Abstract
Tissue-resident memory T (TRM) cells have emerged as immune sentinels that patrol the tissue microenvironment and orchestrate localized antitumor immunity in various solid cancers. Recent studies have revealed that TRM cells are key players in cancer immunosurveillance, and their involvement has been linked to favorable responses to immunotherapy as well as general better clinical outcome in cancer patients. In this review, we provide an overview of the major advances and recent findings regarding TRM cells phenotype, transcriptional and epigenetic regulation in cancer with a special focus on gastrointestinal tumors. Finally, we highlight the exciting clinical implication of TRM cells in these types of tumors.
Keywords: gastrointestinal neoplasms, immunity, immunotherapy, immunologic memory
Introduction
The tumor microenvironment (TME) represents a complex and dynamic structure with several actors from tumor-fighting cells such as cytotoxic CD8+T cells, T helper 1 (TH1) cells to immunosuppressive ones such as regulatory T cells (Treg), myeloid-derived suppressive cells, tumor-associated macrophages to cancer-associated fibroblasts.1 The Composition of the TME dictates not only the patient’s prognosis but also treatment response. Based on the immune contexture of the TME, cancers are now classified into two major groups: ‘cold’ tumors (with low to absent T cells infiltration) and ‘hot’ tumors (with high T cell infiltration).2 Gastrointestinal (GI) cancers are one of the most prevalent and death-leading cancers followed by lung and breast cancers (BC). The majority of GI tumors are essentially classified as cold tumors with the minor exception of tumors with microsatellite instability (MSI). Understanding the interplay between the immune system and the tumor cells has helped tremendously better cancer stratification as well as therapeutic decisions for each patient. An important proof of concept observation in highlighting the key role of the immune infiltrates in the control of tumor, is the positive correlation between tumor-infiltrating lymphocytes (TILs) levels and cancer prognosis observed in a large spectrum of solid cancers including GI tumors. One example is the tremendous success of the ‘immunoscore’ in colon cancer treatment. Based on CD3+ and CD8+ T cells density in the tumor, Galon et al were able to outline the immune fitness of a given tumor, to better stratify patients according to their prognosis, and to predict their response to therapies.3 4 Remarkably, the immunoscore exceeded MSI status in predicting patients’ survival and disease recurrence.5 Taking together, these observations highlight the key role played by the immune system in shaping cancer progression, and suggest that a comprehensive characterization of the composition of the immune infiltrate in GI cancers is ever-growingly advocated, and is crucial for better treatment decision making.
Maintaining a memory from a previously encountered antigen is one of the most fundamental features of the immune system. On encountering their cognate antigen, naive T cells go through clonal expansion and become effector T cells. Subsequently, these cells undergo a bifurcation in their differentiation leading to the generation of a major subset of short-lived terminally differentiated effector T cells and a minor subset of memory T cell precursors. Generally, memory T cells are subdivided into two major subpopulations: central memory T cells (TCM) and effector memory T cells (TEM). Classification of these subsets relies on the expression levels of the lymphoid homing molecules CD62L and CCR7.6 While TCM express both CD62L and CCR7, TEM on the other hand express neither of these markers. Lately, a novel subpopulation of memory T cells named ‘Tissue-resident memory T cells’ (TRM) has been described. In contrary to TCM and TEM that are mainly found recirculating between the blood and peripheral tissues; TRM cells persist in peripheral tissues.7 8
TRM cells can induce potent imminent localized immune responses against diverse pathogens. In the central nervous system (CNS), peripheral immunizations led to the generation of robust TRM cells conferring long-term protection against CNS infection.9 Additionally, in the brain during a secondary challenge, TRM cells autonomously cleared lymphocytic choriomeningitis virus (LCMV) independently of the presence of circulating memory T cells.10 Along with mouse models, TRM cells specific to a wide spectrum of viruses such as CMV, hepatitis B virus (HBV), Epstein-Barr virus, and HIV were also detected in diverse human tissues.11–15 Such findings identify TRM cells as a potent, first-line, self-sufficient adaptive immune response in non-lymphoid tissues.
TRM cells have been reported in several human tissues such as the pancreas, liver, intestine, skin, kidney, brain, lung, as well as lymph nodes, adipose tissues, and salivary glands.16–26 Various studies reported a core transcriptional residency program of TRM cells characterized by high expression of adhesion and retention biomarkers as well as down-regulation of tissue egress ones (S1PR1, CCR7, CD62L).23 27 Expression of CD103 and/or CD69 are considered bona fide markers of TRM cells. CD103 is essentially restricted to CD8+ TRM cells, with little expression on CD4+ TRM cells. CD103 or Integrin αE pairs with integrin β7 to form a heterodimeric receptor that binds to E-cadherin and enables tissue retention of CD103-expressing T cells.28 29 Similarly, CD69, a type II C-lectin counteracts tissue egress by down-regulating the expression of sphingosine-1-phosphate receptor-1 (S1PR1).30 31 Consequently, the expression of these biomarkers by TRM cells sustains their peripheral tissue retention. Furthermore, a recent study conducted on over 50 000 activated and resting human T cells from lung, lymph nodes, bone marrow, and blood revealed that both resting and activated CD4+ and CD8+ TRM expressed the canonical biomarkers CXCR6 and CD49a.32 CXCR6 is a chemokine that promotes extra-lymphoid tissue homing for lymphocytes and was reported to regulate TRM cells’ localization to the airways.33 CD49a (also known as Integrin α1 subunit) pairs with integrin β7 to constitute VLA-1 (Very Late Antigen 1) which binds to collagen IV.34 35 CD49a was described as a regulator of cutaneous TRM cells persistence and is a biomarker for highly cytotoxic TRM cells.36 37 This TRM signature was largely enriched in lung tissue followed by the lymphoid site.38 Interestingly, although TRM cells are defined as memory T cells expressing tissue residency biomarkers, these cells display phenotypic as well as functional tissue-specific heterogeneity dictated by the tissue environment, which will be discussed in detail later in this review.
One of the major limitations in studying TRM cells in human tissues is the exclusive use of the phenotypic characterization for TRM cells identification, which reliability is highly questioned as certain of the tissue residency markers mentioned above (eg, CD69, CD49a) could also be expressed by circulating T cells. Results from solid-organ transplantation and antibody-depletion studies allowed the identification of human TRM cells based on their functional characteristics. In a study conducted on patients who had received pancreatic-duodenal transplantation (Tx), Zuber et al reported the presence of donor CD8 T cells for more than 600 days in some non-rejected transplants.39 In parallel, by studying normal and transplanted human small intestine (SI), Bartolomé-Casado et al demonstrated that the majority of both intraepithelial (IE) and lamina propria (LP) T cells were tissue-resident and persisted over a year in the graft.40 Interestingly, the IE and LP TRM cells were phenotypically, clonally, and functionally distinct, with LP CD8+ TRM cells exhibiting higher polyfunctionality and more potent cytokine production (GZMB, perforin) (table 1). Another study showed that the use of low-dose alemtuzumab, an anti-CD52 antibody that depletes circulating blood T cells and effectively treated patients with leukemic cutaneous T cell lymphoma, induced depletion of TCM cells but sessile, non-circulating skin TEM cells were spared and showed higher IFN-γ production.41 Additionally, alemtuzumab-treated patients presented lower marked infections despite the total absence of circulating T cells. These observations provided evidence of the existence of non-recirculating skin resident T cells, that are capable of providing frontline protection against infection even in the absence of circulating T cells recruitment.
Table 1.
Phenotypic and functional heterogeneity of TRM cells across organs
| Localization | Phenotype | Functional characteristics | Reference | |
| CD8 TRM | ||||
| Gut | mice | Id3low Blimp1high | 50 | |
| Id3high Blimp1 low | ||||
| Human | CD103+CD69+ | Highly polyfunctional (IFN-γ+/IL-2+/TNF-α+) |
51 | |
| β2integrin+CD103−CD69+ | Highly cytotoxic (GZMB+) | |||
| IE | CD103+ KLRG1- CD28 low 2B4 high CD161high CD127high PD-1low |
Damped polyfunctionality Damped cytotoxicity |
40 | |
| LP | CD103+ KLRG1- CD28low 2B4 high CD161high CD127 high PD-1low |
Highly polyfunctional (IFN-γ+/IL-2+/TNF-α+) GZMB+, Perforin+ |
||
| CD103- KLRG1- CD28high CD161low CD127low PD-1 high NKG2D high |
Polyfunctional (IFN-γ+/IL-2+/TNF-α+) |
|||
| CD103- KLRG1+ CD28 high CD161 low CD127 low PD-1 high NKG2D high |
Highly cytotoxic (GZMB+, Perforin+) |
|||
| Liver | mice | CD69+ CD103- | Highly polyfunctional (IFN-γ+/IL-2+/TNF-α+) |
55 |
| Human | CD69+CD103+ CXCR6+ CXCR3+ | High IL-2 production | 11 | |
| Pancreas | Human | CD69+ CD103+ CD49a+ CD101+ PD-1+ |
GZMB+ Highly polyfunctional (IFN-γ+/IL-2+) |
16 |
| Skin | mice | CD69+ CD103+ | Damped polyfunctionality (IFN-γ+/IL-2+/TNF-α+) |
55 |
| CD69+ CD103+ CD49a+ | Increased IFN-γ production | 37 | ||
| Salivary gland | mice | CD69+ CD103- CD69+ CD103+ |
Highly polyfunctional (IFN-γ+/IL-2+/TNF-α+) Damped polyfunctionality (IFN-γ+/IL-2+/TNF-α+) |
55 |
| Lymph nodes | human | CD69+ CD103- | IFN-γ+, IL-2+ | 16 |
| CD4 TRM | ||||
| Liver | human | CD69 high CXCR6+ CD49a+ PD-1+ S1PR1- CD69 int CXCR3+ CX3CR1+ CXCR1+ |
TH1 cytokine production (IL-2, IFN-γ, IL-21) TH2 cytokine production (IL-4) |
56 |
| Small intestine | human | CD69+ CD103+ 2B4+ CD49a+ CXCR6+ CD101+ CD161+ CD28low CD69+ CD103- KLRG1+ CD49a+ CXCR6+ CD101+ CD161+ CD28low |
TH1 cytokine production (IFN-γ+/IL-2+/TNF-α+) GZMB+, Perforin+, IL-17+ TH1 cytokine production (IFN-γ+/IL-2+/TNF-α+) Perforin+ |
57 |
IE, Intra-epithelial; LP, lamina propria.
Most of our understanding of TRM cell biology stems from studies conducted on TRM cells discovered in healthy tissues. Understanding the underlying mechanisms of TRM cells’ differentiation, maintenance, and function in the context of cancer is an ongoing process. Here, we provide an overview of major advances and recent findings regarding TRM cells phenotype, transcriptional and epigenetic regulation in cancer with a special focus on GI tumors. Finally, we highlight the exciting clinical implication of TRM cells in these cancers.
Hallmark features of TRM cells in the GI tract
TRM cells’ presence was described in a wide range of GI tissues.16 42–45 In the intestine, TRM cells have been widely studied in mice and were reported to be essentially lodged in the IE niches, expressing CD103 and CD69.46–49 Recently, single-cell RNA sequencing (scRNAseq) analyses identified distinct intestinal antigen-specific TRM cells subsets based on the expression of transcription factors (TF) Blimp1 and Id3 at different memory time points post LCMV infection.50 These subsets shared transcriptional features of effector like TEM cells and TCM cells and they strikingly presented inter and intratemporal heterogeneity. While Id3low Blimp1high cells were present at high levels early after LCMV infection, the frequency of Id3high Blimp1 low cells increased by 10-fold during the course of LCMV infection and was associated with an enriched memory gene expression signature.
In addition, the Id3high cell subset had a more long-lived transcriptional profile and expressed higher levels of CD69 corresponding to a TRM phenotype, suggesting that this Id3high Blimp1low cell subset represents TRM cells or TRM precursors. In contrast, in the human SI, TRM cells represented the majority of CD8+ T cells accumulated in both the LP and the epithelium, expressing CD103 and CD69 and demarcated by long-term persistence and polyfunctionality (IFN-γ+ IL-2+TNF-α+).40 Using scRNAseq, Fitz Patrick et al recently identified two major CD8+ human intestinal TRM subsets with distinct phenotypic and functional features; CD103+CD69+ TRM cells and β2-integrin+CD103−CD69+ TRM cells; CD103+CD69+ TRM displayed greater polyfunctionality, whereas β2-integrin+CD103−CD69+ TRM cells had higher granzyme expression.51
In the liver, TRM cells patrol the hepatic sinusoids, which allow them to interact with a vast variety of circulating cells and antigens.52 Contrarily to gut TRM cells, liver TRM were essentially identified as CD69+ cells lacking CD103 expression. Liver TRM do not require CD103 for tissue adhesion but rather LFA-1 expression.53 However, a small subset of liver TRM cells was identified as CD69+CD103+CXCR6+CXCR3+ cells poised for non-cytolytic antiviral effector functions.11 54 In line with the observed heterogeneity in the intestine, a novel study revealed that liver and skin TRM illustrate two extremes of phenotypic variations.55 While liver TRM were essentially CD103- CD69+, skin TRM were exclusively CD103+ CD69+ cells exhibiting high PD-1 expression. Strikingly, this observed phenotypic variation was associated with a functional heterogeneity mirrored by high polyfunctionality and an increased proliferative potential of liver TRM cells, while skin TRM were less polyfunctional but with higher longevity.55 Interestingly, the investigation of TRM cells phenotype and function in the salivary glands revealed the presence of two TRM subsets CD103-CD69+ and CD103+CD69+ endowed with the same functional characteristics as liver and skin TRM, respectively. Hence, the salivary gland model proved the existence of an intraorgan phenotypic as well as a functional heterogeneity of TRM cells in addition to the previously discussed inter-organ variability. CD8+ CD69+CD103+PD-1high TRM cells were also reported in the pancreas, showing an almost exclusive lodgment in the exocrine areas.16 Consistently with previously discussed results, pancreatic TRM cells were reported to be phenotypically and functionally distinct from neighboring GI sites (jejunum) and lymphoid tissues. TRM cells in the pancreas showed higher expression of PD-1 and CD49a compared with TRM cells in the jejunum and pancreas-draining lymph nodes, in addition to the significantly higher expression level of GZMB.16
Although little is known about hepatic CD4+ TRM characteristics, a recent study has identified two phenotypically and functionally heterogeneous intrahepatic CD4+ TRM subsets according to CD69 expression.56 CD69high TRM cells occupied sinusoidal and periportal niches and were characterized by the expression of CXCR6, CD49a, PD-1, and the production of TH1 cytokine. CD69int TRM cells had a distinct profile and were characterized by the expression of CX3CR1, CXCR3, and CXCR1, as well as by the production of IL-4. In transplanted duodenum in humans, CD4 +TRM cells also presented intraorgan heterogeneity with the vast majority of CD4+ TRM being CD69+CD103-, however, a small fraction expressed CD103+ and was exclusively lodged in the LP.57 Although both CD4+ TRM subsets displayed a TH1 cytokine profile, CD103+ cells produced significantly higher levels of GZMB and contained a small fraction of TRM cells that produced IL-17.
Although TRM cells share a common transcriptional foundation to establish tissue residency (figure 1), recent studies gave great insight into their phenotypic heterogeneity which was completely independent of the infection model but stringently tied to their tissue of lodgment. Interestingly, whether TRM are localized in the GI tract or the skin or the salivary glands, they did exhibit a phenotypic heterogeneity that conferred heterogenous functional properties to these cells (summarized in table 1).
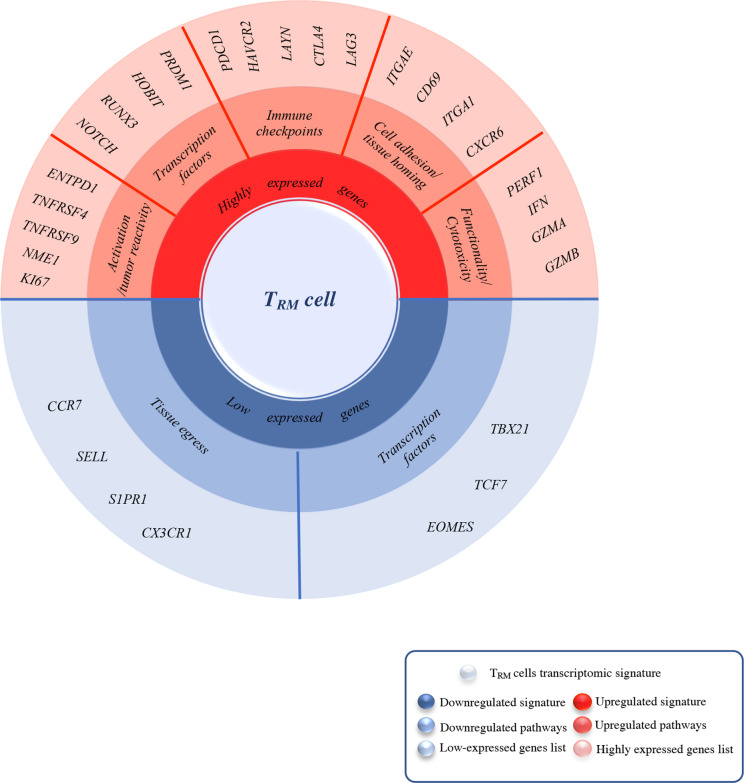
Figure 1.
Summary of the most upregulated/ downregulated genes in TRM cells identified by Bulk/Sc-RNA sequencing. This spin chart summarizes the core residency signature specific to TRM cells compared with TEM and TCM identified by bulk and/or single-cell analysis. TRM signature is mainly characterized by high expression of genes encoding for tissue residency and immune checkpoints (in red), both quite tissue-specific, in this figure, we also focused on those GI cancers specific such as CD103, CXCR6, PD-1, and LAG-3. Another remarkable feature of TRM cells signature is the upregulated expression of cytotoxicity and functionality encoding genes compared with their memory counterparts TEM and TCM. Along with this upregulation, certain genes signaling are either completely shut down (in blue) such as tissue egress ones (SELL, CCR7) and TCF1 which inhibit ITGAE (CD103) expression or downregulated such as TBX21 (T-bet) for IL-15R expression maintenance. GI, gastrointestinal; TCM, central memory T cells; TEM, effector memory T cells; TRM, tissue-resident memory T cells.
Finally, recent findings unveiled tissue-specific metabolic requirements of TRM cells. Although fatty acid-binding proteins ‘FABP4’ and ‘FABP5’ were reported to be important for skin TRM cells persistence, a novel study by Frizzell et al demonstrated that the expression of FABP encoding genes in TRM cells is dictated by their tissue of lodgment.58 While skin TRM cells showed high expression of Fabp4 and Fabp5, TRM cells lodged in the epithelium of the SI (SI-IEL) showed high expression of Fabp1, Fabp2, and Fabp6, whereas liver TRM cells expressed high levels of Fabp1. Interestingly, the expression of FABP encoding genes was driven by tissue-specific mediators rather than a residency program.
TRM phenotype in GI cancer: a field of ongoing investigations
Several independent studies reported the presence of TRM cells in various cancer types including colon cancer,44 pancreatic cancer,42 melanoma,59 lung cancer,60 head and neck squamous cell carcinoma,61 and bladder cancer (figure 2).62 63 Remarkably, in the majority of these studies, TRM cells were reported to express the canonical tissue residency biomarkers CD103 and/or CD69. However, it is important to note that CD103 has also been reported to be a hallmark for tumor-infiltrating Treg and is expressed on dendritic cells (DCs)64 65 and that CD69 is a well-established T cell activation marker.31 Therefore, taken solely these biomarkers does not represent an exclusive hallmark of tissue residency. Consequently, the use of a single biomarker to identify TRM cells is overly simplistic, and in-depth phenotypic and functional investigations are warranted to identify novel, more refined biomarkers for TRM characterization.
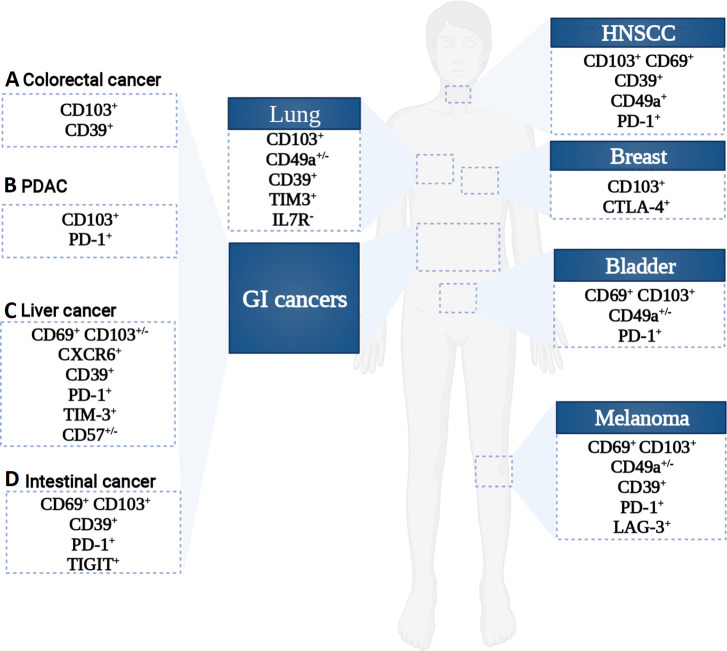
Figure 2.
Phenotypic heterogeneity of CD8 TRM cells across cancer types. An overview of the reported CD8 TRM cells phenotype in GI and non-GI cancers showing an inter and intra-organ heterogeneity. HNSCC, head and neck squamous cell carcinomas; GI, gastrointestinal; PDAC, pancreatic ductal adenocarcinoma.
Although TRM cells phenotype differs between tissue sites and organs, in GI cancers, TRM cells were essentially identified as CD103+, notably in colorectal cancer (CRC), pancreatic cancer, gastric cancer, esophageal cancer, and hepatocellular carcinoma (HCC).42 44 66–68 The high expression of CD103 by TRM cells is possibly resulting from the epithelial origin of these cancers. A subset of CD8+CD103+CD39+ TRM has also been described in CRC.69 Transcriptomic profiling of this subset unveiled a TRM signature characterized by high expression of HAVCR2, CTLA4, and LAYN, while still exhibiting high expression of cytotoxic proteins (GZMA/GZMB/GZMH, TNF, Perforin 1, and IFN-γ). Luckily, the field of TRM phenotyping is gaining huge momentum since the emergence of sc-RNAseq technology, which helps to deciphering the underlying heterogeneity of TRM cells in the context of cancer as well as identifying novel TRM subsets. A recent single-cell study conducted on TRM cells in lung cancer enabled the identification of a novel uncharacterized tumor-associated antigen specific CD8+ TRM subset defined as CD103+TIM-3+IL-7R-.38 Data analysis of this TRM subset, showed enrichment in transcripts encoding for high cytotoxic activity, as well as inhibitory molecules such as CTLA4 and especially PDCD1 transcripts. Expression of these biomarkers is known to be the most emblematic hallmark of ‘T cell exhaustion’, a state of functionally compromised T cells (reviewed in70 71). Contrarily to effector T cells, expression of these checkpoints wasn’t related to an exhausted state in TRM, but rather to a functionally active one.38 In line with these observations, single-cell profiling of TILs in BC revealed a CD8+ CD103+ T cell subset with a core transcriptional profile of tissue residency.72 This subset also exhibited high expression levels of immune checkpoints and presented high levels of transcripts encoding for cytotoxic molecules.
Despite the emergence of few studies investigating the transcriptional landscape of TILs in certain GI tumors such as CRC and liver cancer by single-cell sequencing, transcriptional profiling of TRM cells has not been completely elucidated on a single cell level yet. Nevertheless, dimensionality reduction and clustering analysis of CD8+ TILs in HBV-induced HCC revealed five CD69+PD-1+ TRM subsets that could be separated according to CD103 and CD57 expression.73 Consistently, ScRNAseq of HBV specific CD8+ T cells revealed two major TRM subsets coexpressing CD103 and CD69, and despite PD-1 expression, these cells do not display characteristics of exhausted T cells.73 Strikingly, the presence of these intratumoral HBV-specific TRM cells was correlated with a better prognosis of HBV-induced HCC patients, which might strongly be attributable to their role in controlling tumor growth. Taken together, these transcriptomic analyses helped to mature our understanding of the transcriptional landscape of TRM cells in certain types of solid tumors as well as the identification of novel tumor-specific subsets of TRM sharing the expression of residency biomarkers as well as a sustained activation, and tumor reactivity (figure 1). However, further investigations on the single-cell level of GI ones are warranted to decipher the intra-tumoral phenotypic heterogeneity of TRM cells which may help to improve TRM subsets identification according to tumor type.
TRM cells differentiation and maintenance
Cues promoting TRM cell ontogeny and differentiation remain poorly understood. Two models explaining the generation of committed precursors for memory T cells populations including TRM cells were proposed: The ‘One cell, one fate’ model, which speculates that naïve T cells are predetermined to give rise to either memory or effector T cells. In this model, TCR-MHC interaction strength may dictate differentiation into TCM, TEM, or TRM cells. The second proposed model is the ‘One cell, multiple fates’ model during which asymmetric cell division occurs allowing the generation of effector and memory T cells from a single naïve T cell. (Reviewed by Enamorado et al).74
On cognate antigen recognition, naïve T cells priming in the secondary lymphoid organs (SLOs) leads to their differentiation into TRM precursors. Priming-wise, certain cell types have been described to be involved in TRM cells generation. DC3s, a subset of human DCs CD1c+CD163+ CD88- drove the differentiation of CD103+CD8+ as well as CD103+CD4+ T cell in vitro via TGF-β production.75 76 Crosspriming by murine DNGR-1+ Batf3-dependent DC was reported to be required for committed TRM precursors generation in the LN (lymph node). DNGR-1 mediated cross-presentation provides type 2 (CD24) and 3 (IL-15, IL-12) signals that lead to T-bet induction promoting TRM cells precursors generation and retention in the LN.77 Additionally, migratory αV-expressing DCs activate and present TGF-β to naïve T cells in the LN, leading to epigenetic preconditioning of these cells to differentiate into epithelial TRM.78 Monocytes also contribute to TRM cells generation. In an autocrine manner, monocytes produce IL-10 that will be fixed on their cell surface IL-10 receptor.79 80 Consequently, IL-10 stimulation will lead to TGF-β release resulting in CD103 expression on T cells (figure 3). Recently, novel findings revealed the importance of type 1 Treg in TRM cells development.81 CXCR3+T-bet+ Treg generates bioactive TGF-β necessary for TRM cells differentiation. However, it’s important to note that a minor proportion of TRM cells still could be generated in type 1 Treg deficient mice which leads to the hypothesis that other cells may play a role in TGF-β production.81
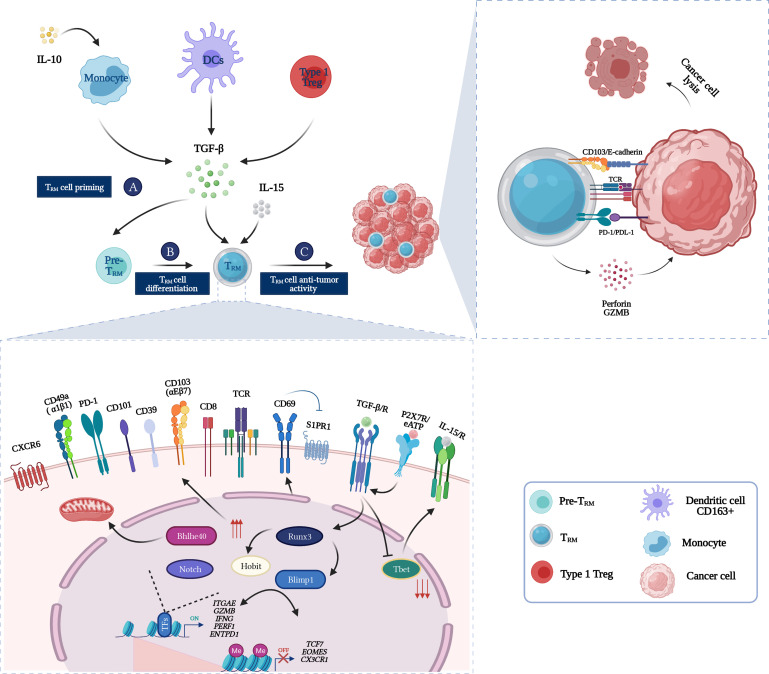
Figure 3.
From Zero to Hero: TRM cells in the cancer-immunity cycle. (A) TRM cells precursors are primed in the tumor-draining lymph node by DCs. (B) Once in the tumor microenvironment and in the presence of TGF-β and IL-15, TRM cells precursors will differentiate into TRM cells. Induction of a core residency signature such as expression of tissue retention molecules (CD103, CD69, CD49a, CXCR6) and acquisition of high cytotoxic activity (GZMB, perforin) is under epigenetic regulation and key transcription factors instructions (Blimp1, Hobit, Runx3, Bhlhe40, and Notch). (C) Once fully differentiated, tumor-specific TRM cells can enact cancer cells eradication via an arsenal of cytotoxic molecules such as perforin and granzyme. CD103 expression enables TRM cells biding to E-cadherin expressing tumor cells which sustains the immunological synapse, hence triggering lytic granules polarization and exocytosis. DC, dendritic cell; TRM, tissue-resident memory T cells.
An emerging proof of concept suggests that cytokine milieu might drive TRM cell signature. TGF-β and IL-15 were reported to be key regulators of TRM cells’ development and survival (figure 3). IL-15 was essentially reported to assure TRM cell maintenance and persistence.82 Additionally, IL-15 was shown to promote TRM cells recruitment from SLOs into mucosal sites through the mTOR signaling pathway activation.83 TGF-β was reported to be a key regulator of TRM cell differentiation and migration (figure 3). TGF-β induces CD103 expression on TRM cells precursors in the gut, SI, lung, and skin.84–87 During TRM cells development, TGF-β downregulates T-bet expression to a residual level, which is necessary for maintaining IL-15 receptor β-chain (CD122) expression and therefore essential to their long-term persistence.82 Falling in line with these observations, sequential exposure of T cells to IL-15 and TGF-β induced the development of CD103+ CD8+ TRM cells in the liver.11 Interestingly, notwithstanding the evident role of TGF-β in the differentiation and retention of TRM cells in peripheral tissues, it has been demonstrated that in SLOs, TGF-β inhibited TRM cells migration and gut homing via α4β7 expression downregulation.84 Apart from these well-studied cytokines, other inflammatory stimuli such as pro-inflammatory cytokines IL-33, TNF-α, and type I IFN were reported to induce TRM cells differentiation in the peripheral tissues.74 Interestingly, these stimuli are highly organ-specific, dictated by the inflammatory microenvironment leading to the generation of TRM subsets with different phenotypes and functions. In the GI tract, IL-21 drove the differentiation of highly activated and cytotoxic TRM cells from circulatory precursors only during graft-versus-host disease but not under homeostatic conditions.88 In murine polyomavirus (MuPyV) brain infection, IL-21 produced by MuPyV high-affinity TCRs CXCR5high PD-1high CD4+ T cells drove CD8+ TRM cells differentiation in response to viral infection.17 Additionally, although TRM cells in the skin required TGF-β signaling for their differentiation, the development of TRM cells in the liver was TGF-β independent.55 Stimuli driving TRM cells’ survival and maintenance are also tissue-specific, although IL-2/IL-15 signaling is important for intrahepatic TRM survival, long-term persistence of antigen-specific TRM cells in the epidermal niche is TGF-β dependent.89
Taken together, seeing from afar, TRM cells share a core residency program as well as the same differentiation mechanisms regardless of their location, nonetheless, it is now evident that stimuli driving their differentiation and maintenance are dictated by their tissue microenvironment which explains the observed phenotypic, functional, and metabolic heterogeneity as well as persistence across organs.
Transcriptional regulation of TRM cells
Gene expression profiling conducted on TRM cells revealed a unique transcriptional program implicated in the differentiation and maintenance of TRM cells.19 Six major transcriptional factors have been reported to play a key role in TRM cells transcriptional regulation; Hobit, Blimp1, Runx3, Notch, T-bet, and recently Bhlhe40 (figure 3). Hobit and Blimp1 have been identified as TRM-specific TF that instructs TRM cells differentiation and maintenance in the liver, gut, kidney, and skin by silencing tissue egress genes such as S1pr1, Ccr7, and Tcf7.27 90–92 Runx3 was also reported to play a role in TRM cells maintenance in a wide range of tissues including the SI. Both in human and mouse, upregulation of Runx3 expression lead to the induction of the core residency signature including expression of tissue retention biomarkers such as CD103 and downregulation of tissue egress ones.93 Another important TF in TRM regulation is the T-box family member T-bet. Remarkably, T-bet was reported to be down-regulated in TRM cells, which is needed for optimal CD103+CD8+ TRM maturation. Further, the total shutdown of T-bet leads to decreased survival of TRM cells, which highlights its implication in TRM maintenance. Lung CD103+ TRM cells exhibited an upregulated Notch signaling signature, reported to be required for their differentiation and maintenance.27 94 95 Interestingly, Blimp1, Runx3, T-bet, and Notch are also key TF implicated in the terminal differentiation of effector T cells and the exhibition of effector functions. This highlights the importance of maintaining an effector function through differentiation into a memory state for TRM cells.
In contrast to TCM, T cell factor 1 (TCF1) is downregulated in TRM cells. Recently, it has been demonstrated that TCF1 binds to the Itgae locus and inhibits CD103 expression.96 Using a global gene expression analysis of Tcf7 sufficient and Tcf7 deficient P14 CD8+ T cells, WU et al reported an increased expression of CD103 in Tcf7 deficient cells associated with a decreased expression of tissue egress genes such as Sell and Ccr7.96 Finally, Bhlhe40 is another TF that has been recently described.97 Bhlhe40 or Basic helix-loop-helix family member E40 is a stress-responsive TF that fosters the development, commitment, fitness, and polyfunctionality of TRM cells via metabolic and epigenetic programming.97 Li et al reported that Bhlhe40 drove the expression of several residency genes such as Cxcr6 and Itgae and that loss of Bhlhe40 expression decreased TRM cells in mice as well as TILs survival without impacting circulating T cells function following B16 melanoma challenge.97 98 Taken together, these observations demonstrate that TRM cells’ differentiation and maintenance are controlled by a hybrid transcriptional program of memory and effector cells.
Epigenetic regulation of TRM cells
Changes in the gene expression profile of T cells are mirrored by a modification in their functional properties. It is well established that epigenetic mechanisms regulate gene expression patterns and dictate whether a gene is expressed or silenced. Of relevance, investigating TRM cells’ epigenetic profile will offer a deeper understanding of their phenotype, function, differentiation, and maintenance.
In line with transcriptomic analysis, Assay for Transposase Accessible Chromatin analysis of TRM cells in lung cancer revealed greater chromatin accessibility of CD103 gene promoter (ITGAE).38 Notably, TIM-3+IL-7R- TRM subset exhibited increased chromatin accessibility of genes encoding the effector molecules IFN-γ as well as granzyme B along with increased accessibility of TIM-3 (HAVCR2) and PD-1 (PDCD1) loci.38 Moreover, a study investigating the DNA methylation profile of PRF1 gene on CD8+ TRM cells in urinary bladder cancer revealed that these cells exhibit signs of exhaustion yet are epigenetically cytotoxic, explained by a low DNA methylation in the reporter CpG site located in the enhancer of the PRF1 locus.63
Genome-wide DNA methylation analysis of tumor-reactive CD8+CD103+CD39+ T cells in CRC has been recently conducted.69 Interestingly, these cells exhibited hypomethylated regions (HypoMRs) that affected both ENTPD1 and ITGAE along with exhaustion markers encoding genes (PDCD1, LAYN, and HAVCR2). Paired with these observations, TFs binding motifs enrichment analysis of CD103+CD39+ cells revealed 85 significantly enriched TFs binding motifs. Notably, five TFs were particularly overrepresented: BATF, NR4A1, RUNX1, EGR2, and VDR. Unsurprisingly, these TFs are largely associated with T cell exhaustion and CD103 expression. In summary, these epigenetic observations fall in line with previous transcriptomic results, showing hypomethylation of the highly expressed genes encoding for tissue residency, activation, and T cell exhaustion markers, coupled with hypermethylation of tissue egress genes. Altogether, this demonstrates plausible evidence that epigenetic regulation shapes TRM cells molecular features.
TRM are strictly non-recirculating cells: time to think twice!
TRM cells are a population of immune cells defined as permanent resident cells in non-lymphoid organs without recirculation through SLOs or the blood. However, over the last few years, certain studies have demonstrated that some CD8+ TRM cells in the LN are derived from cells that exit non-lymphoid tissue (NLT),99 thereby enhancing the accumulation of antigen-specific CD8+ TRM cells in the draining LN. In addition, a recent study conducted on a murine model reported that reactivated TRM cells can indeed rejoin the circulating pool.100 Strikingly, they reported that TRM cells are not terminally differentiated, and are endowed with certain developmental plasticity that allows them to give rise to TCM and TEM cells. These exciting results give rise to a new ‘outside-in’ model of protective immune response and reveal an inter-conversion between TRM and TCM cells. In line with these observations, using Hobit lineage tracer mice, Behr et al demonstrated that on antigen reencounter, intestinal TRM cells downregulated Hobit giving rise to an ex-Hobit+ circulatory memory subset.101 These TRM cells-derived offspring referred to as ‘ex-TRM’ acquired a KLRG1+ CX3CR1+ TEM phenotype that was shown to be transcriptionally and functionally distinct from non-TRM-derived TEM cells. Interestingly, ex-TRM secondary TEM cells presented higher protective potential compared with their non-TRM-derived counterparts. This work gave new insight into the role of TRM cells in shaping not only local but also systemic T cells responses on reinfection. Another intriguing work by Mackay’s group showed that TRM cells’ plasticity is intertwined with the tissue-specific microenvironment which eventually dictates TRM cells’ malleability.55 In fact, in contrast to CD103+CD69+ skin TRM cells, CD103-CD69+ liver TRM cells were able to trans-differentiate into skin TRM cells on relocation. Additionally, ex-liver TRM cells also showed the capacity to repopulate the circulatory memory pools on restimulation. This is indicative of a restrained trans-differentiation capacity of skin TRM cells and higher plasticity of liver TRM cells. Whereas liver ex-TRM cells showed the capacity to differentiate to both skin and liver TRM, skin ex-TRM cells were less malleable, showing the ability to only differentiate into skin TRM on adoptive transfer. TGF-β was the major driver of this lack of plasticity of skin TRM cells since as discussed above, liver TRM cells do not exhibit TGF-β imprinting. Finally, this TRM cells’ developmental plasticity further supports the ‘one cell multiple fate’ hypothesis previously described. However, these interesting findings need to be validated in the cancer setting, meanwhile, they should be taken with a grain of salt.
TRM cells: local key players in GI cancer immunosurveillance
Cancer immunosurveillance is a process whereby the immune system suppresses cancer development and progression.102 TRM cells’ presence has been described in the majority of solid tumors as a sub-population of TILs. Evidence merging from animal models as well as studies of human cancers supported a central role of TRM cells in cancer immunosurveillance. Data from a recent study conducted in a transplantable epicutaneous melanoma mouse model showed that around 40% of mice didn’t develop macroscopic lesions for a long period following epicutaneous inoculation.103 Since the epidermis is hardly accessible by circulating T cells (Tcirc), Park et al speculated that TRM cells are responsible for controlling tumor growth independently of Tcirc cells presence. Indeed, they reported the generation of CD8+CD103+CD69+ TRM cells following epicutaneous inoculation in macroscopic lesions-free mice that was correlated with disease control. Whereas depletion of these TRM cells resulted in tumor growth highlighting the key role of TRM cells in keeping cancer cells dormant and maintaining immune equilibrium.
As previously described, TRM cells are endowed with a cytotoxic activity reported to be higher than non TRM cells. A growing body of evidence supports the hypothesis that TRM cells are able to enact tumor eradication via an arsenal of cytotoxic molecules such as perforin and granzyme (figure 3). In line with these speculations, in lung cancer, ex vivo analysis of TRM cells showed higher co-expression of PD-1 and GZMA and GZMB compared with non-TRM cytotoxic T cells (CTLs).38 Similarly, the transcriptomic signature of CD8+CD103+CD39+ TRM cells in CRC exhibited high expression of genes encoding for cytotoxic proteins such as GZMA/GZMB and PRF1, probably echoing TRM cells capacity to kill CRC cells and thus controlling tumor growth.69 In HCC, single-cell analysis revealed that TRM cells represented up to 90% of intrahepatic and intratumoral HBV- specific CD8+ T cells.73 Interestingly, these subsets were clonally expanded, showed no enrichment in T cell exhaustion signaling pathways, and most importantly their presence was correlated with prolonged relapse-free survival in HCC patients. These results suggest the existence of an anti-tumor immune response imposed by TRM cells. Broadly, these observations evidenced the role of TRM cells in cancer elimination and in maintaining immune equilibrium in certain solid tumors including CRC and HCC.
Prognostic value of TRM cells in GI cancers
The prognostic value of TRM cells is now well established in several cancer types including melanoma, NSCLC, bladder, ovarian and cervical cancer. The presence of CD8+CD103+ TRM cells in the TME was associated with a higher overall survival (OS) rate in these cancers.72
Unfortunately, the clinical implication of TRM cells in GI cancers remains rudimentary at best. Notwithstanding, a positive correlation between TRM cells infiltration and a favorable prognosis has been reported in certain GI cancers. In a study conducted on a cohort of 165 pancreatic ductal adenocarcinoma patients, IE CD8+CD103+ over total CD8+CD103+ cells ratio was significantly associated with improved disease-free survival (DFS) (p=0.022) as well as an improved OS (p=0.009).42 In CRC, a higher number of CD8+CD103+ TRM cells was reported to be significantly associated with a better OS rate. In addition, a higher number of CD8+CD103+ cells was reported to be inversely and significantly associated with distant metastasis.104 Investigations conducted on HBV-related HCC revealed an enrichment of CD8+CD103+ TRM cells in the TME, associated with improved OS.68
Paired with these results, a recent study conducted on three independent CRC cohorts’ datasets revealed that ITGAE+CD8+ infiltrating lymphocytes were associated with a significantly improved OS (p<0.001). Interestingly, uni and multivariate analysis identified ITGAE as an independent prognostic factor for both OS and DFS in CRC patients.105
Taken together, these results highlight the key role of TRM cells in mediating improved clinical outcomes of numerous solid cancers including GI ones.
TRM cells in GI cancer immunotherapy: new hope or more hype
TRM cells: a novel target for checkpoint inhibitors
As outlined above, one common hallmark of TRM cells in healthy tissue as well as in cancer independently of tumor type is the high expression of a wide range of immune checkpoints, such as PD-1, TIM-3, CTLA-4, or LAG-3. This suggests that TRM cells could be a prominent target of immune checkpoint immunotherapy (ICI) therapy and rightfully so. Evaluation of gene expression dataset of nivolumab treated melanoma patients revealed that responders’ samples were significantly enriched in TRM signature at baseline.72 Interestingly, a significant increase of the TRM signature was observed during nivolumab treatment in these patients. Results of the phase 2 NEOSTAR trial conducted on non-small cell lung cancer patients treated with nivolumab or nivolumab plus ipilimumab in the neoadjuvant setting, revealed a considerable increase of CD103+CD8+ as well as CD103+CD4+ TRM cells in the patient’s group receiving nivolumab plus ipilimumab compared with nivolumab alone.106 Similar results were observed in the retrospective DISCOVERY cohort involving advanced NSCLC patients treated with anti-PD-1 therapy; accumulation of CD103+CD69+CD8+ TRM cells was noticed following anti-PD-1 administration only in responders associated with an improved overall outcome.107 In line with these observations, analysis of 19 lung cancer biopsies treated with anti-PD-1 therapy showed a significant increase of CD8+CD103+TIM-3+IL-7R- TRM cells in responders compared with non-responders and treatment-naive patients.38 Paired single-cell transcriptomics with TCR analysis of cytotoxic lymphocytes of responders to anti-PD-1 in post-treatment samples revealed enrichment of ITGAE expression as well as CD38, GZMB, and GZMH along with TCR clonal expansion. Parallelly, coupled scRNA-seq-TCR-seq of in vitro expanded mutation-associated neoantigens (MANA) specific TILs revealed that 90% of MANA-specific T cell clones in NSCLC treated with neoadjuvant anti-PD-1 were TRM cells.108 These MANA-specific TRM were characterized by high expression of CD103, and HOBIT, however, they showed low expression of IL-7R. These results strongly support the hypothesis that anti-PD-1 therapy leads to the expansion of TRM cells endowed with high tumor-neoantigen specificity.
Although the use of ICI is less common in GI cancers compared with melanoma and lung cancers, some emerging studies highlight the predictive value of TRM cells density to ICI. Remarkably, a recent study conducted on esophageal squamous cell carcinoma (ESCC) revealed that tumor-reactive CD8+CD103+ TRM cells secreted higher levels of IFN-γ and IL-2 after anti-PD-1 blockade when cultured with tumor cells.67 Additionally, using an ESCC mouse model, they demonstrated a higher infiltration with CD8+CD103+ TRM in the TME following anti-PD-1 blockade compared with the control group, which indicates a possible resurrection of TRM cells by the use of checkpoint inhibitors. TRM cells metabolism relies on the oxidation of fatty acids, thus in the TME tumor cells outcompete TRM cells for lipid uptake which induces their apoptosis. In gastric adenocarcinoma the use of anti-PD-L1 in a murine model, not only increased CD8+CD103+ TRM cells cytotoxicity, it also decreased FABP4 and FABP5 expression on tumor cells, while increasing their expression on TRM cells leading to a metabolic switch and enhanced lipid uptake.66 This leads us to speculate that along with enhancing TRM cells’ anti-tumor effect in gastric adenocarcinoma, PD-L1 blockade reverses the metabolic reprogramming of TRM dictated by tumor cells leading to their improved survival.
All in all, checkpoint inhibitors administration increases TRM cells cytotoxicity and induces their accumulation in several cancer types. There is a growing body of evidence suggesting that TCF1+ PD-1high TILs represent progenitor subsets that proliferate following ICI. However, TCF1 is downregulated in TRM cells, which leads us to question whether TRM accumulation following anti-PD-1 administration stems from TRM cells reinvigoration or a clonal replacement due to de novo TRM induction remains unclear and needs to be investigated.
Noteworthy, TRM cells’ presence could represent a double-edged sword when it comes to ICI use. Recent studies revealed that TRM cells play a key role in ICI-induced colitis (ICI-colitis), one of the most common immune-related adverse events. Investigating normal and inflamed colon of anti-PD-1/CTLA-4 treated melanoma patients, Luoma et al reported a striking accumulation of highly cytotoxic CD8+ T cells in ICI-colitis.109 Interestingly, the use of coupled scRNA-seq-TCR-seq revealed that ICI-colitis is associated with a shift of TRM cells to effector T cells with high immune checkpoints expression and high cytotoxicity. In addition, a recent study revealed that activated CD8+ TRM cells represented the key effector cells in ICI-colitis.110 The presence of TRM cells was correlated with endoscopic and clinical ICI-colitis severity. These TRM cells were characterized by high production of IFN-γ associated with high expression of immune checkpoints such as PD-1, CTLA-4, LAG-3, and TIM-3.
In summary, ICI use is emerging as a promising therapeutic strategy for ‘hot’ GI cancers mainly characterized by having a MSI and anti-PD-1 treatments are currently FDA approved in the metastatic setting.111 We previously discussed that TRM cells represent an important subset of TILs in CRC, HCC, and pancreatic cancer. Therefore, TRM cells can represent an interesting population to target in these cancers and the presence of TRM cells might be used in a predictive setting in order to stratify responders and non-responders to this therapeutic strategy (figure 4).
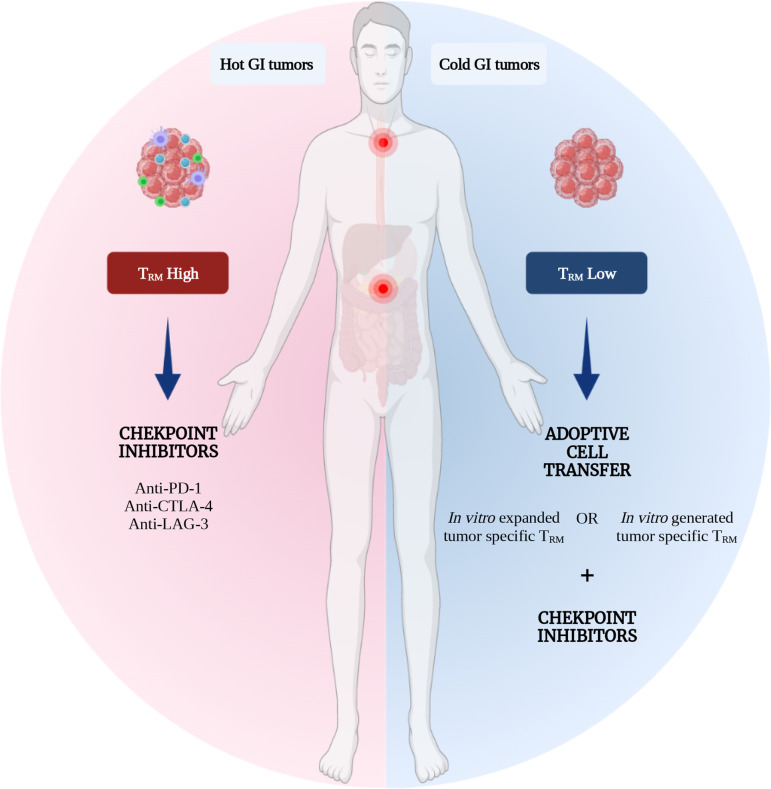
Figure 4.
TRM cells as a tool for GI cancers immunotherapy. TRM cells are emerging as long persisting potent cytotoxic T cells destined for tissue residency, making them a hot target for cancer immunotherapy in general and GI cancers in particular. This is a representation of the potential therapeutic strategies where TRM cells could be incorporated into GI cancers standard treatment paradigms. For immune infiltrated or the so-called ‘hot’ GI tumors, TRM cells could serve as a predictive factor as well as a target for checkpoint inhibitors. For the therapeutically challenging ‘cold’ GI tumors, in vitro expanded or generated CXCR6+ tumor-specific TRM cells could be adoptively transferred in combination or not with checkpoint inhibitors. GI, gastrointestinal; TRM, tissue-resident memory T cells.
TRM in adoptive cells transfer
GI cancers are considered cold tumors with limited access to checkpoint inhibitors.112 Therefore, adoptive cell transfer (ACT) is being considered as one of the most promising therapeutic strategies for patients with cold cancers. One of the major challenges for ACT is the ability of T cells to infiltrate the tumor and to be able to persist long-term, both of which constitute the hallmark features of TRM cells. Additionally, due to their observed in vitro functionality and cytotoxicity, TRM cells are gaining the researcher’s attention as a potent candidate for ACT. A recent study showed that in vitro generated NY-ESO1/SSX2 specific CD8+CD103+ presented a faster cancer recognition as well as cytotoxicity compared with CD8+CD103- non TRM cells.113 Interestingly, they were also endowed with high energetic potential. These data gave new evidence for TRM cells’ antitumor efficacy compared with non TRM cells which makes them a promising candidate for ACT, especially in GI cancers (figure 4). Although ACT of in vitro expanded TILs showed promising results, only a minor fraction of treated patients achieved durable responses. As previously discussed, TILs represent an heterogenous T cells populations encountering tumor-specific as well as bystander T cells which may explain the limited success of the use of the unfractionated TILs populations for ACT.114 Recent studies revealed that whether in HBV-induced liver cancer or NSCLC, TRM cells represented the tumor antigen-specific population of TILs, therefore tetramer sorting and in vitro expansion of these cells with potent anti-tumor properties may represent a better therapeutic strategy for solid cancers.114 115 One of the major challenges impeding the use of CAR-T cells in solid tumors is their restrained trafficking and persistence in the TME. TRM on the other hand represent highly cytotoxic, long-lived T cells endowed with the expression of tissue homing biomarkers and the downregulation of tissue egress ones, which represent highly desired properties when designing T cell-based therapies for solid tumors, GI included. Additionally, to overcome tissue homing challenges, beyond intravenous transfusion, intratumoral injection of TRM cells could be considered. Last but not least, since our understanding of TRM cells generation has evolved substantially over the last years, in vitro generation of tumor antigen-specific TRM cells from the patient’s peripheral blood could represent an easily accessible therapeutic strategy. Furthermore, since TRM cells phenotype is tissue-specific, the generation of TRM cells with organ-specific homing biomarkers (CXCR6+ TRM for liver cancer/metastasis, CD103+ TRM for CRC…) would represent an intriguing approach for improving clinical responses. TRM cells have emerged as the predominant tumor antigen-specific TILs population, they are endowed with high cytotoxicity, tissue-homing ability, and long-term persistence. The success of ACT is built on these duly warranted hallmarks, clinical studies using tumor antigen-specific TRM cells are critically needed especially for cold tumors such as GI cancers.
Conclusions and future perspectives
TRM research has been gaining huge momentum lately. It’s now well established that TRM cells patrol the TME, and they can promote both cancer elimination and equilibrium. Remarkably, immune infiltrated GI tumors were enriched in TRM cells which represented a better indicator of the patient’s prognosis. Moreover, they were predictive of checkpoint inhibitors’ response. However, the importance of leveraging this proof of concept into cancer treatment will require further understanding of TRM function, the molecular network that drives their development and maintenance in the TME, the transcriptional regulation behind their exhaustion status, and first and foremost identification of solid biomarkers that enable to distinguish between TRM cells from conventional TILs subsets. Of note, single-cell analysis helped to gain a more comprehensive understanding of TRM cells heterogeneity, therefore, the use of this technology should be widespread to a larger panel of GI cancers. Once we got exhaustive answers to these questions, capitalizing on TRM cells will be an exciting therapeutic approach to turning ‘cold’ GI tumors ‘hot’.
Footnotes
Contributors: Conceptualization: CB and SAb. Writing: SAb and SAr. Review and editing: CB, SAb, MK, RL, MBK, AV, AD, and BH.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Jin M-Z, Jin W-L. The updated landscape of tumor microenvironment and drug repurposing. Sig Transduct Target Ther 2020;5:1–16. 10.1038/s41392-020-00280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan Q, Zhang H, Zheng J, et al. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 2020;6:605–18. 10.1016/j.trecan.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Fridman W-H, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res 2007;67:1883–6. 10.1158/0008-5472.CAN-06-4806 [DOI] [PubMed] [Google Scholar]
- 5.Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016;44:698–711. 10.1016/j.immuni.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999;401:708–12. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 7.Gebhardt T, Wakim LM, Eidsmo L, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009;10:524–30. 10.1038/ni.1718 [DOI] [PubMed] [Google Scholar]
- 8.Masopust D, Choo D, Vezys V, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 2010;207:553–64. 10.1084/jem.20090858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urban SL, Jensen IJ, Shan Q, et al. Peripherally induced brain tissue-resident memory CD8+ T cells mediate protection against CNS infection. Nat Immunol 2020;21:938–49. 10.1038/s41590-020-0711-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbach K, Vincenti I, Kreutzfeldt M, et al. Brain-resident memory T cells represent an autonomous cytotoxic barrier to viral infection. J Exp Med 2016;213:1571–87. 10.1084/jem.20151916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pallett LJ, Davies J, Colbeck EJ, et al. IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med 2017;214:1567–80. 10.1084/jem.20162115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaid A, Mackay LK, Rahimpour A, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci U S A 2014;111:5307–12. 10.1073/pnas.1322292111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masopust D, Jiang J, Shen H, et al. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol 2001;166:2348–56. 10.4049/jimmunol.166.4.2348 [DOI] [PubMed] [Google Scholar]
- 14.Hogan RJ, Usherwood EJ, Zhong W, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol 2001;166:1813–22. 10.4049/jimmunol.166.3.1813 [DOI] [PubMed] [Google Scholar]
- 15.Kiniry BE, Li S, Ganesh A, et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8+ T-cells in chronic infection. Mucosal Immunol 2018;11:909–20. 10.1038/mi.2017.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg SP, Carpenter DJ, Chait M, et al. Tissue-Resident memory T cells mediate immune homeostasis in the human pancreas through the PD-1/PD-L1 pathway. Cell Rep 2019;29:3916–32. 10.1016/j.celrep.2019.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren HM, Kolawole EM, Ren M, et al. Il-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci Immunol 2020;5:eabb5590. 10.1126/sciimmunol.abb5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottois H, Ngollo M, Hammoudi N, et al. Klrg1 and CD103 expressions define distinct intestinal tissue-resident memory CD8 T cell subsets modulated in Crohn's disease. Front Immunol 2020;11:896. 10.3389/fimmu.2020.00896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013;14:1294–301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 20.Liao W, Liu Y, Ma C, et al. The downregulation of IL-18R defines bona fide kidney-resident CD8+ T cells. iScience 2021;24:101975. 10.1016/j.isci.2020.101975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolders J, Heutinck KM, Fransen NL, et al. Tissue-Resident memory T cells populate the human brain. Nat Commun 2018;9:4593. 10.1038/s41467-018-07053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piet B, de Bree GJ, Smids-Dierdorp BS, et al. Cd8⁺ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest 2011;121:2254–63. 10.1172/JCI44675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar BV, Ma W, Miron M, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep 2017;20:2921–34. 10.1016/j.celrep.2017.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S-J, Glatman Zaretsky A, Andrade-Oliveira V, et al. White adipose tissue is a reservoir for memory T cells and promotes protective memory responses to infection. Immunity 2017;47:1154–68. 10.1016/j.immuni.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thom JT, Weber TC, Walton SM, et al. The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep 2015;13:1125–36. 10.1016/j.celrep.2015.09.082 [DOI] [PubMed] [Google Scholar]
- 26.Stolp B, Thelen F, Ficht X, et al. Salivary gland macrophages and tissue-resident CD8+ T cells cooperate for homeostatic organ surveillance. Sci Immunol 2020;5:eaaz4371. 10.1126/sciimmunol.aaz4371 [DOI] [PubMed] [Google Scholar]
- 27.Behr FM, Chuwonpad A, Stark R, et al. Armed and ready: transcriptional regulation of tissue-resident memory CD8 T cells. Front Immunol 2018;9:9. 10.3389/fimmu.2018.01770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 1994;372:190–3. 10.1038/372190a0 [DOI] [PubMed] [Google Scholar]
- 29.Wagner N, Löhler J, Kunkel EJ, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 1996;382:366–70. 10.1038/382366a0 [DOI] [PubMed] [Google Scholar]
- 30.Shiow LR, Rosen DB, Brdicková N, et al. Cd69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 2006;440:540–4. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- 31.Cibrián D, Sánchez-Madrid F. Cd69: from activation marker to metabolic gatekeeper. Eur J Immunol 2017;47:946–53. 10.1002/eji.201646837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo PA, Levitin HM, Miron M, et al. Single-Cell transcriptomics of human T cells reveals tissue and activation signatures in health and disease. Nat Commun 2019;10:4706. 10.1038/s41467-019-12464-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wein AN, McMaster SR, Takamura S, et al. Cxcr6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med 2019;216:2748–62. 10.1084/jem.20181308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AI, Brolin RE, Ebert EC. Integrin alpha1beta1 (VLA-1) mediates adhesion of activated intraepithelial lymphocytes to collagen. Immunology 1999;97:679–85. 10.1046/j.1365-2567.1999.00812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly EC, Lambert Emo K, Buckley PM, et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc Natl Acad Sci U S A 2020;117:12306–14. 10.1073/pnas.1915681117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromley SK, Akbaba H, Mani V, et al. CD49a Regulates Cutaneous Resident Memory CD8+ T Cell Persistence and Response. Cell Rep 2020;32:108085. 10.1016/j.celrep.2020.108085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheuk S, Schlums H, Gallais Sérézal I, et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017;46:287–300. 10.1016/j.immuni.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarke J, Panwar B, Madrigal A, et al. Single-Cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med 2019;216:2128–49. 10.1084/jem.20190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuber J, Shonts B, Lau S-P, et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci Immunol 2016;1:eaah3732. 10.1126/sciimmunol.aah3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartolomé-Casado R, Landsverk OJB, Chauhan SK, et al. Resident memory CD8 T cells persist for years in human small intestine. J Exp Med 2019;216:2412–26. 10.1084/jem.20190414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med 2012;4:117ra7. 10.1126/scitranslmed.3003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohneis P, Sinn M, Bischoff S, et al. Cytotoxic tumour-infiltrating T lymphocytes influence outcome in resected pancreatic ductal adenocarcinoma. Eur J Cancer 2017;83:290–301. 10.1016/j.ejca.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 43.Ling K-L, Dulphy N, Bahl P, et al. Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol 2007;178:2908–15. 10.4049/jimmunol.178.5.2908 [DOI] [PubMed] [Google Scholar]
- 44.Quinn E, Hawkins N, Yip YL, et al. CD103+ intraepithelial lymphocytes--a unique population in microsatellite unstable sporadic colorectal cancer. Eur J Cancer 2003;39:469–75. 10.1016/s0959-8049(02)00633-0 [DOI] [PubMed] [Google Scholar]
- 45.Ghilas S, Valencia-Hernandez A-M, Enders MH, et al. Resident memory T cells and their role within the liver. Int J Mol Sci 2020;21:E8565. 10.3390/ijms21228565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel JM, Fraser KA, Beura LK, et al. T cell memory. resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 2014;346:98–101. 10.1126/science.1254536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheridan BS, Pham Q-M, Lee Y-T, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 2014;40:747–57. 10.1016/j.immuni.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behr FM, Beumer-Chuwonpad A, Kragten NAM, et al. Circulating memory CD8+ T cells are limited in forming CD103+ tissue-resident memory T cells at mucosal sites after reinfection. Eur J Immunol 2021;51:151–66. 10.1002/eji.202048737 [DOI] [PubMed] [Google Scholar]
- 49.Konjar Špela, Frising UC, Ferreira C, et al. Mitochondria maintain controlled activation state of epithelial-resident T lymphocytes. Sci Immunol 2018;3:eaan2543. 10.1126/sciimmunol.aan2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Milner JJ, Toma C, He Z, et al. Heterogenous Populations of Tissue-Resident CD8+ T Cells Are Generated in Response to Infection and Malignancy. Immunity 2020;52:808–24. 10.1016/j.immuni.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.FitzPatrick MEB, Provine NM, Garner LC, et al. Human intestinal tissue-resident memory T cells comprise transcriptionally and functionally distinct subsets. Cell Rep 2021;34:108661. 10.1016/j.celrep.2020.108661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 2016;13:267–76. 10.1038/cmi.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McNamara HA, Cai Y, Wagle MV, et al. Up-Regulation of LFA-1 allows liver-resident memory T cells to patrol and remain in the hepatic sinusoids. Sci Immunol 2017;2:eaaj1996. 10.1126/sciimmunol.aaj1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stelma F, de Niet A, Sinnige MJ, et al. Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci Rep 2017;7:6172. 10.1038/s41598-017-06352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christo SN, Evrard M, Park SL, et al. Discrete tissue microenvironments instruct diversity in resident memory T cell function and plasticity. Nat Immunol 2021;22:1140–51. 10.1038/s41590-021-01004-1 [DOI] [PubMed] [Google Scholar]
- 56.Wiggins BG, Pallett LJ, Li X, et al. The human liver microenvironment shapes the homing and function of CD4+ T-cell populations. Gut 2021. doi: 10.1136/gutjnl-2020-323771. [Epub ahead of print: 21 Sep 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartolomé-Casado R, Landsverk OJB, Chauhan SK, et al. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol 2021;14:402–10. 10.1038/s41385-020-0315-5 [DOI] [PubMed] [Google Scholar]
- 58.Frizzell H, Fonseca R, Christo SN, et al. Organ-Specific isoform selection of fatty acid-binding proteins in tissue-resident lymphocytes. Sci Immunol 2020;5:eaay9283. 10.1126/sciimmunol.aay9283 [DOI] [PubMed] [Google Scholar]
- 59.Edwards J, Wilmott JS, Madore J, et al. CD103 + Tumor-Resident CD8 + T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti–PD-1 Treatment. Clinical Cancer Research 2018;24:3036–45. 10.1158/1078-0432.CCR-17-2257 [DOI] [PubMed] [Google Scholar]
- 60.Ganesan A-P, Clarke J, Wood O, et al. Tissue-Resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol 2017;18:940–50. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duhen T, Duhen R, Montler R, et al. Co-Expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun 2018;9:2724. 10.1038/s41467-018-05072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Wu S, Zeng H, et al. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J Urol 2015;194:556–62. 10.1016/j.juro.2015.02.2941 [DOI] [PubMed] [Google Scholar]
- 63.Hartana CA, Ahlén Bergman E, Broomé A, et al. Tissue-Resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin Exp Immunol 2018;194:39–53. 10.1111/cei.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anz D, Mueller W, Golic M, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer 2011;129:2417–26. 10.1002/ijc.25902 [DOI] [PubMed] [Google Scholar]
- 65.Shekhar S, Yang X. Pulmonary CD103+ dendritic cells: key regulators of immunity against infection. Cell Mol Immunol 2020;17:670–1. 10.1038/s41423-020-0397-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin R, Zhang H, Yuan Y, et al. Fatty Acid Oxidation Controls CD8+ Tissue-Resident Memory T-cell Survival in Gastric Adenocarcinoma. Cancer Immunol Res 2020;8:479–92. 10.1158/2326-6066.CIR-19-0702 [DOI] [PubMed] [Google Scholar]
- 67.Han L, Gao Q-L, Zhou X-M, et al. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunol Immunother 2020;69:1493–504. 10.1007/s00262-020-02562-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019;68:916–27. 10.1136/gutjnl-2018-316510 [DOI] [PubMed] [Google Scholar]
- 69.Yang R, Cheng S, Luo N, et al. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol 2019;21:2. 10.1186/s13059-019-1921-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blank CU, Haining WN, Held W, et al. Defining 'T cell exhaustion'. Nat Rev Immunol 2019;19:665–74. 10.1038/s41577-019-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Franco F, Jaccard A, Romero P, et al. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab 2020;2:1001–12. 10.1038/s42255-020-00280-9 [DOI] [PubMed] [Google Scholar]
- 72.Savas P, Virassamy B, Ye C, et al. Single-Cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 2018;24:986–93. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 73.Cheng Y, Gunasegaran B, Singh HD, et al. Non-terminally exhausted tumor-resident memory HBV-specific T cell responses correlate with relapse-free survival in hepatocellular carcinoma. Immunity 2021;54:1825–40. 10.1016/j.immuni.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 74.Enamorado M, Khouili SC, Iborra S, et al. Genealogy, Dendritic Cell Priming, and Differentiation of Tissue-Resident Memory CD8+ T Cells. Front Immunol 2018;9:9. 10.3389/fimmu.2018.01751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bourdely P, Anselmi G, Vaivode K, et al. Transcriptional and functional analysis of CD1c+ human dendritic cells identifies a CD163+ subset priming CD8+CD103+ T cells. Immunity 2020;53:335–52. 10.1016/j.immuni.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu CI, Becker C, Wang Y, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-β. Immunity 2013;38:818–30. 10.1016/j.immuni.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iborra S, Martínez-López M, Khouili SC, et al. Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1+ Dendritic Cells. Immunity 2016;45:847–60. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mani V, Bromley SK, Äijö T, et al. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 2019;366:eaav5728. 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson EA, Darrah PA, Foulds KE, et al. Monocytes acquire the ability to prime tissue-resident T cells via IL-10-Mediated TGF-β release. Cell Rep 2019;28:1127–35. 10.1016/j.celrep.2019.06.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chu K-L, Batista NV, Girard M, et al. Monocyte-Derived cells in tissue-resident memory T cell formation. J Immunol 2020;204:477–85. 10.4049/jimmunol.1901046 [DOI] [PubMed] [Google Scholar]
- 81.Ferreira C, Barros L, Baptista M, et al. Type 1 Treg cells promote the generation of CD8+ tissue-resident memory T cells. Nat Immunol 2020;21:766–76. 10.1038/s41590-020-0674-9 [DOI] [PubMed] [Google Scholar]
- 82.Mackay LK, Wynne-Jones E, Freestone D, et al. T-Box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 2015;43:1101–11. 10.1016/j.immuni.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 83.Sowell RT, Goldufsky JW, Rogozinska M, et al. Il-15 complexes induce migration of resting memory CD8 T cells into mucosal tissues. J Immunol 2017;199:2536–46. 10.4049/jimmunol.1501638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 2013;39:687–96. 10.1016/j.immuni.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee Y-T, Suarez-Ramirez JE, Wu T, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 2011;85:4085–94. 10.1128/JVI.02493-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Asady R, Yuan R, Liu K, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 2005;201:1647–57. 10.1084/jem.20041044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qiu Z, Chu TH, Sheridan BS. TGF-β: Many Paths to CD103+ CD8 T Cell Residency. Cells 2021;10:989. 10.3390/cells10050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tkachev V, Kaminski J, Potter EL, et al. Spatiotemporal single-cell profiling reveals that invasive and tissue-resident memory donor CD8+ T cells drive gastrointestinal acute graft-versus-host disease. Sci Transl Med 2021;13:eabc0227. 10.1126/scitranslmed.abc0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirai T, Yang Y, Zenke Y, et al. Competition for active TGFβ cytokine allows for selective retention of antigen-specific tissue- resident memory T cells in the epidermal niche. Immunity 2021;54:84–98. 10.1016/j.immuni.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackay LK, Minnich M, Kragten NAM, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016;352:459–63. 10.1126/science.aad2035 [DOI] [PubMed] [Google Scholar]
- 91.Behr FM, Kragten NAM, Wesselink TH, et al. Blimp-1 Rather Than Hobit Drives the Formation of Tissue-Resident Memory CD8+ T Cells in the Lungs. Front Immunol 2019;10:400. 10.3389/fimmu.2019.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zundler S, Becker E, Spocinska M, et al. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol 2019;20:288–300. 10.1038/s41590-018-0298-5 [DOI] [PubMed] [Google Scholar]
- 93.Milner JJ, Toma C, Yu B, et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 2017;552:253–7. 10.1038/nature24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hombrink P, Helbig C, Backer RA, et al. Programs for the persistence, vigilance and control of human CD8+ lung-resident memory T cells. Nat Immunol 2016;17:1467–78. 10.1038/ni.3589 [DOI] [PubMed] [Google Scholar]
- 95.Szabo PA, Miron M, Farber DL. Location, location, location: tissue resident memory T cells in mice and humans. Sci Immunol 2019;4:eaas9673. 10.1126/sciimmunol.aas9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu J, Madi A, Mieg A, et al. T cell factor 1 suppresses CD103+ lung tissue-resident memory T cell development. Cell Rep 2020;31:107484. 10.1016/j.celrep.2020.03.048 [DOI] [PubMed] [Google Scholar]
- 97.Li C, Zhu B, Son YM, et al. The Transcription Factor Bhlhe40 Programs Mitochondrial Regulation of Resident CD8+ T Cell Fitness and Functionality. Immunity 2019;51:491–507. 10.1016/j.immuni.2019.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park SL, Mackay LK. Bhlhe40 keeps resident T cells too fit to quit. Immunity 2019;51:418–20. 10.1016/j.immuni.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 99.Beura LK, Wijeyesinghe S, Thompson EA, et al. T cells in nonlymphoid tissues give rise to Lymph-Node-Resident memory T cells. Immunity 2018;48:327–38. 10.1016/j.immuni.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fonseca R, Beura LK, Quarnstrom CF, et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat Immunol 2020;21:412–21. 10.1038/s41590-020-0607-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behr FM, Parga-Vidal L, Kragten NAM, et al. Tissue-resident memory CD8+ T cells shape local and systemic secondary T cell responses. Nat Immunol 2020;21:1070–81. 10.1038/s41590-020-0723-4 [DOI] [PubMed] [Google Scholar]
- 102.O'Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151–67. 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- 103.Park SL, Buzzai A, Rautela J, et al. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 2019;565:366–71. 10.1038/s41586-018-0812-9 [DOI] [PubMed] [Google Scholar]
- 104.Hu W, Sun R, Chen L, et al. Prognostic significance of resident CD103+CD8+T cells in human colorectal cancer tissues. Acta Histochem 2019;121:657–63. 10.1016/j.acthis.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 105.Hu X, Li Y-Q, Li Q-G, et al. ITGAE defines CD8+ tumor-infiltrating lymphocytes predicting a better prognostic survival in colorectal cancer. EBioMedicine 2018;35:178–88. 10.1016/j.ebiom.2018.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cascone T, William WN, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504–14. 10.1038/s41591-020-01224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corgnac S, Malenica I, Mezquita L, et al. CD103+CD8+TRM Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep Med 2020;1:100127. 10.1016/j.xcrm.2020.100127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caushi JX, Zhang J, Ji Z, et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 2021;596:126–32. 10.1038/s41586-021-03752-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luoma AM, Suo S, Williams HL, et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 2020;182:655–71. 10.1016/j.cell.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sasson SC, Slevin SM, Cheung VTF, et al. Interferon-Gamma-Producing CD8+ Tissue Resident Memory T Cells Are a Targetable Hallmark of Immune Checkpoint Inhibitor-Colitis. Gastroenterology 2021;161:1229–44. 10.1053/j.gastro.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riedl JM, Stotz M, Gerger A. Role of immune checkpoint inhibitors in gastrointestinal cancer treatment. Memo 2019;12:71–6. 10.1007/s12254-019-0470-0 [DOI] [Google Scholar]
- 112.Lu Z, Peng Z, Liu C, et al. Current status and future perspective of immunotherapy in gastrointestinal cancers. Innovation 2020;1:100041. 10.1016/j.xinn.2020.100041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abd Hamid M, Colin-York H, Khalid-Alham N, et al. Self-Maintaining CD103+ Cancer-Specific T Cells Are Highly Energetic with Rapid Cytotoxic and Effector Responses. Cancer Immunol Res 2020;8:203–16. 10.1158/2326-6066.CIR-19-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beumer-Chuwonpad A, Taggenbrock RLRE, Ngo TA, et al. The potential of tissue-resident memory T cells for adoptive immunotherapy against cancer. Cells 2021;10:2234. 10.3390/cells10092234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okła K, Farber DL, Zou W. Tissue-Resident memory T cells in tumor immunity and immunotherapy. J Exp Med 2021;218:e20201605. 10.1084/jem.20201605 [DOI] [PMC free article] [PubMed] [Google Scholar]