Learning objectives.
By reading this article, you should be able to:
-
•
Define chronic post-surgical pain (CPSP) according to the ICD-11 (International Classification of Diseases, Eleventh Revision) classification and describe its impact on surgical patients.
-
•
Specify typical risk factors for the development of CPSP to identify patients at risk and initiate adequate preventative strategies.
-
•
Discuss possible treatment strategies and anaesthetic techniques to reduce the risk of CPSP.
Key points.
-
•
CPSP is one of the most common complications of surgery.
-
•
Risk factors for CPSP occur before, during and after surgery.
-
•
Anaesthetic techniques, particularly the reduction of opioid use and regional anaesthesia may reduce the incidence of CPSP.
Chronic post-surgical pain (CPSP) is one of the most frequent complications after surgery, with an important negative impact on patients' quality of life that constitutes significant economic and healthcare burdens. The median incidence of chronic pain at 6–12 months after surgery is 20–30% with a slight decrease over time.1,2 The incidence can differ depending on the type of surgery. However, the wide variability in the incidence (5–85%) is largely attributable to methodological differences caused by different methods of data collection and variable definitions of CPSP (Table 1).3 Chronic post-surgical pain is likely to remain a challenge with the increasing number of surgeries worldwide. Obesity, inflammatory disease and increased life expectancy have resulted in an increasing volume of surgeries such as hip and knee arthroplasty that are associated with a high risk of CPSP. This article summarises the current knowledge of the risk factors for CPSP in adults and preventative strategies. CPSP in children is described in other reviews (see Supplementary material).
Table 1.
Incidences of CPSP for different types of surgery. Data adapted from several studies.1,3,5,6,12 Severe CPSP is defined as pain ratings of ≥5 on a scale from 0 (no pain) to 10 (worst possible pain).1,11 CPSP, chronic post-surgical pain; NP, neuropathic pain.
| Type of surgery | Incidence of all CPSP (%) | Incidence of severe CPSP (>5/10) | Chronic pain up to 12 months | Proportion of NP |
|---|---|---|---|---|
| Abdominal surgery (bowel and colorectal) | 17–21 | Not reported | Not reported | Not reported |
| Amputation | 30–85 | 5–10% | 75% (lower limbs) | 80% |
| Caesarean section | 6–55 | 5–10% | Not reported | 50% |
| Cholecystectomy | 3–56 | Not reported | Not reported | Not reported |
| Craniotomy | 7–65 | 25% | Not reported | Not reported |
| Dental surgery | 5–13 | Not reported | Not reported | Not reported |
| Hip arthroplasty | 7–23 | 6% | 28% | 1–2% |
| Inguinal herniotomy | 5–63 | 2–4% | 30% | 80% |
| Knee arthroplasty | 13–44 | 15% | 18% | 6% |
| Mastectomy | 11–57 | 5–10% | 43–56% (breast cancer surgery) | 65% |
| Sternotomy | 7–50 | 5–10% | 27% | 13% |
| Thoracotomy | 5–71 | 10% | 41% | 45% |
| Vasectomy | 0–37 | Not reported | Not reported | Not reported |
Definition of CPSP
The definition of CPSP (i.e. chronic postsurgical or post-traumatic pain) was standardised in 2019 after the inclusion in the new International Classification of Diseases, Eleventh Revision (ICD-11)1:
-
-
Pain develops or increases in intensity after a surgical procedure or a tissue injury.
-
-
Pain persists beyond the healing process, that is ≥3 months after the triggering event.
-
-
Localisation: either at the surgical/area of injury, or projected onto the innervation area of a nerve in this area, or related to a dermatome or Head's zone (after surgery or injury to deep somatic and visceral tissue).
-
-
Other causes of pain (e.g. pre-existing pain conditions, infection, malignancy) are excluded.
-
-
Chronic post-surgical pain can often show characteristics of neuropathic pain.
-
-
It is distinguished between tissue trauma arising from a controlled procedure in the delivery of healthcare (surgery) and forms of uncontrolled accidental damage (other traumas).
The inclusion of CPSP in the ICD-11 is a major step forward that will allow future studies to report the incidence of CPSP more accurately. Furthermore, the inclusion in the ICD-11 raises awareness of the condition as a disease rather than merely a symptom, and the need for specific treatment and management.1
Mechanisms and characteristics
Chronification of pain is complex and the underlying patho-physiology includes peripheral (at the site of injury) as well as central (spinal and supraspinal) sensitisation. The inflammatory and immune response to axonal and tissue damage, including the release of neurotransmitters peripherally as well as in the spinal cord, leads to (micro)glial activation, ectopic neural activity and altered activity in the dorsal horn. There are also changes in the supraspinal processing including brain network connectivity and changes in endogenous, descending pain modulation. The molecular mechanisms of CPSP have been described in several reviews.4, 5, 6, 7
As a consequence of the central sensitisation processes, patients developing CPSP often report typical clinical symptoms such as hyperalgesia (increased painful sensation caused by a noxious stimulus), allodynia (painful sensation caused by a usually non-painful stimulus), dysaesthesia (unpleasant touch perception or tingling) indicating nerve damage and central sensitisation very early after surgery.8 Some of these changes can be assessed and tested, for example by using quantitative sensory testing (QST) early after surgery. Hyperalgesia detected early after surgery might predict prolonged, chronic neuropathic pain after surgery.9 Although 35–57% of patients with CPSP show signs of neuropathic pain, the incidence for different surgeries varies widely and is highest after thoracotomy, mastectomy and amputation. This indicates procedure-specific aspects such as injury to the subcostal nerves during thoracic surgery and injury to the brachial nerve/axillary plexus during mastectomy (Table 1).1 Patients with neuropathic CPSP report more intense pain, greater limitations in activities of daily life and reduced quality of life.1,10 Other mechanisms such as visceral, inflammatory and neuroplastic pain might play a role in procedures such as knee surgery, hip surgery and abdominal surgery that are less likely to be associated with neuropathic CPSP.10,11
Risk factors
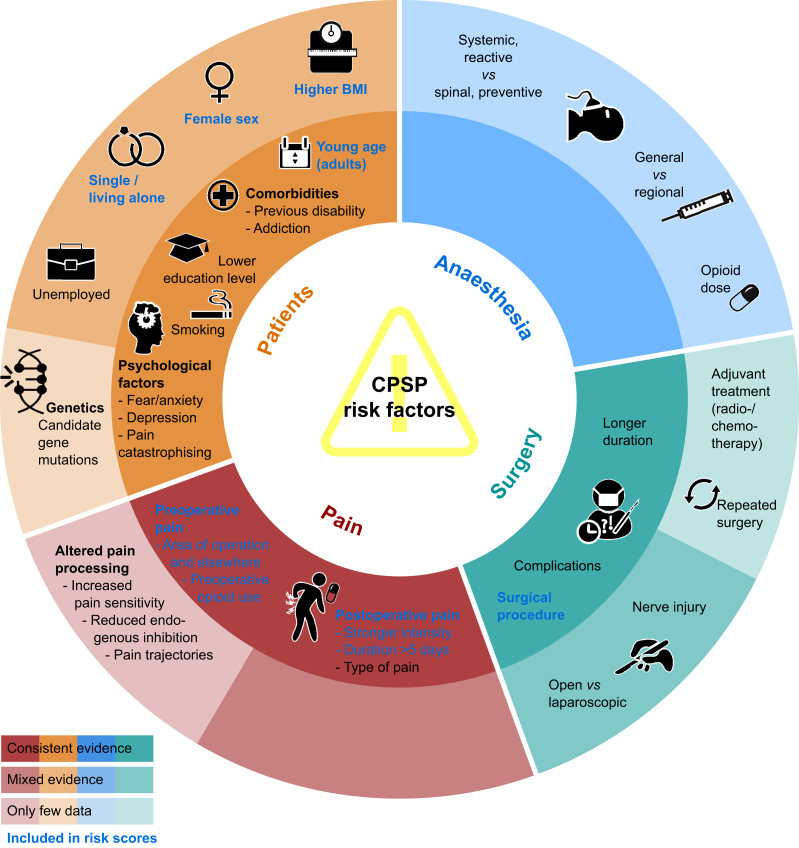
The early identification of risk factors can allow risk stratification and the implementation of preventative treatment strategies. Several factors from the periods before, during and after surgery have been identified across different studies, but the evidence is not conclusive (Fig. 1). Some of the recent studies on predictive models for CPSP are summarised in Table 2 and in more detail in Supplementary Table S1.
Fig 1.
Risk factors for CPSP. Proposed risk factors for development of CPSP and grade of consistency of evidence. Figure adapted from data of Glare and colleagues,5 Lavand'homme11 and Steyaert and Lavand'homme.12 Dark colours relate to consistent evidence from studies, light colours refer to mixed or low evidence (see colour legend). Text in blue refers to risk factors included in risk scores (Table 2). CPSP, chronic post-surgical pain.
Table 2.
Predictive models developed by using different risk factors. Description of the validation studies of two proposed risk scores for chronic post-surgical pain (CPSP) in different surgical patient populations. Discriminative value is given by the area under the receiver operating characteristic (ROC-AUC) according to the original publications, and, if applicable, sensitivity and specificity of the risk scores. For a detailed version of this table, including the development studies and additional risk scores, see Supplementary Table S1. SF-12: Short Form Health Survey-12.
| Study | Type of Surgery | Study type/n | Predictive values/risk factors | Characteristics, quality, comments |
|---|---|---|---|---|
| Meretoja and colleagues 201720 | Breast cancer surgery | Multicentre, n=231+453 |
Four items
|
Performed well in the Danish (ROC-AUC=0.739) and Scottish (ROC-AUC=0.740) cohorts Online tool for risk score, easily applicable Includes postoperative pain, hence preoperative screening is not possible |
| Montes and colleagues 202022 | Four surgical procedures (inguinal hernia repair, hysterectomy (vaginal or abdominal), thoracotomy) | Multicentre, n=1088 (validation)22 |
Six items
|
Sensitivity of 58.9%, specificity of 68.4%; positive predictive value 32.6%; negative predictive value 86.5%; ROC-AUC=0.694 Only preoperative factors are involved, hence preoperative screening is possible. |
Surgical risk factors
The type of surgery is predictive of CPSP. Although the extent of the surgical trauma and duration of surgery are predictive factors, the type of tissue injured is also relevant. This could explain why minimally invasive surgery does not always reduce the risk of CPSP. For example a laparoscopic approach can reduce the risk in certain types of surgery, such as hernia surgery and cholecystectomy, but not after gastrointestinal surgery and nephrectomy.12 Postoperative complications, reoperation, infection or adjuvant therapies, such as radio- or chemotherapy, are additional surgery- and disease-related risk factors for CPSP (Fig. 1).
Acute postoperative pain
The severity of acute postoperative pain is a consistent predictor of CPSP. Although the intensity of acute pain, particularly movement evoked or worst pain intensity, is an important risk factor for CPSP, some studies suggest that the duration of intense pain and pain trajectories are equally important.2,11,12,13 Postoperative pain of neuropathic-like symptoms within the first days after surgery is strongly associated with neuropathic CPSP; screening by using QST (to assess hyperalgesia) or the Douleur Neuropathique 4 (DN4) questionnaire might help to predict neuropathic CPSP.11,14 Acute visceral pain is more predictive than incisional pain after abdominal surgery.9,15
Preoperative chronic pain
Patients with preoperative chronic pain, particularly at the surgical site, are at increased risk of developing CPSP (Fig. 1).5,11 Pre-existing chronic painful conditions (e.g. fibromyalgia, migraine, low back pain) increase the risk of CPSP.5,11 It is likely that coexisting psychosocial risk factors, comorbidities and long-term use of opioids, contribute to the high incidence of CPSP in these patients.5,6
Psychosocial risk factors
Psychological risk factors, such as psychological distress, anxiety, catastrophising, reduced ability to cope with pain, depression and hypervigilance, increase the risk of CPSP (Fig. 1) and long-term opioid use.7,16 A recent meta-analysis highlights state anxiety as the main psychological risk factor for CPSP (and to a lesser degree depression, catastrophising, kinesiophobia and impaired self-efficacy).17
Patient-related risk factors
Young (adult) age, female sex and high BMI are associated with increased risk. Similarly, education status and socioeconomic factors have been identified as risk factors in some studies (Table 2). Although some of these factors are not consistently reported between studies, their association with acute as well as chronic postoperative pain is evident.5,11,18 A risk score for prediction of acute postoperative pain based on these factors has been developed.18
While several single gene candidate mutations related to increased pain sensitivity have been identified as possible risk factors, their predictive value is poor and more data are needed. 5,6,11
Risk factors for CPSP are not independent of each other: for example preoperative pain is more common in female patients, and psychological disorders are related to a higher sensitivity to experimental pain as well as to preoperative chronic pain.3,5,17 This may partly explain why the association of risk factors with CPSP are not always consistent between studies.5 Future studies need to investigate risk factors by using the same definition for CPSP, using adequate sample size and include validated patient-reported outcome measures (PROMs). Finally, risk factors involved in the transition to chronic pain probably differ from those involved in the maintenance of CPSP.5,6
Prediction of CPSP
Based on the risk factors discussed above, risk models/ tools have been developed to predict the risk for individual patients (Table 2 and Supplementary Table S1).3,19,20,21,22 However, most of them have major limitations, such as small sample size, limited surgical procedures or lack of validation in a separate cohort. One of the most promising attempts so far was an investigation of more than 30 potential risk factors in four different surgical procedures.21 This prospective multicentre study identified six independent factors: type of surgical procedure, age, physical and mental health (assessed using SF-12), preoperative pain in the surgical field or elsewhere that were included in a risk model.21 The risk score model was later validated in additional cohorts and showed a moderate but consistent predictive value with a sensitivity of 58.9% and specificity of 68.4% (Table 2).22 Future studies should investigate the applicability of this score in other surgical procedures, increase predictive values by incorporating catastrophising in the model and replace the SF-12 with a similar but free version for general applicability in clinical settings, for example the Patient Health Questionnaire and General Anxiety Disorder questionnaire (PHQ-9 and GAD-7) for routine measurement of anxiety and depression.
Identification of subgroups of patients that might benefit from preventative interventions is an important step towards individualised prevention of CPSP. One such approach is prediction of acute or chronic postsurgical pain based on the individual somatosensory profile of patients before surgery by using QST.23,24 Quantitative sensory testing can assess the peripheral and central function of the nervous system including central sensitisation processes. Identification of increased sensitisation before surgery by QST can predict an increased risk of CPSP. In addition, assessment of the descending endogenous inhibition (a mechanism relevant for the inhibition of pain) might be another option; here, a conditioned pain paradigm is used that has been shown to correlate negatively with CPSP.23,24 Although there are promising initial results, these are inconsistent and currently not applicable to the clinical setting.25
Prevention of CPSP
Numerous pharmacological studies studies have investigated the effects of preventive strategies on the development of CPSP. The results are disappointing as reflected in the latest meta-analysis.26 Methodological deficiencies in most RCTs might be part of the explanation. More conclusive results might be achieved by including only patients at risk, longer (or more individualised) durations of treatment and standardised assessment of endpoints as well as improved quality of reporting. A Cochrane analysis investigating the effect of regional anaesthesia techniques on CPSP indicates more promising effects but is limited by the same methodological deficiencies.26
Pharmacological interventions
Ketamine
Perioperative ketamine may reduce opioid consumption and acute postoperative pain; it is one of the most promising drugs available for reducing the prevalence of CPSP.26 Interestingly, this effect might be more apparent in patients receiving opioids before surgery. Therefore, ketamine can be considered especially for perioperative use in patients on high-dose opioids.27,28 However, the recent updated systematic review on drug treatments for the prevention of CPSP, including 2,757 patients in 27 studies, found no certain effect of ketamine on the prevalence of CPSP after 3, 6 and 12 months.26 The data available include small study sizes; variations in dosage, timing and duration of treatment; lack of stratification of patients and type of surgeries; and variations in outcome measures. Therefore, the effects of ketamine on CPSP are currently inconclusive.
Gabapentinoids
A recent meta-analysis showed that perioperative use of gabapentinoids does not reduce the incidence of CPSP.26 However, as gabapentinoids can reduce neuropathic pain, they may be appropriate to use in certain patients undergoing procedures that can result in nerve damage, such as complex spinal surgery. Recently, a treatment effect of pregabalin on CPSP after 3 months was described in patients who underwent cardiac surgery or total knee arthroplasty.26 However, more and better designed large studies are needed to make a firm conclusion.
I.V. lidocaine
Few studies have evaluated the impact of i.v. lidocaine on CPSP. Lidocaine i.v. for 24 h or less potentially helps to reduce CPSP in patients undergoing breast surgery.26,29 However, no clear recommendation can be made, possible beneficial effects have to be confirmed, and an adequate dose and duration must be found.29
Other drugs
Duloxetine, a serotonin–noradrenaline reuptake inhibitor, improved a reduced CPM in patients with diabetic polyneuropathy, indicating an impact on descending pain modulation.23 In patients with an enhanced pain sensitivity, it could reduce postoperative pain 12 weeks after surgery.30 The effects of drugs such as clonidine, dexmedetomidine and nefopam have not yet been studied adequately.26 However, no recommendation for the prevention of CPCP for any of these drugs is currently possible.
Regional anaesthesia
Perioperative regional anaesthesia has been shown to reduce the risk of CPSP in most types of surgery studied so far.31 By blocking nociceptive input to the central nervous system, central sensitisation processes can be reduced if not prevented. Epidural analgesia reduces the incidence of CPSP in patients undergoing thoracotomy.31 Paravertebral blocks can reduce CPSP after thoracotomy and possibly have an impact on development of neuropathic CPSP in breast cancer surgery.31 Continuous wound infiltration may reduce chronic pain after Caesarean section and harvesting of bone grafts from the iliac crest. However, the level of evidence is low. In most studies, only single-shot procedures are evaluated, although longer treatment duration might increase the effectiveness.25
Reduction of opioid use
Preoperative opioid consumption including chronic opioid use is a risk factor for persistent postoperative pain.16,32 Potential mechanisms include induction of opioid tolerance, changes in the central reward system, facilitated central sensitisation and altered descending inhibition.6 Activation of N-methyl-d-aspartate (NMDA) receptors and (micro-)glia are some of the molecular mechanisms and might explain the effect of ketamine in patients with preoperative chronic pain. A reduction of preoperative opioid consumption by more than 50% has been shown to improve outcomes 6–12 months after knee surgery.12 There are some indications for high intraoperative remifentanil dosage being associated with a higher incidence of CPSP; however, compared with sevoflurane-based anaesthesia, a TIVA with propofol and lower dosed remifentanil led to lower incidences of CPSP.33 Reducing the use of opioids by using multimodal analgesia, regional analgesia techniques or by using ketamine could potentially reduce the incidence of CPSP.8 Also, the implementation of nociception monitors, such as the pupil diameter or indices including, for example the heart rate variability or the pulse wave amplitude, such as the surgical plethysmographic index, the analgesia nociception index and the nociception level index, may help to reduce the intraoperative exposure to opioid, although more evidence is needed.34
Non-pharmacological intervention and transitional pain services
Chronification of pain is not only a biological but also a highly individual process involving psychosocial and temporal aspects. Considering the complex underlying mechanisms leading to chronification of pain, a focus solely on the perioperative phase might be too narrow.12 A multidisciplinary approach, including non-pharmaceutical therapies such as physiotherapy and psychological support might be far more effective.35 An interdisciplinary team follows up the patient at risk not only perioperatively but also for several weeks after surgery. These ‘transitional pain services’ aim to close the gap between acute postoperative pain management and pain management after discharge from hospital. Initial observational studies of this approach showed reduced long-term analgesic and opioid use.5 However, there is currently little evidence to support specific multidisciplinary approaches, and there are no RCTs showing their efficacy. Thus, to implement such approaches in practice, further evidence is needed.
Conclusions
CPSP is an important clinical and socioeconomic challenge that is now included in the new ICD-11. Procedure-specific incidence rates and surgery, as well as patient-related risk factors, have been identified, and some prediction models have been developed. The evidence for most pharmacological and non-pharmacological interventions is limited. However, it makes intuitive sense to reduce the intensity of acute pain by adopting multimodal strategies and regional techniques and limiting the use of pre- and perioperative opioids. Multidisciplinary concepts targeting the biopsychosocial aspects of the pain chronification process are promising but warrant further evaluation before integrating them routinely into clinical practise.
Declaration of interests
DCR has no conflicts of interest. EPZ has received financial support to her institution from Mundipharma and Grunenthal for research activities and advisory and lecture fees from Grünenthal, Mundipharma and Novartis.
MCQs
The associated MCQs (to support CME/CPD activity) will be accessible at www.bjaed.org/cme/home by subscribers to BJA Education.
Biographies
Daniela C. Rosenberger MSc is an assistant physician in physiology and anaesthesiology and research associate in Professor Pogatzki-Zahn's laboratory.
EstherM.Pogatzki-Zahn is an anaesthesiologist, pain specialist and professor in the Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Muenster (UKM). She leads the pain service at UKM, lectures on pain and is principal investigator of the Translational Pain Research Group at the University of Muenster, which focuses on peripheral and central mechanisms relevant to postoperative, neuropathic and cancer pain. She is currently council member of the International Association for the Study of Pain (IASP) and secretary of the German Pain Society (chapter of the IASP).
Matrix codes: 1A02, 2E03, 3E00
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjae.2021.11.008.
Supplementary Material
The following are the Supplementary data to this article:
References
- 1.Schug S.A., Lavand’homme P., Barke A., Korwisi B., Rief W., Treede R.-D. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160:45–52. doi: 10.1097/j.pain.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher D., Stamer U.M., Pogatzki-Zahn E., et al. Chronic postsurgical pain in Europe: an observational study. Eur J Anaesthesiol. 2015;32:725–734. doi: 10.1097/EJA.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 3.Schug S.A., Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2:e627. doi: 10.1097/PR9.0000000000000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pogatzki-Zahn E., Segelcke D., Zahn P. Mechanisms of acute and chronic pain after surgery: update from findings in experimental animal models. Curr Opin Anaesthesiol. 2018;31:575–585. doi: 10.1097/ACO.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 5.Glare P., Aubrey K.R., Myles P.S. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–1546. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 6.Richebé P., Capdevila X., Rivat C. Persistent postsurgical pain: pathophysiology and preventative pharmacologic considerations. Anesthesiology. 2018;129:590–607. doi: 10.1097/ALN.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 7.Chapman C.R., Vierck C.J. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017;18:359.e1–359.e38. doi: 10.1016/j.jpain.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Pogatzki-Zahn E.M., Segelcke D., Schug S.A. Postoperative pain—from mechanisms to treatment. Pain Rep. 2017;2:e588. doi: 10.1097/PR9.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez V., Ammar S.B., Judet T., Bouhassira D., Chauvin M., Fletcher D. Risk factors predictive of chronic postsurgical neuropathic pain: the value of the iliac crest bone harvest model. Pain. 2012;153:1478–1483. doi: 10.1016/j.pain.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Haroutiunian S., Nikolajsen L., Finnerup N.B., Jensen T.S. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154:95–102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Lavand’homme P. Transition from acute to chronic pain after surgery. Pain. 2017;158:S50–S54. doi: 10.1097/j.pain.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 12.Steyaert A., Lavand’homme P. Prevention and treatment of chronic postsurgical pain: a narrative review. Drugs. 2018;78:339–354. doi: 10.1007/s40265-018-0866-x. [DOI] [PubMed] [Google Scholar]
- 13.Lavand’homme P.M., Grosu I., France M.-N., Thienpont E. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: an observational study. Clin Orthop Relat Res. 2014;472:1409–1415. doi: 10.1007/s11999-013-3389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beloeil H., Sion B., Rousseau C., et al. Early postoperative neuropathic pain assessed by the DN4 score predicts an increased risk of persistent postsurgical neuropathic pain. Eur J Anaesthesiol. 2017;34:652–657. doi: 10.1097/EJA.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 15.Blichfeldt-Eckhardt M.R., Ording H., Andersen C., Licht P.B., Toft P. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155:2400–2407. doi: 10.1016/j.pain.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Pagé M.G., Kudrina I., Zomahoun H.T.V., et al. A systematic review of the relative frequency and risk factors for prolonged opioid prescription following surgery and trauma among adults. Ann Surg. 2020;271:845–854. doi: 10.1097/SLA.0000000000003403. [DOI] [PubMed] [Google Scholar]
- 17.Giusti E.M., Lacerenza M., Manzoni G.M., Castelnuovo G. Psychological and psychosocial predictors of chronic postsurgical pain: a systematic review and meta-analysis. Pain. 2021;162:10–30. doi: 10.1097/j.pain.0000000000001999. [DOI] [PubMed] [Google Scholar]
- 18.Schnabel A., Yahiaoui-Doktor M., Meissner W., Zahn P.K., Pogatzki-Zahn E.M. Predicting poor postoperative acute pain outcome in adults: an international, multicentre database analysis of risk factors in 50,005 patients. Pain Rep. 2020;5:e831. doi: 10.1097/PR9.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papadomanolakis-Pakis N., Uhrbrand P., Haroutounian S., Nikolajsen L. Prognostic risk prediction models for chronic postsurgical pain in adults. Pain. 2021;162:2644–2657. doi: 10.1097/j.pain.0000000000002261. [DOI] [PubMed] [Google Scholar]
- 20.Meretoja T.J., Andersen K.G., Bruce J., et al. Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. J Clin Oncol. 2017;35:1660–1667. doi: 10.1200/JCO.2016.70.3413. [DOI] [PubMed] [Google Scholar]
- 21.Montes A., Roca G., Sabate S., et al. Genetic and clinical factors associated with chronic postsurgical pain after hernia repair, hysterectomy, and thoracotomy: a two-year multicenter cohort study. Anesthesiology. 2015;122:1123–1141. doi: 10.1097/ALN.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 22.Montes A., Roca G., Cantillo J., Sabate S. Presurgical risk model for chronic postsurgical pain based on 6 clinical predictors: a prospective external validation. Pain. 2020;161:2611–2618. doi: 10.1097/j.pain.0000000000001945. [DOI] [PubMed] [Google Scholar]
- 23.Yarnitsky D., Granot M., Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155:663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Ruscheweyh R., Viehoff A., Tio J., Pogatzki-Zahn E.M. Psychophysical and psychological predictors of acute pain after breast surgery differ in patients with and without pre-existing chronic pain. Pain. 2017;158:1030–1038. doi: 10.1097/j.pain.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 25.Sangesland A., Støren C., Vaegter H.B. Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand J Pain. 2017;15:44–52. doi: 10.1016/j.sjpain.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Carley M.E., Chaparro L.E., Choinière M., et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults: an updated systematic review and meta-analysis. Anesthesiology. 2021;135:304–325. doi: 10.1097/ALN.0000000000003837. [DOI] [PubMed] [Google Scholar]
- 27.Loftus R.W., Yeager M.P., Clark J.A., et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–646. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 28.Schwenk E.S., Viscusi E.R., Buvanendran A., et al. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American society of regional anesthesia and pain medicine, the American academy of pain medicine, and the American society of anesthesiologists. Reg Anesth Pain Med. 2018;43:456–466. doi: 10.1097/AAP.0000000000000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weibel S., Jelting Y., Pace N.L., et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. doi: 10.1002/14651858.CD009642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh I.J., Kim M.S., Sohn S., Song K.Y., Choi N.Y., In Y. Duloxetine reduces pain and improves quality of recovery following total knee arthroplasty in centrally sensitized patients: a prospective, randomized controlled study. J Bone Jt Surg Am. 2019;101:64–73. doi: 10.2106/JBJS.18.00347. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein E.J., Levene J.L., Cohen M.S., et al. Local anaesthetics and regional anaesthesia versus conventional analgesia for preventing persistent postoperative pain in adults and children. Cochrane Database Syst Rev. 2018;4:CD007105. doi: 10.1002/14651858.CD007105.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown C.R., Chen Z., Khurshan F., Groeneveld P.W., Desai N.D. Development of persistent opioid use after cardiac surgery. JAMA Cardiol. 2020;5:889–896. doi: 10.1001/jamacardio.2020.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gulik L., Ahlers S.J.G.M., van de Garde E.M.W., et al. Remifentanil during cardiac surgery is associated with chronic thoracic pain 1 yr after sternotomy. Br J Anaesth. 2012;109:616–622. doi: 10.1093/bja/aes247. [DOI] [PubMed] [Google Scholar]
- 34.Meijer F.S., Niesters M., van Velzen M., et al. Does nociception monitor-guided anesthesia affect opioid consumption? A systematic review of randomized controlled trials. J Clin Monit Comput. 2020;34:629–641. doi: 10.1007/s10877-019-00362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinrib A.Z., Azam M.A., Birnie K.A., Burns L.C., Clarke H., Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 2017;11:169–177. doi: 10.1177/2049463717720636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.