Abstract
Introduction
Chronic musculoskeletal pain (CMSP) severely affects the individual’s quality of life, functioning and ability to work, and comes with significant societal costs for sick leave and productivity loss. After rehabilitation, patients with CMSP often experience lack of support when responsibility for the return-to-work process is taken over by the employer. Therefore, we aim to evaluate the effectiveness of a digital support (Sustainable WorkEr digital support for Persons with chronic Pain and their Employers (SWEPPE)) for promoting a sustainable return-to-work for persons with CMSP and to facilitate the employers’ supportive role and responsibilities in the process.
Methods and analysis
In this registry-based multicentre randomised controlled trial, 360 patients with CMSP will be randomised to either receive the smartphone application SWEPPE (n=180) or to a control group (n=180). The intervention group will use SWEPPE for 1 year and the control group will not receive any intervention for return to work (RTW). Participants will be recruited from approximately 10 specialist and primary care level units connected to the Swedish National Quality Registry for Pain Rehabilitation providing Interdisciplinary Pain Rehabilitation Programmes (IPRP) for CMSP. Eligibility criteria are age 18–65 years and a need for support in RTW or continued support at work for creating a sustainable work situation. Baseline data will be collected when the participants have completed the IPRP. Final assessment will be performed after 12 months. The primary outcome will be a number of days with sickness cash benefit. Secondary outcomes and explanatory variables including important domains affected by CMSP such as health-related quality of life, functioning and work ability will be collected.
Ethics and dissemination
The Swedish Ethics Review Board approved the study (Dnr 2020-01593, Dnr 2021-01854). The study findings will be disseminated through publication, national and international conferences, and meetings to be available for patients, healthcare providers or stakeholders.
Trial registration number
Keywords: Clinical trials, PAIN MANAGEMENT, REHABILITATION MEDICINE
Strengths and limitations of this study.
Using a shared smartphone application (Sustainable WorkEr digital support for Persons with chronic Pain and their Employers) to facilitate self-management, and communication and collaboration between persons with chronic musculoskeletal pain and their employer during the return-to-work process is a novel intervention with the potential to support a sustainable work situation.
A registry-based multicentre randomised trial will provide rigorous evidence regarding clinical effectiveness of the intervention.
In this trial the primary and secondary outcomes are based on recommendation from the Swedish Social Insurance Agency regarding outcomes for return-to-work which ensures capturing relevant aspects of sick-leave.
It is important to be aware of the risk for selection bias due to patients’ self-confidence or willingness to use smartphone applications.
A study limitation is the lack of blinding to group allocation and the control groups awareness of not receiving the intervention.
Introduction
Chronic musculoskeletal pain (CMSP) (ie, pain duration >3 months) such as chronic neck/shoulder and back pain or generalised widespread pain (including fibromyalgia) has a prevalence from 10.4%1 to 20% among adults.1–3 CMSP negatively impact quality of life, functioning and the ability to work.2 CMSP also causes considerable costs for the society in terms of sick leave expenses and loss of productivity.1 2 4–8 Many patients with CMSP participate in Interdisciplinary Pain Rehabilitation Programmes (IPRP) to enable self-management of pain and increase the ability to work.9–11 After completing a rehabilitation programme for persons with CMSP the patients can experience lack of support when the employer takes over the responsibility for the return to work (RTW) process.12 The employers have a crucial role in a successful RTW process13–15 but may lack knowledge regarding chronic pain and its consequences16 and how to support the employee with CMSP in the best way during RTW.12 Barriers for RTW for persons with CMSP are for example lack of support at the workplace, not finding the right fit between the employee’s physical abilities and work tasks, or problems with relationships with supervisors or coworkers.17 18 Key factors for a successful RTW are communication and collaboration between the employer and the employee.19 Further, employers also need to use active listening skills which means enhancing conversation using open questions and demonstrate effective listening by summary statements.20 To facilitate the important interaction between employer and employee21 22 a shared smartphone application may be a tool for increasing a successful outcome in the RTW process.
A primary aim of IPRP is to reach RTW.9 10 To fill the gap patients with CMSP experience when the RTW process continues after completing IPRP,12 the digital support a Sustainable WorkEr digital support for Persons with chronic Pain and their Employers (SWEPPE) was systematically developed.23 SWEPPE is a smartphone application for persons with CMSP with the possibility to invite and share information with the employer. SWEPPE was developed by a multidisciplinary project team consisting of healthcare researchers, a user representative and a software team. A user-centred agile approach24 was used with continuous involvement of two reference groups consisting of persons with CMSP and employers providing feedback on the functions and the interface in SWEPPE. Smart phone applications as digital support has shown promising results for persons with chronic pain25–27 and can be helpful especially in an out-clinic setting.28 They are easily accessed, can enable management of the condition29 and reduce pain interference.30 An evidence-based content and a simple design are key parts for providing a successful digital support.31 Information provided via apps can improve the level of knowledge among patients.32 Focusing on self-management and empowerment are other important parts of successful digital support.33 Self-management among persons with chronic pain include self-monitoring31 34 and pain education in relation to the neuroscience of pain, medication, stress, depression and sleep management.35 Self-monitoring can contribute to learning about consequences of actions and behaviours in daily life.36 This can lead to making changes in daily activities and a sense of control and motivation for continued use of self-management strategies.37 38
Although positive effects of digital support have been shown there are limitations related to low overall quality of smartphone apps for CMSP and lack of rigorous assessment of their effectiveness.39 40 SWEPPE was found to be useful for self-management for persons with CMSP and for supporting employers, with relevant content, logical and easy to use, and with a nice and clean interface.23 However, the clinical effectiveness of SWEPPE as a digital support for employees and employers to decrease sick leave in persons with CMSP need to be investigated. The aim of this paper is to report the study design, aim, outcome assessment and procedures for a planned registry-based multicentre randomised controlled trial (R-RCT). The overall objective of the R-RCT is to evaluate the clinical effectiveness of a digital support (SWEPPE) for promoting a sustainable RTW for persons with CMSP and to facilitate the employers’ supportive role and responsibilities in the process.
Methods and analysis
Trial design and study setting
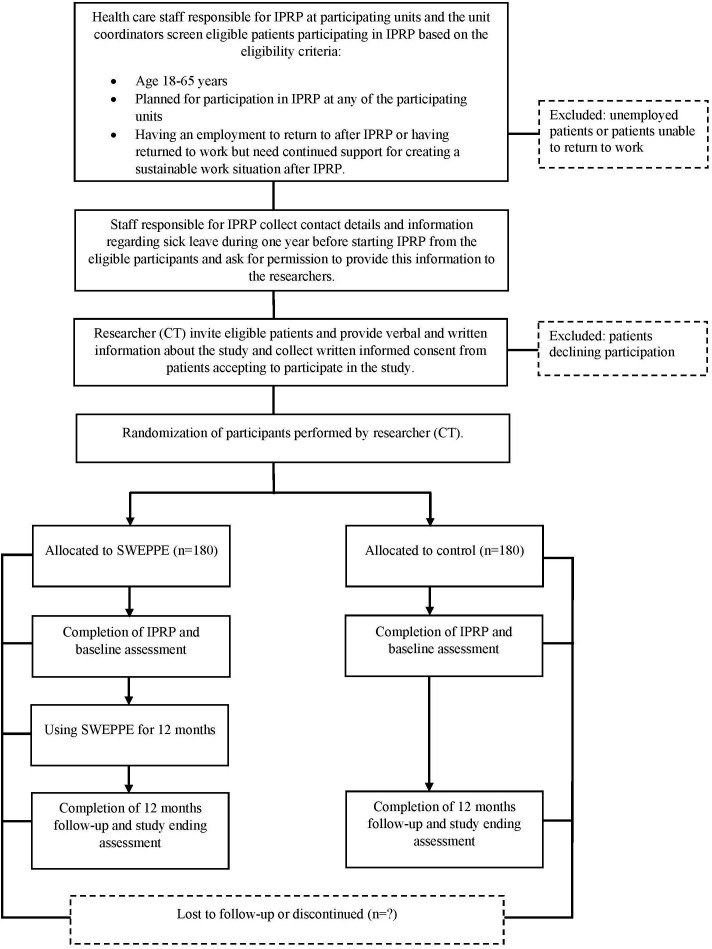
This protocol is reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT).41 The R-RCT will conform with the Consolidated Standards of Reporting Trials.42 43 This is a two-armed multi-centre registry-based randomised controlled trial. The study will be conducted in specialised and primary level clinics in Sweden providing IPRP and reporting to the Swedish National Quality Registry for Pain Rehabilitation (SQRP). Approximately 360 (n=180 intervention group, n=180 control group) patients with CMSP will be recruited to participate in the study. Study design and enrolment details are presented in figure 1. A completed SPIRIT checklist and the WHO trial registration data set44 can be found in online supplemental files S1 and S2.
Figure 1.
Time schedule of enrolment, interventions and assessments. IPRP, Interdisciplinary Pain Rehabilitation Programmes; SWEPPE, Sustainable WorkEr digital support for Persons with chronic Pain and their Employers.
bmjopen-2021-060452supp001.pdf (247.9KB, pdf)
bmjopen-2021-060452supp002.pdf (169.9KB, pdf)
Patient and public involvement
Patients with CMSP were not involved in formulating the research question or setting the research design for the planned study. However, patients with CMSP who had undergone IPRP and employers participated in design and development of the intervention, the digital smart phone application SWEPPE. In addition, a user representative from the Swedish Rheumatism Association participated as a research partner in the development process of SWEPPE.23
Participants
Eligibility criteria for the study is that patients entering the trial must have completed an IPRP for CMSP. The principal inclusion criteria for IPRP in Sweden are persistent or intermittent pain lasting ≥3 months, pain affecting daily activities to a large extent, completed systematic assessment and non-pharmacological optimisation, and screening for psychosocial risk factors and differential diagnosis completed. In this trial, patients with CMSP will be recruited based on the following criteria: age between 18 and 65 years, completed IPRP at any of the participating units, having an employment to return to after IPRP or having returned to work but need continued support for creating a sustainable work situation after IPRP. Patients who have completed IPRP but are unemployed or unable to RTW will be excluded.
Recruitment
Units in specialised and primary care level in Sweden providing IPRP based on individualised needs and who are reporting to the SQRP will be included in the study. Two of the researchers (CT, MB) will invite healthcare staff (primarily occupational therapists and physiotherapists but also psychologist/counsellor, nurses, etc) at the participating units to online digital information meetings to present the study. A contact person will be appointed at each unit. One researcher (CT) will have continuous contact with the participating units regarding the planned IPRP groups and screening of eligible patients for the study.
Patients with CMSP participating in IPRP at any of the study units and who is meeting the inclusion criteria will be asked to take part in the study. The recruitment process will start with screening of eligible participants in the IPRP groups. Screening of eligible participants will be performed by the healthcare staff providing the IPRP and will be discussed with one of the researchers (CT). The healthcare staff will collect contact details and information regarding previous sick leave during 1 year before starting IPRP from the eligible participants and ask for permission to provide this information to the researchers. The participants will at the end of IPRP receive verbal and written information about the study from one of the researchers (CT) and written informed consent (S3) will be collected for those willing to participate in the study. The participants will receive detailed information regarding voluntary participation and the right to withdraw from the study at any time.
Intervention
The participants will be assigned to the intervention group or the control group. Participants in both groups will follow the plan for RTW that has been established at the end of IPRP.
Intervention group receiving SWEPPE
Participants randomised to the intervention group will receive the smartphone application SWEPPE to use as a digital support during the RTW process. SWEPPE consist of six modules to support self-management33: the action plan, daily self-rating of health aspects, self-monitoring graphs of health aspects and goals, the coach, the library and shared information with the employer. The action plan includes setting a work-related goal, identification of barriers for RTW, strategies to handle the barriers, identification of support needed from the employer and weekly evaluation. SWEPPE address pain education35 and the library provides evidence-based information about CMSP, self-management strategies and information and tools for RTW.
The intervention starts after completed IPRP with self-rating of work conditions and goal setting in SWEPPE. The participants will use SWEPPE for 12 months. Data registered in SWEPPE by the participant about their goal, work condition and self-rating will be stored in the application and used for self-monitoring and visualising progress for the participant. The participant invites his/her employer/employers to access the web application SWEPPE depending on what information the participant wants to share with the employer. The employer will receive email reminders to use SWEPPE.
Control group
Participants randomised to the control group will not be blinded to treatment allocation as it is not feasible. They will follow the regular procedure at any specific unit and as there is no standardised intervention for RTW after IPRP, that will mean the participants follow their planned RTW process without further support from the IPRP team. However, the patient can initiate and seek other types of healthcare or support during their RTW process based on their needs.
Allocation/randomisation
The unit of randomisation will be the individual participants who have approved to participate in the study. One of the researchers (CT) will enrol and randomise participants who have given informed consent (S3) to participate in the study to either the intervention or control group. As sick leave history is a strong predictor for future sick leave45 participants will be stratified into high or low sick leave history based on self-reported number of sick leave days during the year before IPRP. It has been shown that patients with low sick leave history to a larger extent are younger, have an employment, higher education and are more confident regarding recovery.45 Participants will therefore be divided in high (total number of gross sick leave days ≥70) or low sick leave absence45 and then randomised to intervention or control group. Allocation of the participants to intervention or control group will be conducted using a block randomisation design with varying block sizes of 2–6.46–48 The allocation sequence will be computer generated and sealed sequentially numbered opaque sealed envelopes will be prepared by one of the researchers (GL).
Blinding
Due to the nature of the intervention the participants will not be blinded to group allocation. As the intervention is a smartphone application it is not feasible to give a sham intervention to the control group. Randomisation to intervention or control group is performed at the completion of IPRP and the participants will not have further contact with the healthcare staff responsible for IPRP or other patients. However, the participants will also be instructed by the healthcare staff not to reveal their group allocation to the healthcare staff responsible for IPRP or other patients if they would have further contact.
Sample size
The null hypothesis in this trial is that there will be no difference between the intervention group and the control group concerning the primary outcome sick leave. Since there is no established minimal clinical important difference regarding sick leave, the sample size calculation was inspired by previous research regarding patterns of sick leave after IPRP.49 50 It has been shown that the distribution of sick leave among persons with chronic pain change over time from full-time to partial sick leave after IPRP49 and sick leave are reduced with approximately 16 net days from 1 year before to 2 years after IPRP.50 Therefore, an estimated difference between the groups of 20 net days with an SD of 60 and an effect-size of 0.333 was set for rejection of the null hypothesis. To detect this difference with a power of 80% and a significance level of 0.05 a total sample size of approximately 300 participants (150/group) are needed. With an allowance for 20% of participants lost to follow-up we aim to recruit a total sample size of 360 participants (n=180 intervention, n=180 control). To reach the target sample size, participants will be recruited from multiple special and primary care level healthcare providing IPRP for patients with chronic pain.
Outcomes
Outcome assessments in the present trial are intended to capture the complexity of pain51 based on the biopsychosocial model52 namely medical, psychological and social (total life situation) factors impacting on the work situation. It has been recommended to include multiple outcomes in clinical trials for persons with CMSP to capture important domains affected by symptoms such as functioning and health-related quality of life.53 54
The primary and secondary outcomes are collected for evaluation of the clinical effectiveness of SWEPPE, and the complementary variables will be collected based on their effect on the outcome. Personal characteristics of the participants will be collected from the SQRP for specialist and primary care respectively and from supplementary questions regarding sex, age, education, currently working/studying (yes, no), work importance in addition to the importance of income (five alternatives: (1) very important, (2) important, (3) partially important, (4) hardly no importance or (5) no importance), diagnosis and pain duration, sick leave during 1 year before IPRP and type of work.
Primary outcomes
Primary outcome is days with sickness cash benefit measured according to the Swedish Social Insurance Agency’s (SSIA’s) proposal of outcome measures of RTW55:
Number of gross and net days with sickness cash benefit during the follow-up period (mean and median values).
Secondary outcomes
Secondary outcomes will be collected from SSIA, the SQRP for specialist and primary care, respectively, supplementary questionnaires and SWEPPE. An overview of the outcome assessments and data sources is presented in table 1.
Table 1.
Overview of the study period, measurement time points (t), primary and secondary outcome assessments and explanatory variables, and data sources (italics)
| Study period | ||||
| Time point | Enrolment | Allocation | Postallocation | |
| −t1 | 0 | Baseline | t1 | |
| Enrolment | X | |||
| Eligibility screen | X | |||
| Written and verbal study information | X | |||
| Informed consent | X | |||
| Allocation/randomisation | X | |||
| Interventions | ||||
| Intervention, SWEPPE (12 months) | X | X | ||
| Control (12 months) | X | X | ||
| Outcome assessments | ||||
| Personal characteristics | ||||
| Sex, age (SQRP sc and pc) | X | |||
| Education (SQRP sc and pc) | X | |||
| Employment, work importance and type of work (SQRP sc, supplementary questions for pc) | X | |||
| Diagnosis, pain duration (SQRP sc and pc) | X | |||
| Sick leave during 1 year before IPRP (supplementary questionnaire) | X | |||
| Primary outcomes | ||||
| Number of gross and net days with sickness cash benefit during the follow-up period (SSIA) | X | X | ||
| Secondary outcomes | ||||
| Return to work (partially or full time) every month (SSIA) | X | X | ||
| Number of sick-leave spells (per month) (SSIA) | X | X | ||
| Proportions of a group who returns to full-time or part-time work (per month) (SSIA) | X | X | ||
| Number of days in work before new sick leave during study period (SSIA) | X | X | ||
| Proportion of a group back to work >28 days (full-time or part time) before a new sick-leave spell occurs (SSIA) | X | X | ||
| Number of sick-leave spells during study period (SSIA) | X | X | ||
| Length of total sick leave during study period (SSIA) | X | X | ||
| Pain intensity (last 7 days), NRS (SQRP sc and pc) | X | X | ||
| Consequences of pain on daily life, MPI-S (SQRP sc and pc) | X | X | ||
| Overall emotional distress, HADS (SQRP sc and pc) | X | X | ||
| Physical and mental health, RAND-36, (SQRP sc, supplementary questionnaire for pc) | X | X | ||
| Goal fulfilment and satisfaction (supplementary questionnaire) | X | X | ||
| Explanatory variables | ||||
| Self-reported fatigue (last 7 days), NRS (supplementary question) | X | X | ||
| Self-reported level of sleep disturbance, ISI (SQRP sc, supplementary questionnaire for pc) | X | X | ||
| Self-reported fear of movement, TSK (SQRP sc, supplementary questionnaire for pc) | X | X | ||
| Self-reported physical activity, (SQRP sc, supplementary questionnaire for pc) |
X | X | ||
| Pain catastrophising, PCS (SQRP sc and pc) | X | X | ||
| Perceived work ability, WAI (SQRP sc and pc) | X | X | ||
| Self-reported demands, control and support at the workplace, DCSQ (supplementary questionnaire) | X | X | ||
| Physical work environment (supplementary questionnaire) | X | X | ||
| Perceived life satisfaction, LiSat (optional questionnaire in SQRP for sc units, supplementary questionnaire for sc units not using it and for pc units) | X | X | ||
| Self-reported work situation during the study period (supplementary questions) | X | X | ||
| Self-reported workload during the study period (supplementary questions) | X | X | ||
−t1=prerecruitment period, t1=completed study period and follow-up 12 months after completed interdisciplinary pain rehabilitation programme.
DCSQ, The Swedish Demand-Control-Support Questionnaire; HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; LiSat, Life Satisfaction Scale; MPI-S, Multidimensional Pain Inventory Swedish version; NRS, Numeric Pain/Fatigue Rating Scale; pc, primary care level; PCS, Pain Catastrophizing Scale; sc, specialist care level; SQRP, Swedish National Quality Registry for Pain Rehabilitation; SSIA, Swedish Social Insurance Agency; SWEPPE, Sustainable WorkEr digital support for Persons with chronic Pain and their Employers; TSK, Fear-avoidance Tampa Scale for Kinesiophobia; WAI, Work Ability Index.
Secondary outcomes from SSIA:
Frequencies of individuals in a group who return to full-time or part-time work.
Number of sick-leave spells (per month).
Proportions of a group who returns to full-time or part-time work (per month).
Number of days at work before a new sick-leave spell >14 days occurs (in current diagnosis and in total for all diagnoses).
Proportions of a group who is back to work >28 days (full-time or part time) before a new sick-leave spell occurs.
Number of new sick-leave spells during the study period.
Duration of new sick-leave spells per person (gross and net days).55
Secondary outcomes from SQRP for specialist and primary care, and supplementary questionnaires:
Pain intensity during the last 7 days estimated with the Numeric Pain Rating Scale (0–10).56
Consequences of pain on daily life measured with the Multidimensional Pain Inventory Scale Swedish version, section 1 and 2 (0–6).57 58
Overall emotional distress assessed with the Hospital Anxiety and Depression Scale.59–61
Health-related quality of life measured with the RAND-36 health survey.60 62 63
Goal fulfilment inspired by the Canadian Occupational Performance Measure.64 The participants will at baseline be asked to report their work-related goal of full-time or part-time work for the coming twelve months and rate their present goal fulfilment and satisfaction on a scale ranging from 0, equalling ‘far from reaching my goal’/‘not satisfied at all’, to 10, equalling ‘my goal is fulfilled’/‘very satisfied’. At twelve months they will be asked to rate their goal fulfilment and satisfaction again.
Explanatory variables
The following explanatory variables, consistent with a biopsychosocial perspective, will be collected from SQRP for specialist and primary care, and supplementary questionnaires:
Self-reported fatigue during the last 7 days estimated with the Numeric Fatigue Rating Scale (0–10).65–67
Patient-reported insomnia measured with the Insomnia Severity Index.68 69
Fear of movement assessed with Fear-avoidance Tampa scale for Kinesiophobia (17 items).70
Physical activity estimated with the National Board of Health and Welfare’s three questions on physical activity (0–>300 min/week), exercise (0–>120 min/week) and sedentary behaviour (0–15 hours).71
Pain related catastrophising assessed with the Pain Catastrophizing Scale.72
Perceived work ability measured with the Work Ability Index (0–10).73
Job characteristics influencing psychological well-being estimated with the The Swedish Demand-Control-Support Questionnaire.74
Self-reported physical work environment using a questionnaire inspired by the Swedish Work Environment Authority ergonomics checklist.75 76
Perceived life satisfaction (1–6) measured with the Life Satisfaction Scale.77 78
Self-reported perceived work situation regarding barriers for RTW, strategies to handle barriers and need of support from the employer.
Self-reported total workload where the participants register number of hours per day for paid work and unpaid household work.79–81
Data collected from SWEPPE
Mobile app usage, for example, number of participants using the app, performing daily self-rating, sharing information with the employer or asking questions to the coach will be retrieved from SWEPPE.
Data collection methods
Data collection for the present trial will start during 2022. Baseline data will be collected when the IPRP is completed and study ending will be at 12 months follow-up after IPRP. Data will be collected from SSIA, the SQRP, supplementary questionnaires to the SQRP, and data registered in SWEPPE (table 1). Data collection for the SQRP is routinely performed when the IPRP is completed and at 12 months follow-up at both primary and specialised care units in Sweden providing IPRP. The supplementary questionnaires will be added to these routine data collections for the SQRP.
Data management
Data will be retrieved from SSIA and from the SQRP and connected to individual-level data retrieved from SWEPPE. The procedure is initiated by sending a file with the participants social security numbers and a consecutive number key to the SSIA who will fill in the ordered data for each participant. The SSIA will then send the file to SQRP for addition of registry data. The principal investigator will receive the file with consecutive numbered data from SQRP. All data collected in the study will be stored on a safe server at Linköping University. A data management plan (DMP) will be developed by the principal investigator and coworkers and will include a description of research data, information about documentation and quality control of research data, storage and back-up copying of research data, legal and ethical aspects, accessibility and long-term preservation of research data, and responsibility and resources related to the research data.
Data monitoring
All data in the trial will be monitored regularly. Since no sponsors or competing interests exists, monitoring of data will be performed independently. To ensure proper handling and storing of data (structure, organisation, file naming), the DMP will be reviewed regularly by the principal investigator and co-workers.
Statistical analysis
A statistical analysis plan will be developed with details of statistical analyses, handling of missing data and any additional analyses, for example, subgroup and adjusted analyses. Descriptive statistical analyses will be performed for transparent reporting of participant characteristics. The clinical effectiveness off SWEPPE will be analysed using effect-size and univariate and multivariate statistical analyses as a preliminary plan. Data from primary and secondary outcomes will be analysed according to intention-to-treat. Data from SWEPPE will be analysed using repeated measures analyses. All p values will be presented and a p value of <0.05 will be considered significant.
Harms
SWEPPE can be assumed not to create adverse events and is considered a safe intervention. Nevertheless, all participants will be encouraged to report any adverse events or unintended effects of trial intervention or trial conduct such as unexpected side effects or deterioration of symptoms.82
Auditing
To facilitate adherence to the study protocol,83 the project coordinator (CT) will have regular contact (every second week) with the unit coordinators during the study period. Processes to be reviewed are participant screening and eligibility. Documentation of the recruitment and randomisation/allocation process, for example, eligible patients asked to participate, the number of patients included, excluded or declining participation, performed by CT will be reviewed by the researchers (GL, MB).
Ethics and dissemination
The study is approved by the Swedish Ethics Review Board (Dnr 2020-01593, Dnr 2021-01854). Any important modifications of the study protocol will be communicated to the Swedish Ethics Review Board and to the participants. Informed consent (S3) will be collected from all participants by one of the researchers (CT). The consent form is design based on the Ethics committee recommendation and includes written information about the study.
Confidentiality will be protected by coding of individual participants’ collected data. Data will be stored at a password protected project server at Linköping University and will not be accessed by unauthorised persons. The study results will be submitted to peer-review journals for publication and will be presented in national and international research networks, clinical settings and patient associations. There is no present plan regarding public access of participant-level data set or statistical code.
Supplementary Material
Footnotes
Twitter: @ChristinaTures1
Contributors: MB and GL formed the original research concept. CT, MB and GL contributed to the study design and CT will coordinate the project in cooperation with MB and GL. CT, ML and LV will be responsible for the unit coordinators at each participating unit and the inclusion of participants. All authors will collect and manage data during the trial. CT, MB and GL have written and revised this protocol with critical input from ML and LV. All authors have contributed important intellectual content to the manuscript.
Funding: This study was supported by the Swedish Research Council for Health, Working Life and Welfare (Dnr 2019-01264).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Harker J, Reid KJ, Bekkering GE, et al. Epidemiology of chronic pain in Denmark and Sweden. Pain Res Treat 2012;2012:371248 10.1155/2012/371248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011;27:449–62. 10.1185/03007995.2010.545813 [DOI] [PubMed] [Google Scholar]
- 3.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:1001–6. 10.15585/mmwr.mm6736a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez C, Margarit C, Sánchez-Magro I, et al. Chronic pain features relate to quality of life more than physiopathology: a cross-sectional evaluation in pain clinics. Pain Pract 2017;17:866–78. 10.1111/papr.12533 [DOI] [PubMed] [Google Scholar]
- 5.Gerdle B, Björk J, Henriksson C, et al. Prevalence of current and chronic pain and their influences upon work and healthcare-seeking: a population study. J Rheumatol 2004;31:1399–406. [PubMed] [Google Scholar]
- 6.Cáceres-Matos R, Gil-García E, Barrientos-Trigo S, et al. Consequences of chronic non-cancer pain in adulthood. scoping review. Rev Saude Publica 2020;54:39. 10.11606/s1518-8787.2020054001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asmundson GJG, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009;26:888–901. 10.1002/da.20600 [DOI] [PubMed] [Google Scholar]
- 8.Shupler MS, Kramer JK, Cragg JJ, et al. Pan-Canadian estimates of chronic pain prevalence from 2000 to 2014: a repeated cross-sectional survey analysis. J Pain 2019;20:557–65. 10.1016/j.jpain.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: cochrane systematic review and meta-analysis. BMJ 2015;350:h444. 10.1136/bmj.h444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waterschoot FPC, Dijkstra PU, Geertzen JHB. Dose or content? effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Author reply. Pain 2014;155:1902–3. [DOI] [PubMed] [Google Scholar]
- 11.Scascighini L, Toma V, Dober-Spielmann S, et al. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology 2008;47:670–8. 10.1093/rheumatology/ken021 [DOI] [PubMed] [Google Scholar]
- 12.Svanholm F, Liedberg GM, Löfgren M, et al. Factors of importance for return to work, experienced by patients with chronic pain that have completed a multimodal rehabilitation program - a focus group study. Disabil Rehabil 2022;44:1–9. 10.1080/09638288.2020.1780479 [DOI] [PubMed] [Google Scholar]
- 13.Toye F, Seers K, Allcock N, et al. A synthesis of qualitative research exploring the barriers to staying in work with chronic musculoskeletal pain. Disabil Rehabil 2016;38:566–72. 10.3109/09638288.2015.1049377 [DOI] [PubMed] [Google Scholar]
- 14.Liedberg GM, Björk M, Dragioti E, et al. Qualitative evidence from studies of interventions aimed at return to work and staying at work for persons with chronic musculoskeletal pain. J Clin Med 2021;10:1247:1247. 10.3390/jcm10061247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkie R, Bjork M, Costa-Black KM, et al. Managing work participation for people with rheumatic and musculoskeletal diseases. Best Pract Res Clin Rheumatol 2020;34:101517. 10.1016/j.berh.2020.101517 [DOI] [PubMed] [Google Scholar]
- 16.Glavare M, Löfgren M, Schult M-L. Between unemployment and employment: experience of unemployed long-term pain sufferers. Work 2012;43:475–85. 10.3233/WOR-2012-1394 [DOI] [PubMed] [Google Scholar]
- 17.Saunders SL, Nedelec B. What work means to people with work disability: a scoping review. J Occup Rehabil 2014;24:100–10. 10.1007/s10926-013-9436-y [DOI] [PubMed] [Google Scholar]
- 18.Grant M, O-Beirne-Elliman J, Froud R, et al. The work of return to work. challenges of returning to work when you have chronic pain: a meta-ethnography. BMJ Open 2019;9:e025743. 10.1136/bmjopen-2018-025743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magalhães L, Chan C, Chapman AC, et al. Successful return to work of individuals with chronic pain according to health care providers: a meta-synthesis. Cadernos Brasileiros de Terapia Ocupacional 2017;25:825–37. 10.4322/2526-8910.ctoAR2063 [DOI] [Google Scholar]
- 20.Cohen D, Allen J, Rhydderch M, et al. The return to work discussion: a qualitative study of the line manager conversation about return to work and the development of an educational programme. J Rehabil Med 2012;44:677–83. 10.2340/16501977-0996 [DOI] [PubMed] [Google Scholar]
- 21.Scott W, Chilcot J, Guildford B, et al. Feasibility randomized-controlled trial of online acceptance and commitment therapy for patients with complex chronic pain in the United Kingdom. Eur J Pain 2018;22:1473–84. 10.1002/ejp.1236 [DOI] [PubMed] [Google Scholar]
- 22.Coutu M-F, Légaré F, Durand M-J, et al. Acceptability and feasibility of a shared decision-making model in work rehabilitation: a mixed-methods study of stakeholders' perspectives. J Occup Rehabil 2019;29:128–39. 10.1007/s10926-018-9770-1 [DOI] [PubMed] [Google Scholar]
- 23.Turesson C, Liedberg G, Björk M. Development of a digital support application with evidence-based content for sustainable return to work for persons with chronic pain and their employers: a user-centered agile design approach. JMIR Hum Factors 2022;9:e33571. 10.2196/33571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brhel M, Meth H, Maedche A, et al. Exploring principles of user-centered agile software development: a literature review. Inf Softw Technol 2015;61:163–81. 10.1016/j.infsof.2015.01.004 [DOI] [Google Scholar]
- 25.Braillard O, Cedraschi C, Jesaimani A, et al. [Chronic noncancer pain and patient education: a place for e-learning?]. Rev Med Suisse 2015;11:1400:2–5. [PubMed] [Google Scholar]
- 26.Solem IKL, Varsi C, Eide H. A user-centered approach to an evidence-based electronic health pain management intervention for people with chronic pain: design and development of EPIO. JMIR 2020;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristjánsdóttir Ólöf Birna, Fors EA, Eide E, et al. A smartphone-based intervention with diaries and therapist feedback to reduce catastrophizing and increase functioning in women with chronic widespread pain. Part 2: 11-month follow-up results of a randomized trial. J Med Internet Res 2013;15:e72. 10.2196/jmir.2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurnheer SE, Gravestock I, Pichierri G, et al. Benefits of mobile apps in pain management: systematic review. JMIR Mhealth Uhealth 2018;6:e11231. 10.2196/11231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garg S, Garg D, Turin TC, et al. Web-based interventions for chronic back pain: a systematic review. J Med Internet Res 2016;18:e139. 10.2196/jmir.4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slattery BW, Haugh S, O'Connor L, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res 2019;21:e11086. 10.2196/11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ledel Solem IK, Varsi C, Eide H, et al. Patients' needs and requirements for eHealth pain management interventions: qualitative study. J Med Internet Res 2019;21:e13205. 10.2196/13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmers T, Janssen L, Kool RB, et al. Educating patients by providing timely information using smartphone and tablet apps: systematic review. J Med Internet Res 2020;22:e17342. 10.2196/17342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granja C, Janssen W, Johansen MA. Factors determining the success and failure of eHealth interventions: systematic review of the literature. J Med Internet Res 2018;20:e10235. 10.2196/10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devan H, Hale L, Hempel D, et al. What works and does not work in a self-management intervention for people with chronic pain? qualitative systematic review and meta-synthesis. Phys Ther 2018;98:381–97. 10.1093/ptj/pzy029 [DOI] [PubMed] [Google Scholar]
- 35.Devan H, Farmery D, Peebles L, et al. Evaluation of self-management support functions in apps for people with persistent pain: systematic review. JMIR Mhealth Uhealth 2019;7:e13080. 10.2196/13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruckenstein M. Visualized and interacted life: personal analytics and Engagements with data doubles. Societies 2014;4:68–84. 10.3390/soc4010068 [DOI] [Google Scholar]
- 37.Mamykina L, Smaldone AM, Bakken SR. Adopting the sensemaking perspective for chronic disease self-management. J Biomed Inform 2015;56:406–17. 10.1016/j.jbi.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morton K, Dennison L, May C, et al. Using digital interventions for self-management of chronic physical health conditions: a meta-ethnography review of published studies. Patient Educ Couns 2017;100:616–35. 10.1016/j.pec.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado GC, Pinheiro MB, Lee H, et al. Smartphone apps for the self-management of low back pain: A systematic review. Best Pract Res Clin Rheumatol 2016;30:1098–109. 10.1016/j.berh.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 40.Coe-O'Brien R, Joseph L, Kuisma R, et al. Outcome measures used in the smartphone applications for the management of low back pain: a systematic scoping review. Health Inf Sci Syst 2020;8:5. 10.1007/s13755-019-0097-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672–7. 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 44.WHO . WHO trial registration data set (version 1.3.1): World Health organization, 2021. Available: https://www.who.int/clinical-trials-registry-platform/network/who-data-set
- 45.LoMartire R, Dahlström Örjan, Björk M, et al. Predictors of sickness absence in a clinical population with chronic pain. J Pain 2021;22:1180–94. 10.1016/j.jpain.2021.03.145 [DOI] [PubMed] [Google Scholar]
- 46.Altman DG, Bland JM. How to randomise. BMJ 1999;319:703–4. 10.1136/bmj.319.7211.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J, Shin W. How to do random allocation (randomization). Clin Orthop Surg 2014;6:103–9. 10.4055/cios.2014.6.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedgwick P. Treatment allocation in trials: block randomisation. BMJ 2014;348:g2409. 10.1136/bmj.g2409 [DOI] [PubMed] [Google Scholar]
- 49.Sennehed CP, Stigmar K, Grahn B, et al. Evaluation of a multimodal pain rehabilitation programme in primary care based on clinical register data: a feasibility study. Prim Health Care Res Dev 2020;21:e2. 10.1017/S1463423619000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rivano Fischer M, Persson EB, Stålnacke B-M, et al. Return to work after interdisciplinary pain rehabilitation: one- and two-year follow-up based on the Swedish quality Registry for pain rehabilitation. J Rehabil Med 2019;51:281–9. 10.2340/16501977-2544 [DOI] [PubMed] [Google Scholar]
- 51.IASP . IASP terminology. Available: https://www.iasp-pain.org/terminology?navItemNumber=576#Pain
- 52.Ballantyne JC, Sullivan MD. Intensity of chronic pain-the wrong metric? N Engl J Med 2015;373:2098–9. 10.1056/NEJMp1507136 [DOI] [PubMed] [Google Scholar]
- 53.Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137:276–85. 10.1016/j.pain.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 54.Patel KV, Amtmann D, Jensen MP, et al. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep 2021;6:e784. 10.1097/PR9.0000000000000784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Försäkringskassan . Förslag på utfallsmått för att mäta återgång i arbete efter sjukskrivning. Försäkringskassan Analys och prognos, 2016. [Google Scholar]
- 56.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005;14:798–804. 10.1111/j.1365-2702.2005.01121.x [DOI] [PubMed] [Google Scholar]
- 57.Bergström G, Jensen IB, Bodin L, et al. Reliability and factor structure of the Multidimensional Pain Inventory--Swedish Language Version (MPI-S). Pain 1998;75:101–10. 10.1016/S0304-3959(97)00210-8 [DOI] [PubMed] [Google Scholar]
- 58.Kerns RD, Turk DC, Rudy TE. The West Haven-Yale multidimensional pain inventory (WHYMPI). Pain 1985;23:345–56. 10.1016/0304-3959(85)90004-1 [DOI] [PubMed] [Google Scholar]
- 59.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 60.LoMartire R, Äng BO, Gerdle B, et al. Psychometric properties of short Form-36 health survey, EuroQol 5-dimensions, and hospital anxiety and depression scale in patients with chronic pain. Pain 2020;161:83–95. 10.1097/j.pain.0000000000001700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 2003;1:29. 10.1186/1477-7525-1-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vetter TR. A primer on health-related quality of life in chronic pain medicine. Anesth Analg 2007;104:703–18. 10.1213/01.ane.0000255290.64837.61 [DOI] [PubMed] [Google Scholar]
- 63.Orwelius L, Nilsson M, Nilsson E, et al. The Swedish RAND-36 Health Survey - reliability and responsiveness assessed in patient populations using Svensson's method for paired ordinal data. J Patient Rep Outcomes 2017;2:4. 10.1186/s41687-018-0030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wressle E, Lindstrand J, Neher M, et al. The Canadian occupational performance measure as an outcome measure and team tool in a day treatment programme. Disabil Rehabil 2003;25:497–506. 10.1080/0963828031000090560 [DOI] [PubMed] [Google Scholar]
- 65.Aaronson LS, Teel CS, Cassmeyer V, et al. Defining and measuring fatigue. Image J Nurs Sch 1999;31:45–50. 10.1111/j.1547-5069.1999.tb00420.x [DOI] [PubMed] [Google Scholar]
- 66.Chuang L-L, Lin K-C, Hsu A-L, et al. Reliability and validity of a vertical numerical rating scale supplemented with a faces rating scale in measuring fatigue after stroke. Health Qual Life Outcomes 2015;13:91. 10.1186/s12955-015-0290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dailey DL, Frey Law LA, Vance CGT, et al. Perceived function and physical performance are associated with pain and fatigue in women with fibromyalgia. Arthritis Res Ther 2016;18:68. 10.1186/s13075-016-0954-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akerstedt T, Anund A, Axelsson J, et al. Subjective sleepiness is a sensitive indicator of insufficient sleep and impaired waking function. J Sleep Res 2014;23:240–52. 10.1111/jsr.12158 [DOI] [PubMed] [Google Scholar]
- 69.Morin CM. Measuring outcomes in randomized clinical trials of insomnia treatments. Sleep Med Rev 2003;7:263–79. 10.1053/smrv.2002.0274 [DOI] [PubMed] [Google Scholar]
- 70.Boersma K, Linton S, Overmeer T, et al. Lowering fear-avoidance and enhancing function through exposure in vivo. A multiple baseline study across six patients with back pain. Pain 2004;108:8–16. 10.1016/j.pain.2003.03.001 [DOI] [PubMed] [Google Scholar]
- 71.Agerberg M. Ställ rätt fråga om fysisk aktivitet. Läkartidningen 2014;48:C9AY. [Google Scholar]
- 72.Sullivan MJL, Bishop SR, Pivik J, Scott R. The pain Catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 73.Lundin A, Leijon O, Vaez M, et al. Predictive validity of the work ability index and its individual items in the general population. Scand J Public Health 2017;45:350–6. 10.1177/1403494817702759 [DOI] [PubMed] [Google Scholar]
- 74.Sanne B, Torp S, Mykletun A, et al. The Swedish Demand-Control-Support questionnaire (DCSQ): factor structure, item analyses, and internal consistency in a large population. Scand J Public Health 2005;33:166–74. 10.1080/14034940410019217 [DOI] [PubMed] [Google Scholar]
- 75.Arbetsmiljöverket . Belastningsergonomi. Arbetsmiljöverkets föreskrifter och allmänna råd om belastningsergonomi. 2. AFS, 2012. [Google Scholar]
- 76.Arbetsmiljöverket . Checklista för bedömning - utifrån föreskrifterna om belastningsergonomi, AFS 2012:2. Stockholm: Arbetsmiljöverket, 2013. [Google Scholar]
- 77.Stålnacke B-M. Life satisfaction in patients with chronic pain - relation to pain intensity, disability, and psychological factors. Neuropsychiatr Dis Treat 2011;7:683. 10.2147/NDT.S25321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fugl-Meyer A, Bränholm I, Fugl-Meyer K. Happiness and domain-specific life satisfaction in adult Northern Swedes. Clin Rehabil 1991;5:25–33. [Google Scholar]
- 79.Krantz G, Berntsson L, Lundberg U. Total workload, work stress and perceived symptoms in Swedish male and female white-collar employees. Eur J Public Health 2005;15:209–14. 10.1093/eurpub/cki079 [DOI] [PubMed] [Google Scholar]
- 80.Kahn R. The forms of women’s work. In: Frankenhaeuser M, Lundberg U, Chesney M, eds. Women, work and health. stress and opportunities. New York Plenum Press, 1991: 65–83. [Google Scholar]
- 81.Mårdberg B, Lundberg ULF, Frankenhaeuser M. The total workload of parents employed in white-collar jobs: construction of a questionnaire and a scoring system. Scand J Psychol 1991;32:233–9. 10.1111/j.1467-9450.1991.tb00873.x [DOI] [Google Scholar]
- 82.Palermo TM, Slack K, Loren D, et al. Measuring and reporting adverse events in clinical trials of psychological treatments for chronic pain. Pain 2020;161:713–7. 10.1097/j.pain.0000000000001772 [DOI] [PubMed] [Google Scholar]
- 83.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-060452supp001.pdf (247.9KB, pdf)
bmjopen-2021-060452supp002.pdf (169.9KB, pdf)