Abstract
Chronic urticaria (CU) is defined by the presence of itchy wheals, sometimes accompanied by angioedema, lasting for at least 6 weeks. CU is treated with second-generation antihistamines, increased up to four times the normal doses for second-line treatment. Omalizumab (a monoclonal antibody anti-IgE) may be recommended as third-line therapy in children aged over 12 years. Few reports have suggested that glucose homeostasis is impaired in some type 2 diabetic patients receiving omalizumab, and even in non-diabetic patients, fasting blood glucose and HOMA-IR values appeared to be significantly increased. We report the case of a 13-year-old girl with diabetes mellitus type 1 and chronic spontaneous urticaria (CSU) refractory to standard recommended therapy that we treated with omalizumab at a standard recommended dose of 300 mg every 4 weeks. We observed a rapid and complete remission of CSU after treatment with this humanized monoclonal antibody without detrimental effects on the patient’s glucose control especially in terms of HbA1c (glycated hemoglobin), time in glycemic range (TIR), and daily insulin needs.
Keywords: chronic spontaneous urticaria, type 1 diabetes, omalizumab, pediatrics, glycemic
Introduction
Chronic urticaria is defined by itchy hives and/or angioedema that recur for at least 6 weeks. It affects 0.1%–0.3% of children. When no specific eliciting trigger is identified, this condition is classified as CSU (1). It is believed that up to 50% of cases are associated with pathogenic autoantibodies most frequently related to thyroiditis and celiac disease (2, 3). It is known that children with T1DM compared with children without T1DM have an increased risk of urticaria (4).
Antihistamines are the first-line treatment for CSU, and if the standard dose is not effective, the dosage can be increased fourfold (5). Unfortunately, 25%–50% of patients do not respond to this treatment regimen and require other therapies, including antileukotrienes, cyclosporine, and more recently omalizumab.
Omalizumab is a recombinant humanized monoclonal anti-IgE antibody that binds free serum IgE, prevents its attachment to the high-affinity receptor on mast cells (FcεRI), and decreases receptor expression. Omalizumab was approved by the US Food and Drug Administration and the European Medicines Agency for antihistamine-refractory patients with CSU who are at least 12 years of age and significantly reduces the signs, symptoms, and burden of CSU (1).
In many studies, omalizumab has been demonstrated to be effective against CSU in children, even under 12 years of age (6–8).
Few reports have suggested that glucose homeostasis is impaired in some type 2 diabetic patients receiving omalizumab, and even in non-diabetic patients, fasting blood glucose and HOMA-IR values appeared significantly increased (9, 10). Another recent study demonstrated the safety of omalizumab in an 8-year-old child with type 1 diabetes mellitus (T1DM) and autoimmune thyroiditis (11).
Case Description
A 13-year-old girl with T1DM was admitted to our hospital for poor glucose control under multiple daily insulin injections.
A review of her medical history and physical examination at presentation suggested that the girl had been suffering from chronic spontaneous urticaria unresponsive to antihistamine therapy. T1DM was diagnosed at the age of 11, appropriate treatment was initiated (multiple daily insulin injection therapy), and other type 1 diabetes-associated autoimmune diseases (celiac disease, autoimmune thyroiditis, and autoimmune atrophic gastritis) were excluded at diagnosis and yearly follow-up. Two years after the diagnosis of T1DM, she developed severe symptoms of chronic urticaria. Treatment with cetirizine (10 mg/day) was initiated, although no significant response was observed. There was no apparent correlation with exposure to common allergens nor physical stimuli. No history of allergy was reported; in particular, she did not report symptoms of oculorhinitis, asthma, or atopic dermatitis. She only referred one episode of urticaria in early childhood after taking amoxicillin-clavulanic acid.
The timing of onset and the frequency and duration of the hives were not consistent with insulin allergy (12, 13). Skin tests with insulin were not performed due to the persistence of hives and ongoing antihistamine treatment and the absence of concrete evidence supporting insulin skin test reliability (14).
Laboratory work-up revealed normal complete blood count and leukocyte formula (mild eosinophilia was detected: 600/mmc, n.v. 0–450) and normal serum total IgE. Specific IgE against common food and inhalant allergens showed weak positivity for only dust mites and ragweed. Liver, kidney, and thyroid function were normal; thyroid autoimmunity (anti-thyroid peroxidase, 11 IU/ml; anti-thyroglobulin, 31 IU/ml; and anti-TSH receptor, <0.8 IU/L) was negative. Celiac disease was also excluded, and inflammatory markers (CRP, ESR) were negative. Screening for other autoantibodies most frequently associated with CSU (ANA and ENA) showed negative results, with the exception of antinuclear antibodies (titer, 1:160). Complement fractions (C3, 1.13 g/L; C4, 0.22 g/L) and immunoglobulin classes were normal. Microbiological tests (including fecal Helicobacter pylori antigen) and stool parasite tests yielded negative results. Streptococcal infection was excluded. Urinalysis was also normal.
Due to treatment failure on standard cetirizine prescription, the dose was increased twofold, with only minimal improvement. We monitored the disease course on a weekly basis with a mean Urticaria Activity Score (UAS) of 36.
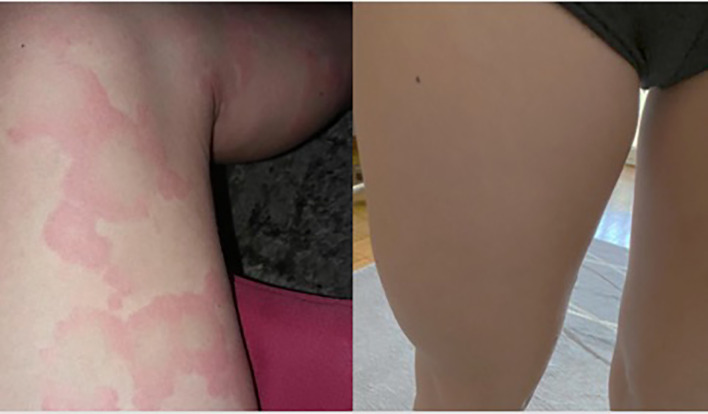
Given the persistence of clinical symptoms and the patient’s poor quality of life, omalizumab was started at a standard recommended dose of 300 mg intramuscular injection every 4 weeks. After the third dose and concomitant oral antihistamine treatment, symptoms significantly improved (UAS, 14), and only small urticarial elements remained on the extremities. After the sixth dose, CSU symptoms completely subsided, and antihistamine treatment was suspended ( Figure 1 , picture before and after treatment). Treatment was completed after 11 administrations of omalizumab with no relapsing CSU to date.
Figure 1.
Cutaneous symptoms before and after treatment with omalizumab.
Glucometric data derived by flash glucose monitoring during treatment is shown in Table 1 . Time spent in specific glucose ranges at baseline and after treatment were compared by chi-squared test. From baseline, time below range (TBR) has shown a slight but statistically significant reduction. However, this improvement has been apparently at the expense of an increased time above range (TAR) of 180–250 mg/dl. Time in range (TIR) did not vary. Baseline and post-treatment HbA1c were 6.8% and 6.9%, respectively, showing a substantial stable average glucose control during the treatment period. Daily insulin doses also did not vary significantly and were 1.1 U/kg at baseline and 0.9 U/kg post-treatment.
Table 1.
Glucometric data derived by flash glucose monitoring during treatment.
| PRE (N = 2,656) | POST (N = 2,590) | p-value | |
|---|---|---|---|
| TAR >250 mg/dl (%) | 8 | 9 | 0.1940 |
| TAR 180–250 mg/dl (%) | 20 | 25 | <0.05 |
| TIR 70–180 mg/dl (%) | 63 | 61 | 0.1357 |
| TBR 54–70 mg/dl (%) | 6 | 4 | <0.05 |
| TBR <54 mg/dl (%) | 3 | 1 | <0.05 |
Discussion
Omalizumab is known to be an effective drug against chronic urticaria in children from 12 years of age. This monoclonal antibody has already proved to be beneficial for the treatment of severe asthma and CSU even when associated with other autoimmune diseases (15, 16).
Our case represents one of the few children with CSU in association with isolated type 1 diabetes who have been treated successfully and safely with omalizumab to date. Contrary to previous large-scale studies that suggested an increase in insulin resistance, assessment of flash glucose monitoring data in our patient suggests that treatment with this humanized monoclonal antibody did not determine detrimental effects on the patient’s glucose control especially in terms of HbA1c, TIR, and daily insulin needs (9, 10, 17). Further randomized controlled studies of CSU in pediatric patients with T1DM are warranted in order to more clearly assess the impact of omalizumab treatment on glucometrics and insulin resistance in this particular population. This is of particular concern especially for patients undergoing multiple daily insulin injections, as the ever increasing popularity of hybrid closed loops systems may prevent any omalizumab-related increased glucose variability.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the minor’s legal guardian for the publication of any potentially identifiable images or data included in this article.
Author Contributions
PB, FT, GF and MG conceived the idea of the clinical case and wrote the manuscript. RB and GB supervised the findings of this work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Agache I, Rocha C, Pereira A, Song Y, Alonso-Coello P, Solà I, et al. Efficacy and Safety of Treatment With Omalizumab for Chronic Spontaneous Urticaria: A Systematic Review for the EAACI Biologicals Guidelines. Allergy Eur J Allergy Clin Immunol (2021) 76(1):59–70. doi: 10.1111/all.14547 [DOI] [PubMed] [Google Scholar]
- 2. Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic Urticaria and Autoimmunity: Associations Found in a Large Population Study. J Allergy Clin Immunol (2012) 129(5):1307–13. doi: 10.1016/j.jaci.2012.01.043 [DOI] [PubMed] [Google Scholar]
- 3. Sahiner UM, Civelek E, Tuncer A, Yavuz ST, Karabulut E, Sackesen C, et al. Chronic Urticaria: Etiology and Natural Course in Children. Int Arch Allergy Immunol (2011) 156(2):224–30. doi: 10.1159/000322349 [DOI] [PubMed] [Google Scholar]
- 4. Lin SY, Lin CL, Lin CC, Hsu WH, Hsu CY, Kao CH. Risk of Urticaria in Children With Type 1 Diabetes Mellitus: A Nationwide Cohort Study. Int J Environ Res Public Health (2019) 17(1):176. doi: 10.3390/ijerph17010176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy Eur J Allergy Clin Immunol (2018) 73(7):1393–414. doi: 10.1111/all.13397 [DOI] [PubMed] [Google Scholar]
- 6. Leonardi L, Uva A, Duse M. Chronic Urticaria in a Child Affected by Atopic Dermatitis: Effective Treatment With Omalizumab. J Dermatolog Treat (2018) 29(sup3):17–9. doi: 10.1080/09546634.2018.1543844 [DOI] [PubMed] [Google Scholar]
- 7. Dekkers C, Alizadeh Aghdam M, de Graaf M, Knulst AC, Meijer Y, van den Reek JMPA, et al. Safety and Effectiveness of Omalizumab for the Treatment of Chronic Urticaria in Pediatric Patients. Pediatr Allergy Immunol (2021) 32(4):720–6. doi: 10.1111/pai.13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ari A, Levy Y, Segal N, Maoz-Segal R, Benor S, Broides A, et al. Efficacy of Omalizumab Treatment for Pediatric Chronic Spontaneous Urticaria: A Multi-Center Retrospective Case Series. Pediatr Dermatol (2020) 37(6):1051–4. doi: 10.1111/pde.14360 [DOI] [PubMed] [Google Scholar]
- 9. Hamada S, Kuroe A, Tsukino M. Does Omalizumab Impair Glucose Homeostasis in a Patient With Severe Persistent Asthma and Type 2 Diabetes Mellitus? Rev Port Pneumol (English Ed) (2017) 23(5):303–4. doi: 10.1016/j.rppnen.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 10. Falay Gur T, Savas Erdogan S, Erdemir VA, Doğan B. Effect of Omalizumab Use on Glucose Homeostasis in Non-Diabetic Patients With Chronic Urticaria. Cutan Ocul Toxicol (2020) 39(4):348–53. doi: 10.1080/15569527.2020.1818769 [DOI] [PubMed] [Google Scholar]
- 11. Jesenak M, Ciljakova M, Janickova M, Banovcin P. Omalizumab in an 8-Year-Old Boy With Diabetes Mellitus and Refractory Chronic Spontaneous Urticaria. J Investig Allergol Clin Immunol (2019) 29(2):144–6. doi: 10.18176/jiaci.0351 [DOI] [PubMed] [Google Scholar]
- 12. Mastrorilli C, Rizzuti L, Cangelosi AM, Iovane B, Chiari G, Caffarelli C. Long-Acting Insulin Allergy in a Diabetic Child. Int J Immunopathol Pharmacol (2017) 30(2):174–7. doi: 10.1177/0394632017700431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura T, Fushimi Y, Hayashi H, Tatsumi F, Kanda-Kimura Y, Shimoda M, et al. Insulin Allergy Brought Out 8 Years After Starting Insulin Therapy in a Subject With Type 1 Diabetes Mellitus. Acta Diabetol (2020) 57(8):1025–6. doi: 10.1007/s00592-020-01499-4 [DOI] [PubMed] [Google Scholar]
- 14. Haastrup MB, Henriksen JE, Mortz CG, Bindslev-Jensen C, et al. Insulin Allergy can be Successfully Managed by a Systematic Approach. Clin Transl Allergy (2018) 8:35. doi: 10.1186/s13601-018-0223-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yalcin AD, Yalcin AN. A Case of Asthma With Behcet’s Disease: Successful Treatment With Omalizumab and its Effects on Recurrent Aphthous Lesions. Immunopharmacol Immunotoxicol (2020) 42(4):379–82. doi: 10.1080/08923973.2020.1789656 [DOI] [PubMed] [Google Scholar]
- 16. Yalcin AD, Genc GE, Celik B, Gumuslu S. Anti-IgE Monoclonal Antibody (Omalizumab) is Effective in Treating Bullous Pemphigoid and its Effects on Soluble CD200. Clin Lab (2014) 60(3):523–4. doi: 10.7754/clin.lab.2013.130642 [DOI] [PubMed] [Google Scholar]
- 17. Yalcin AD, Gorczynski RM, Cilli A, Strauss L. Omalizumab (Anti-IgE) Therapy Increases Blood Glucose Levels in Severe Persistent Allergic Asthma Patients With Diabetes Mellitus: 18 Month Follow-Up. Clin Lab (2014) 60(9):1561–4. doi: 10.7754/clin.lab.2013.130302 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.