Abstract
Purpose
Treatment of severe asthma has made great strides thanks to rapid progress in understanding immune response and inflammatory pathways. This led to the advent of the first biologic for severe allergic asthma (SAA), omalizumab. Although the long-term efficacy and safety of omalizumab has been confirmed, increasingly longer follow-up data can further reinforce this evidence and potentially provide new ones, for example on any loss of efficacy or the appearance of unexpected side effects. This study reports omalizumab treatment-related outcomes after 16 years of follow-up.
Patients and Methods
In this real-life retrospective study, an extension of a previous 9-year follow-up study on patients initially recruited in a clinical trial, we enrolled 8 adult patients with SAA followed-up from November 2005 to December 2021. Study subjects were selected based on omalizumab eligibility criteria.
Results
Exacerbation rate significantly decreased from 3.6 ± 2.1 events in year before index date to 0.1 ± 0.4 after 32 weeks of treatment (p < 0.0001). Mean annual number of mild-to-moderate exacerbations at 16 years was 0.88 compared with 1.8 in the year before the index date and 1.1 at 32 weeks. No hospitalizations were documented during the 16-year follow-up compared to 0.3 hospitalizations/patient in the year before the index date. Respiratory function also progressively and significantly improved. Regarding patient-reported outcomes (PROs), The AQLQ and ACT significantly improved from baseline throughout the follow-up, particularly up to 9 years of follow-up. During the study, an overall reduction in doses of asthma medications was observed, with a significant OCS-sparing effect.
Conclusion
Our study, the longest clinical follow-up on patients treated with anti-IgE, confirms and amplifies the results of the studies carried out so far, as they are maintained over a very long interval of time without drops in efficacy without any type of side effect.
Keywords: severe allergic asthma, biologics, omalizumab, exacerbations, side effects, oral corticosteroids
Introduction
Treatment of severe asthma has made great strides thanks to rapid progress in understanding the complex interactions between genetic and environmental factors, immunological pathways, and different phenotypes and endotypes. More than 15 years ago, this led to the development of omalizumab, the first biological treatment for severe asthma.1 Since then, a lot of information has emerged both on the complex mechanisms of action of this drug and on its efficacy and safety. Omalizumab is a humanized anti-IgE monoclonal antibody (mAb) approved by the European Union and the United States for the treatment of allergic asthma. This agent prevents the binding of free IgE to the high-affinity receptor (FcƐRI) present on mast cells and basophils, thus inhibiting the release of proinflammatory mediators.2,3 Some authors have shown that omalizumab downregulates the expression of FcƐRI on the cell membrane, further reducing inflammation.3 An important study showed that omalizumab can reduce exacerbations during peak viral seasons, thanks to the inhibition of IgE cross-linking and the consequent restoration of interferon (IFN)-α production toward rhinoviruses.4 A large amount of data has confirmed the efficacy of this mAb in the treatment of allergic asthma, both in randomized controlled trials (RCTs) and real-life studies (REFS). A Cochrane review evaluated 25 RCTs, showing a 25% reduction in exacerbations compared to placebo, reduced hospitalizations, and reduced dose of inhaled corticosteroids (ICS).5 Even real-world studies have shown a reduction in exacerbations, hospitalizations with omalizumab and oral corticosteroids (OCS)-sparing effect even in very long follow-ups (up to about 11 years), sometimes even more than RCTs.6–10 In addition to efficacy, an excellent safety profile has also been confirmed over time, even in fragile patients and under particular conditions, so much so that omalizumab can even be administered to pregnant women.11,12
Our previous disease-related outcomes study of 8 patients with severe persistent allergic asthma (SAA) treated with omalizumab for a total of 9 years had shown a significant improvement in quality of life (QoL), respiratory function and almost complete zeroing of exacerbations and of admissions without safety concerns.7
Although the long-term efficacy and safety of omalizumab have been confirmed, increasingly longer follow-up data can further reinforce this evidence and potentially provide new ones, for example, on any loss of efficacy or the appearance of unexpected side effects. The longer period of follow-up published so far refers to 12 years. The aim of the present study was to evaluate the persistence of the efficacy and safety of omalizumab after 16 years of treatment in a real-world setting.
Materials and Methods
Ethical Approval
This study was approved by the Local Ethics Committee (Vast Emilia Nord Area Ethics Committee - AUSL IRCCS, Reggio Emilia), protocol number 2011/0006211/03-01-2011.
This study was conducted in accordance with the Good Clinical Practice (ICH Harmonized Tripartite Guidelines for Good Clinical Practice 1996; Directive 91/507. CEE, the rules governing medical products produced in the European Community) and in full compliance with Italian national laws (Legislative Decree No. 211 of June 24, 2003; Legislative Decree No. 200 of November 6, 2007; Ministerial Decree of December 21, 2007).
Study Population and Design
In this real-life retrospective study, data of 8 adult patients with SAA according to the ERS/ATS guidelines and GINA13,14 were analyzed (Table 1).
Table 1.
Demographics
| No. of patients | 8 |
| Age at baseline, mean ± SD | 42.6 ± 9.1 |
| Males, n (%) | 5 (62.5) |
| Former smokers, n (%) | 1 (12.5) |
| Comorbidities, n (%) | |
| Atopy | 8 (100) |
| Multi-sensitivity | 3 (37) |
| Rhinitis | 5 (62) |
| Nasal polyposis | 4 (50) |
| Dermatitis | 3 (37) |
Abbreviation: SD, standard deviation.
The present study represents an extension of a previous 9-year study,7 and reports omalizumab treatment-related outcomes on 8 patients after 16 years of follow-up in a real-world setting. This study was an extension of a first 4-year follow-up study.15 Initially, 11 patients were enrolled by our Center in a randomized, open label, parallel-group, international, multicenter study evaluating persistency of response to omalizumab during 32 weeks treatment given as add on to optimized asthma therapy in adult and adolescent patients with severe persistent allergic asthma, who remain inadequately controlled despite GINA (2004) step 4 therapy (November 2005/September 2008) [ClinicalTrials.gov Identifier: NCT00264849]. Of these 11 patients, 8 continued the follow-up up to 16 years and 3 discontinued for various reasons (2 transferred to another city and 1 due to ineffectiveness) after 4 years.
The patients were followed up on at the Pulmonology Unit of AUSL-IRCSS, Reggio Emilia, from November 2005 to December 2021. All the patients signed informed consent forms to participate in this study. The patients received omalizumab treatment according to the Italian Medicines Agency’s (AIFA) indications: age ≥ 6 and ≤ 75 years; total blood IgE levels 76 ≤ IgE ≤ 1500 IU/mL; ≥ 1 exacerbations of asthma that required systemic corticosteroids (SCS), emergency department (ED) visits, or hospital admissions in the previous 12 months with respect to index date; positive skin prick test or specific serum IgE for at least one perennial allergen; and forced expiratory volume (FEV1) < 80% pre-bronchodilator (pre-BD).
The primary outcome was the persistence of response to omalizumab treatment during 16 years of follow-up, and the secondary outcome was safety and tolerability throughout the follow-up period.
Efficacy Evaluation
To assess the persistence of response to treatments, the following clinical outcomes were evaluated at 32 weeks, 4 years, 9 years, and 16 years after initiation of omalizumab treatment (index date): exacerbation rate, level of asthma control, and asthma medications.
Severe exacerbations were defined as those requiring SCS, hospitalization, or ED visits. Mild and moderate exacerbations were classified as those requiring home or primary care. Lung function was assessed by measuring FEV1 and FEV1/forced vital capacity (FVC) ratio.
Patient-reported outcomes (PROs) were also included in the outcomes. In particular, asthma control was defined using the asthma control test (ACT). It is a self-administered tool for the purpose of identifying patients with poor control, consisting of 5 items (symptoms, daily activities, use of rescue medications, overall self-assessment of asthma impact). Scores can range from 5 (poor asthma control) to 25 (well-controlled asthma). A score of > 19 indicates optimal asthma control.16 QoL assessment was performed using the Juniper Asthma-Related QoL Questionnaire (AQLQ).17
The dosage variations of asthma medications (inhaled corticosteroids/long-acting β-adrenergic receptor agonists (ICS/LABA), leukotriene receptor antagonists (LTRA), long-acting muscarinic antagonists (LAMA), theophylline, and SCS were also assessed.
Any local or systemic omalizumab treatment-related adverse events were monitored throughout the study.
Statistical Analysis
Descriptive statistics were performed in order to show our data. The continuous variables were reported as mean ± standard deviation (SD), while categorical variables were reported as absolute values and percentages. In order to compare two groups of continuous variables, Welch’s t-tests, or Mann–Whitney tests were used where appropriate; for three or more groups of variables, the Brown–Forsythe test, Welch’s ANOVA, or the Kruskal–Wallis test were applied where appropriate. For categorical variables, the chi-square test and Fisher’s exact test were used. All statistical analyses were conducted using the software GraphPad Prism version 8 for Mac (GraphPad Software, San Diego, California, USA, www.graphpad.com).
Results
Exacerbations
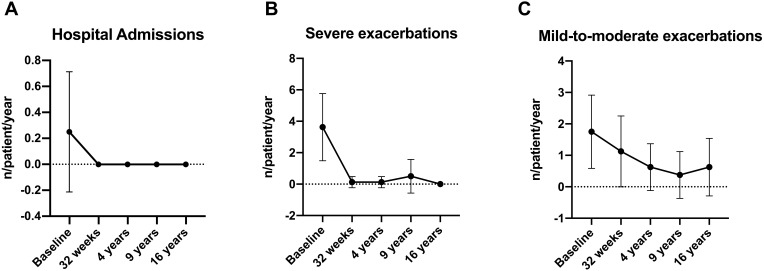
The exacerbation rate significantly decreased from 3.6 ± 2.1 events in year before index date to 0.1 ± 0.4 after 32 weeks of treatment (p < 0.0001). The very low exacerbation rate remained stable throughout the 16-year follow-up. The mean annual number of mild-to-moderate exacerbations at 16 years was 0.88 compared with 1.8 in the year before the index date and 1.1 at 32 weeks. No hospitalizations were documented during the 16-year follow-up compared to 0.3 hospitalizations/patient in the year before the index date (Table 2 and Figure 1).
Table 2.
Clinical, Functional and Laboratory Indexes Variations
| Baseline | 32 Weeks | 4 Years | 9 Years | 16 Years | p value | |
|---|---|---|---|---|---|---|
| ACT, mean ± SD | 12.6 ± 2.2 | 15.4 ± 3.1 | 17.5 ± 5 | 21.4 ± 3.1 | 21.5 ± 2.6 | 0.0002 |
| AQLQ, mean ± SD | 2.9 ± 0.5 | 4.2 ± 0.5 | 5.1 ± 0.9 | 5.8 ± 0.8 | 5.9 ± 0.6 | <0.0001 |
| Hospital admissions (n/patient/year), mean ± SD | 0.3 ± 0.5 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.08 |
| Severe exacerbations (n/patient/year), mean ± SD | 3.6 ± 2.1 | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.5 ± 1.1 | 0 ± 0 | <0.0001 |
| Mild-to-moderate exacerbations (n/patient/year), mean ± SD | 1.8 ± 1.2 | 1.1 ± 1.1 | 0.6 ± 0.7 | 0.4 ± 0.7 | 0.4 ± 0.7 | 0.054 |
| FEV1/FVC (%), mean ± SD | 61.9 ± 10.4 | 67 ± 7.9 | 72.7 ± 11.1 | 70 ± 8.2 | 69.7 ± 7.2 | 0.24 |
| FEV1 (%), mean ± SD | 54.8 ± 13.5 | 72.8 ± 21.4 | 76.6 ± 13.3 | 83.6 ± 18.8 | 83.1 ± 14.9 | 0.02 |
| Total IgE (IU/mL), mean ± SD | 386.9 ± 196.5 | 285 ± 201.7 | 959.5 ± 1018 | 614.4 ± 448.5 | 641.3 ± 383.9 | 0.14 |
Abbreviations: ACT, asthma control test; SD, standard deviation; AQLQ, asthma quality of life questionnaire; FEV1, forced expiratory volume in 1 second; FEV1/FVC, forced expiratory volume in 1 second/forced vital capacity; IgE, immunoglobulin E.
Figure 1.
(A) hospital admission variation during omalizumab therapy. (B) severe exacerbations variation during omalizumab therapy. (C) mild-to-moderate exacerbations variation during omalizumab therapy.
Respiratory Function
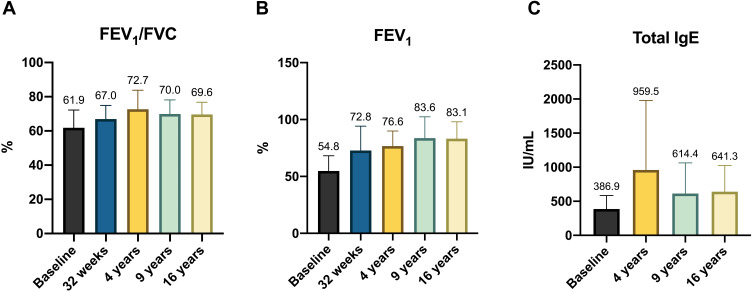
Respiratory function also progressively and significantly improved throughout the follow-up period. At the baseline, FEV1 was 54.8 ± 13.5%, while at 16 years, it was 83.1 ± 14.9% (Figure 2).
Figure 2.
(A) FEV1/FVC (%) variation during omalizumab therapy. (B FEV1 (%) variation during omalizumab therapy. (C) total IgE levels (IU/mL) variation during omalizumab therapy.
Abbreviation: FEV1, forced expiratory volume in 1 second; FEV1/FVC, forced expiratory volume in 1 second/forced vital capacity; IgE, immunoglobulin E.
Patients’ Reported Outcomes
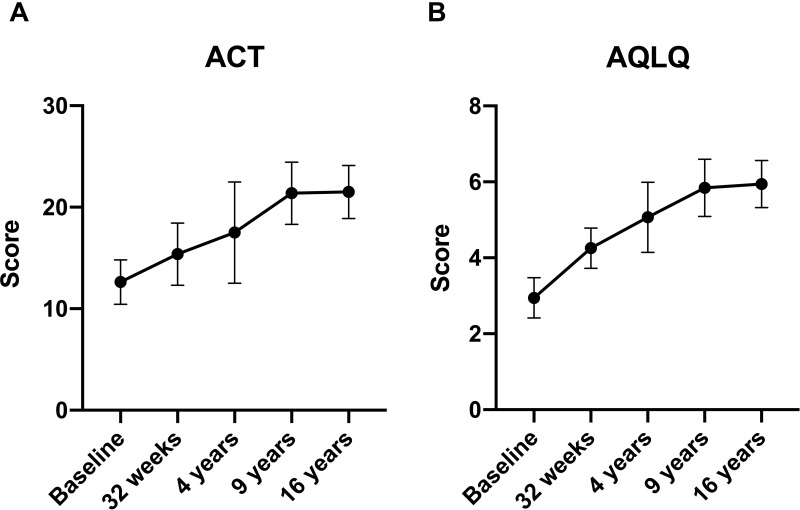
The AQLQ and ACT significantly improved from baseline throughout the follow-up, particularly up to 9 years of follow-up (Table 2 and Figure 3). The mean baseline AQLQ and ACT values were 2.9 ± 0.5 and 12.6 ± 2.2 and 5.9 ± 0.6 (p < 0.0001) and 21.5 ± 2.6 (P = 0.0002) at 16 years, respectively.
Figure 3.
(A) ACT variation during omalizumab therapy. (B) AQLQ variation during omalizumab therapy.
Abbreviation: ACT, asthma control test; AQLQ, asthma quality of life questionnaire.
Use of Asthma Medications
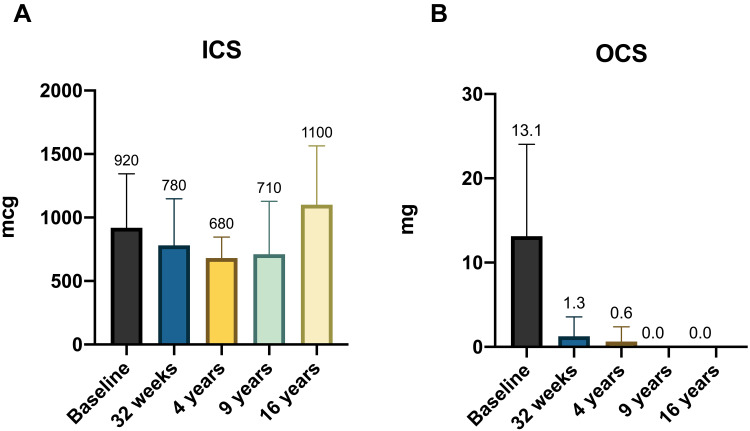
During the study, an overall reduction in the use and doses of asthma medications was observed (Tables 3 and 4). In particular, the chronic use of OCS was significantly reduced, and the use of OCS was discontinued in all the patients throughout the follow-up (Figure 4). The use of as-needed SABAs was also progressively reduced and then completely suspended starting from the 4th year of anti-IgE treatment. The mean ICS dose at the end of the study was slightly higher than the baseline, although not statistically significant (920 mcg/day at baseline and 1100 mcg/day after 16 years) (Figure 4).
Table 3.
Drugs Variation
| Medication | Baseline | 32 Weeks | 4 Years | 9 Years | 16 Years | p value |
|---|---|---|---|---|---|---|
| ICS | ||||||
| High dose | 8 | 2 | 2 | 1 | 4 | 0.031 |
| Medium dose | 0 | 6 | 5 | 2 | 4 | 0.018 |
| Low dose | 0 | 0 | 1 | 5 | 0 | 0.001 |
| OCS | 7 | 1 | 0 | 1 | 0 | <0.0001 |
| LABAs | 8 | 8 | 8 | 7 | 7 | 0.53 |
| LAMAs | 5 | 2 | 2 | 2 | 2 | 0.39 |
| LTRAs | 4 | 1 | 0 | 2 | 3 | 0.15 |
| Theophylline | 1 | 0 | 0 | 0 | 0 | 0.39 |
| SABAs | 8 | 7 | 3 | 2 | 2 | 0.002 |
Abbreviations: ICS, inhaled corticosteroids; OCS, oral corticosteroids; LABAs, long-acting beta-agonists; LAMAs, long-acting muscarinic antagonists; LTRAs, leukotriene receptor antagonists; SABAs, short-acting beta agonists.
Table 4.
Drugs Dosage Variation
| Medication | Baseline | 32 Weeks | 4 Years | 9 Years | 16 Years | p value |
|---|---|---|---|---|---|---|
| ICS (mcg/day), mean ± SD | 920 ± 425.5 | 780 ± 367.2 | 680 ± 175.6 | 710 ± 417.7 | 1100 ± 464 | 0.47 |
| OCS (mg/day), mean ± SD | 13.1 ± 10.9 | 1.3 ± 2.3 | 1.6 ± 1.8 | 0 ± 0 | 0 ± 0 | 0.003 |
| SABAs (mcg/day), mean ± SD | 137.5 ± 130.2 | 50 ± 75.6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.006 |
| LTRAs (mg/day), mean ± SD | 6.3 ± 7.4 | 2.5 ± 4.6 | 1.3 ± 3.5 | 3.1 ± 4.6 | 3.8 ± 5.2 | 0.52 |
Abbreviations: ICS, inhaled corticosteroids; SD, standard deviation; OCS, oral corticosteroids; SABAs, short-acting beta agonists; LTRAs, leukotriene receptor antagonists.
Figure 4.
(A) ICS equivalent dosage variation during omalizumab therapy. (B) OCS equivalent dosage during omalizumab therapy.
Abbreviation: ICS, inhaled corticosteroids; OCS, oral corticosteroids.
Blood IgE Levels
Regarding the total serum IgE level, the baseline value was 386.9 ± 196.5 IU/mL, with a growth trend up to control at 4 years (959.5 ± 1018) and a decrease at the end of the follow-up period (641.3 ± 383.9) (Table 2 and Figure 2).
Safety Data
Throughout the study period, no systemic or local adverse effects of omalizumab were detected and no issues related to immunological pathologies, infections, or neoplasms emerged.
Comorbidities
All patients were atopic and three of them (37%) were multi-sensibilized. Five patients (62%) were affected by rhinitis and four patients (50%) had nasal polyposis. Furthermore, three patients (37%) had also dermatitis (Table 1).
Discussion
In this study, we found that the efficacy of omalizumab treatments persists during a very long follow-up period (16 years), with an excellent safety profile. Indications for biological treatments frequently overlap. Understanding which is the best therapeutic option for the right patients is challenging in severe asthma. Virtually, all these new therapeutic options are for patients with a type 2 high (T2) endotype.18 T2 high phenotypes are classified into three main groups: early-onset allergic asthma, late-onset eosinophilic asthma, and aspirin-exacerbated respiratory disease (AERD).19 Frequently, patients may present with overlapping phenotypic characteristics that make them potentially eligible for different treatment options.20 As head-to-head comparisons between omalizumab, anti-IL5, and anti-IL4/IL13 are not available and do not yet have predictive biomarkers of response, the initial choice is often not easy. Therefore, the clinician should rely on the combined use of the patient’s clinical characteristics, comorbidities, and biomarkers. It may be simplistic to think that an outdated drug, such as omalizumab, may be less effective than newer and potentially more interesting agents. For these reasons, obtaining data that not only confirm the known efficacy of the anti-IgE mAb but also amplify its results over a very long period, confirming at the same time its great safety, is very important.
A recent meta-analysis based on 86 real-world evidence studies (RWE) confirmed that anti-IgE therapy enabled consistent improvements in the global evaluation of treatment effectiveness (GETE), respiratory function, PROs, reductions in asthma exacerbations, OCS dose, and health care resource utilization.21 A systematic review evaluated 24 RWE studies conducted in 32 countries on a total of 4117 patients followed for up to 4 years. The data highlighted the short- and long-term benefits of omalizumab in terms of asthma control, lung function, and reduced use of healthcare resources.22 Similar results emerged from eXpeRience, a 2-year international observational registry that assessed the efficacy, safety, and real-life use of omalizumab in 943 SAA patients.23 Patients enrolled in the studies examined did not highlight any particular safety issues, albeit with the limitation of not particularly long follow-up periods.
To try to overcome the limitations of shorter follow-up periods, a recent RWE study examined the response to anti-IgE treatment and its safety in a group of patients treated for about 11 years.6 Omalizumab produced significant but also lasting improvements in exacerbations, asthma control, and lung function, thereby demonstrating a significant OCS sparing effect and a reassuring safety profile. A recent RWE study examined the response to anti-IgE treatment and its safety in a group of patients treated for about 11 years. Omalizumab produced significant but also lasting improvements in exacerbations, asthma control, and lung function, also demonstrating a significant OCS sparing effect and a reassuring safety profile.
The results of a previous study conducted in our center with a follow-up of 9 years were comparable,7 so we decided to extend the observation even longer to answer still open questions on the response and safety to omalizumab over a much longer period. In the present study, omalizumab showed great efficacy in terms of almost complete elimination of severe, mild-to-moderate exacerbations and hospitalizations. The most important aspect is the stability of these results until the end of the follow-up period, without loss of efficacy, with complete control of asthma also confirmed by PROs, such as ACT and AQLQ scores. This highlights that the efficacy of omalizumab on asthma control in terms of QoL remains stable, even in the very long term, without the appearance of signs of deterioration. Respiratory function also progressively improved, and after reaching the plateau at the 9-year time point, it stabilized up to 16 years of treatment. The impact of omalizumab on all outcomes also had a positive impact on QoL, as confirmed by the improvement in the AQLQ score, which increased significantly over time and up to the end of the study. It is interesting to note that over time, the mean OCS dose decreased, resulting in complete weaning at 9 years, which was confirmed until the end of the follow-up. This further reinforces the already positive data on asthma control and PROs obtained in our small cohort of patients, and also supports the use of anti-IgE therapy to reduce the impact of OCS and improve the health status of patients with SAA.
The efficacy of omalizumab in terms of the OCS sparing effect has long been controversial, even if recent data confirm the efficacy of this biologic also for this outcome. Data on OCS use on very large series (about 3000 patients overall) evaluated in meta-analysis studies show how the probability of having to take OCS decreases over the course of treatment with omalizumab, with a reduction that stood at 52% after 2 years.24 A European multicenter study of 346 patients showed that following anti-IgE treatment > 16 weeks, 50.6% patients on OCS therapy at baseline reduced/stopped OCS. Among these, 20.5% of patients had been weaned from OCS and 30.1% reduced OCS dose.25 Several literature data and extensive retrospective analyses have demonstrated a significant dose–response relationship between continuous or intermittent long-term SCS use and the risk of developing comorbidities and complications related to long-term SCS treatment.26,27 These also lead to major increases in costs and burdens for health systems.28 This study demonstrates, together with the extensive clinical experience of omalizumab lasting over 15 years, that this agent can significantly improve asthma control and thus reduce the impact of OCS. Even in the context of the current landscape of T2 asthma treatments, it should still be considered the first choice for SAA patients.
In the context of globally positive results obtained in our study, the only countertrend is the slight increase in the ICS dosage compared to baseline and after a reduction in intermediate time points, in particular up to 9 years with an increase seen at 16 years. However, this result was not statistically significant, and could be explained by the weaning of the OCS obtained in parallel, which as a consequence required an adjustment (albeit minimal) of the average dose of the ICS without consequences on the study outcomes. Another possible explanation is linked to a greater adherence to inhalation therapy due to the regular follow-up of these patients. A loss of asthma control, however slight, does not seem conceivable given the clinical and functional parameters obtained.
The excellent anti-IgE results in the present study are confirmed by a composite score that includes PROs, exacerbations, hospitalizations, overall reduction in doses of asthma medications (particularly OCS), and lung function. This score gradually improved up to 9 years, stabilizing thereafter until the end of the study with no loss of asthma control over a really prolonged period of time.
In our patient group, no side effects occurred despite the very long treatment period. Omalizumab has a good safety profile, and the most common adverse reactions are local reactions at the drug injection site. More rarely, headache, asthenia, or nausea have been reported but with a similar pattern to placebo.29 Anaphylactoid events were also very sporadic, and the drug has been used for years in home administration settings with excellent results.30,31 In addition, the post-marketing observational study “Evaluating Clinical Effectiveness and Long-term Safety (EXCELS)” was excluded, after a careful analysis of data on 3342 patients treated with omalizumab, the possibility of an increased risk of acute cardiovascular and cerebrovascular events.32 Finally, anti-drug antibodies are not usually found in subjects treated with this agent.33 Our study confirms this excellent safety profile, with no evidence of adverse events even after 16 years of continuous treatment.
In view of the long follow-up period, biological changes associated with aging and environmental exposure factors such as allergens, air pollution and possible occupational exposures should also be considered. These factors could have affected some parameters, such as the respiratory function which decreased slightly after the first 4 years of treatment, even if not significantly (Table 2 and Figure 2). However, it is not easy to correlate these factors with the response to omalizumab over time.
This study has some limitations. The first is the small sample size, even if the very long duration of the follow-up justifies the sample size. Another limitation is the lack of a control group, as is often the case in retrospective studies. The absence of the control group makes it difficult to know precisely whether the magnitude of the observed improvements would hold up if it had been compared with a control group, but it is however evident that the comparison with the literature data makes the results obtained from the present study significant and generalizable in clinical practice. Finally, another limitation is that patients enrolled are all Caucasian and of Italian origin and are followed up on at a single center. The patients were initially selected based on the restrictive criteria in a clinical trial setting, including origin and ethnicity. These aspects represent biases to be taken into consideration, but the results obtained show a sustained clinical efficacy and safety profile of omalizumab in the long term. All these factors should be taken into consideration when selecting the appropriate biological therapy.
Conclusion
The discussion regarding the positioning of biologics for the treatment of severe asthma remains a hot topic, especially since there are no head-to-head comparison studies. Even in the case of a biologic such as omalizumab, whose development represented a major innovation in the treatment of SAA and which has been available for almost 20 years, the arrival of new competitors has made it less easy to identify the correct patient. This is also due to the fact that no RCTs have been carried out for many years now, which is why RWE data are of crucial importance to obtain confirmation of its safety and efficacy but also to consolidate its role in a much more complex current scenario than that existing at the time of its advent.
This study, despite its limitations, confirms and strengthens data relating to the sustained improvement in asthma control and the OCS-sparing effect, resulting in a reduction in the impact of OCS-related complications. These include infections, diabetes, osteoporosis, and psychiatric disorders, which they are more frequent in patients with long-term exposure to OCS and leading to increased use and costs of healthcare resources in patients with uncontrolled steroid-dependent/resistant asthma. Furthermore, in our small cohort omalizumab had a reassuring safety profile. The most interesting aspect of our study concerns the duration of the follow-up period, much longer than what has emerged so far in the literature. This aspect, together with what has been said previously, should be considered in the choice of a therapeutic option in the field not only of precision medicine but also of the safety of fragile and complex patients. Omalizumab therefore continues to play a key role in the current landscape of severe asthma treatments and represents a concrete and reliable weapon available to clinicians.
Funding Statement
There is no funding to report.
Abbreviations
mAb, monoclonal antibody; FceRI, high affinity receptor; IFN-α, interferon-α; RCTs, randomized controlled trials; OCS, oral corticosteroids; ICS, inhaled corticosteroids; SAA, severe allergic asthma; SCS, systemic corticosteroids; ED, emergency department; FEV1, forced expiratory volume; pre-BD, pre-bronchodilator; FVC, forced vital capacity; PROs, patient-reported outcomes; ACT, asthma control test; QoL, quality of life; AQLQ, asthma-related QoL questionnaire; ICS/LABA, inhaled corticosteroids/long-acting β-adrenergic receptor agonists; LTRA, leukotriene receptor antagonists; LAMA, muscarinic antagonists a long-acting; BDP, beclomethasone dipropionate; SABAs, short-acting beta-agonists; T2 high, type 2 high; RWE, real-world evidence studies, GETE, global evaluation of treatment effectiveness.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author.
Consent for Publication
Informed consent was obtained from all subjects involved in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article; they gave final approval of the version to be published, they agreed on the journal to which the article has been submitted and they agreed to be accountable for all aspects of the work.
Disclosure
Francesco Menzella has received research grants from AstraZeneca, Novartis, and Sanofi; lecture fees and advisory board fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis, and Sanofi. Marco Contoli reports grants and/or personal fees from GlaxoSmithKline, Chiesi, AstraZeneca, Alk Abello, and Boehringer Ingelheim, outside the submitted work. The other authors have no conflicts of interest to declare in this work.
References
- 1.First biologic for allergy-related asthma. FDA Consum. 2003: 5. [PubMed]
- 2.Manka LA, Wechsler ME. Selecting the right biologic for your patients with severe asthma. Ann Allergy Asthma Immunol. 2018;121(4):406–413. doi: 10.1016/j.anai.2018.07.033 [DOI] [PubMed] [Google Scholar]
- 3.McCracken JL, Tripple JW, Calhoun WJ. Biologic therapy in the management of asthma. Curr Opin Allergy Clin Immunol. 2016;16(4):375–382. doi: 10.1097/ACI.0000000000000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teach SJ, Gill MA, Togias A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015;136(6):1476–1485. doi: 10.1016/j.jaci.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559. doi: 10.1002/14651858.CD003559.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papaioannou AI, Mplizou M, Porpodis K, et al. Long-term efficacy and safety of omalizumab in patients with allergic asthma: a real-life study. Allergy Asthma Proc. 2021;42(3):235–242. doi: 10.2500/aap.2021.42.210014 [DOI] [PubMed] [Google Scholar]
- 7.Menzella F, Galeone C, Formisano D, et al. Real-life Efficacy of Omalizumab After 9 Years of Follow-up. Allergy Asthma Immunol Res. 2017;9(4):368–372. doi: 10.4168/aair.2017.9.4.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niven RM, Saralaya D, Chaudhuri R, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open. 2016;6(8):e011857. doi: 10.1136/bmjopen-2016-011857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casale TB, Luskin AT, Busse W, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract. 2019;7(1):156–164.e1. doi: 10.1016/j.jaip.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 10.Bhutani M, Yang WH, Hébert J, de Takacsy F, Stril JL. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX observational study. PLoS One. 2017;12(8):e0183869. doi: 10.1371/journal.pone.0183869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Ahmad M, Arifhodzic N, Nurkic J, et al. “Real-life” efficacy and safety aspects of 4-year omalizumab treatment for asthma. Med Princ Pract. 2018;27(3):260–266. doi: 10.1159/000487482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namazy J, Cabana MD, Scheuerle AE, et al. The Xolair pregnancy registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol. 2015;135(2):407–412. doi: 10.1016/j.jaci.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 13.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European respiratory society/American thoracic society guideline. Eur Respir J. 2020;55(1):1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Asthma. Available from: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf. Accessed January 29, 2022.
- 15.Menzella F, Facciolongo N, Piro R, et al. Clinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-up. Ther Adv Respir Dis. 2012;6(2):87–95. DOI: 10.1177/1753465811429478 [DOI] [PubMed] [Google Scholar]
- 16.Schuler M, Faller H, Wittmann M, Schultz K. Asthma control test and asthma control questionnaire: factorial validity, reliability and correspondence in assessing status and change in asthma control. J Asthma. 2016;53(4):438–445. doi: 10.3109/02770903.2015.1101134 [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, Guyatt GH, Ferrie PJ, Griffith LE. Measuring quality of life in asthma. Am Rev Respir Dis. 1993;147(4):832–838. doi: 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- 18.Bakakos A, Loukides S, Usmani OS, Bakakos P. Biologics in severe asthma: the overlap endotype - opportunities and challenges. Expert Opin Biol Ther. 2020;20(12):1427–1434. doi: 10.1080/14712598.2020.1809651 [DOI] [PubMed] [Google Scholar]
- 19.Kuruvilla ME, Lee FE, Lee GB, Phenotypes UA. Endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi: 10.1007/s12016-018-8712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albers FC, Müllerová H, Gunsoy NB, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. doi: 10.1080/02770903.2017.1322611 [DOI] [PubMed] [Google Scholar]
- 21.Bousquet J, Humbert M, Gibson PG, et al. Real-World Effectiveness of omalizumab in severe allergic asthma: a meta-analysis of observational studies. J Allergy Clin Immunol Pract. 2021;9(7):2702–2714. doi: 10.1016/j.jaip.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 22.Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71(5):593–610. doi: 10.1111/all.12815 [DOI] [PubMed] [Google Scholar]
- 23.Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107(8):1141–1151. doi: 10.1016/j.rmed.2013.04.017 [DOI] [PubMed] [Google Scholar]
- 24.Faulkner KM, MacDonald K, Abraham I, Alhossan A, Lee CS. ‘Real-world’ effectiveness of omalizumab in adults with severe allergic asthma: a meta-analysis. Expert Rev Clin Immunol. 2021;17(1):73–83. doi: 10.1080/1744666X.2020.1856658 [DOI] [PubMed] [Google Scholar]
- 25.Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010;104(9):1381–1385. doi: 10.1016/j.rmed.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 26.Dalal AA, Duh MS, Gozalo L, et al. Dose-Response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22(7):833–847. doi: 10.18553/jmcp.2016.22.7.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–1495. doi: 10.1016/j.jaci.2015.07.046 [DOI] [PubMed] [Google Scholar]
- 28.Menzella F, Ghidoni G, Fontana M, et al. The role of systemic corticosteroids in severe asthma and new evidence in their management and tapering. Expert Rev Clin Immunol. 2021;17(12):1283–1299. doi: 10.1080/1744666X.2021.20041 [DOI] [PubMed] [Google Scholar]
- 29.Holgate ST, Djukanović R, Casale T, Anti-immunoglobulin BJ. E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35(4):408–416. doi: 10.1111/j.1365-2222.2005.02191.x [DOI] [PubMed] [Google Scholar]
- 30.Harrison RG, MacRae M, Karsh J, Santucci S, Yang WH. Anaphylaxis and serum sickness in patients receiving omalizumab: reviewing the data in light of clinical experience. Ann Allergy Asthma Immunol. 2015;115(1):77‐78. doi: 10.1016/j.anai.2015.04.014 [DOI] [PubMed] [Google Scholar]
- 31.Kim HL, Leigh R, Omalizumab: BA. Practical considerations regarding the risk of anaphylaxis. Allergy Asthma Clin Immunol. 2010;6(1):32. doi: 10.1186/1710-1492-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iribarren C, Rahmaoui A, Long AA, et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: results from EXCELS, a prospective cohort study in moderate to severe asthma. J Allergy Clin Immunol. 2017;139(5):1489–1495.e5. doi: 10.1016/j.jaci.2016.07.038 [DOI] [PubMed] [Google Scholar]
- 33.Pelaia C, Calabrese C, Terracciano R, de Blasio F, Vatrella A, Pelaia G. Omalizumab, the first available antibody for biological treatment of severe asthma: more than a decade of real-life effectiveness. Ther Adv Respir Dis. 2018;12:1753466618810192. doi: 10.1177/1753466618810192 [DOI] [PMC free article] [PubMed] [Google Scholar]