Abstract
Purpose of Review
Telehealth and telerehabilitation in spinal cord injury (teleSCI) is a growing field that can improve access to care and improve health outcomes in the spinal cord injury population. This review provides an overview of the recent literature on the topic of teleSCI and provides insights on current evidence, future directions, and considerations when using teleSCI for clinical care.
Recent Findings
TeleSCI is used most often for preventive health; management of chronic pain, anxiety, and depression; and rehabilitation-related interventions. As video telehealth becomes mainstream, growth in wearable monitors, bio and neurofeedback mechanisms, and app-based care is expected.
Summary
TeleSCI is growing in prevalence, demonstrates positive impact on health outcomes, and requires ongoing study to identify, refine, and implement best practices.
Keywords: Telehealth, Telerehabilitation, Telemedicine, Spinal cord injury, TeleSCI
Introduction
Living with a spinal cord injury (SCI) is challenging and complex, requiring significant support and effort from the healthcare community to ensure people living with disability from SCI achieve positive health and wellness outcomes. Individuals with SCI are two to five times more likely to die a premature death and have poorer overall health than their nonimpaired counterparts [1–3]. Common causes of premature mortality in SCI include respiratory disease (e.g., pneumonia and flu), cardiovascular diseases, and neurological disorders [4]. Additionally, many secondary complications of SCI, such as functional decline, urinary tract infections, and pressure injuries, significantly decrease satisfaction with life and overall health [1, 5]. Individuals with SCI also have poorer mental health outcomes. For example, a diagnosis of SCI is associated with higher rates of mental illness, with one in five suffering from depression, significantly higher rates of anxiety, posttraumatic stress disorder, and death by suicide [6, 7]. People living with SCI face unique challenges regarding functional independence, access to care, and complex health issues, often requiring comprehensive specialty care [8, 9]; as a result, telehealth provides a unique opportunity to augment established SCI care.
The term telehealth has been used broadly to encompass many remote clinical modalities such as telephone, web-based care and communication (e.g., email or text), and video-telehealth, with which clinical support is rendered using communication modes other than traditional in-person encounters [10–12]. Further refining that concept, telerehabilitation denotes telehealth used in rehabilitation contexts in a myriad of ways. For example, newer trends in rehabilitation have incorporated the use of web-based apps, wearables, virtual reality, and robotics to support ongoing recovery and rehabilitation, which may blur the lines of traditional telehealth conceptualizations [13–15]. However, provided that these alternate modalities allow healthcare teams to monitor patient progress and tailor programming to patient needs, the core concept remains applicable. In the context of SCI, the term teleSCI has been coined by the International Spinal Cord Society to refer to telehealth modalities used to provide healthcare to the SCI community [16]. Throughout this article, we refer to telehealth and telerehabilitation in the context of SCI as teleSCI.
Reasons to Use TeleSCI
Effectiveness and satisfaction data demonstrate that teleSCI benefits individuals with SCI and that patients generally view it favorably. In a recent review on effectiveness of teleSCI, nearly 80% of studies demonstrated significant improvements in the primary outcome of interest (e.g., mortality, mood disorders, functional improvements, self-management, and quality of life) [17]; and there is moderately strong evidence of high satisfaction with teleSCI modalities [18, 19, 20•]. Video telehealth modalities are especially helpful in general SCI care as an efficient and convenient mode for follow-ups, discussing bowel and bladder concerns, or addressing spasticity and chronic pain issues [19, 21]. Video modalities can enhance remote care by allowing patients to see their provider, supporting clinical rapport, and building interpersonal relationships and therapeutic alliance, while affording clinicians an opportunity to see home environments to gain insights regarding challenges impacting care [22].
The benefits of using telehealth to augment traditional care are also salient when discussing transitions of care from inpatient care to community settings. Acute hospital and inpatient rehabilitation stays are shortening as reimbursement becomes the primary driver of length of stays and often does not permit time for patients and families to achieve maximum independence after a SCI [3, 23]. Telehealth has been found to be useful as a cost-effective supplement to in-person SCI care, preventing re-hospitalizations and promoting better disease management [11, 17, 24]. It also has the potential to support caregivers of individuals with SCI during transitions from acute rehabilitation to home [25].
TeleSCI can expand access to care for rural patients by providing access to SCI expertise without traveling long distances to be seen [26–28]. It can supplement in-person care for those with complex sacral wounds by reducing the number of visits that require complex transportation planning [24, 29, 30]. TeleSCI can also provide added safety and comfort for those at elevated risk during flu season or the COVID-19 pandemic by reducing in-person appointments when clinically appropriate [31, 32]. However, not all patients or situations are conducive for teleSCI, for example, patients without access to high-speed broadband or internet-capable devices (e.g., highly rural or low income) as well as those requiring physical assessment or intervention (e.g., neurological assessments or required lab work) or in-person support. For this reason, teleSCI serves to augment (not replace) in-person care. Clinicians and patients should collaboratively determine when teleSCI is appropriate by considering whether a clinical need cannot be addressed remotely, if the cost/risk of an in-person visit outweighs the benefit (e.g., times of disaster), and whether the patient has adequate access to needed resources to facilitate successful engagement, while considering patient preference.
Despite growing interest in telehealth modalities and reported benefits to the SCI population, teleSCI adoption has remained relatively low [17]. This is likely in part because research in teleSCI lags behind other fields [33•] and because of the disability digital divide [34–38]. Furthermore, individuals with SCI may require complex intervention (e.g., training, equipment) related to upper-limb impairment. Similarly, for those with low technology literacy and/or comfort, hesitancy may further compound difficulties where structural barriers (e.g., lack of adaptive equipment, caregiver support, or poor broadband infrastructure) already exist [28]. Some mitigating forces include using adaptive equipment [39], increasing broadband infrastructure, provision of iPads to patients without internet-enabled devices [19], and creating clinic-to-clinic agreements in rural communities to extend SCI specialty care in under-resourced areas [26, 32, 40].
In the last decade [41], remote medical care and wellness support using telehealth and mobile health (mHealth) have become more prevalent in the SCI literature [42]. However, research supporting best practices in TeleSCI is still an emerging science [17, 40], lagging behind telehealth research in behavior change literature [33•] and even similar diagnoses requiring rehabilitation, such as stroke [13, 14]. Telehealth expands access to care for those requiring specialty care and, when used effectively, can reduce cost and effort of providing clinical support and allow more frequent (but brief) encounters (e.g., coaching) that could better support patient engagement in active self-management [17, 43].
Recent Trends in TeleSCI
In our review of the current literature, we surveyed articles pertaining to telehealth and telerehabilitation and SCI, published 2016–present, while using reference lists to perform snowball searches for missed literature. We found that recent teleSCI literature represented four main concepts: preventive health and wellness after SCI; management of chronic pain, anxiety, and depression; restorative and rehabilitation care; and disaster planning.
Preventive Health and Wellness
After initial hospitalization for SCI, there is a significant adjustment period during which patients and families must integrate complex compensatory strategies into their routine to prevent long-term complications. Common issues include adjustment difficulties, ongoing challenges with pain and spasticity, and bowel and bladder management, as well as skin care and wound prevention. Even after integrating these skills, patients who have paraplegia or tetraplegia remain at high risk for long-term complications of physical inactivity, such as obesity, diabetes mellitus, and physical deconditioning, leading to an increased risk for pneumonia, cardiovascular events, and osteoporosis-related frailty. As a result, much recent teleSCI research focuses on preventive health and general wellness interventions primarily involving self-management of SCI [43–45], physical exercise [18, 46–49], and nutrition and weight management [50].
Three studies focused on self-management of SCI-related care such as self-directed goal attainment in bladder and bowel management [44], empowerment and engagement in medical care [45], and daily care such as medications and skin checks [43]. Efficacy for the self-management studies demonstrated mixed results. One study using the Interactive Mobile Health and Rehabilitation (iMHere) phone application was able to significantly reduce urinary tract infection incidence (P = 0.03) using content and reminders related to bladder management [43]. Another study, using a publicly available web program called SCI & U (available at https://www.sci-and-u.com/users/sign_in), did not find a significant impact on desired outcomes. However, participants qualitatively voiced an overall positive impact [44]. The last study, which used telephone coaching to promote active involvement in medical care, found significant improvements in patient activation (P = 0.03), social/role activity limitation (P = 0.04), and service/resource awareness and utilization (P = 0.02) over control groups [45].
Five studies that had a primary focus on physical exercise and endurance had mixed results [18, 46–49]. All interventions used some form of coaching to promote exercise. One team used web-based didactic content, assigned exercise homework, and then evaluated exercise diaries remotely every 2 weeks to adjust exercise regimens via email or phone [18]. Another provided a home exercise tool kit and exercises via a mailed DVD but had a psychologist provide telephone coaching to encourage physical activity every 1 to 2 weeks [47]. Three interventions used video telehealth either for coaching check-ins and/or real-time exercise monitoring [46, 48, 49]. Additionally, one intervention employed the use of a bioharness to gather physical activity data in real time, while using video teleconference to provide synchronous coaching and feedback about exercise form and intensity [48].
Three studies demonstrated large effects on physical activity and tolerance outcomes [46, 47, 49], but only one study demonstrated statistically significant improvements in exercise tolerance (p ≤ 0.05) [49]. The last study conducted a feasibility case series that found improvements in physical activity and exercise tolerance. Interestingly, while several interventions did not significantly impact physical activity, several demonstrated significant impact on secondary outcomes such as satisfaction with life [48] and quality of life [18], depression [18, 47], anxiety [18], health participation [47], and meaningful life experience [46], suggesting physical activity interventions may have wider applicability in this population. Only one of these studies focused on nutrition, which found significant improvement in secondary outcomes regarding choice of balanced meals, reading food labels, logging meals, and monitoring food portions [50].
Chronic Pain, Anxiety, and Depression
Approximately 60–70% of individuals with SCI experience chronic pain and spasticity as a result of their neurologic injury [51, 52]. Furthermore, there is a well-established association between untreated chronic pain and anxiety and depression that often impacts functional independence and results in overall lower quality of life [53, 54].
Four studies addressed at least one or more components of chronic pain or associated mood disorders in SCI [20•, 55–57]. Three studies used web-based programs with self-guided content using psychotherapy techniques involving relaxation such as mindfulness, psychoeducation, and suggested skills practice between sessions. Two studies targeted chronic pain as a primary outcome while evaluating depression and anxiety as secondary indicators of improved quality of life after the pain intervention [55, 56]. Both found significant improvements in at least one pain-related measure, as well as in anxiety, depression, and pain catastrophizing. Another study aimed to reduce depression and anxiety while seeking to improve well-being after providing 10 weeks of Electronic Personal Administration of Cognitive Therapy (ePACT), which consisted of web-based modules, homework, and email/phone support from a clinician [57]. However, the intervention had equivocal benefit with a significant change in the intervention group from pre- and postintervention but no significant differences when comparing the intervention group to the control group, possibly due to systematic differences between the groups at baseline.
A newer trend using brain-computer interfacing to provide neurofeedback to patients with central neuropathic pain from SCI, using portable electroencephalograms (EEGs), has demonstrated promise. In this intervention, patients and caregivers were trained on proper placement and set-up of the equipment, visited after 2 weeks to assess proper use, and provided check-ins in person and by phone as needed. Using the home EEG and a tablet, participants could view graphical representations of their brain waves on a bar graph or through a race car game. This graphical feedback allowed participants to selectively train their brains to increase alpha waves (i.e., brain waves produced when one is calm and relaxed), while attempting to downregulate theta and beta bands (i.e., EEG signatures associated with pain). At completion, 80% of participants were able to achieve alpha-wave upregulation and had significant improvement in reported pain; and 53% had a greater than 30% improvement in pain. Though this modality shows early promise, more studies are needed to determine the effectiveness of this approach before wider clinical implementation can begin.
Restorative and Rehabilitation Care
SCI rehabilitation is a highly specialized field that requires experience and expertise that are often unavailable outside academic medical centers, often located in urban settings. Studies show that patients achieve better functional outcomes when they are treated by trained SCI rehabilitation clinicians [58–60]. For this reason, expansion of teleSCI programs could significantly expand reach and access of SCI expertise, promoting greater equity in care. Four studies addressed some component of rehabilitation care, such as physiotherapy [61], transfer training [62], discharge to home from inpatient rehabilitation transitional support [63], and vocational rehabilitation for job-seeking adults [64]. They are described below.
Strength and Skills Training
Two studies involved locomotion, which is a key factor in achieving greater functional independence after injury [61, 62]. One study used a website for transfer training that included detailed education and training around safe wheelchair transfers in various situations, accompanied by pictures, videos, and quizzes to ensure comprehension [62]. There were significant improvements in the Transfer Assessment Instrument from baseline to completion of the course. These improvements did not significantly differ from those of participants who were trained using the in-person equivalent. Since only 40% of wheelchair users with SCI report being trained by a professional [65], this freely available intervention (available at: http://www.upmc-sci.pitt.edu/book/independent-transfers-training) could greatly improve transfer technique and safety where SCI expertise is limited.
Another study employed virtual reality (VR) to conduct a home-based training program aimed at improving lower-limb strength, balance, and mobility [61]. Participants were set up with VR systems and foot sensors that were used to simulate movement of avatar feet within a virtual environment. Over 4 weeks, participants completed 16–20 sessions asynchronously. A therapist visited the home weekly to assess training data and increased repetitions/difficulty as participants progressed. Participants had five games that trained ankle dorsal flexion, knee extension, leg abduction, and leg adduction in both seated and upright positions. After 4 weeks of regular use, participants demonstrated significant improvements in lower extremity muscle strength, balance (Berg Balance Scale), and functional mobility (Timed Up and Go).
Transition to Community
After discharge from inpatient rehabilitation, the first several weeks at home following an SCI are challenging for most patients and their families. In addition to psychosocial adjustment, they usually encounter numerous unexpected challenges that can compound the stress of transition [66]. However, living away from urban centers where specialty care is often centered usually results in lower service utilization [40]. TeleSCI care is one way to bridge this gap. One study evaluated the use of telephone check-ins with newly diagnosed SCI patients during the transition from inpatient rehabilitation to the home setting in Bangladesh [63]. Participants were contacted every 2 weeks for 1 year after discharge and monthly during the second year. Healthcare providers assessed for complications and provided case coordination as needed. However, no effectiveness analyses were performed; and the primary outcome of mortality was equal between experimental and standard-of-care groups, suggesting that additional work is needed before this intervention can be recommended.
One case report described challenges of community transitions in low- and middle-income countries and provided two cases where check-ins comprised a combination of email, text, or videos sent to rural patients with a return of pictures/videos/text to clinicians for feedback. After an initial stabilization period of 4 weeks, the team began providing new videos of customized exercises five times a week. Live video visits with the care team were done as needed for each patient, with demonstrable success [67].
Vocational Rehabilitation
A review of literature reveals that individuals living with SCI and returning to work are younger with less severe injury and more functional independence. Common barriers identified were physical limitations, education, and training. Many of these individuals were employed prior to the injury, however, and face many challenges postinjury secondary to organizational-, structural-, and system-level barriers, offering a unique opportunity for home-based telework [68–70].
One study examined the impact of web-based content to aid those with SCI in job-seeking, interviewing, and career development [64]. Participants were emailed standardized email prompts to complete modules on a weekly basis for 4 weeks. Though there was no observable impact on the primary outcome (job procurement self-efficacy), there was a small positive impact on optimism.
Disaster Preparedness
Within the SCI professional community, there is a growing focus on planning for disasters [71]. Access to transportation, medical supplies, and caregivers is often impacted during natural disasters and pandemics. Telehealth presents another opportunity to support individuals living with SCI in the community during difficult events. Three case studies discussing specific challenges encountered during disaster events identified various ways telehealth served patients with SCI.
One report described how telehealth was used during a very large month-long wildfire that claimed the home of a recently discharged patient [72]. Although the patient was able to evacuate in time to avoid injury, some essentials were lost in the fire. Telehealth allowed the patient to maintain contact with clinical care, while the team re-ordered durable medical equipment and supplies that were lost in the fire.
Two case studies describe how telehealth supported individuals during the COVID-19 pandemic. In one case, a previously independent patient with paraplegia was hospitalized due to worsening of his condition resulting in new-onset tetraplegia [73]. Soon after the pandemic started, the patient was discharged home but faced significant barriers in using his previous supports, resulting in financial hardship, food insecurity, two visits to the emergency room for bowel impaction, and depression due to isolation from his family. Telehealth allowed the care team to serve as a safety net while coordinating additional community resources. In another case study, a patient living in a rural area in the northeast part of the USA experienced extreme difficulty coordinating in-home caregivers during the pandemic [74]. His inability to find reliable care resulted in a decision to move cross-country to be closer to family who would help. Telehealth visits were then used between SCI teams from his departing and arriving location to coordinate care and a warm handoff, while facilitating safe transportation for this patient.
Optimizing TeleSCI Programs
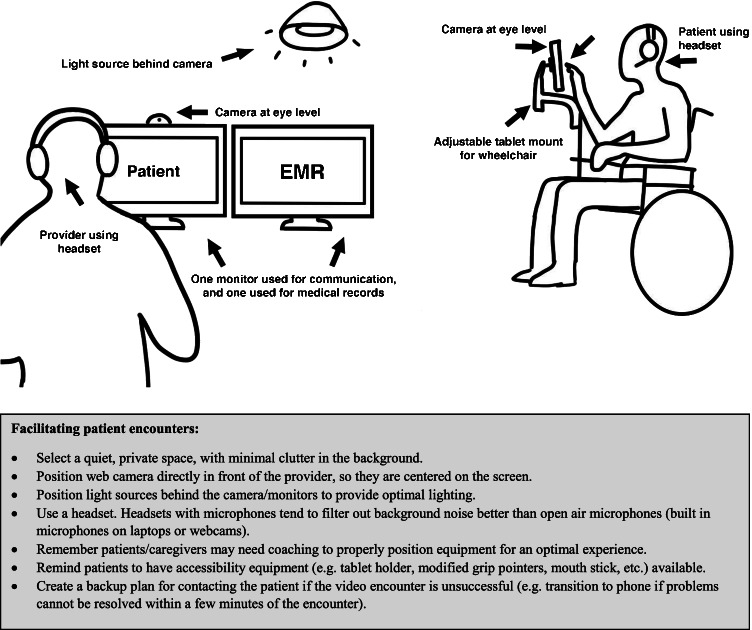
A small subset of studies used telephones as the primary mode for contact with participants [45, 47, 63], but evidence suggests better interactions occur when video components are included during the clinical visit [22, 75, 76]. Phone communications should be reserved for times when video conferencing is not feasible or practical (e.g., bad video connection). Figure 1 provides an ideal configuration to promote effective communication during a clinical visit. Additionally, providing suggestions to the patient and family may aid them to better prepare for a telehealth session that best meets their needs. For example, while telehealth can be done on a smartphone, a larger screen provides easier viewing angles for patients. Tablets and/or laptops are easier to prop up, while devices such as pointers and tablet holders may aid those with limited hand function [32]. Additionally, being aware of and cueing patients to use commonly available accessibility features, such as blue-tooth hearing aids, e-readers, and voice commands, can significantly improve the patient experience.
Fig. 1.
Optimizing your set-up for a patient encounter
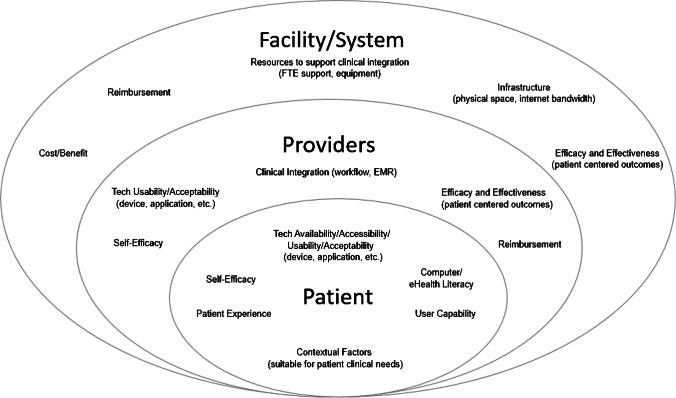
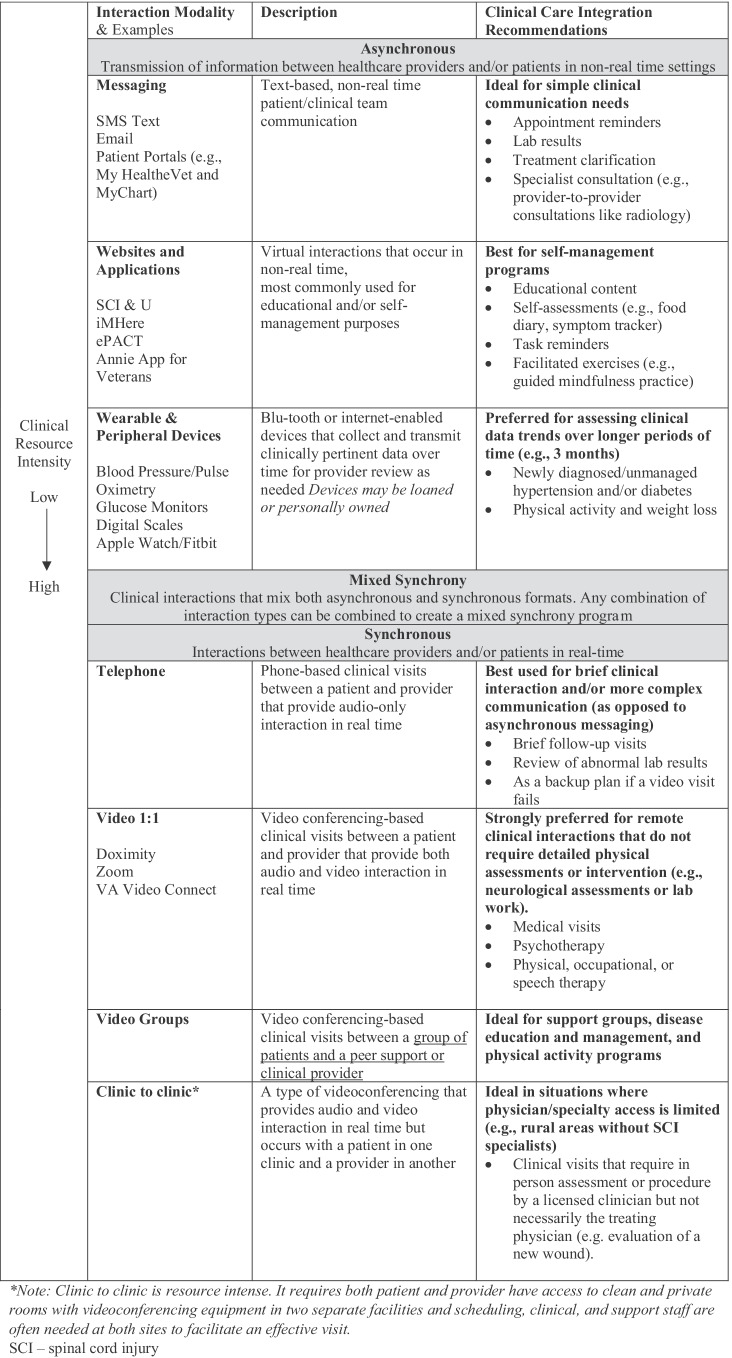
There are additional factors to consider when deciding to use teleSCI in clinical practice (Fig. 2). Table 1 provides examples of how different solutions may be used in clinical care and Table 2 maps strategies to programs discussed in this review. Thoughtful consideration should be given to the cost/benefit of synchrony versus asynchrony. For therapist time, asynchronous monitoring of exercise with therapist feedback/prescription may provide a cost-effective rehabilitation intervention; but if the skill and motivational level of the participant are low, adherence will likely impact success. When using web-based content and applications, automated versus personalized coaching should be used judiciously to maximize impact. Automated coaching is an excellent way to provide task reminders or positive messaging when a new achievement is reached; but personalized feedback, encouragement, and support are likely more effective for big-picture program outcomes. Another consideration is the use of peers or professionals in interventions that use coaching. Both peers and professionals serve important roles and interventions, such as the MyCareMyCall program’s demonstration of positive results when combining efforts [45]. Lastly, when determining pacing, consider whether a scheduled pace or a self-directed pace best serves the patient’s needs. Scheduled sessions may work better in early recovery when structured therapy is needed [21], while self-directed engagement may serve those who are actively engaged in care but looking for more education. However, it is important to caution that self-directed probably does not mean set it and forget it. Patients will likely still need prompts, suggested activities, and check-ins to maximize benefit.
Fig. 2.
Contextual factors to consider when implementing TeleSCI programs
Table 1.
Key concepts and approaches in TeleSCI
Table 2.
TeleSCI programs and components
| Author | Asynchronous | Synchronous | |||||
|---|---|---|---|---|---|---|---|
| Messaging | Web/App | Wearable/Peripheral | Telephone | Video | Group | Clinic to Clinic | |
| Coulter et al., 2017 [18] | X | X | X | ||||
| Al-Taleb et al., 2019 [20•] | X | ||||||
| Kryger et al., 2019 [43] | X | X | |||||
| Allin et al., 2020 [44] | X | X | X | X | |||
| Houlihan et al., 2017 [45] | X | X | |||||
| Chemtob et al., 2019 [46] | X | ||||||
| Bombardier et al., 2021 [47] | X | ||||||
| Lai et al., 2016 [48] | X | X | X | ||||
| Costa et al., 2021 [49] | X | X | |||||
| Wood et al., 2021 [50] | X | X | |||||
| Dear et al., 2018 [55] | X | X | X | ||||
| Hearn & Finlay, 2018 [56] | X | X | |||||
| Migliorini et al., 2016 [57] | X | X | |||||
| Villiger et al., 2017 [61] | X | X | |||||
| Worobey et al., 2018 [62] | X | ||||||
| Hossain et al., 2017 [63] | X | ||||||
| Dorstyn et al., 2019 [64] | X | X | |||||
| Tyagi et al., 2019 [67] | X | X | X | ||||
| Pasipanodya & Shem, 2020 [72] | X | ||||||
| Siddiqui et al., 2021 [73] | X | ||||||
| Huang et al., 2021 [74] | X | X | |||||
Discussion
A challenge to creating strong and effective teleSCI programs is the limited literature. Other issues that pose a threat to widespread adoption include insufficient infrastructure within institutions to support regular utilization of teleSCI modalities (e.g., adoption willingness, technology proficiency, policy/procedures, and communication/education to support full integration of teleSCI into clinical care). Within the teleSCI literature, there is little replication and homogeneity of outcome measures to create a strong evidence base of best practices, although there are similar neurological conditions, such as stroke, that have a significant presence in the telehealth and telerehabilitation literature. Using evidence from similar rehabilitation conditions can help guide development, but more concentrated work needs to support full maturation of teleSCI care [77, 78].
Unsurprisingly, COVID-19 propelled the telehealth movement forward in clinical settings, making telehealth salient and accessible, and eliminating many regulatory barriers. However, the pandemic also highlighted several important issues concerning equitable access to care and resources [22, 32, 74]. The digital divide widened, as people living with disability, lower socioeconomic status, and in remote communities with poor highspeed broadband infrastructure were disproportionately left behind in the rapid transition to remote care [34–37]. Though the issue of the digital divide is not new, as video telehealth becomes a standard of care, greater efforts need to ensure that people living with SCI have access and are involved in development of programs, raising an issue of equity when portions of our population are left behind in new care models [39, 79].
TeleSCI care ultimately provides a convenient, accessible, and engaging way to receive care. Prior to the COVID-19 pandemic, SCI providers reported relatively low adoption of telemedicine (18%), but 77% expressed an interest in learning more [71]. TeleSCI options should be integrated into standard of care as an option to promote access to care, enhance self-management skills, and encourage engagement in care. Specific modalities, such as video telehealth, should be used as a supplement to clinical visits that require in-person care (such as times when physical exams require components that are not easily replicated remotely or labs/imaging is needed, evaluation of new pressure injuries, and specialty procedures such as refilling baclofen pumps). Recent work highlights effective ways to conduct virtual care in these settings [29, 80••], and there is growing availability of supportive peripheral devices such as wearable monitors, electronic stethoscopes, and digital cameras that can accurately measure wounds in 3D.
Conclusions
TeleSCI care will advance and expand as remote and digital treatment tools become more broadly accessible. Moving forward, we expect to see a significant rise in guided usage of VR simulation, games, and neurofeedback methods for both rehabilitation and therapeutic management of chronic issues such as pain. With virtual interventions, there is likely a desire for immersive, engaging, and social experiences to support ongoing needs of patients; but we were unable to find any literature at this time. Early evidence for efficacy of various teleSCI modalities is promising, but the benefits of engagement are clear: increased adherence and lower attrition [81], which, ultimately, support better clinical outcomes when using effective treatments. Additionally, we anticipate growth in the use of wearables and other ways to gather patient-generated data, given the benefits of having directly accessible feedback for patients and additional data points for providers doing remote care. Using patient-generated data could help clinicians decide when asynchronous sessions are appropriate, when to assign and monitor practice between synchronous sessions, and when to slowly wean to independence.
Funding
This work is supported in part by funding from the Office of Rural Health Veterans Rural Health Resource Center in Salt Lake City (#16032) and in part by the use of facilities and resources of the Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN13-413) and the VA South Central Mental Illness Research, Education and Clinical Center, which played no role in the preparation of this manuscript.
Declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The opinions expressed are those of the authors and do not necessarily reflect those of the Department of Veterans Affairs, the US government, or Baylor College of Medicine.
Footnotes
This article is part of the Topical Collection on Spinal Cord Injury Rehabilitation
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.World Health Organization. International Perspectives on Spinal Cord Injury (IPSCI).; 2013. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=70724512. Accessed 7 Nov 2021.
- 2.Hiremath SV, Hogaboom NS, Roscher MR, Worobey LA, Oyster ML, Boninger ML. Longitudinal prediction of quality-of-life scores and locomotion in individuals with traumatic spinal cord injury. Arch Phys Med Rehabil. 2017;98(12):2385–2392. doi: 10.1016/j.apmr.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Middleton A, Downer B, Haas A, Lin YL, Graham JE, Ottenbacher KJ. Functional status is associated with 30-day potentially preventable readmissions following skilled nursing facility discharge among medicare beneficiaries. J Am Med Dir Assoc. 2018;19(4):348–354.e4. doi: 10.1016/j.jamda.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagen EM, Lie SA, Rekand T, Gilhus NE, Gronning M. Mortality after traumatic spinal cord injury: 50 years of follow-up. J Neurol Neurosurg Psychiatry. 2010;81(4):368–373. doi: 10.1136/jnnp.2009.178798. [DOI] [PubMed] [Google Scholar]
- 5.Lidal IB, Snekkevik H, Aamodt G, Hjeltnes N, Stanghelle JK, Biering-Sørensen F. Mortality after spinal cord injury in Norway. J Rehabil Med. 2007;39(2):145–151. doi: 10.2340/16501977-0017. [DOI] [PubMed] [Google Scholar]
- 6.Williams R, Murray A. Prevalence of depression after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2015;96(1):133–140. doi: 10.1016/j.apmr.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Post MWM, Van Leeuwen CMC. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50(5):382–389. doi: 10.1038/sc.2011.182. [DOI] [PubMed] [Google Scholar]
- 8.McColl MA, Aiken A, McColl A, Sakakibara B, Smith K. Primary care of people with spinal cord injury: scoping review. Can Fam Physician. 2012;58(11):1207–1216, e626–35. http://www.ncbi.nlm.nih.gov/pubmed/23152456/0A/http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3498012. Accessed 7 Oct 2021. [PMC free article] [PubMed]
- 9.Kroll T, Jones GC, Kehn M, Neri MT. Barriers and strategies affecting the utilisation of primary preventive services for people with physical disabilities: a qualitative inquiry. Heal Soc Care Community. 2006;14(4):284–293. doi: 10.1111/j.1365-2524.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 10.Tenforde AS, Hefner JE, Kodish-Wachs JE, Iaccarino MA, Paganoni S. Telehealth in physical medicine and rehabilitation: a narrative review. PM & R. 2017;9(5):S51–S58. doi: 10.1016/j.pmrj.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Irgens I, Rekand T, Arora M, et al. Telehealth for people with spinal cord injury: a narrative review. Spinal Cord. 2018;56(7):643–655. doi: 10.1038/s41393-017-0033-3. [DOI] [PubMed] [Google Scholar]
- 12.Policy C for CH. What is Telehealth? Published 2017. http://www.cchpca.org/what-is-telehealth. Accessed 8 Jan 2021.
- 13.Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. 2020;2020(1). 10.1002/14651858.CD010255.pub3 [DOI] [PMC free article] [PubMed]
- 14.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2017;2017(11). 10.1002/14651858.CD008349.pub4 [DOI] [PMC free article] [PubMed]
- 15.Brochard S, Robertson J, Médée B, Rémy-Néris O. What’s new in new technologies for upper extremity rehabilitation? Curr Opin Neurol. 2010;23(6):683–687. doi: 10.1097/WCO.0b013e32833f61ce. [DOI] [PubMed] [Google Scholar]
- 16.Alexander M, Irgens I, Marshall R, Houlihan B, Arora M, Liu N. Around the world with TeleSCI.
- 17.Lee S, Kim J, Kim J. Substantiating clinical effectiveness and potential barriers to the widespread implementation of spinal cord injury telerehabilitation: a systematic review and qualitative synthesis of randomized trials in the recent past decade. Telemed Reports. 2021;2(1):64–77. doi: 10.1089/tmr.2020.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulter EH, McLean AN, McLean AN, et al. The effectiveness and satisfaction of web-based physiotherapy in people with spinal cord injury: A pilot randomised controlled trial. Spinal Cord. Published online 2017. 10.1038/sc.2016.125 [DOI] [PubMed]
- 19.Sechrist S, Lavoie S, Khong C-M, Dirlikov B, Shem K. Telemedicine using an iPad in the spinal cord injury population: a utility and patient satisfaction study. Spinal Cord Ser Cases. Published online 2018. 10.1038/s41394-018-0105-4 [DOI] [PMC free article] [PubMed]
- 20.Al-Taleb MKH, Purcell M, Fraser M, Petric-Gray N, Vuckovic A. Home used, patient self-managed, brain-computer interface for the management of central neuropathic pain post spinal cord injury: usability study. J Neuroeng Rehabil. 2019;16(1):1–24. doi: 10.1186/s12984-019-0588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Straaten MG, Cloud BA, Morrow MM, Ludewig PM, Zhao KD. Effectiveness of home exercise on pain, function, and strength of manual wheelchair users with spinal cord injury: a high-dose shoulder program with telerehabilitation. Arch Phys Med Rehabil. 2014;95(10):1810–1817.e2. doi: 10.1016/j.apmr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zulman DM, Verghese A. Virtual care, telemedicine visits, and real connection in the era of COVID-19. JAMA. 2021;325(5):26–38. doi: 10.1037/amp0000751. [DOI] [PubMed] [Google Scholar]
- 23.Thompson LR, Ifejika NL. The transition from the hospital to an inpatient rehabilitation setting for neurologic patients. Nurs Clin North Am. 2019;54(3):357–366. doi: 10.1016/j.cnur.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Woo C, Guihan M, Frick C, Gill CM, Ho CH. What’s happening now! Telehealth management of spinal cord injury/disorders. J Spinal Cord Med. 2011;34(3):322–331. doi: 10.1179/2045772311Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin PB, McDonald SD, Watson JD, Pierce BS, Elliott TR. Telehealth transition assistance program for acute spinal cord injury caregivers: Protocol for a mixed-methods, randomized controlled trial. JMIR Res Protoc. 2021;10(3):1–8. doi: 10.2196/28256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho C, Atchison K, Noonan VK, et al. Models of Care Delivery from rehabilitation to community for spinal cord injury: a scoping review. J Neurotrauma. 2021;38(6):677–697. doi: 10.1089/neu.2020.7396. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton R, Driver S, Noorani S, Callender L, Bennett M, Monden K. Utilization and access to healthcare services among community-dwelling people living with spinal cord injury. J Spinal Cord Med. 2017;40(3):321–328. doi: 10.1080/10790268.2016.1184828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatch MN, Martinez RN, Etingen B, et al. Characterization of telehealth use in veterans with spinal cord injuries and disorders. PM & R. 2021;13(10):1094–1103. doi: 10.1002/pmrj.12515. [DOI] [PubMed] [Google Scholar]
- 29.Walia S, Wolfe D, Keast D, et al. Facilitators and barriers for implementing an internet clinic for the treatment of pressure injuries. Telemed e-Health. 2019;25(12):1237–1243. doi: 10.1089/tmj.2018.0196. [DOI] [PubMed] [Google Scholar]
- 30.Tung JY, Stead B, Mann W, Ba’Pham, Popovic MR. Assistive technologies for self-managed pressure ulcer prevention in spinal cord injury: A scoping review. J Rehabil Res Dev. 2015;52(2):131-146. 10.1682/JRRD.2014.02.0064 [DOI] [PubMed]
- 31.Stillman MD, Capron M, Alexander M, Di Giusto ML, Scivoletto G. COVID-19 and spinal cord injury and disease: results of an international survey. Spinal cord Ser cases. 2020;6(1):21. doi: 10.1038/s41394-020-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrows D, Goldstein B. Virtual care in the Veterans Affairs spinal cord injuries and disorders system of care during the COVID-19 National Public Health Emergency. Phys Med Rehabil Clin N Am. 2021;32(2):207–221. doi: 10.1016/j.pmr.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dugas M, Gao G, Agarwal R. Unpacking mHealth interventions: a systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digit Heal. 2020;6:1–16. doi: 10.1177/2055207620905411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duplaga M. Digital divide among people with disabilities: analysis of data from a nationwide study for determinants of Internet use and activities performed online. PLoS ONE. 2017;12(6):1–19. doi: 10.1371/journal.pone.0179825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson S, Gulliksen J, Gustavsson C. Disability digital divide: the use of the internet, smartphones, computers and tablets among people with disabilities in Sweden. Univers Access Inf Soc. 2020;(0123456789). 10.1007/s10209-020-00714-x
- 36.Dobransky K, Hargittai E. Unrealized potential: Exploring the digital disability divide. Poetics. 2016;58:18–28. doi: 10.1016/j.poetic.2016.08.003. [DOI] [Google Scholar]
- 37.Pew Research Center. Disabled Americans are less likely to use technology. Fact Tank News Numbers. Published online 2017:1–8. http://www.pewresearch.org/fact-tank/2018/10/19/5-charts-on-global-views-of-china/
- 38.Smith A. Older Adults and Technology Use | Pew Research Center. Pew Res Center. Published online 2014:2. Accessed May 19, 2020. https://www.pewresearch.org/internet/2014/04/03/older-adults-and-technology-use/
- 39.Hogan TP, Hill JN, Locatelli SM, et al. Health information seeking and technology use among veterans with spinal cord injuries and disorders. PM&R. Published online 2016. 10.1016/j.pmrj.2015.06.443 [DOI] [PubMed]
- 40.Van De Pol E, Lucas K, Geraghty T, et al. The delivery of specialist spinal cord injury services in Queensland and the potential for telehealth. BMC Health Serv Res. 2016;16(1):1–10. doi: 10.1186/s12913-016-1256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalczewski J, Chong SL, Galea M, Prochazka A. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair. 2011;25(5):412–422. doi: 10.1177/1545968310394869. [DOI] [PubMed] [Google Scholar]
- 42.Zulman DM, Wong EP, Slightam C, et al. Making connections: nationwide implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323–329. doi: 10.1093/jamiaopen/ooz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kryger MA, Crytzer TM, Fairman A, et al. The effect of the interactive mobile health and rehabilitation system on health and psychosocial outcomes in spinal cord injury: Randomized controlled trial. J Med Internet Res. 2019;21(8):1–14. doi: 10.2196/14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allin S, Shepherd J, Thorson T, et al. Web-based health coaching for spinal cord injury: results from a mixed methods feasibility evaluation. JMIR Rehabil Assist Technol. 2020;7(2):1–15. doi: 10.2196/16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houlihan B, Brody M, Everhart-Skeels S, et al. Randomized trial of a peer-led, telephone-based empowerment intervention for persons with chronic spinal cord injury improves health self-management. Arch Phys Med Rehabil. Published online 2017. 10.1016/j.apmr.2017.02.005 [DOI] [PubMed]
- 46.Chemtob K, Rocchi M, Arbour-Nicitopoulos K, Kairy D, Fillion B, Sweet SN. Using tele-health to enhance motivation, leisure time physical activity, and quality of life in adults with spinal cord injury: A self-determination theory-based pilot randomized control trial. Psychol Sport Exerc. 2018;2019(43):243–252. doi: 10.1016/j.psychsport.2019.03.008. [DOI] [Google Scholar]
- 47.Bombardier CH, Dyer JR, Burns P, et al. A tele-health intervention to increase physical fitness in people with spinal cord injury and cardiometabolic disease or risk factors: a pilot randomized controlled trial. Spinal Cord. 2021;59(1):63–73. doi: 10.1038/s41393-020-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai B, Rimmer J, Barstow B, Jovanov E, Bickel CS. Teleexercise for persons with spinal cord injury: a mixed-methods feasibility case series. JMIR Rehabil Assist Technol. 2016;3(2). 10.2196/rehab.5524 [DOI] [PMC free article] [PubMed]
- 49.Costa RRG, Dorneles JR, Veloso JHCL, Gonçalves CWP, Neto FR. Synchronous and asynchronous tele-exercise during the coronavirus disease 2019 pandemic: comparisons of implementation and training load in individuals with spinal cord injury. J Telemed Telecare. Published online 202110.1177/1357633X20982732 [DOI] [PMC free article] [PubMed]
- 50.Wood S, Khong CM, Dirlikov B, Shem K. Nutrition counseling and monitoring via tele-nutrition for healthy diet for people with spinal cord injury: a case series analyses. J Spinal Cord Med. 2021;0(0):1–9. doi: 10.1080/10790268.2021.1871824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burchiel KJ, Hsu KFP. Pain and spasticity after spinal cord injury: mechanisms and treatment. Spine (Phila Pa 1976) 2001;26(24 SUPPL.):146–160. doi: 10.1097/00007632-200112151-00024. [DOI] [PubMed] [Google Scholar]
- 52.Andresen SR, Biering-Sørensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB. Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord. 2016;54(11):973–979. doi: 10.1038/sc.2016.46. [DOI] [PubMed] [Google Scholar]
- 53.Hartoonian N, Hoffman JM, Kalpakjian CZ, Taylor HB, Krause JK, Bombardier CH. Evaluating a spinal cord injury-specific model of depression and quality of life. Arch Phys Med Rehabil. 2014;95(3):455–465. doi: 10.1016/j.apmr.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 54.Putzke JD, Richards JS, Hicken BL, DeVivo MJ. Interference due to pain following spinal cord injury: Important predictors and impact on quality of life. Pain. 2002;100(3):231–242. doi: 10.1016/S0304-3959(02)00069-6. [DOI] [PubMed] [Google Scholar]
- 55.Dear BF, Nicholson Perry K, Siddall P, et al. The pain course: exploring the feasibility of an internet-delivered pain management programme for adults with spinal cord injury. Spinal Cord. 2018;56(10):931–939. doi: 10.1038/s41393-018-0146-3. [DOI] [PubMed] [Google Scholar]
- 56.Hearn JH, Finlay KA. Internet-delivered mindfulness for people with depression and chronic pain following spinal cord injury: a randomized, controlled feasibility trial. Spinal Cord. 2018;56(8):750–761. doi: 10.1038/s41393-018-0090-2. [DOI] [PubMed] [Google Scholar]
- 57.Migliorini C, Sinclair A, Brown D, Tonge B, New P. A randomised control trial of an Internet-based cognitive behaviour treatment for mood disorder in adults with chronic spinal cord injury. Spinal Cord. 2016;54(9):695–701. doi: 10.1038/sc.2015.221. [DOI] [PubMed] [Google Scholar]
- 58.Parent S, Barchi S, LeBreton M, Casha S, Fehlings MG. The impact of specialized centers of care for spinal cord injury on length of stay, complications, and mortality: a systematic review of the literature. J Neurotrauma. 2011;28(8):1363–1370. doi: 10.1089/neu.2009.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Emerich L, Parsons K, Stein A. Competent Care for persons with spinal cord injury and dysfunction in acute inpatient rehabilitation. Top Spinal Cord Inj Rehabil. 2012;18(2):149–166. doi: 10.1310/sci1802-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whalley HK. Experience of rehabilitation following spinal cord injury: a meta-synthesis of qualitative findings. Spinal Cord. 2007;45(4):260–274. doi: 10.1038/sj.sc.3102034. [DOI] [PubMed] [Google Scholar]
- 61.Villiger M, Liviero J, Awai L, et al. Home-based virtual reality-augmented training improves lower limb muscle strength, balance, and functional mobility following chronic incomplete spinal cord injury. Front Neurol. 2017;8(11). 10.3389/fneur.2017.00635 [DOI] [PMC free article] [PubMed]
- 62.Worobey LA, Rigot SK, Hogaboom NS, Venus C, Boninger ML. Investigating the efficacy of web-based transfer training on independent wheelchair transfers through randomized controlled trials. Arch Phys Med Rehabil. 2018;99(1):9–16.e10. doi: 10.1016/j.apmr.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 63.Hossain MS, Harvey LA, Rahman MA, et al. A pilot randomised trial of community-based care following discharge from hospital with a recent spinal cord injury in Bangladesh. Clin Rehabil. 2017;31(6):781–789. doi: 10.1177/0269215516654207. [DOI] [PubMed] [Google Scholar]
- 64.Dorstyn D, Roberts R, Murphy G, et al. Work and SCI: a pilot randomized controlled study of an online resource for job-seekers with spinal cord dysfunction. Spinal Cord. 2019;57(3):221–228. doi: 10.1038/s41393-018-0200-1. [DOI] [PubMed] [Google Scholar]
- 65.Fliess-Douer O, Vanlandewijck YC, Van Der Woude LHV. Most essential wheeled mobility skills for daily life: an international survey among paralympic wheelchair athletes with spinal cord injury. Arch Phys Med Rehabil. 2012;93(4):629–635. doi: 10.1016/j.apmr.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 66.Weber L, Voldsgaard NH, Holm NJ, Schou LH, Biering-Sørensen F, Møller T. Exploring the contextual transition from spinal cord injury rehabilitation to the home environment: a qualitative study. Spinal Cord. 2021;59(3):336–346. doi: 10.1038/s41393-020-00608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyagi N, Goel SA, Alexander M. Improving quality of life after spinal cord injury in India with telehealth. Spinal cord Ser cases. Published online 2019. 10.1038/s41394-019-0212-x [DOI] [PMC free article] [PubMed]
- 68.Lidal IB, Huynh TK, Biering-Sørensen F. Return to work following spinal cord injury: a review. Disabil Rehabil. 2007;29(17):1341–1375. doi: 10.1080/09638280701320839. [DOI] [PubMed] [Google Scholar]
- 69.Frieden L, Winnegar AJ. Opportunities for research to improve employment for people with spinal cord injuries. Spinal Cord. 2012;50(5):379–381. doi: 10.1038/sc.2012.38. [DOI] [PubMed] [Google Scholar]
- 70.Barclay L, Lalor A, Migliorini C, Robins L. A comparative examination of models of service delivery intended to support community integration in the immediate period following inpatient rehabilitation for spinal cord injury. Spinal Cord. 2020;58(5):528–536. doi: 10.1038/s41393-019-0394-x. [DOI] [PubMed] [Google Scholar]
- 71.Alexander M, Alexander J, Arora M, Slocum C, Middleton J. A bellweather for climate change and disability: educational needs of rehabilitation professionals regarding disaster management and spinal cord injuries. Spinal Cord Ser Cases. 2019;5(1). 10.1038/s41394-019-0239-z [DOI] [PMC free article] [PubMed]
- 72.Pasipanodya EC, Shem K. Provision of care through telemedicine during a natural disaster: A case study. Spinal cord Ser cases. Published online 2020. 10.1038/s41394-020-0309-2 [DOI] [PMC free article] [PubMed]
- 73.Siddiqui S, Huang D, Touchett H, Skelton F. A clinical vignette on community transition after inpatient rehabilitation for a veteran with new spinal cord injury–related disability during the COVID-19 pandemic. Am J Phys Med Rehabil. 2021;100(7):631–632. doi: 10.1097/phm.0000000000001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang D, Siddiqui SA, Touchett HN, Skelton F. Protecting the most vulnerable among us: Access to care and resources for persons with disability from spinal cord injury during the COVID-19 pandemic. PM&R. 2021;(1):1–5. 10.1002/pmrj.12576 [DOI] [PMC free article] [PubMed]
- 75.Lindsay JA, Hogan JB, Ecker AH, Day SC, Chen P, Helm A. The importance of video visits in the time of COVID-19. J Rural Heal. 2021;37(1):242–245. doi: 10.1111/jrh.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slightam C, Gregory AJ, Hu J, et al. Patient perceptions of video visits using Veterans Affairs telehealth tablets: survey study. J Med Internet Res. 2020;22(4):e15682. doi: 10.2196/15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maresca G, Maggio MG, De Luca R, et al. Tele-neuro-rehabilitation in Italy: state of the art and future perspectives. Front Neurol. 2020;11(September):1–12. doi: 10.3389/fneur.2020.563375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. 2017;4(2):1–9. doi: 10.2196/rehab.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinez RN, Hogan TP, Balbale SN, et al. Sociotechnical perspective on implementing clinical video telehealth for veterans with spinal cord injuries and disorders. Telemed J E-health. Published online 2017. 10.1089/tmj.2016.0200 [DOI] [PMC free article] [PubMed]
- 80.Verduzco-Gutierrez M, Bean AC, Tenforde AS, Tapia RN, Silver JK. How to conduct an outpatient telemedicine rehabilitation or prehabilitation Visit. PM R. 2020;12(7):714–720. doi: 10.1002/pmrj.12380. [DOI] [PubMed] [Google Scholar]
- 81.Wilroy JD, Lai B, Davlyatov G, Mehta T, Thirumalai M, Rimmer JH. Correlates of adherence in a home-based, self-managed exercise program tailored to wheelchair users with spinal cord injury. Spinal Cord. 2021;59(1):55–62. doi: 10.1038/s41393-020-0497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]