzed for Sb(III) content after 24 h.
To determine the kinetics of antimony reduction in model conditions of physiological state, solutions containing meglumine antimoniate (antimony concentration, 2 mM) and 10 mM GSH were prepared in 0.15 M KCl at pH 5 or 7.2 and at 25 or 37°C. The amount of Sb(III) was determined after different times of incubation.
Determination of Sb(III).
The procedure used to determine Sb(III) was described in detail previously (18). It is based on the specific interaction of Sb(III) with the chromogen BPR. The absorbance of BPR at 560 nm decreases proportionally to the amount of Sb(III) in the analyte solution, as a consequence of the formation of the 1:1 BPR-Sb(III) complex. Briefly, 2.5 ml of analyte solution was prepared from 0.5 ml of 0.1 M phosphate, 0.05 ml of 5% (wt/vol) tartrate, 0.25 ml of 350 μM BPR solution in 1:1 (vol/vol) water-ethanol, and 1.7 ml of water. The pH was then adjusted to 6.8. The absorbance was registered at 560 nm before (A0) and after (Am) the addition of 5 to 25 μl of the sample to be analyzed, so as to obtain a final antimony concentration of 20 μM. For each experiment, a calibration curve was established, using potassium antimony tartrate as the source of Sb(III), by plotting the difference in absorbance (A0 − Am) as a function of Sb(III) concentration. We checked that neither Sb(V) nor GSH interfered with the colorimetric test. Moreover, we observed that the presence of GSH in the analyte solution did not interfere with the formation of the BPR-Sb(III) complex.
RESULTS
Reduction of Sb(V) into Sb(III) as a function of GSH/Sb(V) ratio.
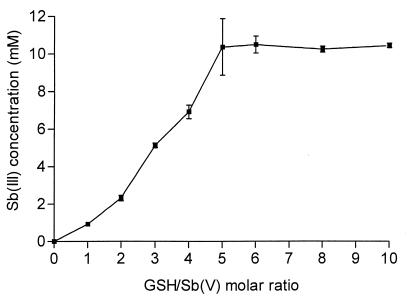
The fraction of Sb(III) present in freshly prepared meglumine antimoniate (4) as well as in commercial meglumine antimoniate was found to be less than 0.2% of the total antimony, indicating that antimony was initially in the pentavalent form. Meglumine antimoniate was incubated (Sb concentration, 10 mM) in the presence of GSH at a concentration varying from 10 mM to 100 mM at pH 3.5. After a week of incubation at 25°C in an argon atmosphere, the samples were analyzed for Sb(III) content. The results, displayed in Fig. 1, show that GSH induced the reduction of Sb(V) in a dose-dependent manner, indicating that an oxidation-reduction reaction between Sb(V) and GSH occurred. When GSH was absent from the incubation medium, the amount of antimony reduced in the same period of time was insignificant. It is noteworthy that, from a GSH/Sb ratio of 5:1, all antimony was encountered in the reduced form, indicating a stoichiometry of 5:1 between GSH and Sb(V) in the reaction. Cysteine, in the same experimental conditions, was also found to promote the reduction of Sb(V) into Sb(III) (data not shown); however, it was not possible to perform quantitative analysis due to the appearance of a precipitate in the incubation medium.
FIG. 1.
Level of Sb(III) produced in aqueous solutions of meglumine antimoniate and GSH, as a function of the GSH/Sb(V) molar ratio. The antimony concentration was maintained at 10 mM. Solutions were kept at 25°C for 7 days. The results are expressed as means ± standard deviations (error bars) (n = 3).
Reduction of Sb(V) into Sb(III) as a function of pH.
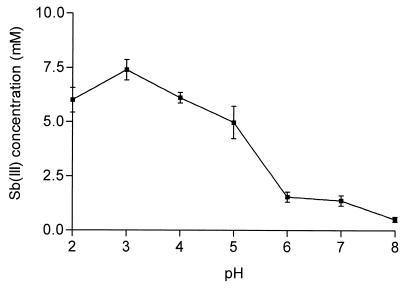
Meglumine antimoniate was incubated (Sb concentration, 10 mM) with 50 mM GSH for 24 h at 25°C in an argon atmosphere, at a pH varying from 2 to 8. The results displayed in Fig. 2 indicate that the level of Sb(III) produced in these conditions decreased when the pH increased from 3 to 8. The reaction between Sb(V) and GSH at pH 7 was found to procced more than five times as slowly as that at pH 3.
FIG. 2.
Effect of pH on the concentration of Sb(III) produced in an aqueous solution of meglumine antimoniate and GSH. Solutions were kept at 25°C for 24 h. Initial Sb(V) concentration, 10 mM; initial GSH concentration, 50 mM. The results are expressed as means ± standard deviations (error bars) (n = 3).
Kinetics of antimony reduction in model conditions of physiological state.
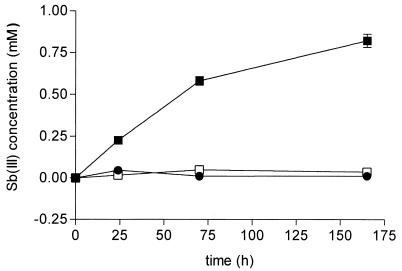
To get insight into the physiopharmacological significance of this reaction, we performed evaluations using the different conditions of pH and temperature to which Sb(V) is expected to be exposed while migrating to its site of action. Hence, pH 7.2 would mimic the pH of external medium and that of cytosol, whereas pH 5 would mimic the pH of the phagolysosome. Moreover, a GSH concentration of 10 mM was chosen, so as to be close to the average cellular concentration, and the Sb(V) concentration was kept at a lower value (2 mM). Finally, the reaction was performed in a solution of 0.15 M KCl in an atmosphere of either argon or air. Figure 3 displays the kinetics of reduction of Sb(V) in an argon atmosphere under different conditions of temperature and pH. The results clearly established that the reaction occurred at pH 5 (and 37°C); however, no significant amount of Sb(III) was detected at pH 7.2. Temperature was also found to have a strong influence on the rate of reduction. About 30% of the Sb(V) was converted to Sb(III) at 37°C following a 3-day incubation at pH 5, but less than 4% was converted to Sb(III) when the reaction was performed at 25°C. Moreover, no significant increase of the amount of Sb(III) was observed between day 3 and day 7 of incubation at 25°C. The same behavior was observed when samples were kept under an atmosphere of air instead of argon, and the results did not differ significantly (data not shown).
FIG. 3.
Kinetics of production of Sb(III) in aqueous solutions of meglumine antimoniate and GSH in different conditions of pH and temperature, as follows: pH 7.2 at 37°C (●), pH 5 at 37°C (■), and pH 5 at 25°C (□). Initial Sb(V) concentration, 2 mM; initial GSH concentration, 10 mM. Solutions were maintained in an argon atmosphere. The results are expressed as means ± standard deviations (error bars) (n = 3).
DISCUSSION
Our data clearly demonstrate that GSH promotes the reduction of Sb(V) to Sb(III) and that the oxidation-reduction reaction is greatly favored at an acidic pH level and an elevated temperature. It is noteworthy that the stoichiometry of 1:5 could be determined in the reaction between Sb(V) and GSH. A similar reaction was previously reported in the case of arsenate [As(V)] and GSH (20), and the stoichiometry of 1:5 was attributed to the formation of As(GS)3 complex between As(III) and GSH. By analogy, and considering that Sb and As have very similar chemical properties, the formation of an Sb(GS)3 complex can also be proposed. This interpretation is also supported by a recent study that established the formation of Sb(GS)3 from Sb(III) and GSH (23). Thus, in order to reduce one equivalent of Sb(V), five equivalents of GSH are needed, including three equivalents that would form the Sb(GS)3 complex.
Such a conversion was previously suggested by Dey et al. (5), following the observation that sodium stibogluconate became an effective inhibitor of As(GS)3 transport after an overnight incubation with GSH. Our findings, however, constitute the first direct evidence for the occurrence of this oxidation-reduction reaction.
It can be assumed that (i) the antimony complex must first dissociate to allow for Sb(V) reduction, (ii) the main soluble forms of Sb(V) and Sb(III) in the pH range of 2 to 8 are SbO3− and HSbO2, respectively (16), and (iii) the half-reaction for antimony reduction occurs as follows (see reference 16):
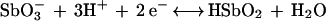 |
1 |
Based on these assumptions, the following oxidation-reduction reaction can be proposed:
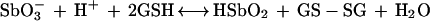 |
2 |
where GS-SG is the oxidized form of glutathione. According to Pitman et al. (16), the redox potential for half-reaction 1 at 25°C can be expressed by the equation EHSbO2 = 0.678 − 0.0886(pH) + 0.0295{log ([SbO3]/[HSbO2])} (here, as below, pH serves as a variable representing an unspecified pH level). On the other hand, the redox potential for the glutathione reduction half-reaction at 25°C can be expressed by the equation E°GSH = E°GSH − 0.059(pH) + 0.0295{log [(concentration of GS-SG)/(concentration of GSH)2]}. Since the apparent standard redox potential for the glutathione reduction half-reaction at pH 7 and 25°C (E°′GSH) is −0.240 V (2), one can deduce the value of the standard redox potential as follows: E°GSH = 0.173 V. Taking this data into consideration, the apparent standard redox potential for reaction 2 can be expressed as a function of the pH at 25°C by the following equation:
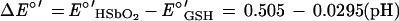 |
According to this equation, in the range of pH levels studied, the reaction equilibrium is expected to be almost completely displaced towards the formation of Sb(III). Therefore, the strong dependence of Sb(III) production on the pH level cannot be explained on the basis of thermodynamics, but rather on the basis of kinetic and/or mechanistic considerations. The formation of the Sb(GS)3 complex may be described by the following reaction:
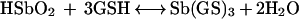 |
3 |
Therefore, from reactions 2 and 3, the following general reaction can be proposed:
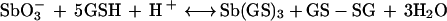 |
According to our results, the conversion of Sb(V) to Sb(III) should not occur in the host cell cytosol due to its neutral pH, even if this compartment contains a high concentration of GSH. On the other hand, antimony reduction may occur in the macrophage acidic organelles, such as lysosomes and endosomes, and also in the parasitophorous vacuole, whose pH lies between 4.7 and 5.2, in which Leishmania resides (1). However, GSH, or another related thiol compound, should be present at a high concentration in these organelles so that the reaction can occur. This seems to be the case because high concentrations of reduced thiol were found to be required for the reduction of protein disulfide bonds in the course of antigen processing (10). However, it is not clear which thiol compound (cysteine, cysteinyl-glycine, or GSH) is the most abundant in these organelles (8, 11).
On the basis of our data, the following model can be proposed for the mechanism of action of antimonials. As a first step, Sb(V) may reach the parasitophorous vacuole, either by drug diffusion across the plasma and lysosome membranes of macrophage or via the endocytic pathway, assuming, for instance, the transfer of Sb(V) from its original ligand to some carbohydrates of the host cell surface. As a second step, Sb(V) would be reduced into Sb(III) in the presence of thiols coming from the host cell or even from the parasite. As a third step, Sb(III) would accumulate inside the phagolysosome, penetrate inside the parasite, and interact with key leishmanial sulfhydryl groups, resulting in parasite death. Given that after a short exposure to antimonial drugs macrophages were found to retain antimony for several days (19), one can expect that Sb(V) reduction at pH 5 and 37°C is fast enough to generate a sufficient amount of Sb(III) to kill the parasite. It is noteworthy that, according to this model, host cells would be relatively protected from the toxic effects of Sb(III), especially if Sb(V) enters the host cells by phagocytosis, since its intracellular location would be restricted mainly to the lysosome-endosome compartments.
As another implication, our data may explain why promastigotes, contrary to intracellular amastigotes, are insensitive to Sb(V). Indeed, the conditions of pH (neutral) and temperature (25°C) usually employed to assess the activity of antileishmanial agents against promastigotes may not favor the reduction of Sb(V) into Sb(III).
On the other hand, our model for the mechanism of action of antimonials seems contradictory to the recent conclusion of Ephros et al. (6) that Sb(V) would enter the parasite cells and subsequently exert its antileishmanial effect. This conclusion was based on the observation that axenic amastigotes, derived from promastigotes differentiated at an acidic pH level (pH 5.5) and elevated temperature (37°C), were highly sensitive to Sb(V). In order to rule out the possibility that Sb(V) was reduced to Sb(III) by the growth medium, the authors showed that neither acidic pH nor elevated temperature alone resulted in increased toxicity of Sb(V) to promastigotes. However, it was not possible to assess the effects of the combination of the acidic pH and the elevated temperature. The lack of significant reduction of Sb(V) either at pH 5 (and 25°C) or at 37°C (and pH 7.2) reported in our study is in good agreement with their report. However, we did observe a significant level of Sb(V) reduction when incubating meglumine antimoniate and GSH at pH 5 and 37°C, which are precisely the conditions used to promote the transformation of promastigotes to axenic amastigotes. Therefore, the conversion of Sb(V) into Sb(III) in the conditions used by these investigators cannot be ruled out. The reduced thiol required for the reduction of Sb(V) into Sb(III) may have come from the growth medium used in this assay or may have been produced and excreted by the parasites in the course of their differentiation. Nevertheless, alternative models, such as (i) the catalysis of the reaction by a reductase such as that recently identified for Saccharomyces cerevisiae, which promoted the reduction of arsenate to arsenite (14); (ii) the reduction of Sb(V) by trypanothione in the parasite cytosol (7); and (iii) the reduction of Sb(V) within acidic compartments of the parasite (17), cannot be excluded.
In conclusion, we demonstrated that GSH promotes the reduction of Sb(V) to Sb(III) and characterized the oxidation-reduction reaction. We observed that this reaction is much faster at an acidic pH level than at a neutral pH level, suggesting that GSH and/or related thiol compounds are involved in the reduction of Sb(V) in vivo in the phagolysosome of macrophages.
ACKNOWLEDGMENTS
This work was supported by grants from CNPq (521010/97-7), FAPEMIG (CBS2418/96), and PRONEX (Brazil).
We thank Jean Michel Pernaut for his helpful suggestions during the preparation of the manuscript.
REFERENCES
- 1.Alexander J, Russel D G. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 2.Aslung F, Berndt K D, Holmgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem. 1997;272:30780–30786. doi: 10.1074/jbc.272.49.30780. [DOI] [PubMed] [Google Scholar]
- 3.Burguera J L, Burguera M, Petit de Pena Y, Lugo A, Anez N. Selective determination of antimony(III) and antimony(V) in serum and urine and of total antimony in skin biopsies of patients with cutaneous leishmaniasis treated with meglumine antimoniate. Trace Elem Med. 1993;10:66–70. [Google Scholar]
- 4.Demicheli C, de Figueiredo T L, Carvalho S, Sinesterra R D, Lopes J C, Frézard F. Physico-chemical characterization of meglumine antimoniate. Biometals. 1999;12:63–66. doi: 10.1023/a:1009200330741. [DOI] [PubMed] [Google Scholar]
- 5.Dey S, Ouellette M, Lightbody J, Papadopoulou B, Rosen B P. An ATP-dependent As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc Natl Acad Sci USA. 1996;93:2192–2197. doi: 10.1073/pnas.93.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ephros M, Binun A, Shaked P, Waldman E, Zilberstein D. Stage-specific activity of pentavalent antimony against Leishmania donovani axenic amastigotes. Antimicrob Agents Chemother. 1999;43:278–282. doi: 10.1128/aac.43.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fairlamb A H, Cerami A. Metabolism and functions of trypanothione in kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 8.Gainey D, Short S, McCoy K L. Intracellular location of cysteine transport activity correlates with productive processing of antigen disulfide. J Cell Physiol. 1996;168:248–254. doi: 10.1002/(SICI)1097-4652(199608)168:2<248::AID-JCP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin L C, Page J E. A study of the excretion of organic antimonials using a polarographic procedure. Biochem J. 1943;22:236–240. doi: 10.1042/bj0370198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd J B. Disulphide reduction in lysosomes. Biochem J. 1986;237:271–272. doi: 10.1042/bj2370271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mego J L. Stimulation of intralysosomal proteolysis by cysteinyl-glycine, a product of the action of gamma-glutamyl transpeptidase on glutathione. Biochim Biophys Acta. 1985;841:139–144. doi: 10.1016/0304-4165(85)90014-5. [DOI] [PubMed] [Google Scholar]
- 12.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 13.Mottram J C, Coombs G H. Leishmania mexicana: enzyme activities of amastigotes and promastigotes and their inhibition by antimonials and arsenicals. Exp Parasitol. 1985;59:151–160. doi: 10.1016/0014-4894(85)90067-0. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay R, Rosen B P. Saccharomyces cerevisiae ACR2 gene encodes an arsenate reductase. FEMS Microbiol Lett. 1998;168:127–136. doi: 10.1111/j.1574-6968.1998.tb13265.x. [DOI] [PubMed] [Google Scholar]
- 15.Petit de Pena Y, Gallignani M, Burguera M, Burguera J L, Anez N, Lugo Y. Selective determination of antimony(III) and antimony(V) in blood serum and urine by hydride generation and atomic absorption spectrometry. J Braz Chem Soc. 1990;1:72–75. [Google Scholar]
- 16.Pitman A L, Pourbaix M, de Zoubov N. Potential-pH diagram of the antimony-water system. Its applications to properties of the metal, its compounds, its corrosion, and antimony electrodes. J Electrochem Soc. 1957;104:594–600. [Google Scholar]
- 17.Rabinovitch M. Leishmanicidal activity of amino acid and peptide esters. Parasitol Today. 1989;5:299–301. doi: 10.1016/0169-4758(89)90024-0. [DOI] [PubMed] [Google Scholar]
- 18.Rath S, Jardim W F, Dórea J G. A simple spectrophotometric procedure for the determination of antimony(III) and (V) in antileishmanial drugs. Fresenius' J Anal Chem. 1997;358:548–550. [Google Scholar]
- 19.Roberts W L, Berman J D, Rainey P M. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob Agents Chemother. 1995;39:1234–1239. doi: 10.1128/aac.39.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott N, Hatlelid K M, MacKenzie N E, Carter D E. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- 21.Sereno D, Lemesre J L. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother. 1997;41:972–976. doi: 10.1128/aac.41.5.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sereno D, Cavaleyra M, Zemzoumi K, Maquaire S, Ouaissi A, Lemesre J L. Axenically grown amastigotes of Leishmania infantum used as an in vitro model to investigate the pentavalent antimony mode of action. Antimicrob Agents Chemother. 1998;42:3097–3102. doi: 10.1128/aac.42.12.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Yan S C, Cheng W S. Interaction of antimony tartrate with the tripeptide glutathione. Eur J Biochem. 2000;267:5450–5457. doi: 10.1046/j.1432-1327.2000.01605.x. [DOI] [PubMed] [Google Scholar]