Abstract
Introduction
Heart failure (HF) is common in chronic kidney disease (CKD); identifying patients with CKD at high risk for HF may guide clinical care. We assessed the prognostic value of cardiac biomarkers and echocardiographic variables for 10-year HF prediction compared with a published clinical HF prediction equation in a cohort of participants with CKD.
Methods
We studied 2147 Chronic Renal Insufficiency Cohort (CRIC) participants without prior HF with complete clinical, cardiac biomarker (N-terminal brain natriuretic peptide [NT-proBNP] and high sensitivity troponin-T [hsTnT]), and echocardiographic data (left ventricular mass [LVM] and left ventricular ejection fraction [LVEF] data). We compared the discrimination of the 11-variable Atherosclerosis Risk in Communities (ARIC) HF prediction equation with LVM, LVEF, hsTnT, and NT-proBNP to predict 10-year risk of hospitalization for HF using a Fine and Gray modeling approach. We separately evaluated prediction of HF with preserved and reduced LVEF (LVEF ≥50% and <50%, respectively). We assessed discrimination with internally valid C-indices using 10-fold cross-validation.
Results
Participants’ mean (SD) age was 59 (11) years, 53% were men, 43% were Black, and mean (SD) estimated glomerular filtration rate (eGFR) was 44 (16) ml/min per 1.73 m2. A total of 324 incident HF hospitalizations occurred during median (interquartile range) 10.0 (5.7–10.0) years of follow-up. The ARIC HF model with clinical variables had a C-index of 0.68. Echocardiographic variables predicted HF (C-index 0.70) comparably to the published ARIC HF model, while NT-proBNP and hsTnT together (C-index 0.73) had significantly better discrimination (P = 0.004). A model including cardiac biomarkers, echocardiographic variables, and clinical variables had a C-index of 0.77. Discrimination of HF with preserved LVEF was lower than for HF with reduced LVEF for most models.
Conclusion
The ARIC HF prediction model for 10-year HF risk had modest discrimination among adults with CKD. NT-proBNP and hsTnT discriminated better than the ARIC HF model and at least as well as a model with echocardiographic variables. HF clinical prediction models tailored to adults with CKD are needed. Until then, measurement of NT-proBNP and hsTnT may be a low-burden approach to predicting HF in this population, as they offer moderate discrimination.
Keywords: biomarkers, cardiovascular disease, chronic kidney disease, echocardiogram, heart failure
Graphical abstract
Heart failure (HF) is a leading cause of morbidity and mortality in the United States, and both HF incidence and prevalence are predicted to increase substantially in coming decades.1 Patients with chronic kidney disease (CKD) are estimated to have a 3-fold higher risk of incident HF than those without CKD.2 Among patients with CKD, HF has been associated with greater risk of death, recurrent hospitalizations, and worse health-related quality of life.2, 3, 4, 5, 6, 7 Predicting future HF may help clinicians identify patients with CKD who will benefit from primary treatment for cardiovascular disease and more intensive surveillance.
HF prediction equations have been previously developed and validated in the general population. As an example, the ARIC Study HF equation discriminated the 10-year risk of HF well (C-index 0.773) using 11 clinical characteristics including age, Black race/ethnicity, sex, heart rate, systolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, current and former smoking, and body mass index.8 However, the performance of the ARIC HF prediction model has not been evaluated in a dedicated CKD population, which has both a higher burden of traditional HF risk factors than in the general population as well as CKD-specific metabolic and hemodynamic derangements that can contribute to HF.9
Furthermore, studies have shown that echocardiographic abnormalities and elevations in clinically available, circulating cardiac biomarkers, such as NT-proBNP and hsTnT, are strongly associated with incident HF in patients with CKD.10, 11, 12 As such, published equations developed in community-based populations have explored the contributions of cardiac biomarkers and echocardiography to predict incident HF and have found benefit in incorporating these variables with clinical information.13, 14, 15, 16 Yet, it remains unknown whether these clinically available diagnostic tools should be used to predict HF in patients with CKD.
Therefore, we evaluated the performance of the previously published ARIC HF clinical risk prediction equation, clinically available cardiac biomarkers (NT-proBNP and hsTnT), and echocardiographic measures (LVM and LVEF) to predict 10-year incident HF in persons with CKD. We hypothesized that (i) the ARIC HF model would have poor discrimination for incident HF in a CKD population, (ii) cardiac biomarkers and echocardiographic measures alone would not perform as well as a clinical model in this population, and (iii) combining all measures would have improved discrimination compared with the published ARIC HF clinical model in a cohort of patients with CKD.
Methods
Study Design and Population
The study population included participants in the CRIC study, an ongoing, prospective, multicenter cohort study that recruited 3939 adult participants with mild to moderate CKD (eGFR of 20–70 ml/min per 1.73 m2) from 7 clinical centers in the United States between June 2003 and August 2008. Further details on study design have been published previously.17,18 Participants on maintenance dialysis, with a kidney transplant, or with advanced HF, defined as New York Heart Association functional class III or IV, on study entry were not included. Study participants had annual in-person study visits, which included questionnaires, physical examination, laboratory measures, and cardiovascular testing; participants were contacted every 6 months to obtain updated information on medical history or hospitalizations. Institutional review board approval was obtained from all participating institutions, and informed consent was obtained from all participants.
For the present analysis, we excluded participants with a self-reported history of HF at cohort entry (n = 382) and those without available cardiac biomarkers at study enrollment (n = 120). Because follow-up time for this analysis of incident HF began at the year 1 examination, we also excluded 428 participants who experienced HF, died, withdrew from the study, or were lost to follow-up between study enrollment and the year 1 examination. Finally, we excluded those who had missing clinical or echocardiographic covariates at the Year 1 examination (n = 862), leaving a final analytical population of 2147 (Supplementary Figure S1).
Clinical Predictor Variables
We considered 3 types of candidate predictors: clinical variables, cardiac biomarker variables, and echocardiogram variables. The 11 clinical variables that we considered were those included in a previously published ARIC HF prediction equation: age, Black race/ethnicity, sex, heart rate, systolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, current and former smoking, and body mass index. The ARIC HF equation was chosen as comparator for its previously demonstrated good discrimination and external validity in a general population8 and for its use of clinically available predictors. For this analysis, we used assessments for all clinical predictors that occurred at the year 1 evaluation to coincide with the year 1 echocardiograms.
Information on socio-demographic characteristics, medical history, medication use, and lifestyle behaviors was provided by participants at each visit. Race/ethnicity was self-reported and categorized as non-Hispanic White, non-Hispanic Black, Hispanic, or other. Heart rate was obtained from a twelve-lead electrocardiogram.19 Blood pressure was obtained in a standardized setting by trained coordinators, using the mean of 3 seated resting blood pressure readings. Use of antihypertensive medications, including angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, β-blockers, calcium channel blockers, and diuretics, was ascertained by detailed review with participants at each clinic visit. Diabetes mellitus was defined as a fasting glucose of >126 mg/dl, a nonfasting glucose of >200 mg/dl, or use of insulin or other antidiabetic medications. Medical history (including HF, myocardial infarction, coronary revascularization, stroke, peripheral vascular disease, and chronic obstructive pulmonary disease) was determined by self-report; coronary heart disease was defined as a history of myocardial infarction or coronary revascularization. Self-reported tobacco use was categorized as current, former, or never. Body mass index was derived as weight (kg) divided by height (m) squared. eGFR was calculated from serum creatinine using the CKD Epidemiology Collaboration equation,20 where serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC central laboratory and standardized to isotope dilution mass spectrometry–traceable values.21,22
Cardiac Biomarker Variables
Two clinically available cardiac biomarkers, sampled from participants at study enrollment, were also considered as candidate predictors: NT-proBNP and hsTnT.10,11 Laboratory measurement details can be found in the Supplementary Methods.
Echocardiographic Variables
Research echocardiograms were performed at the year 1 evaluation and provided information on LVEF and LVM index, which were evaluated as candidate predictors of HF.23 Methods used to assess cardiac structure and function have been described previously.24,25 Briefly, assessments were performed using 2-dimensional images and a standard imaging protocol according to American Society of Echocardiography guidelines26 and quantified centrally by a single highly trained registered diagnostic cardiac sonographer who was unaware of participant identity or characteristics. LVM index was defined using Cornell criteria and indexed to height (in m) raised to the power of 2.7. LVEF was calculated using diastolic and systolic left ventricular volumes measured using the single plane Simpson rule method: LVEF = ([diastolic volume − systolic volume]/diastolic volume) × 100.
Incident HF Outcome Definitions
The primary outcome was incident HF over 10 years from the year 1 examination through 2019. Study participants were asked semiannually whether they had been hospitalized, and electronic health records from selected hospitals or healthcare delivery systems were also examined for qualifying encounters. The first 30 discharge codes were identified for all hospitalizations; study personnel obtained medical records for centralized adjudicated review if codes relevant to HF were present. At least 2 study physicians reviewed all HF events and deaths using medical records; clinical HF adjudication was based on clinical symptoms, physical examination of the heart and lungs, and central venous hemodynamic monitoring data and echocardiographic imaging, when available. HF was confirmed when both reviewers agreed on a “probable” or “definite” occurrence of HF on the basis of modified clinical Framingham criteria.27
As a secondary outcome, we classified incident HF events as HF with preserved ejection fraction (HFpEF), HF with reduced ejection fraction (HFrEF), or neither. HFpEF was defined as LVEF ≥50% and HFrEF as LVEF <50%, following guideline suggestions but collapsing into 2 categories owing to power considerations.28 LVEF was preferentially ascertained from clinical echocardiograms performed during the index HF hospitalization (available in 195 [60%] incident HF hospitalizations). If an echocardiogram was not performed during the hospitalization, we instead used EF from CRIC research echocardiograms up to 1 year before or after the index hospitalization (44 [14%] incident HF hospitalizations in our analyses). Research echocardiograms were performed at years 1, 4, and 7 and when eGFR declined to <20 ml/min per 1.73 m2 in a subset of participants. Previous research in CRIC has indicated that EF is relatively stable over time in this population.25,29 Finally, if EF categorization could not be determined, the HF event was termed “unclassified” (85 [26%] index HF hospitalizations) and was not included in this secondary analysis.
Statistical Analysis
We summarized participant demographic and clinical characteristics with descriptive measures such as mean and SD for continuous variables and frequency and percent for categorical variables. The primary outcome was the time to incident HF, measured from the year 1 examination and censored for loss to follow-up or end of study; follow-up time was truncated at 10 years after the year 1 examination. Secondary outcomes were the time to incident HFpEF and HFrEF; these were censored for loss to follow-up, end of study, or other subtypes of HF than that of interest. Predictions derived from standard Cox regression models have been shown to substantially overestimate predicted probabilities in the face of strong competing risks.30,31 Because death was common among CRIC participants, we used a Fine and Gray subdistribution hazard modeling approach in all models to account for the competing risk of death and to estimate the actual risk of HF as accurately as possible.32 We excluded participants with missing predictors, so there were complete data available for the 2147 participants included in this analysis.
We evaluated the performance of a series of models to predict incident HF, including (i) a previously published HF prediction equation that was developed in the ARIC cohort, which predicted the 10-year risk of HF using either clinical characteristics alone (ARIC HF clinical) or with the addition of NT-proBNP (ARIC HF + NT-proBNP).8 In this study, we examined the performance of these equations using both published coefficients and re-estimated coefficients using the CRIC data, because ARIC was a general population cohort and may not be applicable to a CKD population. We further examined the predictive performance of (ii) NT-proBNP alone, (iii) hsTnT alone, (iv) both cardiac biomarkers, and (v) echocardiographic variables (LVM index and LVEF) alone, as well as the performance of each of these subsets of predictors in conjunction with the clinical variables used in the ARIC HF model. Finally, we considered a full model that used all available clinical, biomarker, and echocardiographic variables together. Cardiac biomarkers were entered both linearly and log-transformed; remaining covariates were untransformed. In a secondary analysis, we evaluated the ability of each of these sets of predictors to predict incident HFpEF and HFrEF. Another secondary analysis examined the performance of each model compared with the ARIC HF clinical model by category of eGFR (<30, 30–44, 45–59, or ≥60 ml/min per 1.73 m2).
We assessed the discriminatory ability of each model via the concordance probability (C-index) for right-censored survival data, together with inverse probability of censoring weighting to account for the competing risk of death.30,33 We obtained CIs for the C-index and difference in C-indices via the nonparametric bootstrap with 2000 replicates.34 For models requiring estimation (i.e., all models except ARIC HF models with previously published coefficients), we evaluated discriminatory performance using 10-fold cross-validation, which can avoid overoptimistic assessment of the model and has been shown to make more efficient use of data compared with splitting data into training and validation sets.35 Calibration of selected models was evaluated graphically by plotting the predicted versus the observed 10-year probability of incident HF and by estimating the intercept and slope of the calibration plot.36,37 Finally, we examined the incidence rates of HF by deciles of predicted probability for top models.
In a sensitivity analysis, we evaluated the performance of models with respect to 2 reclassification statistics, the net reclassification index (NRI) and integrated discrimination index.38,39 We evaluated both the categorical NRI (with categories of <10%, 10%–20%, and >20%), which summarizes the proportions of cases and noncases (which are then summed) moving up or down in risk strata, and the continuous NRI, which evaluates how risk increases for cases and decreases for noncases, comparing a new model to the referent. As recommended, we obtained CIs for these measures though nonparametric bootstrapping with 2000 replicates.40
The TRIPOD checklist for reporting development and validation of prediction models can be found in Supplementary Table S4. All analyses were conducted using the R 3.6.2 software environment (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Characteristics
Among the 2147 eligible participants, the mean (SD) age was 59 (11) years, 47% were women, and 43% self-identified as non-Hispanic Black. The mean (SD) eGFR was 44 (16) ml/min per 1.73 m2, with a median (interquartile range) urine albumin excretion of 41 (8–390) mg/day (Table 1). There were 324 incident HF events that occurred during a median (interquartile range) of 10.0 (5.7–10.0) years of follow-up for an incidence rate of 1.9 (95% CI: 1.7–2.1) events per 100 person-years; 135 and 104 HF events were classified as HFpEF and HFrEF, respectively (Supplementary Table S1). A total of 361 participants died without developing HF, and 644 participants developed end-stage kidney disease (471 initiated hemodialysis, 102 initiated peritoneal dialysis, 2 initiated an unspecified dialysis modality, and 69 received a kidney transplant) before developing HF or censoring.
Table 1.
Characteristics of CRIC analytical population (N = 2147)
| Characteristics | Value |
|---|---|
| Age (yr) | 58.6 (10.9) |
| Men | 1129 (53) |
| Race/ethnicity | |
| White | 1098 (51) |
| Black | 932 (43) |
| Other | 117 (5) |
| Diabetes | 957 (45) |
| History of coronary heart disease | 389 (18) |
| History of atrial fibrillation | 329 (15) |
| History of stroke | 211 (10) |
| History of PVD | 132 (6) |
| History of COPD | 88 (4) |
| Antihypertensive medications | 1944 (91) |
| ACEi/ARB | 1464 (68) |
| Beta blockers | 996 (46) |
| Calcium channel blockers | 877 (41) |
| Diuretics | 1188 (55) |
| Height (cm) | 168.8 (9.6) |
| Weight (kg) | 89.5 (21.7) |
| BMI (kg/m2) | 31.4 (7.3) |
| Systolic blood pressure (mm Hg) | 125.9 (21.0) |
| Diastolic blood pressure (mm Hg) | 70.0 (12.4) |
| Heart rate (bpm) | 65.3 (11.4) |
| eGFR (CKD-EPI), ml/min per 1.73 m2 | 43.8 (15.7) |
| eGFR category (ml/min per 1.73 m2) | |
| ≥60 | 332 (15) |
| 45–59 | 637 (30) |
| 30–44 | 713 (33) |
| <30 | 426 (20) |
| ESRD | 21 (1) |
| 24-hour urine albumin (mg), median (IQR) | 41 (8-390) |
| Hemoglobin (g/dl) | 12.9 (1.8) |
| LDL (mg/dl) | 100.8 (34.2) |
| HDL (mg/dl) | 49.7 (16.0) |
| LV mass index, Cornell criteria (g/m2.7) | 49.9 (13.0) |
| Left ventricular ejection fraction (%) | 55.3 (7.3) |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CKD-EPI, CKD-Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; LV, left ventricular; PVD, peripheral vascular disease.
Entries are mean (SD) for continuous variables and n (%) for categorical variables, except as noted. Table reflects participant characteristics at the year 1 examination, which was the beginning of follow-up for this analysis.
Performance of ARIC HF Equation for Prediction of Incident HF in CKD
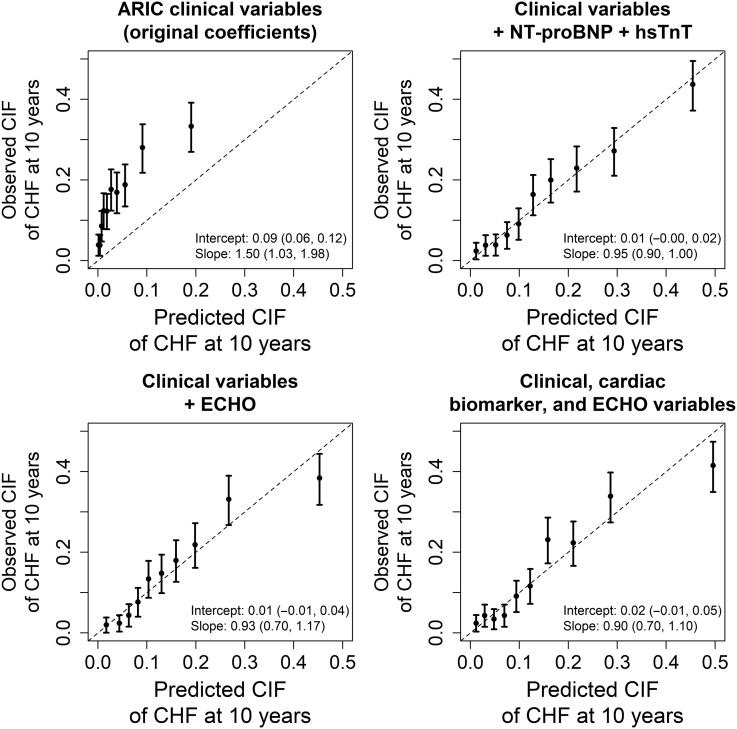
ARIC HF clinical prediction equations that used the original and re-estimated coefficients had C-indices of 0.680 (95% CI: 0.652–0.708) and 0.702 (95% CI 0.673–0.731), respectively (Table 2). The ARIC HF clinical model was poorly calibrated in this cohort, with a calibration intercept of 0.09 (95% CI: 0.06–0.12) and a calibration slope of 1.50 (95% CI: 1.03–1.98) (Figure 1). Discriminatory ability of the ARIC HF clinical model was substantially poorer among participants with eGFR <45 ml/min per 1.73 m2 (Supplementary Table S2) overall.
Table 2.
Discriminatory ability of models to predict incident HF, compared with the ARIC HF clinical model
| Model | C-index (95% CI) | Difference in C-index (95% CI), compared with ARIC clinical model with published coefficients | Difference in C-index (95% CI), compared with ARIC clinical model with re-estimated coefficients |
|---|---|---|---|
| ARIC clinical model (published coefficients) | 0.680 (0.652–0.708) | Reference | NA |
| ARIC + NT-proBNP model | 0.740 (0.714–0.765) | 0.060 (0.043–0.077) | NA |
| NT-proBNP alone | 0.703 (0.676–0.731) | 0.024 (−0.013 to 0.060) | 0.002 (−0.035 to 0.039) |
| hsTnT alone | 0.679 (0.650–0.707) | −0.001 (−0.035 to 0.033) | −0.023 (−0.057 to 0.011) |
| NT-proBNP + hsTnT | 0.728 (0.701–0.755) | 0.048 (0.015–0.082) | 0.026 (−0.008 to 0.061) |
| LV mass + LV ejection fraction | 0.701 (0.672–0.730) | 0.021 (−0.013 to 0.056) | −0.001 (−0.034 to 0.032) |
| Clinical variables (re-estimated coefficients) | 0.702 (0.673–0.731) | 0.022 (0.002–0.043) | Reference |
| Clinical variables + NT-proBNP | 0.750 (0.724–0.777) | 0.071 (0.044–0.098) | 0.048 (0.028–0.069) |
| Clinical variables + hsTnT | 0.726 (0.699–0.754) | 0.046 (0.022–0.071) | 0.024 (0.008–0.040) |
| Clinical variables + NT-proBNP + hsTnT | 0.753 (0.726–0.779) | 0.073 (0.045–0.100) | 0.051 (0.029–0.072) |
| Clinical variables + LV mass + LV ejection fraction | 0.742 (0.715–0.769) | 0.062 (0.036–0.089) | 0.040 (0.022–0.058) |
| Clinical variables + NT-proBNP + hsTnT + LV mass + LV ejection fraction | 0.765 (0.739–0.791) | 0.086 (0.058–0.114) | 0.064 (0.041–0.086) |
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; HF, heart failure; hsTNT, high sensitivity troponin-T; LV, left ventricular; NA, not applicable; NT-proBNP, N-terminal brain natriuretic peptide.
Entries for ARIC clinical and ARIC + NT-proBNP models are C-index and associated 95% bootstrap CIs; all other entries are 10-fold cross-validated C-indices or difference in C-indices compared with ARIC clinical model and associated 95% bootstrap CIs. Bolded entries indicate statistical significance at the 5% level. ARIC model predicts 10-year risk of HF from age, Black race/ethnicity, sex, heart rate, systolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, current smoking, former smoking, and BMI.
Figure 1.
Calibration plots of models to predict incident heart failure. Figures show predicted and observed CIFs of incident CHF at 10 years, by decile of predicted probability. CHF, congestive heart failure; CIF, cumulative incidence fraction; ECHO, echocardiogram; NT-proBNP, N-terminal brain natriuretic peptide.
Performance of Cardiac Biomarker and Echocardiographic Models for Prediction of Incident HF
Echocardiographic variables (C-index 0.701; 95% CI: 0.672–0.730) had discrimination that was comparable to the ARIC HF clinical model (Table 2). NT-proBNP, in conjunction with hsTnT, had significantly higher discrimination (C-index 0.728; 95% CI: 0.701–0.755) than the ARIC HF clinical model.
The addition of NT-proBNP to clinical information improved discrimination further (C-index 0.750; 95% CI: 0.724–0.777), while a model including all clinical, cardiac biomarker, and echocardiographic variables had a C-index of 0.765 (95% CI: 0.7392–0.791). Calibration appeared adequate for top models (Figure 1), with higher incidence rates observed in higher predicted probability deciles (Supplementary Figure S2); final coefficient estimates for top models can be found in Table 3.
Table 3.
Final coefficient estimates of top regression models to predict incident heart failure
| Predictor | Clinical variables + cardiac biomarkers | Clinical variables + cardiac biomarkers + ECHO variables |
|---|---|---|
| Age (yr) | 0.00809 | 0.0107 |
| Black race/ethnicity | 0.165 | 0.141 |
| Male sex | −0.0811 | −0.194 |
| Heart rate (bpm) | 0.00691 | 0.00422 |
| Systolic blood pressure (mm Hg) | 0.00777 | 0.00581 |
| Antihypertensives | 1.26 | 1.27 |
| Diabetes | 0.328 | 0.399 |
| CHD | 0.358 | 0.253 |
| Current smoking | 0.301 | 0.261 |
| Former smoking | 0.368 | 0.351 |
| BMI (kg/m2) | 0.0235 | 0.00762 |
| NT-proBNP (pg/ml) | −0.0000964 | −0.00018 |
| hsTnT (pg/ml) | −0.0037 | −0.00321 |
| Log (NT-proBNP [pg/ml]) | 0.379 | 0.338 |
| Log (hsTnT [pg/ml]) | 0.398 | 0.311 |
| LV mass (g/m2.7) | 0.0196 | |
| LV ejection fraction (%) | −0.0302 | |
| Mean linear predictor | 6.854 | 4.921 |
BMI, body mass index; bpm, beats per minute; CHD, coronary heart disease; ECHO, echocardiogram; hsTNT, high sensitivity troponin-T; LV, left ventricular; MLP, mean linear predictor; NT-proBNP, N-terminal brain natriuretic peptide.
Predicted 10-year risk of incident heart failure can be calculated as 1 – 0.84490e(ΣXβ - MLP), where β is the regression coefficient, X is the level for each risk factor, and MLP is the value of the mean linear predictor listed above.
Discriminatory ability of all models was substantially poorer among participants with lower eGFR (Supplementary Table S2). The largest improvements in discrimination to the ARIC HF clinical model among participants with eGFR <45 ml/min per 1.73 m2 occurred in models that used clinical variables, cardiac biomarkers, and echocardiographic variables to predict incident HF (difference in C-index compared with ARIC HF among eGFR 30–45 ml/min per 1.73 m2, 0.113; 95% CI: 0.064–0.162).
Performance of Models for Prediction of Incident HFpEF and HFrEF
The ARIC HF clinical model with original coefficients provided better discrimination of incident HFrEF (C-index 0.690; 95% CI: 0.637–0.744) compared with HFpEF (C-index 0.664; 95% CI: 0.622–0.706; Table 4). While models involving hsTnT performed similarly for both outcomes, the inclusion of NT-proBNP or echocardiographic variables resulted in better discrimination of HFrEF compared with HFpEF. Models that incorporated all clinical, cardiac biomarker, and echocardiographic variables provided moderate discrimination of HFpEF (C-index 0.729; 95% CI: 0.682–0.776) and HFrEF (C-index 0.775; 95% CI: 0.729–0.822). The HFpEF but not the HFrEF model was adequately calibrated, with calibration intercepts of 0.01 (95% CI: −0.00 to 0.03) and 0.01 (95% CI: 0.00–0.01), respectively, and calibration slopes of 0.82 (95% CI: 0.58–1.06) and 0.85 (95% CI: 0.75–0.96), respectively.
Table 4.
Discriminatory ability of models to predict incident HFpEF and HFrEF, compared with the ARIC HF clinical model
| Model | Incident HFpEF |
Incident HFrEF |
||||
|---|---|---|---|---|---|---|
| C-index (95% CI) | Difference in C-index (95% CI), compared with ARIC clinical model with published coefficients | Difference in C-index (95% CI), compared with ARIC clinical model with re-estimated coefficients | C-index (95% CI) | Difference in C-index (95% CI), compared with ARIC clinical model with published coefficients | Difference in C-index (95% CI), compared with ARIC clinical model with re-estimated coefficients | |
| ARIC clinical model (published coefficients) | 0.664 (0.622–0.706) | Reference | NA | 0.690 (0.637–0.744) | Reference | NA |
| ARIC + NT-proBNP model | 0.719 (0.679–0.759) | 0.055 (0.027–0.083) | NA | 0.749 (0.703–0.795) | 0.058 (0.031–0.086) | NA |
| NT-proBNP alone | 0.674 (0.628–0.720) | 0.010 (−0.047 to 0.066) | −0.018 (−0.079 to 0.043) | 0.714 (0.666–0.762) | 0.023 (−0.043 to 0.090) | 0.026 (−0.048 to 0.099) |
| hsTnT alone | 0.667 (0.622–0.712) | 0.003 (−0.053 to 0.059) | −0.025 (−0.084 to 0.035) | 0.658 (0.604–0.712) | −0.033 (−0.087 to 0.021) | −0.030 (−0.088 to 0.027) |
| NT-proBNP + hsTnT | 0.700 (0.653–0.747) | 0.036 (−0.018 to 0.090) | 0.008 (−0.049 to 0.066) | 0.724 (0.675–0.773) | 0.034 (−0.026 to 0.093) | 0.036 (−0.030 to 0.102) |
| LV mass + LV ejection fraction | 0.674 (0.632–0.716) | 0.010 (−0.043 to 0.062) | −0.018 (−0.073 to 0.037) | 0.731 (0.679–0.782) | 0.040 (−0.023 to 0.103) | 0.042 (−0.024 to 0.109) |
| Clinical variables only (re-estimated coefficients) | 0.694 (0.643–0.744) | 0.027 (−0.014 to 0.068) | Reference | 0.688 (0.630–0.746) | −0.002 (−0.034 to 0.030) | Reference |
| Clinical variables + NT-proBNP | 0.722 (0.674–0.770) | 0.058 (0.012–0.103) | 0.030 (0.001–0.059) | 0.752 (0.706–0.799) | 0.062 (0.015–0.109) | 0.064 (0.023–0.106) |
| Clinical variables + hsTnT | 0.715 (0.668–0.762) | 0.051 (0.007–0.095) | 0.024 (−0.002 to 0.049) | 0.699 (0.639–0.758) | 0.008 (−0.027 to 0.044) | 0.011 (−0.012 to 0.033) |
| Clinical variables + NT-proBNP + hsTnT | 0.728 (0.681–0.775) | 0.064 (0.018–0.110) | 0.037 (0.005–0.068) | 0.750 (0.702–0.798) | 0.060 (0.012–0.108) | 0.062 (0.020–0.104) |
| Clinical variables + LV mass + LV ejection fraction | 0.704 (0.656–0.751) | 0.039 (−0.003 to 0.082) | 0.012 (−0.007 to 0.031) | 0.754 (0.704–0.804) | 0.064 (0.017–0.111) | 0.066 (0.027–0.105) |
| Clinical variables + NT-proBNP + hsTnT + LV mass + LV ejection fraction | 0.729 (0.682–0.776) | 0.065 (0.019–0.111) | 0.037 (0.005–0.070) | 0.775 (0.729–0.822) | 0.085 (0.033–0.137) | 0.087 (0.041–0.134) |
ARIC, Atherosclerosis Risk in Communities; BMI, body mass index; HF, heart failure; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; hsTnT, high sensitivity troponin-T; LV, left ventricular; NA, not applicable; NT-proBNP, N-terminal brain natriuretic peptide.
Entries for ARIC clinical and ARIC + NT-proBNP models are C-index and associated 95% bootstrap CIs; all other entries are 10-fold cross-validated C-indices or difference in C-indices compared with ARIC clinical model and associated 95% bootstrap CIs. Bolded entries indicate statistical significance at the 5% level. ARIC model predicts 10-year risk of HF from age, Black race/ethnicity, sex, heart rate, systolic blood pressure, use of antihypertensive medications, diabetes, coronary heart disease, current smoking, former smoking, and BMI.
Reclassification Metrics for Prediction of Incident HF
Compared with a model using clinical variables only, models that used cardiac biomarkers or echocardiographic variables alone did not have significant improvements in NRI or integrated discrimination index (Supplementary Table S3). In contrast, models that added ≥1 cardiac biomarkers, echocardiographic variables, or both to clinical variables had significantly higher continuous and categorical NRI and integrated discrimination index.
Discussion
In this analysis, we found that an HF prediction model of clinical variables developed in a general population had poor discrimination in a large, well-characterized CKD cohort. In contrast, a model consisting of NT-proBNP and hsTnT alone had significantly better discrimination than the published model among CKD participants and was comparable to a model that used 2 echocardiographic variables (LVM and LVEF) alone. Models that added clinically available cardiac biomarkers and/or echocardiographic variables to the 11 routinely available clinical variables further improved prediction of HF. We further found that discrimination of HF with preserved LVEF was lower than for HF with reduced LVEF in most models. These data suggest that 2 clinically available cardiac biomarkers (NT-proBNP and hsTnT) are better than or at least comparable to a validated published HF prediction model or echocardiographic measures in a CKD population. Furthermore, differences in discrimination across the range of eGFR highlight the need for CKD-specific HF prediction models, particularly for HFpEF.
The published ARIC HF clinical model performed poorly in the CRIC cohort overall, and we further found that the ARIC HF clinical model was poorly calibrated in this CKD population. Our analysis also showed poorer discrimination among participants with lower eGFR (below eGFR <45 ml/min per 1.43 m2). This variation in predictive performance could reflect the complex pathophysiology of HF in CKD, which differs from the general population.9 For example, a 2020 evaluation of the ARIC HF clinical model using data from the UK Biobank found that the addition of urine albumin-to-creatinine ratio significantly enhanced HF prediction in the general population, which suggests a role for CKD-specific factors.41 Furthermore, other important CKD-specific risk factors such as alterations in mineral metabolism, anemia, or inflammation have been strongly associated with HF and should be tested in de novo prediction models in patients with CKD.42, 43, 44, 45, 46, 47, 48
We found that NT-proBNP and hsTnT together had better discrimination to predict incident HF in this CKD population compared with the published clinical model and were comparable to a model that included LVEF and LVM. Furthermore, the combination of clinically available cardiac biomarkers and echocardiographic measures improved discrimination for incident HF in this population, although the performance was poorer at lower eGFR. These data align with some findings from other populations. A study conducted in the ARIC cohort found that adding hsTnT and NT-proBNP to the ARIC HF clinical model was found to improve the C-index, similar to what we found in this study.16 In the Cardiovascular Health Study, a community-based cohort study of older adults, discrimination of 5-year risk of HF was improved by 0.027 and 0.031 by adding an NT-proBNP or an echocardiographic score including LVEF and LVM, respectively, to a base clinical model, improvements that were smaller than those we observed.14 Finally, a HF prediction model developed from 735 variables in the Multi-Ethnic Study of Atherosclerosis included NT-proBNP, troponin-T, LVEF, and end-diastolic LV mass among its top 20 predictors and a 12-year C-index of 0.84, with NT-proBNP the top overall predictor.13 Overall, our results suggest that NT-proBNP and hsTnT alone may be a reasonable and low-burden alternative to clinical or echocardiogram-based models to predict incident HF among patients with CKD until CKD-specific models can be developed.
Our evaluation of HFpEF prediction is especially relevant in the therapeutic context of CKD, in which HFpEF is relatively more common.49,50 Among a subset of participants in whom the type of HF could be ascertained, the tested models of clinical variables, cardiac biomarkers, and echocardiographic variables generally had better discrimination of HFrEF compared with HFpEF, although there was still moderate discrimination of HFpEF. Our findings are broadly consistent with those reported by Ho et al.15 among 4 community-based cohorts. That work derived separate models for HFpEF and HFrEF outcomes; the HFrEF model included electrocardiogram-derived predictors in addition to clinical variables, whereas the HFpEF model did not. In our study, we found that the addition of NT-proBNP and echocardiographic variables to clinical variables increased the discrimination of both HF subtypes, and the addition of hsTnT to clinical variables improved prediction of HFpEF only. HFpEF and HFrEF have increasingly been recognized as reflecting distinct pathologic processes, both of which merit greater study of risk factors and potential therapies51,52 and may need separate prediction equations. In particular, our findings underscore the poorly understood pathophysiology of HFpEF, which may be related to aging, inflammation, or impaired sodium handling.53, 54, 55, 56 Moreover, HFpEF itself has been shown to be a heterogeneous condition,57, 58, 59 which could benefit from a more granular “precision medicine” approach in future research, particularly in patients with CKD.
Prediction models for HF in patients with CKD could have several important applications. Clinical risk scores could be used for risk stratification, guiding primary prevention strategies such as better blood pressure management or lifestyle modifications, or could prompt clinicians to assess symptoms of subclinical HF more frequently for patients with CKD at high risk of HF. Clinical models to identify patients with CKD at high risk of HF might be used for enrichment of clinical trials of HF therapies, especially as future therapies may target HF subphenotypes or CKD-specific pathology.60 Because the underlying burden of cardiovascular disease is much higher in patients with CKD than in the general population, implementation of risk-based management strategies could have a proportionally greater impact among this high-risk population. Until prediction models in patients with CKD are developed and validated, use of these widely available cardiac biomarkers may help guide clinical management and may be a low-burden approach to predict HF, particularly if echocardiography is not readily available. Furthermore, randomized clinical trials have demonstrated that BNP guided pharmacotherapy may be effective in the prevention of incident and recurrent HF,61, 62, 63, 64 a strategy that has not been tested in patients with CKD and may be an important next step to further apply cardiac biomarkers into practice.
Strengths of this study include a thoroughly characterized, longitudinal, multicenter CKD cohort and physician adjudication of HF events. We focused on clinically available predictors, which make resulting models more likely to be routinely implemented. Unlike some previous research,8,13,14,41 we accounted analytically for the competing risk of death. We also recognize a few limitations. While we provided internally valid estimates of discrimination in a CKD cohort, we were unable to externally validate our findings, as there are few longitudinal CKD cohorts with similar data available. Although the use of race in predictive modeling is controversial, we included self-reported race as a potential predictor for comparability with the published ARIC HF model. Nonetheless, future models should evaluate the relative contributions of genetic factors (e.g., APOL1) versus social determinants of health instead of traditional social constructs of race. We considered a limited number of clinical variables from a single published model; however, neither this nor other general population HF prediction models have been tested in CKD. Cardiac biomarkers were measured approximately 1 year before other predictors. A large proportion of CRIC participants were missing echocardiograms, for unclear reasons; it is unknown whether this missingness introduced bias into the results. Our outcome only included HF hospitalizations, so we were unable to assess how these models predict HF not requiring hospitalization or HF-related death occurring outside the hospital setting. Were such data available, a similar approach could be used in future. HF subtype classification by ejection fraction was missing in a large proportion of hospitalizations, so a combination of clinical echocardiograms and research echocardiograms were used to classify HF subtype. Even with these approaches, the data were not complete, and there may have been interim changes in ejection fraction between the research echocardiogram and the hospitalization. The present study evaluated 10-year risk of HF on the basis of published models. Unfortunately, we were not able to evaluate shorter intervals because the ARIC HF prediction model was specifically developed and validated to predict 10-year risk. Future studies in CKD patients should consider predicting HF risk over shorter time periods. Finally, the CRIC cohort consisted of research volunteers and may differ from the broader CKD population in ways relevant to prediction of HF.
In conclusion, among a cohort of patients with CKD, a published HF prediction model derived in the general population had only modest discrimination of 10-year HF risk, particularly among those with lower eGFR and for the HFpEF subphenotype. In contrast, 2 cardiac biomarkers, NT-proBNP and hsTnT, alone provided moderate discrimination of HF and performed similarly to echocardiographic variables alone. Future studies are needed to develop and validate HF prediction models specifically in patients with CKD; until then, use of NT-proBNP and hsTnT may be a low-burden approach to predicting HF in this population.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by R01 DK103612 (Bansal). This research was supported in part by an unrestricted gift from the Northwest Kidney Centers to the Kidney Research Institute. Roche Diagnostics provided partial funding for the NT-proBNP and hsTnT assays.
Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, U01DK060902 and U24DK060990). In addition, this work was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH / NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH / NCRR UCSF-CTSI UL1 RR-024131, and Department of Internal Medicine, University of New Mexico School of Medicine Albuquerque, NM R01DK119199.
Footnotes
Supplemental Methods. Measurement of cardiac biomarkers.
Table S1. Characteristics of CRIC analytic population by subtype of heart failure.
Table S2. Discriminatory ability of models to predict incident heart failure by eGFR category.
Table S3. Reclassification statistics of models to predict incident heart failure.
Table S4. TRIPOD checklist.
Figure S1. CONSORT diagram of analytic population.
Figure S2. Incidence rates of heart failure by decile of predicted probability.
Contributor Information
Leila R. Zelnick, Email: lzelnick@uw.edu.
CRIC Study Investigators:
Lawrence J. Appel, Jing Chen, Debbie Cohen, Harold I. Feldman, Alan S. Go, James P. Lash, Robert G. Nelson, Mahboob Rahman, Panduranga S. Rao, Vallabh O. Shah, and Mark L. Unruh
Appendix
List of CRIC Study Investigators
Lawrence J. Appel, MD, MPH
Jing Chen, MD, MMSc, MSc
Debbie Cohen, MD
Harold I. Feldman, MD, MSCE
Alan S. Go, MD
James P. Lash, MD
Robert G. Nelson, MD, PhD, MS
Mahboob Rahman, MD
Panduranga S. Rao, MD
Vallabh O. Shah, PhD, MS
Mark L. Unruh, MD, MS
Supplementary Material
Supplemental Methods. Measurement of cardiac biomarkers.
Table S1. Characteristics of CRIC analytic population by subtype of heart failure.
Table S2. Discriminatory ability of models to predict incident heart failure by eGFR category.
Table S3. Reclassification statistics of models to predict incident heart failure.
Table S4. TRIPOD checklist.
Figure S1. CONSORT diagram of analytic population.
Figure S2. Incidence rates of heart failure by decile of predicted probability.
References
- 1.Savarese G., Lund L.H. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kottgen A., Russell S.D., Loehr L.R., et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 3.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med. 2008;18:4] N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Bansal N., Katz R., Robinson-Cohen C., et al. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2:314–318. doi: 10.1001/jamacardio.2016.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saran R., Robinson B., Abbott K.C., et al. US renal data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(suppl 1):A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Harel Z., Wald R., McArthur E., et al. Rehospitalizations and emergency department visits after hospital discharge in patients receiving maintenance hemodialysis. J Am Soc Nephrol. 2015;26:3141–3150. doi: 10.1681/ASN.2014060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter A.C., Lash J.P., Xie D., et al. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11:1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal S.K., Chambless L.E., Ballantyne C.M., et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuegel C., Bansal N. Heart failure in patients with kidney disease. Heart. 2017;103:1848–1853. doi: 10.1136/heartjnl-2016-310794. [DOI] [PubMed] [Google Scholar]
- 10.Bansal N., Zelnick L., Go A., et al R., et al. Cardiac biomarkers and risk of incident heart failure in chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal N., Zelnick L.R., Soliman E.Z., et al. Change in cardiac biomarkers and risk of incident heart failure and atrial fibrillation in CKD: the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2021;77:907–919. doi: 10.1053/j.ajkd.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin R.F., Deo R., Bansal N., et al. Associations of conventional echocardiographic measures with incident heart failure and mortality: the Chronic Renal Insufficiency Cohort. Clin J Am Soc Nephrol. 2017;12:60–68. doi: 10.2215/CJN.02700316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambale-Venkatesh B., Yang X., Wu C.O., et al. Cardiovascular event prediction by machine learning: the multi-ethnic study of atherosclerosis. Circ Res. 2017;121:1092–1101. doi: 10.1161/CIRCRESAHA.117.311312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalogeropoulos A.P., Georgiopoulou V.V., deFilippi C.R., Gottdiener J.S., Butler J., Cardiovascular Health Study Echocardiography, natriuretic peptides, and risk for incident heart failure in older adults: the Cardiovascular Health Study. JACC Cardiovasc Imaging. 2012;5:131–140. doi: 10.1016/j.jcmg.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho J.E., Enserro D., Brouwers F.P., et al. Predicting heart failure with preserved and reduced ejection fraction: the international collaboration on heart failure subtypes. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.003116. 10.1161/CIRCHEARTFAILURE.115.003116 e003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nambi V., Liu X., Chambless L.E., et al. Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clin Chem. 2013;59:1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman H.I., Appel L.J., Chertow G.M., et al. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 18.Lash J.P., Go A.S., Appel L.J., et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function [published correction appears in Clin J Am Soc Nephrol. 2011;6:2548-2553] Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deo R., Shou H., Soliman E.Z., et al. Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol. 2016;27:559–569. doi: 10.1681/ASN.2014101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inker L.A., Schmid C.H., Tighiouart H., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C [published correction appears in N Engl J Med. 2012;367:681] [published correction appears in N Engl J Med. 2012;367:2060] N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joffe M., Hsu C.Y., Feldman H.I., et al. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey A.S., Coresh J., Greene T., et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 23.Lang R.M., Bierig M., Devereux R.B., et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Park M., Hsu C.Y., Li Y., et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal N., Roy J., Chen H.Y., et al. Evolution of echocardiographic measures of cardiac disease from CKD to ESRD and risk of all-cause mortality: findings from the CRIC Study [published correction appears in Am J Kidney Dis. 2020;75:817] Am J Kidney Dis. 2018;72:390–399. doi: 10.1053/j.ajkd.2018.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiller N.B., Shah P.M., Crawford M., et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 27.Ho K.K., Anderson K.M., Kannel W.B., Grossman W., Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 28.Yancy C.W., Jessup M., Bozkurt B., et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Bansal N., Keane M., Delafontaine P., et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8:355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolbers M., Koller M.T., Witteman J.C., Steyerberg E.W. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 31.Austin P.C., Lee D.S., Fine J.P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine J., Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 33.Wolbers M., Blanche P., Koller M.T., Witteman J.C., Gerds T.A. Concordance for prognostic models with competing risks. Biostatistics. 2014;15:526–539. doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efron B., Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statist Sci. 1986;1:54–75. doi: 10.1214/ss/1177013815. [DOI] [Google Scholar]
- 35.James G., Witten D., Hastie T., Tibshirani R. Springer; 2013. An Introduction to Statistical Learning: With Applications in R. [Google Scholar]
- 36.May S., Hosmer D.W. A cautionary note on the use of the Grønnesby and Borgan goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 2004;10:283–291. doi: 10.1023/b:lida.0000036393.29224.1d. [DOI] [PubMed] [Google Scholar]
- 37.Grønnesby J.K., Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 38.Uno H., Tian L., Cai T., Kohane I.S., Wei L.J. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pencina M.J., D’Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr K.F., McClelland R.L., Brown E.R., Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak C., Ärnlöv J. Kidney disease biomarkers improve heart failure risk prediction in the general population. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.006904. [DOI] [PubMed] [Google Scholar]
- 42.Scialla J.J., Xie H., Rahman M., et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ix J.H., Katz R., Kestenbaum B.R., et al. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–207. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wollert K.C., Kempf T., Wallentin L. Growth differentiation Factor 15 as a biomarker in cardiovascular disease. Clin Chem. 2017;63:140–151. doi: 10.1373/clinchem.2016.255174. [DOI] [PubMed] [Google Scholar]
- 45.Kalogeropoulos A., Georgiopoulou V., Psaty B.M., et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonelli M., Sacks F., Pfeffer M., Gao Z., Curhan G. Cholesterol And Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease [published correction appears in Circulation. 2007;116:e556] Circulation. 2005;112:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 47.Wannamethee S.G., Welsh P., Papacosta O., Lennon L., Whincup P.H., Sattar N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ Heart Fail. 2014;7:732–739. doi: 10.1161/CIRCHEARTFAILURE.114.001272. [DOI] [PubMed] [Google Scholar]
- 48.He J., Shlipak M., Anderson A., et al. Risk factors for heart failure in patients with chronic kidney disease: the CRIC (Chronic Renal Insufficiency Cohort) study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinberg B.A., Zhao X., Heidenreich P.A., et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 50.Bansal N., Zelnick L., Bhat Z., et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73:2691–2700. doi: 10.1016/j.jacc.2019.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmonds S.J., Cuijpers I., Heymans S., Jones E.A.V. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9:242. doi: 10.3390/cells9010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah S.J., Kitzman D.W., Borlaug B.A., et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gevaert A.B., Shakeri H., Leloup A.J., et al. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003806. [DOI] [PubMed] [Google Scholar]
- 54.Glezeva N., Baugh J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail Rev. 2014;19:681–694. doi: 10.1007/s10741-013-9405-8. [DOI] [PubMed] [Google Scholar]
- 55.Sabbah M.S., Fayyaz A.U., de Denus S., et al. Obese-inflammatory phenotypes in heart failure with preserved ejection fraction. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.119.006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller W.L., Mullan B.P. Volume overload profiles in patients with preserved and reduced ejection fraction chronic heart failure: are there differences? A pilot study. JACC Heart Fail. 2016;4:453–459. doi: 10.1016/j.jchf.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Hedman Å.K., Hage C., Sharma A., et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart. 2020;106:342–349. doi: 10.1136/heartjnl-2019-315481. [DOI] [PubMed] [Google Scholar]
- 58.Shah A.M., Solomon S.D. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1716–1717. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 59.Shah S.J., Katz D.H., Deo R.C. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10:407–418. doi: 10.1016/j.hfc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark K.A.A., Velazquez E.J. Heart failure with preserved ejection fraction: time for a reset. JAMA. 2020;324:1506–1508. doi: 10.1001/jama.2020.15566. [DOI] [PubMed] [Google Scholar]
- 61.Pfisterer M., Buser P., Rickli H., et al. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–392. doi: 10.1001/jama.2009.2. [DOI] [PubMed] [Google Scholar]
- 62.Jourdain P., Jondeau G., Funck F., et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–1739. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 63.Ledwidge M., Gallagher J., Conlon C., et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA. 2013;310:66–74. doi: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 64.Felker G.M., Hasselblad V., Hernandez A.F., O’Connor C.M. Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.