Abstract
Those living with coexistent frailty and cognitive impairment are at risk of poorer health outcomes. Research often focuses on identifying biological factors. This review sought to identify the association psychological and social factors have with coexisting physical and cognitive decline. Six databases were systematically searched in July 2020. Studies included individuals aged 60 years or older identified as being both frail and cognitively impaired. A narrative synthesis examined patterns within the data. Nine studies were included, most employed a cross-sectional design. Depression was investigated by all nine studies, those with coexistent frailty and cognitive impairment had higher levels of depressive symptoms than peers. Findings were mixed on social factors, although broadly indicate lower education, living alone and lower material wealth were more frequent in those living with coexistent decline. Further research is needed to explore potentially modifiable psychological and social factors which could lead to the development of supportive interventions.
Keywords: dementia, older people, depression, anxiety, education
Introduction
Increases to life expectancy have led to elevated risk of age-related illness and associated disability (Lunenfeld & Stratton, 2013). Therefore, ensuring healthy ageing is a priority for both healthcare providers and policy makers worldwide (WHO, 2015). This includes improved understanding of factors linked to age-related illness and improving healthcare provision for older people (WHO, 2015). Linked to this there is increasing interest in the concept of biological ageing as there is evidence that some individuals appear to develop age-related ill health earlier than others (Vernon, 2020). Differences in the occurrence of age-related ill health has been linked to the accumulation of deficits, such as symptoms, disease states and functional impairments, in later life a concept strongly linked with frailty (Mitnitski et al., 2013). Social inequalities and psychosocial factors may play a role in this chronological discrepancy given there is an established association with poor health outcomes across the life course and into old age (Braveman & Gottlieb, 2014). To address these inequalities in age-related health, the focus has been on identifying factors linked to poorer outcomes that might be amendable by intervention to promote healthy ageing. Consequently, one area of interest is the identification and support of those living with frailty (Vernon, 2020).
Frailty syndrome has been conceptualized as a decline over multiple biological systems, resulting in diminished resistance and capacity to return to former functioning following stressor events (Clegg et al., 2013). Frailty is associated with, yet distinct from, ageing, comorbidity and disability (Fried et al., 2001). Two major approaches to measuring frailty persist. The phenotype of frailty includes physiologic components only. These are unintended weight-loss, poor endurance, reduced physical activity, slow gait and weak grip (Fried et al., 2001). Alternatively, the accumulated deficits model implicates the build-up of symptoms, disease states, abnormal test results and disabilities in an index (Mitnitski et al., 2001). This model is more likely to include cognitive, psychological and social aspects. There is no consensus over the best approach to identifying those with frailty; however, both the phenotype criteria and frailty indices are broadly accepted by clinicians (de Vries et al., 2011). Frailty has been linked to adverse outcomes such as increased disability, hospitalization, admission to long-term care and death (Clegg et al., 2016; Fried et al., 2001; Theou et al., 2013). While frailty is long-term and progressive, it has been proposed that the state is modifiable with appropriate support and timely intervention (Travers et al., 2019).
Increasing age is linked to elevated risk of frailty; similarly, changes to cognition are also seen to increase with ageing. Although some cognitive change is anticipated with normal ageing, pathological decline can lead to impaired cognitive function and dementia (Jessen et al., 2014). There appears to be an association between frailty, as captured by the phenotype criteria, and cognitive impairment (Fried et al., 2001). Exploration of the relationship between frailty and impaired cognition indicates there may be shared biological mechanisms between the two (Panza et al., 2006), although as yet this is poorly understood (Kant et al., 2018). There have been attempts to describe this link further, including the conceptualization of cognitive frailty (Kelaiditi et al., 2013). Cognitive frailty is defined as the presence of both frailty and cognitive impairment, rated as questionable dementia (0.5) on the Clinical Dementia Rating (CDR) scale (Hughes et al., 1982). This definition excludes those living with dementia. One of the key aims, in associating cognition with frailty, was to identify reversible cognitive decline in old age, given the proposal that frailty is treatable and modifiable. Studies have established an association between frailty and specific cognitive domains such as executive function (Bunce et al., 2019) and motoric cognitive risk, which associates cognitive decline with slow gait, a measure of the frailty phenotype (Montero-Odasso et al., 2016). These findings indicate a relationship between cognition and physical function, although the exact causal route is uncertain.
Both frailty and impaired cognition are individually associated with adverse health outcomes and poor quality of life (Lim et al., 2018; Lucke et al., 2018; Malmstrom et al., 2014). Furthermore, an accumulative effect of frailty and cognitive impairment is apparent. Those with coexistent frailty and cognitive impairment experience more hospitalizations, more frequent admission to long-term care and a more rapid decline to dependency and death, than healthy peers or those living with frailty or cognitive impairments alone (Avila-Funes et al., 2009; Feng et al., 2016; Panza et al., 2018). Therefore, given the negative outcomes associated with having both frailty and cognitive impairment, it is important to understand the factors that increase an individual’s risk of having coexistent frailty and cognitive impairment in later life. Moreover, it is particularly important to identify modifiable factors which have been linked to both frailty and cognitive impairment, to aid in the development of targeted therapy and support.
The association frailty coexistent with cognitive impairment has with biological factors is well investigated. Clinical markers such as cardiovascular disease and elevated blood pressure or evidence of physiologic change such as chronic inflammation or hormonal changes have been examined (Sargent et al., 2018). However, there may be risk factors that occur earlier in the lifespan. Longitudinal evidence indicates that multiple factors, including those of a social or psychological nature, which have been given far less consideration in research thus far, may present as risks, or provide protection, against frailty (Feng et al., 2017). The need to look at other health determinants, beyond physiology, is implicated in the search for modifiable factors and therapeutic measures. Psychological factors such as depression (Soysal et al., 2017), and social factors such as level of education (Ng et al., 2014), income (Marshall et al., 2015), living arrangement (Makizako et al., 2018) and marital status (Peek et al., 2012) have all been identified as being associated with frailty. Similarly, there is also evidence that psychological and social factors influence the onset and/or trajectory of cognitive decline in older people (da Silva et al., 2013; Kuiper et al., 2015). This suggests that psychological and social factors potentially contribute to vulnerability associated with coexistent frailty and cognitive impairment, warranting further exploration, particularly as these are potentially modifiable factors.
While under-researched in comparison with biological factors, psychological and social factors are being increasingly considered in relation to coexistent frailty and cognitive impairment (Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020). Yet, no previous review has explored the influence of psychological and social factors on this coexistent decline. Previous reviews have focussed on biological risk factors or health outcomes (Facal et al., 2019; Sugimoto et al., 2018). Identification of factors which pose risks for developing coexistent frailty and cognitive impairment, and those which might offer protection, is vital in developing appropriate therapies and supportive interventions. Increased awareness of factors which contribute to coexistent decline may improve treatment suitability for older people living with coexistent frailty and cognitive impairment.
Aim: The aim of this review is to identify, and evaluate the quality of, existing evidence regarding the nature and impacts of psychological and social factors in people living with coexistent frailty and cognitive impairment.
Methods
Search Strategy and Registration
The review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (Page et al., 2021). The search strategy included a systematic search of Medline, CINAHL, Embase, AMED, PsychINFO and Cochrane databases (from January 2001 to July 2020 inclusive). Three key search concepts included ‘frailty’, ‘cognitive impairment’ and ‘psychosocial factors’ with a wide range of associated keywords. These were combined in a full text search to enable a comprehensive and inclusive examination of the existing literature. Table 1 gives an example of the search strategy; modifications were made to implement a comparable search in all databases. Additional searches were conducted in EThoS, NTLTD and Proquest Express to identify theses. Forward and backward citation searching was used to identify additional studies from relevant retrieved papers. The review protocol was registered with PROSPERO: CRD42020196086.
Table 1.
Search Term Example: Medline.
| Concept | Theme | Terms |
|---|---|---|
| 1 | Frailty | frai*; “gait speed”; “sarcopenia”; “frailty index”; “clinical frailty scale”; “motoric cognitive risk” π |
| 2 | Cognitive impairment | cogniti*; mci; “mild cognitive impairment”; “memory impairment”; “memory decline”; cind; “cognitive impairment no dementia”; dementia; Alzheimer*; “motoric cognitive risk” π |
| 3 | Psychological and social factors | “social frailty”; Socioeconomic*; “social network”; “social support”; “social environment”; “social environment”; “social vulnerabilit*”; “social contact”; “marital status”; “living alone”; income; educat*; loneliness; isolat*; neighb#rhood; psychology*; depressi*; wellbeing; well-being; “quality of life”; “low mood”; anxiety; “mental health”; “negative affect”; “positive affect”; “self-rated health”; psycho-social; psychosocial |
π Motoric cognitive risk combines both concept 1 and concept 2.
Search combines concepts 1 AND 2 AND 3.
Limited to publications in English from January 2001 to July 2020 inclusive.
Inclusion and Exclusion Criteria
The search was limited to articles written in English published after 2001, when the landmark Cardiovascular Health Study paper (Fried et al., 2001) was published which defined and identified criteria for the measurement of frailty in older people. Although age associated decline cannot be determined by chronological age, and there is global variation, the World Health Organisation considers 60 as a global threshold for old age (WHO, 2018). As such, this age limit was used as an inclusion criterion within this review. We included only studies which used primary data with the additional criteria to identify comparable studies:
Inclusion Criteria
Studies involving individuals aged 60 years or older identified as being both frail and cognitively impaired.
Where frailty is measured using a standardized measure of either the phenotype approach (Fried et al., 2001) or alternatively using a frailty index which has no cognitive, psychological or social components and developed using pre-defined set criteria (Searle et al., 2008).
Where cognitive impairment is defined as an impairment which is more severe than that which is associated with normal ageing, assessed using self-report or validated measures or clinician diagnosis.
Studies exploring factors that were either psychological (e.g. depression, self-esteem or optimism) or social (e.g. income, education, living arrangement, marital status or social support) in nature.
Studies using randomized, quasi-randomized, cohort and case-controlled designs.
Exclusion Criteria
• Studies where data on those aged ≥60 years cannot be extracted.
• Studies that did not include people with both frailty and cognitive impairment, or this was ambiguously defined or measured.
Review Process
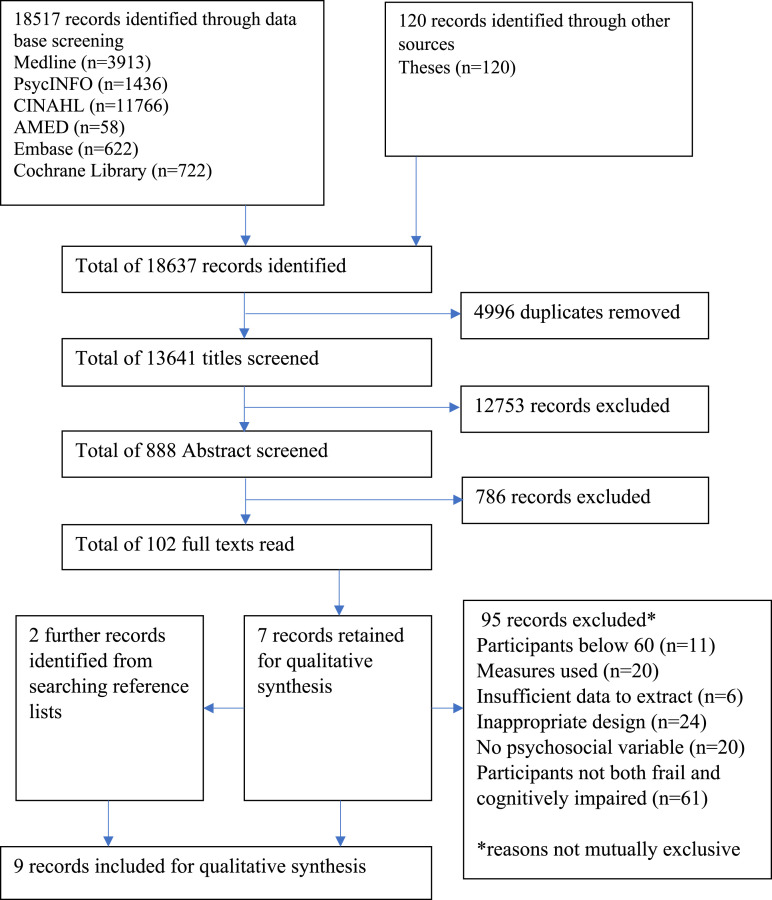
Figure 1 illustrates the literature search process. EndNote Software version X9 was used to facilitate screening. Title, abstract and full text screening was conducted by two reviewers. There was 94.0% agreement on title screening, 81.3% agreement on abstract screening and 93.7% agreement on full text screening. Any disagreements were resolved through discussion until consensus was achieved.
Figure 1:
PRISMA flow chart of records through the screening process.
Data Extraction
For each paper included in the review, information was collected on study design, county, year of data collection, year of publication, the setting, characteristics of the sample (including sample size, age, gender and ethnicity), the instrument to measure frailty, the measure of cognitive impairment and the measure(s) of psychological and social factors. In addition, the main significant associations were extracted. It was noted during extraction that some studies used different thresholds for significance; in this review, a p value of 0.05 was used for the purposes of comparability. Data were extracted by one reviewer and a proportion of papers reviewed by a second.
Study Quality Assessment
Given the heterogeneity of the study designs, quality was assessed using a tool devised to reflect the diversity of analytical approaches included in the review, the 16-item quality assessment tool (QATSDD) (Sirriyeh et al., 2012). Quality was assessed using the following criteria: clear aims, objectives and theoretical basis for the study, sample size rationalized and methods for recruitment and data collection stated and rationalized, analytical methods befitting the research question and evidence of reflection on the study limitations. Each criterion was rated on a four-point scale, with higher scores indicating higher quality, up to a total of 42. Quality assessment was undertaken by two reviewers, and disagreements were resolved through discussion. Studies which were assessed as lower in quality were still included in the review due to the emergent nature of the field, the low number of studies and overall quality.
Results
Study Selection
Figure 1 summarizes study selection. A total of 18,637 records were identified, and 102 were retrieved for full text reading and nine were included for data extraction. The main reasons for excluding studies upon reading the full text were that participants were not identified as both frail and cognitively impaired (n = 61) and the design or analysis was inappropriate for inclusion (n = 24) (see Figure 1).
Study Characteristics
Characteristics of the nine included studies are shown in Table 2. All studies were published between 2016 and 2020, with the majority published since 2019. A total of 24,617 older adults were included. The majority of studies were conducted in Asia, in China (Ge et al., 2020; Kwan et al., 2019), Taiwan (Li et al., 2020; Wu et al., 2020), Malaysia (Rivan et al., 2020; Rivan et al., 2019) and Japan (Shimada et al., 2016). One was conducted in the United States (Aliberti et al., 2019) and one in Mexico (Aguilar-Navarro et al., 2019). Studies were undertaken with those living in the community, although recruitment was through hospital clinics in two studies (Aguilar-Navarro et al., 2019; Wu et al., 2020). There was considerable heterogeneity in study designs (see Table 3). Three studies involved cross-sectional data analysis of cohorts (Aliberti et al., 2019; Rivan et al., 2019; Shimada et al., 2016). One employed a longitudinal cohort design (Rivan et al., 2020). All studies included a population with both physical and cognitive decline with at least one comparison group, and from this they produced prevalence estimates. The reported prevalence of frailty coexistent with cognitive impairment in the study populations was highly variable ranging from 1.2% to 39.6% (Table 3). The majority of studies included three comparison groups, either healthy peers, or those with frailty or cognitive impairment alone (Aguilar-Navarro et al., 2019; Aliberti et al., 2019; Ge et al., 2020; Kwan et al., 2019; Li et al., 2020; Shimada et al., 2016; Wu et al., 2020). Although Aguilar-Navvarro et al. (2019) had three comparison groups, these were used inconsistently in analyses. Rivan et al. (2019) compared those with coexistent frailty and cognitive impairment with only healthy individuals. While in the study by Rivan et al. (2020), the comparison group was less clearly defined and simply described as those who had not developed both frailty and cognitive impairment. Therefore, was likely comprised of a mixed group of healthy individuals and those with frailty or cognitive impairment alone (see Table 3).
Table 2.
Characteristics of Included Studies (n = 9).
| First Author | Publication year | Data collection year(s) | Country | Study setting | Total sample size all participants baseline (and follow up) | Mean age of total sample all participant (±SD) | Gender % female | Quality assessment |
|---|---|---|---|---|---|---|---|---|
| Aguilar-Navarro | 2019 | 2017-2018 | Mexico | Memory clinic | 180 | 72.99 ±6.6 | 75% female | 24 |
| Aliberti | 2019 | 2006-2008 | USA | Community | 7338 | 74.4 ±7.0 | 54.9% female | 29 |
| Ge | 2020 | 2018 | China | Community | 4103 | 67.8 ±5.9 | 58.3% female | 27 |
| Kwan | 2019 | 2018-2018 | China | Community | 185 | 86.2±4.5 | 71.4% female | 24 |
| Li | 2020 | 2013 | Taiwan | Community | 2693 | Not stated | Not stated | 23 |
| Rivan | 2019 | 2012 | Malaysia | Community | 815 | 68.86 ±6.12 | 54.4% female | 29 |
| Rivan | 2020 | 2017 | Malaysia | Community | 490 baseline, 282 Follow-up | Not stated | Not stated | 29 |
| Shimada | 2016 | 2011-2013 | Japan | Community | 8864 | 73.4±5.4 | 52.0% female | 27 |
| Wu | 2020 | 2018 | Taiwan | Geriatric clinic | 157 | 79.4±7.9 | 66.9% female | 20 |
Table 3:
Measures of Included Studies (n = 9).
| First Author, date | Study design | Frailty assessment | Cognitive assessment(s) | Defining mixed group | Comparison groups | Prevalence comorbid group in study population | Psychological factors and assessment methods | Social factors and assessment methods |
|---|---|---|---|---|---|---|---|---|
| Aguilar-Navarro, 2019 e | Cross-sectional | Fried Phenotype a | MCI
c
MRI MMSE NEUROPSI |
Fried 3 or more criteria
a
, MCIv c |
n = 1 healthy |
17% | Depressive symptoms (GDS-15) | None |
| Aliberti, 2019 | Cohort | Fried Phenotype a | HRS validated approach 27-point scale: normal = 12-27; CIND = 7-11; dementia ≤6 | Fried 3 or more criteria
a
, CIND |
n = 3 healthy, frail only CIND only |
5% | Depression (CES-D ≥3 symptoms classed as depression) | Married (yes or no Education level) (completed high school or not) Net worth (total household assets minus current debt) |
| Ge, 2020 | Prospective observational study | Fried Phenotype a | SPMSQ adjusted for education level (+2 for those with high school education or +4 for those with primary education only or lower) | Fried 3 or more criteria, cognitive impairment on SPMSQ a | n = 3 healthy, frail only, cognitive impairment only |
2.9% | Depression (GDS-15 ≥8 symptoms classed as depression) | None |
| Kwan, 2019 | Cross-sectional | FRAIL Scale b | MCI
c
MMSE |
FRAIL 1 or more criteria
b
, MCI c |
n = 3 healthy, frail only, MCI only c |
35.7% | Depressive symptoms (GDS-15) | Education level (primary or below, secondary, tertiary or above) |
| Li, 2020 | Cross-sectional | FRAIL Scale b | MMSE adjusted for education level (24/30 for 7+years education, 20/30 for 1-6 years of education, 17/30 if illiterate) | FRAIL 1 or more criteria b , cognitive impairment on MMSE | n = 3 healthy, frail only, cognitive impairment only |
11% | The presence of anxiety and depression (EQ-5D answers dichotomised yes/no) | Education level (illiterate, 1-6 years, ≥7 years) Marital status (married/living with partner or not) |
| Rivan, 2019 d | Cohort, cross-sectional | Fried Phenotype a | MCI
c
Digit Span or RAVLT MMSE |
Fried 1 or more criteria
a
, MCI c |
n = 1 healthy | 39.6% | Depressive symptoms (GDS-15) | Education level (≤ 6 years or >6 years) Occupation (working or not) Household income (high or low) Marital status (married or not) Social support (MOS-SS) |
| Rivan, 2020 d | Cohort, longitudinal | Fried Phenotype a | MCI
c
, Digit Span or RAVLT MMSE |
Fried 1 or more criteria
a
, MCI c |
n = 1 healthy | 35.5% | Depressive symptoms (GDS-15) | Education level (years) Living alone status (yes/no) Household income |
| Shimada, 2016 | Cohort | Fried Phenotype a | NCGG-FAT 4 domains cognitively impaired if 2 or more limitations | Fried 3 or more criteria
a
, 2-4 limitations on NCGG-FAT |
n = 3 healthy, frail only, cognitive impairment only |
1.2% | Depressive symptoms (GDS-15) | Education Level (years) Living alone status (yes or no) |
| Wu, 2020 | Cross-sectional | FRAIL Scale b | CDR = 1-2 mild to moderate dementia MMSE 10-24 |
FRAIL 3 or more criteria
b
, CDR = 1-2 mild to moderate dementia MMSE 10-24 |
n = 2 cognitive impairment only, cognitive impairment and pre-frailty |
15.9% | Depression (GDS-15 ≥6 points classed as Depression) | Marital status, (Married or not) Living condition (living with family or not) Education Level (years) |
aFried (Fried et al. 2001) frailty phenotype 5 criteria for measurement: weight-loss, poor endurance, reduced physical activity, slow gait, and weak grip. 0 criteria classified as healthy, 1 or 2 criteria classified as pre-frail, 3 criteria classified as frail, based on the Cardiovascular Health Study criteria, studies use the criteria and base cut offs around gender and age specific cut offs
bFRAIL Scale (Morley et al. 2012): Fatigue, Resistance, Ambulation, Illness, Loss of weight) frailty 3-5, pre-frailty 1-2; healthy 0
cMCI: Mild Cognitive Impairment by Petersen Criteria (Petersen 2004) CDR = 0.5 (Berg 1984); intact functional activities; subjective memory complaint
dRivan 2019 and Rivan, 2020 used the same cohort, one as a cross-sectional study the other as longitudinal.
eAguilar-Navarro 2019 had 3 control groups, however when comparing groups on depressive symptoms they compared the mixed group and healthy group.
MRI: Magnetic Resonance Imaging; MMSE: Mini-Mental State Examination; CDR: Clinical Dementia Rating scale; NEUROPSI: neuropsychological evaluation in Spanish; MCIv: Mild Cognitive Impairment with vascular aetiology; GDS-15: Geriatric Depression Scale-15 item; HRS: Health and Retirement Study; CIND: Cognitive Impairment No Dementia; CES-D: Center for Epidemiologic Studies Depression Scale; SPMSQ: Short Portable Mental Status Questionnaire; EQ-5D: EuroQol-5 dimension; RAVLT: Rey Auditory Verbal Learning Test; NCGG-FAT: National Center for Geriatrics and Gerontology Functional Assessment Tool; MOS-SS: Medical Outcomes Study Social Support Survey.
Methodological Quality of Included Studies
The quality of studies was moderate with scores ranging from 20 to 29 out of a possible 42 (see Table 2). The approach to data collection and analysis was generally of a high standard; however, there were no theoretical considerations described in any included study. In addition, there was no described involvement from those living with frailty and cognitive impairment in the conception or design of the studies.
Measures of Frailty and Cognitive Impairment
Frailty was evaluated with relative consistency across the studies. The Cardiovascular Health Study criteria (Fried et al., 2001) were used in six studies (Aguilar-Navarro et al., 2019; Aliberti et al., 2019; Ge et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016) (see Table 3). With this definition frailty is measured through weight-loss, poor endurance, reduced physical activity, slow gait and weak grip. Participants were classified as frail if they meet any three, or more, criteria and pre-frail if they meet one or two. However, studies did vary in how they collected this data and applied established population appropriate cut-off points. The remaining three studies used the FRAIL scale (Morley et al., 2012) (Kwan et al., 2019; Li et al., 2020; Wu et al., 2020) collecting data on fatigue, resistance, ambulation, illness and loss of weight. For this scale having three or more criteria is classified as frail and pre-frail if there was evidence of one or two.
There was greater variation in how cognitive impairment was measured by the studies (see Table 3). Five studies used multiple assessments to establish that participants were cognitively impaired (Aguilar-Navarro et al., 2019; Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019). Assessments included the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) (Aguilar-Navarro et al., 2019; Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Wu et al., 2020); Short Portable Mental Status Questionnaire (SPMSQ) (Pfeiffer, 1975) (Ge et al., 2020); National Centre for Geriatrics and Gerontology-Functional Assessment Tool (NCGG-FAT) (Makizako et al., 2013) (Shimada et al., 2016); Rey Auditory Verbal Learning Test (RAVLT) (Schmidt, 1996) (Rivan et al., 2020; Rivan et al., 2019); Digit Span Forward and Backward test (Rivan et al., 2020; Rivan et al., 2019); neuropsychological evaluation in Spanish (NEUROPSI) (Ostrosky-Solís et al., 2007) (Aguilar-Navarro et al., 2019); Health Retirement Study (HRS) (Sonnega et al., 2014) validated assessment (Aliberti et al., 2019), diagnostic criteria such as Petersen criteria for MCI (Petersen, 2004) (Aguilar-Navarro et al., 2019; Rivan et al., 2020; Rivan et al., 2019) and/or the Clinical Dementia Rating scale (Berg, 1984) (Aguilar-Navarro et al., 2019; Kwan et al., 2019; Wu et al., 2020) and neuroimaging techniques Magnetic Resonance Imaging (MRI) (Aguilar-Navarro et al., 2019). Two studies adjusted cut-off points in line with the educational level of participants for the SPMSQ (Ge et al., 2020) and the MMSE (Li et al., 2020). Four studies (Aguilar-Navarro et al., 2019; Kwan et al., 2019; Rivan et al., 2020; Rivan et al., 2019) used subjective memory complaints as part of an MCI diagnosis but no studies relied upon subjective memory complaints alone.
The ways in which studies identified frail and cognitively impaired groups was variable, with all except one study (Wu et al., 2020) excluding those with dementia from involvement in their study. Furthermore, four studies included those with pre-frailty in their experimental group (Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019).
Psychological Factors
Data on depression or depressive symptoms were collected in all studies included in the review (see Table 3), one of these included anxiety and depression (Li et al., 2020). Three measures were used, and the Geriatric Depression Scale (GDS) (Yesavage, 1988) was collected most frequently (n = 7) (Aguilar-Navarro et al., 2019; Ge et al., 2020; Kwan et al., 2019; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016; Wu et al., 2020), the Centre for Epidemiologic Studies Depression Scale (CES-D) (Turvey et al., 1999) (n = 1) (Aliberti et al., 2019) and the EuroQol-5 dimension (EQ-5D) (Rabin & Charro, 2001) (n = 1) (Li et al., 2020) were also collected. Measures were scored with a cut-off to indicate clinical depression or not (n = 4) (Aliberti et al., 2019; Ge et al., 2020; Li et al., 2020; Wu et al., 2020), or summed to give a score (n = 5) (Aguilar-Navarro et al., 2019; Kwan et al., 2019; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016).
Table 4 outlines the relationships between various factors and coexistent frailty and cognitive impairment. The findings from nine studies (Aguilar-Navarro et al., 2019; Aliberti et al., 2019; Ge et al., 2020; Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016; Wu et al., 2020) show those living with coexistent frailty and cognitive impairment have significantly higher levels of depressive symptoms than the comparison peer groups. Of particular interest is the only study which included individuals with dementia (Wu et al., 2020) with depression being far higher in those with frailty than those with pre-frailty (88% depressed with frailty vs. 56.2% depressed with pre-frailty). Additionally, further analyses uniformly reveal a significant positive association between depression and the coexistence of frailty and cognitive impairment (see Table 5).
Table 4:
Results of Studies Examining Differences between Groups.
| Factor | Citation | Statistical analysis | Finding | interpretation |
|---|---|---|---|---|
| Depressive Symptoms | Aguilar-Navarro, 2019
a
Aliberti, 2018 a Ge, 2020 a Kwan, 2019 a Rivan, 2019 b Rivan, 2020 b Shimada, 2016 a Wu, 2020 c |
Chi-square Chi-square Chi-square t-test, chi-square, Mann-Whitney U t-test t-test t-test one-way ANOVA |
Significant at <0.001 Significant at <0.001 Significant at <0.001 Significant at <0.001 Significant at <0.001 Significant at <0.001 Significant at <0.001 Significant at <0.001 |
Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers Comorbid group greater number of symptoms than peers |
| Depression and anxiety | Li, 2020 a | Chi-square | Significant at <0.0001 | Comorbid group report more problems than peers |
| Marital status | Aliberti, 2018
a
Li, 2020 a Rivan, 2019 b Wu, 2020 c |
Chi-square Chi-square Chi-square Chi-square |
Significant at <0.001 Significant at <0.0001 Not significant =0.936 Not significant +0.619 |
Comorbid group less likely to be married than peers Comorbid group less likely to be married than peers No difference in marital status across groups No difference in marital status across groups |
| Living alone status | Rivan, 2020
b
Shimada, 2016 a Wu, 2020 c |
Chi-square Chi-square Chi-square |
Significant at <0.05 Significant at <0.001 Not Significant =0.910 |
Comorbid group more likely to live alone than peers Comorbid group more likely to live alone than peers No difference in co-residence status across groups |
| Education level | Aliberti, 2018
a
Kwan, 2019 a Li, 2020 a Rivan, 2019 b Rivan, 2020 b Shimada, 2016 a Wu, 2020 c |
Chi-square t-test, chi-square, Mann-Whitney U Chi-square Chi-square t-test t-test One-way ANOVA |
Significant at <0.001 Not significant =0.053 Significant at <0.0001 Significant at <0.001 Significant at <0.001 Significant at <0.001 Not significant =0.102 |
Comorbid group less years in education than peers No difference in education years across groups Comorbid group less years in education than peers Comorbid group less years in education than peers Comorbid group less years in education than peers Comorbid group less years in education than peers No difference in education years across groups |
| Employment status | Rivan, 2019 b | Chi-square | Not significant =0.652 | No difference in employment status across groups |
| Net worth | Aliberti, 2018 a | ANOVA or Kruskal-Wallis | Significant at <0.001 | Comorbid group has less net worth than peers |
| Household income | Rivan, 2019
b
Rivan, 2020 b |
Chi-square t-test |
Significant at <0.05 Significant at =0.001 |
Comorbid group has less income than peers Comorbid group has less income than peers |
| Social Support | Rivan, 2019 b | t-test | Significant at <0.001 | Comorbid group reports less support than peers |
astudy has four groups to compare a healthy group, a frail only group, a cognitively impaired only group and a comorbid group with both frailty and cognitive impairment
bstudy has two groups to compare a healthy group and a comorbid group with both frailty and cognitive impairment
cstudy has three groups to compare one with dementia and no frailty, one with dementia and prefrailty and a comorbid group with dementia and frailty
Table 5.
Relationships Between Various Factors and Co-Existent Frailty and Cognitive Impairment.
| Factor | Citation | Statistical analysis | Finding | interpretation |
|---|---|---|---|---|
| Depressive Symptoms | Aguilar-Navarro, 2019 Ge, 2020 Kwan, 2019 Rivan, 2019 Rivan, 2020 Wu, 2020 |
Multinomial logistic regression Multinomial logistic regression Multinomial logistic regression Hierarchical binary logistic regression Stepwise binary logistic regression Multiple logistic regression |
Significant association 0.007 Significant association <0.001 Adjusted model significant association = 0.002 Significant association <0.001 Significant association = 0.007 Significant association = 0.022 |
Greater number of depressive symptoms associated with comorbidity Presence of depression associated with comorbidity Greater number of depressive symptoms associated with comorbidity Presence of depression associated with comorbidity Greater number of depressive symptoms associated with comorbidity Greater number of depressive symptoms associated with comorbidity |
| Depression and anxiety | Li, 2020, | Poisson regression model | Significant association <0.001 | Reporting of problems with anxiety and depression associated with comorbidity |
| Education level | Wu, 2020 | Multiple logistic regression | Not significant association = 0.924 | Years of education not associated with comorbidity |
| Social Support | Rivan, 2019 | Hierarchical binary logistic regression | Significant association <0.001 | Reported lower levels of social support associated with comorbidity |
Social Factors
A range of social variables were investigated, but with no consistency in approach and in little detail. Education was collected in seven studies, either categorizing level of education (Aliberti et al., 2019; Kwan et al., 2019; Li et al., 2020; Rivan et al., 2019) or years spent in education (Rivan et al., 2020; Shimada et al., 2016; Wu et al., 2020) (Table 3). The findings related to education were mixed (Table 4). Five studies (Aliberti et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016) identified those living with coexistent frailty and cognitive impairment had spent less time in education than comparison groups, while two studies (Kwan et al., 2019; Wu et al., 2020) reported no difference. Only one study (Wu et al., 2020) included education in regression modelling (Table 5); this found for the population they studied, education was not associated with the coexistence of frailty and cognitive impairment.
Marital status was collected in four studies (Aliberti et al., 2019; Li et al., 2020; Rivan et al., 2019; Wu et al., 2020). Two studies (Aliberti et al., 2019; Li et al., 2020) reported higher numbers of unmarried individuals in their group of frail and cognitively impaired individuals. The remaining two (Rivan et al., 2019; Wu et al., 2020) found no significant difference. Living alone was found to be significantly more common in the comorbid group in two studies (Rivan et al., 2020; Shimada et al., 2016) while another (Wu et al., 2020) did not find this association. Lack of social support was only addressed by one study (Rivan et al., 2019), which found that lower social support was associated with the coexistence of frailty and cognitive impairment.
The impact of financial resources was investigated in three studies either by assessing net worth (Aliberti et al., 2019) or household income (Rivan et al., 2020; Rivan et al., 2019). All three studies revealed those living with coexistent frailty and cognitive impairment had consistently fewer financial resources than peers. Having a current occupation was only investigated in one study (Rivan et al., 2019); the results revealing no differences whether people were currently occupied or not between the comorbid group and the comparison group.
Discussion
This review identified only nine studies which investigated the relationship between psychological or social factors and frailty coexistent with cognitive impairment. Relationships with depression were examined most frequently and in greatest depth. All nine studies found those with coexistent frailty and cognitive impairment were more likely to report having depressive symptoms than their peers. Social factors such as education, marital status and financial resources were rarely investigated in the same level of detail, and findings were varied on the difference between groups on these factors.
Measures of Frailty and Cognitive Impairment
Across the included studies frailty was assessed and recorded with consistency. Despite a number of assessments available which measure frailty, only the CHS criteria (Fried et al., 2001) and the FRAIL scale (Morley et al., 2012) were used. This may be attributed to these measures being preferred forms of assessment. Those classified as pre-frail were included within the comorbid group in some studies (Kwan et al., 2019; Rivan et al., 2020; Rivan et al., 2019) but not in others (Aliberti et al., 2019; Ge et al., 2020; Li et al., 2020; Shimada et al., 2016; Wu et al., 2020). In contrast to the consistency in frailty assessment, a range of tools were used to evaluate cognitive impairment in the reviewed studies. This may be partly due to the level of inclusivity within this review, for example, Aguilar-Navarro et al. (2019) were working with a population exclusively with mild cognitive impairment and vascular burden, while (Wu et al., 2020) were investigating only those with diagnoses of dementia. The variability in selection and application of measures for cognition, and the inclusion of pre-frailty in some studies, created complexity and limitations when comparing and synthesizing findings.
Psychological Factors
Depression was explored with the greatest consistency, frequency, and depth in the studies (Aguilar-Navarro et al., 2019; Aliberti et al., 2019; Ge et al., 2020; Kwan et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016; Wu et al., 2020). Depressive symptom levels were variable across studies but consistently greater in the comorbid group. These findings are echoed by the findings of reviews which found depression to be associated with frailty syndrome (Soysal et al., 2017) and with cognitive impairment (da Silva et al., 2013). This suggests there may be connections between depression and later life decline, be that either cognitive or physical or both. In four of the reviewed studies (Aliberti et al., 2019; Kwan et al., 2019; Li et al., 2020; Shimada et al., 2016), there were similar levels of depressive symptoms in those solely diagnosed with frailty. However, in two others (Aguilar-Navarro et al., 2019; Ge et al., 2020) the levels of depressive symptoms were far higher in the comorbid group than any of the comparison groups. These findings may implicate increased risk in those with both physical and cognitive decline. Wu et al. (2020) reported that depression was far higher in those with frailty and dementia than those with pre-frailty and dementia. One interpretation of this could be that physical decline contributes more to low mood than cognitive impairment, although these findings are based on small numbers. Given the higher levels of depressive symptomology evidenced in the comorbid groups in the reviewed studies, this is an area which may benefit from longitudinal investigation. Additionally, all studies focussed on identifying current levels of depressive symptoms and did not consider previous bouts of depression over the life course. Moreover, some studies excluded those diagnosed with depression (Aguilar-Navarro et al., 2019; Shimada et al., 2016) or psychiatric disorders (Rivan et al., 2020; Rivan et al., 2019). It is worth noting that poor endurance or fatigue, a frailty phenotype criterion, is also a symptom of depression. There is some overlap between symptoms of both frailty and cognitive impairment, and those of depression. This may create complexity firstly in diagnosing the etiology of a symptom such as fatigue, and furthermore understanding the relationship between symptoms and different health states. A history of depression has been associated with both physical and cognitive decline in later life, suggesting a long-term relationship (Crimmins, 2020). Evidence indicates that certain individuals may be predisposed to both frailty and depression, and that there may not be a causal link between depression and frailty as formerly thought; however, this requires further investigation (Mayerl et al., 2020). As the studies included in the review primarily used cross-sectional methods, with the exception of (Rivan et al., 2020), this limits the understanding which can be gained about this complex relationship.
It has been proposed that psychological distress (predominantly depression and anxiety) may escalate the development of cognitive complaints in those with frailty (Jing et al., 2020). Therefore, psychological distress may highlight a particular risk of cognitive impairment for those with frailty. Anxiety was considered only in one study (Li et al., 2020), and then only in partnership with depression and with one single self-report question. Anxiety is worthy of greater investigation given the relationship anxiety may have with increased stress hormones such as cortisol, and their contribution to frailty syndrome (Baylis et al., 2013). There are also other psychological factors that might be pertinent to explore in people with coexistent frailty and cognitive impairment. For example, psychological resources such as self-esteem and optimism have been shown to impact positively on ageing (Dumitrache et al., 2019; Hornby-Turner et al., 2017), while positive attitudes to ageing appear to decrease the risk of frailty associated decline (Gale et al., 2018). In addition to this, affective disorders have been linked with dementia risk (da Silva et al., 2013). Further work, beyond depression, is essential in understanding the role psychological factors may play in the onset and trajectory of physical and cognitive decline.
Social Factors
Social determinants can influence the onset and trajectory of decline in later life (Andrew et al., 2008; Crimmins, 2020). This review found that relationships between frailty coexistent with cognitive impairment and education were not consistent across studies. Other work has demonstrated that there are links between frailty development and lower education levels (Dury et al., 2017), and between impaired cognition and lower education levels (Chapko et al., 2017). Considerable variation in the educational levels of study participants was identified across the reviewed studies. However, studies which reported no differences between those with coexistent frailty and cognitive impairment and comparison groups involved participants with low levels of education or high levels of homogeneity. In one study, participants had an average of less than 5 years of schooling (Wu et al., 2020), and in another only 13% of the study population had above primary level education (Kwan et al., 2019).
Instead of purely focussing on education, greater focus should perhaps be placed on lifelong engagement with activity which involves engagement with learning across the life course. Higher childhood education level, adulthood occupation and current engagement with cognitively stimulating activity have been associated with better outcomes for older adults (Ihle et al., 2017). Implicating the benefits of continued engagement with activity that promotes thinking and learning. Such exploration would be of particular value given that current engagement with cognitively stimulating activity may prove more modifiable than educational status in childhood. While there is evidence that lower educational attainment is related to health behaviours such as smoking and alcohol consumption, there may be other ways in which education contributes to later life decline (Braveman & Gottlieb, 2014). The accumulation of wear and tear across multiple physiological systems, which is termed allostatic load, is associated with chronic exposure to stress (McEwen & Stellar, 1993). Lower educational attainment may be a source of lifelong stress, given that higher education is often associated with more lucrative and secure employment in adulthood (Braveman & Gottlieb, 2014). A longitudinal study of cohort data has identified that increased allostatic load may be a possible mechanism by which educational attainment contributes to increased frailty risk (Gale et al., 2016).
Living condition or marital status were collected in six studies (Aliberti et al., 2019; Li et al., 2020; Rivan et al., 2020; Rivan et al., 2019; Shimada et al., 2016; Wu et al., 2020). One study (Wu et al., 2020) collected both. As detailed in the results, findings were mixed across the reviewed studies, suggesting that this would benefit from more in-depth exploration. While marital status may be a proxy measure of increased practical and emotional support and financial security, this is unlikely to always be the case. In the included studies, populations were almost all married and often did not define differences in being widowed, divorced or never married. While frailty syndrome is often linked with being unmarried, potential gender differences and the possible variations between the experiences of those who have never married and those who are widowed or separated from their spouse have raised questions about why this relationship occurs (Kojima et al., 2019). Longitudinal study into the relationship between loneliness and health shows increasing loneliness with ageing and that increased loneliness is associated with poorer health. However, this may be mediated by partner status, those who experience spousal loss experience greater loneliness (Dykstra et al., 2005).
Only one study examined levels of social support, finding those living with frailty and cognitive impairment reported lower levels of support than a matched group of healthy older people, perhaps suggesting social support may offer protection against comorbid decline (Rivan et al., 2019). However, as this study employed a cross-sectional design, this may simply be support which is currently required through necessity. Social support may present as one of the few modifiable factors identified by this review. A UK-based study of cross-sectional data found that cognitive and social activity were protective of cognitive function in later life (Clare et al., 2017). Other investigation implicates the value of social ties in maintaining good health in later life, however, stipulating that the quality of such relationships may be key to this health benefit (Rook & Charles, 2017). The role, which the presence or absence, or the nature of support may play in the onset of physical and cognitive decline would benefit from longitudinal exploration.
This review found that those with both frailty and cognitive impairment had lower net worth or income. This suggests an association between affluence and poor outcomes in later life. The rate at which frailty and cognitive impairment accelerate may well be attenuated by the financial resources an individual has access to (Steptoe & Zaninotto, 2020). Financial security in later life is likely to be beneficial to health and wellbeing, given the links this has with adequate housing, use of safe outdoors environments, capacity to fund healthcare and access to leisure activities (Braveman & Gottlieb, 2014). Within this review, financial resources were shown to be impactful; however, this requires further investigation. While current wealth may be an important consideration, lifelong wealth and social standing appear to play a role in later life decline. Findings from a UK study show that paternal socioeconomic status in childhood impacts on health outcomes in later life, and this was attributed to increased levels of physiologic dysregulation in response to stressor events (Gale et al., 2016). The findings of Gale and colleagues in 2016 were consistent with work in Latin America (Alvarado et al., 2008) and Europe (Landös et al., 2019), where the consequence of childhood poverty on health was still evident in later life. While some impact may be attenuated by improving circumstances throughout adulthood understanding the long-term impact of wealth and social status requires further research.
Strengths and Limitations
This review has many strengths, including measures of frailty and cognitive impairment enabled a wide scope of the existing literature. In addition, searching the full text of publications, rather than keyword, title or abstract search has limited the risk of overlooking a study. However, limiting searches to publications written in English will have restricted the search yield, particularly given the high number of studies identified from countries where English is not a native language. As part of the exclusion criteria for this review we excluded frailty measures that contained cognitive, psychological or social components. This was to try and ensure that there was minimal overlap between the measurement of frailty, the measurement of cognitive impairment and the outcomes of interest. Therefore, frailty indices which included outcomes of interest, such as depression, were excluded from this synthesis; symptoms of frailty and cognitive impairment are shared with conditions such as depression and may contribute to this observed close relationship. Rigour in research is a key factor in advancing knowledge, and while this review aimed to be inclusive, the moderate quality of many of the included studies limits conclusions drawn from this synthesis. However, given the emergent nature of the field, overall quality and low number of studies, inclusion was considered relevant. The current evidence base predominantly originates from Asia; research is required from other continents to ensure cross-cultural application. While the review included papers from 2001, all papers included for synthesis were published since 2016. Suggesting this topic, this is an emergent area of interest.
Future Research and Implications for Practice
The need to investigate the characteristics of individuals living with coexistent frailty and cognitive impairment is highlighted by the cumulative effect of different deficits upon wellbeing (Avila-Funes et al., 2009). Such research is ever more pertinent in the COVID-19 era, where many older adults have been increasingly isolated, and access to healthcare and support services has been limited (Baker & Clark, 2020). While depression was investigated in the reviewed studies, there was far less consideration of the role other psychological factors may play in posing a risk or protection for decline. Turning attention towards an assets-based approach and identifying potential protective factors may lead to improved intervention development. Exploration of the lifelong psychological and social circumstances and experiences of those living with coexistent frailty and cognitive impairment may lead to a greater understanding of this comorbid decline and how it might be ameliorated.
Many of the studies included in this review were seeking to test other hypotheses, such as mortality rates. Socioeconomics and comorbid depression were often secondary analyses, examining the relationship such factors have with comorbid deterioration but with insufficient consideration of how they might influence the nature and course of decline. Furthermore, the examination of modifiable factors must be a focus of research. Physical activity levels, smoking, alcohol intake and nutrition have been reviewed extensively as modifiable risk factors in relation to frailty (Lafortune et al., 2016) and dementia (Beydoun et al., 2014). However, focussing upon behavioural factors does little to fully acknowledge the multidimensional nature of later life decline. While improving educational attainment in childhood may be a long-term goal, improving access to lifelong learning and engagement with cognitively stimulating activity may have more immediate benefits. Treatments and improved identification of depression across the life course are implicated. While other modifiable factors such as social support, access to better housing and safe outdoor spaces and timely access to health and social care require investigation with this population.
Health and social care professionals play a significant role in providing not only healthcare but also support to those who live with frailty and cognitive impairment. Many working in the field acknowledge that psychological and social factors play a role in the onset and trajectory of decline in later life (Colón-Emeric et al., 2020; Gee et al., 2019). However, there is still a heavy focus on the physiological factors which are associated with later life decline (D’Avanzo et al., 2017). Increasingly gerontologists are acknowledging the implications of life course events, social factors and psychological wellbeing (Crimmins, 2020). How far this is understood and taken forward in health and social care practice is less explored and requires investigation. A tailored approach to treating and supporting individuals, with in-depth consideration of psychological and social past and present circumstances, and multidisciplinary input, is likely to be required to enable those living with coexistent frailty and cognitive impairment to live well as they age.
Conclusion
To conclude, this review highlights social and psychological differences between those living with coexistent frailty and cognitive impairment. The findings indicate that depression, lower education, lower material wealth and less social support are related to this comorbid state. Marital status and living alone may also play a role but this is less clear and current occupation is not shown to have an impact. The review identifies a limited number of psychological and social factors that have been explored in this area and highlights further research is required, particularly in the identification of modifiable factors. Understanding the psychological and social factors linked to coexistent frailty and cognitive impairment may aid in the identification and development of interventions to reduce the incidence of physical and cognitive decline in later life and lead to improved support for those living with coexistent frailty and cognitive decline.
Acknowledgements
Dr. Elizabeth Teale, of the University of Leeds, supported with development of the review aim and writing of the protocol. Dr. Michael Cross, of the University of Bradford, provided statistical advice on the full text papers. This PhD studentship is supported by the National Institute for Health Research, Yorkshire and Humber Applied Research Collaborations. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Author Biographies
Alison Ellwood is a PhD student at the University of Bradford. Her doctoral research focuses on older adults with coexistent physical frailty and cognitive impairment. Her previous work in research was focused on improving service provision with older people in care homes, hospitals and community settings.
Catherine Quinn is a Associate Professor whose research focuses on how we can better support people with dementia and their carers, both through gaining a better understanding of what enables people to ‘live well’ and through the development of psychosocial interventions. She also has an interest in positive experiences in providing care.
Gail Mountain is a Professor of Applied Dementia Research, University of Bradford. She also holds a visiting Professorship at University of Sheffield, and is Research Associate within the Lab4living health and design collaboration at Sheffield Hallam University. Professor Mountain worked as an occupational therapist prior to commencing a research career.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the University of Bradford.
ORCID iD
Alison Ellwood https://orcid.org/0000-0001-8632-1830
References
- Aguilar-Navarro S. G., Mimenza-Alvarado A. J., Corona-Sevilla I., Jiménez-Castillo G. A., Juárez-Cedillo T., Ávila-Funes J. A., Román G. C. (2019). Cerebral vascular reactivity in frail older adults with vascular cognitive impairment. Brain sciences, 9(9), 214. 10.3390/brainsci9090214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliberti M. J. R., Cenzer I. S., Smith A. K., Lee S. J., Yaffe K., Covinsky K. E. (2019). Assessing risk for adverse outcomes in older adults: The need to include both physical frailty and cognition. Journal of the American Geriatrics Society, 67(3), 477−483. 10.1111/jgs.15683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado B. E., Zunzunegui M.-V., Béland F., Bamvita J.-M. (2008). Life course social and health conditions linked to frailty in Latin American older men and women. The journals of gerontology. Series A, Biological sciences and medical sciences, 63(12), 1399−1406. 10.1093/gerona/63.12.1399 [DOI] [PubMed] [Google Scholar]
- Andrew M. K., Mitnitski A. B., Rockwood K. (2008). Social vulnerability, frailty and mortality in elderly people. PloS one, 3(5), e2232. 10.1371/journal.pone.0002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Funes J. A., Amieva H., Barberger-Gateau P., Le Goff M., Raoux N., Ritchie K., Carrière I., Tavernier B., Tzourio C., Gutiérrez-Robledo L. M., Dartigues J.-F. (2009). Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: The three-city study. Journal of the American Geriatrics Society, 57(3), 453−461. 10.1111/j.1532-5415.2008.02136.x [DOI] [PubMed] [Google Scholar]
- Baker E., Clark L. L. (2020). Biopsychopharmacosocial approach to assess impact of social distancing and isolation on mental health in older adults. British Journal of Community Nursing, 25(5), 231−238. 10.12968/bjcn.2020.25.5.231 [DOI] [PubMed] [Google Scholar]
- Baylis D., Bartlett D. B., Syddall H. E., Ntani G., Gale C. R., Cooper C., Lord J. M., Sayer A. A. (2013). Immune-endocrine biomarkers as predictors of frailty and mortality: A 10-year longitudinal study in community-dwelling older people. Age, 35(3), 963–971. 10.1007/s11357-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L. (1984). Clinical dementia rating. The British Journal of Psychiatry, 145(3), 339. 10.1192/S0007125000118082 [DOI] [PubMed] [Google Scholar]
- Beydoun M. A., Beydoun H. A., Gamaldo A. A., Teel A., Zonderman A. B., Wang Y. (2014). Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC public health, 14(1), 643. 10.1186/1471-2458-14-643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P., Gottlieb L. (2014). The Social Determinants of health: It’s time to consider the causes of the causes. Public health reports (1974), 129(1_suppl2), 19–31. 10.1177/00333549141291s206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D., Batterham P. J., Mackinnon A. J. (2019). Long-term associations between physical frailty and performance in specific cognitive domains. Journals of Gerontology Series B: Psychological Sciences & Social Sciences, 74(6), 919–926. 10.1093/geronb/gbx177 [DOI] [PubMed] [Google Scholar]
- Chapko D., McCormack R., Black C., Staff R., Murray A. (2017). Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia–A systematic literature review. Aging & mental health, 22(8), 921–932. 10.1080/13607863.2017.1348471 [DOI] [PubMed] [Google Scholar]
- Clare L., Wu Y.-T., Teale J. C., MacLeod C., Matthews F., Brayne C., Woods B., CFAS-Wales study team . (2017). Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study. PLoS medicine, 14(3), e1002259. 10.1371/journal.pmed.1002259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A., Bates C., Young J., Ryan R., Nichols L., Ann Teale E., Mohammed M. A., Marshall T. (2016). Development and validation of an electronic frailty index using routine primary care electronic health record data. Age and ageing, 45(3), 353–360. 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A. D., Young J. P., Iliffe S. P., Rikkert M. O. P., Rockwood K. P. (2013). Frailty in elderly people. The Lancet, 381(9868), 752–762. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Emeric C., Pieper C. F., Schmader K. E., Sloane R., Bloom A., McClain M., Magaziner J., Huffman K. M., Orwig D., Crabtree D. M., Whitson H. E. (2020). Two approaches to classifying and quantifying physical resilience in longitudinal data. The journals of gerontology. Series A, Biological sciences and medical sciences, 75(4), 731–738. 10.1093/gerona/glz097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. (2020). Social hallmarks of aging: Suggestions for geroscience research. Ageing Research Reviews, 63, 101136. 10.1016/j.arr.2020.101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J., Gonçalves-Pereira M., Xavier M., Mukaetova-Ladinska E. B. (2013). Affective disorders and risk of developing dementia: systematic review. British journal of psychiatry, 202(3), 177–186. 10.1192/bjp.bp.111.101931 [DOI] [PubMed] [Google Scholar]
- de Vries N. M., Staal J. B., van Ravensberg C. D., Hobbelen J. S. M., Olde Rikkert M. G. M., Nijhuis-van der Sanden M. W. G. (2011). Outcome instruments to measure frailty: A systematic review. Ageing research reviews, 10(1), 104–114. 10.1016/j.arr.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Dumitrache C. G., Rubio L., Cordón-Pozo E. (2019). Successful aging in Spanish older adults: The role of psychosocial resources. International psychogeriatrics, 31(2), 181–191. 10.1017/S1041610218000388 [DOI] [PubMed] [Google Scholar]
- Dury S., De Roeck E., Duppen D., Fret B., Hoeyberghs L., Lambotte D., Van der Elst M., van der Vorst M., Schols J., Kempen G., Rixt Zijlstra G. A., De Lepeleire J., Schoenmakers B., Kardol T., De Witte N., Verté D., De Donder L., De Deyn P. P., Sebastia E., Dierckx E. (2017). Identifying frailty risk profiles of home-dwelling older people: Focus on sociodemographic and socioeconomic characteristics. Aging & Mental Health, 21(10), 1031–1039. 10.1080/13607863.2016.1193120 [DOI] [PubMed] [Google Scholar]
- Dykstra P. A., van Tilburg T. G., Gierveld J. d. J. (2005). Changes in older adult loneliness: Results from a seven-year longitudinal study. Research on Aging, 27(6), 725–747. 10.1177/0164027505279712 [DOI] [Google Scholar]
- D’Avanzo B., Shaw R., Riva S., Apostolo J., Bobrowicz-Campos E., Kurpas D., Bujnowska M., Holland C. (2017). Stakeholders’ views and experiences of care and interventions for addressing frailty and pre-frailty: A meta-synthesis of qualitative evidence. PLoS One, 12(7), e0180127. 10.1371/journal.pone.0191763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facal D., Maseda A., Pereiro A. X., Gandoy-Crego M., Lorenzo-López L., Yanguas J., Millán-Calenti J. C. (2019). Cognitive frailty: A conceptual systematic review and an operational proposal for future research. Maturitas, 121, 48–56. 10.1016/j.maturitas.2018.12.006 [DOI] [PubMed] [Google Scholar]
- Feng L. P., Zin Nyunt M. S. P., Gao Q. P., Feng L. P., Yap K. B. M., Ng T.-P. M. D. P. (2016). Cognitive frailty and adverse health outcomes: Findings from the singapore longitudinal ageing studies (SLAS). Journal of the American Medical Directors Association, 18(3), 252–258. 10.1016/j.jamda.2016.09.015 [DOI] [PubMed] [Google Scholar]
- Feng Z., Lugtenberg M., Franse C., Fang X., Hu S., Jin C., Raat H. (2017). Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PloS one, 12(6), e0178383. 10.1371/journal.pone.0178383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W. J., Burke G., McBurnie M. A., Cardiovascular Health Study Collaborative Research . (2001). Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences, 56(3), M146–M157. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Gale C. R., Booth T., Starr J. M., Deary l. J. (2016). Intelligence and socioeconomic position in childhood in relation to frailty and cumulative allostatic load in later life: The Lothian birth cohort 1936. Journal of Epidemiology and Community Health, 70(6), 576–582. 10.1136/jech-2015-205789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R., Westbury L., Cooper C. (2018). Social isolation and loneliness as risk factors for the progression of frailty: the English Longitudinal Study of Ageing. Age and ageing, 47(3), 392–397. 10.1093/ageing/afx188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge M., Zhang Y., Zhao W., Yue J., Hou L., Xia X., Zhao Y., Liu X., Dong B., Ge N. (2020). Prevalence and its associated factors of physical frailty and cognitive impairment: Findings from the West China health and aging trend study (WCHAT). Journal of Nutrition, Health & Aging, 24(5), 525–533. 10.1007/s12603-020-1363-y [DOI] [PubMed] [Google Scholar]
- Gee S. B., Cheung G., Bergler U., Jamieson H. (2019). “There’s more to frail than that”: Older New Zealanders and health professionals talk about frailty. Journal of Aging Research, 2019, 2573239. 10.1155/2019/2573239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby-Turner Y. C., Peel N. M., Hubbard R. E. (2017). Health assets in older age: a systematic review. BMJ open, 7(5), e013226. 10.1136/bmjopen-2016-013226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. P., Berg L., Danziger W. L., Coben L. A., Martin R. L. (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry, 140(6), 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Ihle A., Gouveia É. R., Gouveia B. R., Freitas D. L., Jurema J., Odim A. P., Kliegel M. (2017). The relation of education, occupation, and cognitive activity to cognitive status in old age: The role of physical frailty. International Psychogeriatrics, 29(9), 1469–1474. 10.1017/S1041610217000795 [DOI] [PubMed] [Google Scholar]
- Jessen F., Amariglio R. E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G., Dubois B., Dufouil C., Ellis K. A., van der Flier W. M., Glodzik L., van Harten A. C., de Leon M. J., McHugh P., Mielke M. M., Molinuevo J. L., Mosconi L., Osorio R. S., Perrotin A., Subjective Cognitive Decline Initiative Working . (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 10(6), 844–852. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Z., Li J., Wang Y., Ding L., Tang X., Feng Y., Zhou C. (2020). The mediating effect of psychological distress on cognitive function and physical frailty among the elderly: Evidence from rural Shandong, China. Journal of Affective Disorders, 268, 88–94. 10.1016/j.jad.2020.03.012 [DOI] [PubMed] [Google Scholar]
- Kant I. M. J., de Bresser J., van Montfort S. J. T., Aarts E., Verlaan J.-J., Zacharias N., Winterer G., Spies C., Slooter A. J. C., Hendrikse J., BioCog Consortium . (2018). The association between brain volume, cortical brain infarcts, and physical frailty. Neurobiology of aging, 70, 247-253. 10.1016/j.neurobiolaging.2018.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelaiditi E., Cesari M., Canevelli M., Abellan van Kan G., Ousset P. J., Gillette-Guyonnet S., Ritz P., Duveau F., Soto M. E., Provencher V., Nourhashemi F., Salvà A., Robert P., Andrieu, S., Rolland Y., Touchon J., Fitten J. L., Vellas B., Iana/Iagg . (2013). Cognitive frailty: Rational and definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. The Journal of Nutrition, Health & Aging, 17(9), 726–734. 10.1007/s12603-013-0367-2 [DOI] [PubMed] [Google Scholar]
- Kojima G., Walters K., Iliffe S., Taniguchi Y., Tamiya N. (2019). Marital status and risk of physical frailty: A systematic review and meta-analysis. Journal of the American Medical Directors Association, 21(3), 322–330. 10.1016/j.jamda.2019.09.017 [DOI] [PubMed] [Google Scholar]
- Kuiper J. S., Zuidersma M., Oude Voshaar R. C., Zuidema S. U., van den Heuvel E. R., Stolk R. P., Smidt N. (2015). Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing research reviews, 22, 39–57. 10.1016/j.arr.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Kwan R. Y. C., Leung A. Y. M., Yee A., Lau L. T., Xu X. Y., Dai D. L. K. (2019). Cognitive frailty and its association with nutrition and depression in community-dwelling older people. Journal of Nutrition, Health & Aging, 23(10), 943–948. 10.1007/s12603-019-1258-y [DOI] [PubMed] [Google Scholar]
- Lafortune L., Martin S., Kelly S., Kuhn I., Remes O., Cowan A., Brayne C. (2016). Behavioural risk factors in mid-life associated with successful ageing, disability, dementia and frailty in later life: A rapid systematic review. PloS one, 11(2), e0144405. 10.1371/journal.pone.0144405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landös A., von Arx M., Cheval B., Sieber S., Kliegel M., Gabriel R., Orsholits D., van der Linden B. W. A., Blane D., Boisgontier M. P., Courvoisier D. S., Guessous I., Burton-Jeangros C., Cullati S. (2019). Childhood socioeconomic circumstances and disability trajectories in older men and women: A European cohort study. European journal of public health, 29(1), 50–58. 10.1093/eurpub/cky166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.-L., Chang H.-Y., Stanaway F. F. (2020). Combined effects of frailty status and cognitive impairment on health-related quality of life among community dwelling older adults. Archives of Gerontology & Geriatrics, 87, 103999. 10.1016/j.archger.2019.103999 [DOI] [PubMed] [Google Scholar]
- Lim W. S., Cesari M., Canevelli M. (2018). Dementia, Frailty and Aging. Frontiers Media SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucke J. A., de Gelder J., Heringhaus C., van der Mast R. C., Fogteloo A. J., Anten S., Blauw G. J., de Groot B., Mooijaart S. P. (2018). Impaired cognition is associated with adverse outcome in older patients in the emergency department; the acutely presenting older patients (APOP) study. Age and Ageing, 47(5), 679–684. 10.1093/ageing/afx174 [DOI] [PubMed] [Google Scholar]
- Lunenfeld B., Stratton P. (2013). The clinical consequences of an ageing world and preventive strategies. Best Practice & Research Clinical Obstetrics & Gynaecology, 27(5), 643–659. 10.1016/j.bpobgyn.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Doi T., Tsutsumimoto K., Hotta R., Nakakubo S., Makino K., Lee S. (2018). Social frailty leads to the development of physical frailty among physically non-frail adults: A four-year follow-up longitudinal cohort study. International Journal of Environmental Research and Public Health, 15(3), 490. 10.3390/ijerph15030490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Park H., Doi T., Yoshida D., Uemura K., Tsutsumimoto K., Suzuki T. (2013). Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test–retest reliability and validity in community‐dwelling older adults. Geriatrics & Gerontology International, 13(4), 860−866. 10.1111/ggi.12014 [DOI] [PubMed] [Google Scholar]
- Malmstrom T. K., Miller D. K., Morley J. E. (2014). A comparison of four frailty models. Journal of the American Geriatrics Society (JAGS), 62(4), 721−726. 10.1111/jgs.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A., Nazroo J., Tampubolon G., Vanhoutte B. (2015). Cohort differences in the levels and trajectories of frailty among older people in England. Journal of Epidemiology and Community Health, 69(4), 316−321. 10.1136/jech-2014-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerl H., Stolz E., Freidl W. (2020). Frailty and depression: Reciprocal influences or common causes? Social Science & Medicine, 263, 113273. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Stellar E. (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153(18), 2093−2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- Mitnitski A., Song X., Rockwood K. (2013). Assessing biological aging: The origin of deficit accumulation. Biogerontology (Dordrecht), 14(6), 709−717. 10.1007/s10522-013-9446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski A. B., Mogilner A. J., Rockwood K. (2001). Accumulation of deficits as a proxy measure of aging. TheScientificWorldJournal, 1, 323−336. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M. M., Barnes B., Speechley M., Muir Hunter S. W., Doherty T. J., Duque G., Gopaul K., Sposato L. A., Casas-Herrero A., Borrie M. J., Camicioli R., Wells J. L. (2016). Disentangling cognitive-frailty: Results from the gait and brain study. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(11), 1476–1482. 10.1093/gerona/glw044 [DOI] [PubMed] [Google Scholar]
- Morley J. E., Malmstrom T. K., Miller D. K. (2012). A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. The Journal of nutrition, health & aging, 16(7), 601–608. 10.1007/s12603-012-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. P., Feng L., Nyunt M. S. Z., Larbi A., Yap K. B. (2014). Frailty in older persons: Multisystem risk factors and the frailty risk index (FRI). Journal of the American Medical Directors Association, 15(9), 635–642. 10.1016/j.jamda.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Ostrosky-Solís F., Esther Gómez-Pérez M., Matute E., Rosselli M., Ardila A., Pineda D. (2007). Neuropsi attention and memory: A neuropsychological test battery in Spanish with norms by age and educational level. Applied Neuropsychology, 14(3), 156–170. 10.1080/09084280701508655 [DOI] [PubMed] [Google Scholar]
- Page M. J., McKenzie J. E., Bossuyt P. M., Boutron I., Hoffmann T. C., Mulrow C. D., Shamseer L., Tetzlaff J. M., Akl E. A., Brennan S. E., Chou R., Glanville J., Grimshaw J. M., Hróbjartsson A., Lalu M. M., Li T., Loder E. W., Mayo-Wilson E., McDonald S., Moher D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Journal of Clinical Epidemiology, 134, 178–189. 10.1016/j.jclinepi.2021.03.001 [DOI] [PubMed] [Google Scholar]
- Panza F., D’Introno A., Colacicco A. M., Capurso C., Parigi A. D., Capurso S. A., Parigi A. D., Capurso S. A., Caselli R. J., Pilotto A., Scafato E., Solfrizzi V. (2006). Cognitive frailty: Predementia syndrome and vascular risk factors. Neurobiology of Aging, 27(7), 933–940. 10.1016/j.neurobiolaging.2005.05.008 [DOI] [PubMed] [Google Scholar]
- Panza F., Lozupone M., Solfrizzi V., Sardone R., Dibello V., Di Lena L., D’Urs F., Stallone R., Petruzzi M., Giannelli G., Quaranta N., Bellomo A., Greco A., Daniele A., Seripa D., Logroscino G. (2018). Different cognitive frailty models and health- and cognitive-related outcomes in older age: From epidemiology to prevention. Journal of Alzheimer’s disease : JAD, 62(3), 993–1012. 10.3233/JAD-170963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek M. K., Howrey B. T., Ternent R. S., Ray L. A., Ottenbacher K. J. (2012). Social support, stressors, and frailty among older Mexican American adults. The Journals of Gerontology. Series B, Psychological sciences and social sciences, 67(6), 755. 10.1093/eronb/gbs081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C. (2004). Mild cognitive impairment as a diagnostic entity. Journal of internal medicine, 256(3), 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Pfeiffer E. (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society, 23(10), 433–441. [DOI] [PubMed] [Google Scholar]
- Rabin R., Charro F. d. (2001). EQ-SD: A measure of health status from the EuroQol Group. Annals of medicine, 33(5), 337–343. [DOI] [PubMed] [Google Scholar]
- Rivan N. F. M., Shahar S., Rajab N. F., Singh D. K. A., Che Din N., Mahadzir H., Mohamed Sakian N. I., Ishak W. S., Abd Rahman M. H., Mohammed Z., You Y. X. (2020). Incidence and predictors of cognitive frailty among older adults: A community-based longitudinal study. International Journal of Environmental Research and Public Health, 17(5), 1547. 10.3390/ijerph17051547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivan N. F. M., Shahar S., Rajab N. F., Singh D. K. A., Din N. C., Hazlina M., Hamid T. A. T. A. (2019). Cognitive frailty among Malaysian older adults: Baseline findings from the LRGS TUA cohort study. Clinical Interventions in Aging, 14, 1343–1352. 10.2147/CIA.S211027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook K. S., Charles S. T. (2017). Close social ties and health in later life: Strengths and vulnerabilities. The American psychologist, 72(6), 567-–577. 10.1037/amp0000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent L., Nalls M., Starkweather A., Hobgood S., Thompson H., Amella E. J., Singleton A. (2018). Shared biological pathways for frailty and cognitive impairment: A systematic review. Ageing Research Reviews, 47, 149–158. 10.1016/j.arr.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. (1996). Rey auditory verbal learning test: A handbook. Western Psychological Services. [Google Scholar]
- Searle S. D., Mitnitski A., Gahbauer E. A., Gill T. M., Rockwood K. (2008). A standard procedure for creating a frailty index. BMC geriatrics, 8(1), 1–10. 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Makizako H., Lee S., Doi T., Tsutsumimoto K., Harada K., Hotta R., Bae S., Nakakubo S., Harada K., Suzuki T. (2016). Impact of cognitive frailty on daily activities in older persons. The Journal of Nutrition, Health & Aging, 20(7), 729–735. 10.1007/s12603-016-0685-2 [DOI] [PubMed] [Google Scholar]
- Sirriyeh R., Lawton R., Gardner P., Armitage G. (2012). Reviewing studies with diverse designs: the development and evaluation of a new tool. Journal of Evaluation in Clinical Practice, 18(4), 746–752. 10.1111/j.1365-2753.2011.01662.x [DOI] [PubMed] [Google Scholar]
- Sonnega A., Faul J. D., Ofstedal M. B., Langa K. M., Phillips J. W. R., Weir D. R. (2014). Cohort profile: the health and retirement study (HRS). International journal of epidemiology, 43(2), 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soysal P., Veronese N., Thompson T., Kahl K. G., Fernandes B. S., Prina A. M., Solmi M., Schofield P., Koyanagi A., Tseng P. T., Lin P. Y., Chu C. S., Cosco T. D., Cesari M., Carvalho A. F., Stubbs B. (2017). Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Research Reviews, 36, 78–87. 10.1016/j.arr.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Steptoe A., Zaninotto P. (2020). Lower socioeconomic status and the acceleration of aging: An outcome-wide analysis. Proceedings of the National Academy of Sciences of the United States of America, 117(26), 14911–14917. 10.1073/pnas.1915741117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T., Sakurai T., Ono R., Kimura A., Saji N., Niida S., Toba K., Chen L. K., Arai H. (2018). Epidemiological and clinical significance of cognitive frailty: A mini review. Ageing Research Reviews, 44, 1–7. 10.1016/j.arr.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Theou O., Brothers T. D., Mitnitski A., Rockwood K. (2013). Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. Journal of the American Geriatrics Society (JAGS), 61(9), 1537–1551. 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- Travers J., Romero-Ortuno R., Bailey J., Cooney M.-T. (2019). Delaying and reversing frailty: a systematic review of primary care interventions. British Journal of General Practice, 69(678), e61–e69. 10.3399/bjgp18x700241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turvey C. L., Wallace R. B., Herzog R. (1999). A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics, 11(2), 139–148. [DOI] [PubMed] [Google Scholar]
- Vernon M. J. (2020). The politics of frailty. Age and ageing, 49(4), 544–548. 10.1093/ageing/afaa023 [DOI] [PubMed] [Google Scholar]
- WHO . (2015). World Report on Ageing and Health. 9789240694811_eng.pdf (who.int). [Google Scholar]
- WHO . (2018). Ageing and Health. Ageing and health (who.int). [Google Scholar]
- Wu Y. H., Lee H. N., Chang Y. S., Wu C. H., Wang C. J. (2020). Depressive symptoms were a common risk factor for pre-frailty and frailty in patients with Alzheimer’s disease. Archives of Gerontology & Geriatrics, 89, 104067. 10.1016/j.archger.2020.104067 [DOI] [PubMed] [Google Scholar]
- Yesavage J. A. (1988). Geriatric depression scale. Psychopharmacol Bull, 24(4), 709–711. [PubMed] [Google Scholar]