Abstract
Inflammation is a central process in most benign bladder disorders, and its control is a delicate balance between initiating factors and resolving factors. While recent discoveries have shown a central role for the NLRP3 inflammasome in initiation, the resolving pathways remain unexplored. Resolution is controlled by specialized pro-resolution mediators (SPMs) functioning through seven receptors (six in rodents). Here we demonstrate expression of all seven in humans (six in mice) through immunocytochemistry. Expression was universal in urothelia with most also expressed in smooth muscle. We next explored the therapeutic potential of three SPMs; Resolvin E1 (RvE1), Maresin 1 (MaR1), and Protectin D1 (PD1). SPMs promote epithelial wound/barrier repair and RvE1 triggered dose-dependent wound closure in urothelia in vitro (scratch assay) (EC90 = 12.5 nM). MaR1 and PD1 were equally effective at this concentration. In vivo analyses employed a cyclophosphamide (CP) model of bladder inflammation (Day 0-CP [150 mg/kg], Day 1 to 3 SPM [25 µg/kg/day], Day 4 – analysis). All three SPMs reduced bladder inflammation (Evans blue) and bladder weights to control levels. Effects of RvE1 were also examined by urodynamics. CP decreased void volume, increased frequency and decreased bladder capacity while RvE1 restored values to control levels. Finally, SPMs reduce fibrosis and RvE1 reduced urothelial expression of TGF-β and collagen I to control values. Together these results expand the known SPMs active in the bladder tissue and provide promising therapeutic targets for controlling inflammation in a wide variety of inflammation-associated benign bladder diseases.
Keywords: Cystitis, pro-resolution, inflammation
Impact Statement
Many benign bladder disorders are precipitated or exacerbated by inflammation and pathways that resolve inflammation hold great promise for treatment. Here we demonstrate expression of pro-resolution receptors in the human and mouse bladder and the therapeutic potential of three separate specialized pro-resolution mediators (SPMs) in promoting urothelial repair in vitro and reducing bladder inflammation using the mouse cyclophosphamide model. Exploring Resolvin E1 further we show its efficacy in restoring normal bladder functions and suppressing fibrotic gene expression. This study demonstrates that SPMs represent a large, important, and widely unstudied field in bladder physiology that provides very promising therapeutic targets important in many disparate bladder inflammation disorders. This work has the potential to bring real relief to a wide variety of patients in the future.
Introduction
Bladder inflammation is a precipitating or exacerbating factor in many benign urological diseases including bladder outlet obstruction (BOO), 1 diabetic bladder dysfunction,2–4 urinary tract infections,5–7 interstitial cystitis/bladder pain syndrome, 8 and even aging. 9 Thus, understanding the common underlying pathways that control inflammation in the bladder should identify molecular targets that could be modulated to reduce the severity and/or morbidity of many, if not all, of the diverse bladder inflammatory disorders.
Much work has been focused on the pathways that initiate inflammation with the concept that blocking these pathways can prevent inflammation and reduce or eliminate many of the negative consequences. Less than 20 years ago (in 2002), there was a significant breakthrough in the understanding of how inflammation was triggered with the discovery of the inflammasome. 10 Inflammasomes recognize molecules released from damaged or dying cells (called Damage Associated Molecular Patterns or DAMPs) or components of pathogens (Pathogen Associated Molecular Patterns or PAMPs) (for review of inflammasomes see)11–15. These patterns trigger oligomerization of the inflammasome and ultimately activation of caspase-1. Caspase-1, in turn, cleaves pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) into their active forms which are released to act as pro-inflammatory cytokines that precipitate the wider inflammatory process. While vigorous release of DAMPs, or significant infection with PAMPs, can elicit the cardinal signs of inflammation (redness [rubor], swelling [tumor], heat [calor], and pain [dolor]), less aggressive but repetitive or chronic stimuli will elicit low-level inflammation, referred to as meta-inflammation.16–20 It seems logical that vigorous exposure may precipitate acute inflammatory bladder disorders such as urinary tract infections while meta-inflammation would be a critical component of the chronic inflammation-based bladder disorders, such as BOO (typically brought on by years of progressively worsening benign prostatic hyperplasia), diabetic bladder dysfunction, and aging. Recently, our laboratory and others have shown a critical role for the NLRP3 inflammasome in acute stimuli such as chemotherapeutic cystitis 21 (caused by cyclophosphamide [CP] which is the model employed in this study) and urinary tract infections5–7 as well as in the more chronic inflammatory bladder diseases such as BOO, 1 diabetic bladder dysfunction, 4 interstitial cystitis/chronic bladder pain syndrome, 8 and aging. 9 In these studies, pharmacological inhibitors or genetic deletion of NLRP3 showed the utility of targeting this complex in preventing bladder inflammation and the negative consequences of these conditions. However, there are two major problems with this approach that makes it less desirable in all circumstances. First, these studies looked at preventing inflammation, but patients often present to their physician only after symptoms are well in place and inflammation is firmly established. This is particularly true with the low-levels insults such as BOO (due to BPH) and diabetic bladder dysfunction. Second, inhibiting the initiation of inflammation runs the risk of creating immunocompromised individuals. 22
An alternative to targeting inflammation initiation that also circumvents these concerns is to target the resolution of the response. While initially it was thought that simply reducing the initiation stimulus would result in reduction of inflammation, it has been recognized for some time that resolving inflammation is driven by its own set of mediators and pathways. In fact, the first report of pro-resolving mediators (known collectively today as Specialized Pro-resolving Mediators or SPMs) was published in 1984,23,24 nearly 20 years prior to the discovery of inflammasomes. 10 Since their initial discovery, the number of SPMs has expanded to include at least 26 mediators in five main classes.25–27 One of these classes (Annexin-A1) is composed of a single polypeptide while the other four (lipoxins, resolvins, maresins, and protectins) are composed of lipids synthesized from polyunsaturated fatty acids. Despite the large numbers of SPMs, the actions of all the SPMs are thought to be mediated by only seven independent receptors (CHEMR23, BLT-1, LGR6, GPR37, GPR18, FPR2, and GPR32).28,29 Only six are expressed in rodents for there is no GPR32 homolog. 30 Among these receptors, there appears to be significant ligand poly-pharmacology (single SPMs binding to more than one receptor) and receptor pleiotropy (single receptor activated by multiple ligands).28,29
Given the time that has elapsed since the discovery of SPMs and the importance of inflammation in so many bladder diseases, it is surprising that no studies have examined their therapeutic potential in the bladder. Previously, Monastyrskaya et al. examined the expression of many annexin family members, including Annexin-A1, in the human bladder. 31 In that study, they found urothelial expression of Annexin-A1 and noted that it was reduced in interstitial cystitis/bladder pain syndrome patients. In an additional study, a microarray analysis detected an increase in LGR6 in a rat model of BOO but with no context of possible function. 32 Recently, we expounded on a possible role for Annexin-A1 in treating BOO using a rat model. 33 In those studies, we demonstrated the presence of all six SPM receptors in the rat bladder. Expression was predominantly urothelial with some expression in the detrusor and some changes in expression pattern in response to BOO. Importantly, treatment in vivo with a peptide mimetic of Annexin-A1 (Ac2-26) diminished BOO-induced inflammation and normalized bladder dysfunction. This mimetic even promoted faster and more complete functional recovery after surgical de-obstruction. Thus, we have shown the likely presence of multiple resolution pathways in the bladder and the therapeutic possibility of manipulating even a single one.
In this study, we seek to expand this information to look at the therapeutic potential of additional SPMs in treating other inflammation-related bladder diseases using mouse models. We first survey expression of the various SPM receptors in the mouse bladder to compare and contrast to the rat. We also survey expression in human bladder to provide context as to how the mouse and rat data may transfer to humans. We then examine Resolvin E1 (RvE1), Maresin 1 (MaR1), and Protectin D1 (PD1) as representative of three additional classes of SPM. We begin by examining their in vitro ability to promote wound healing in urothelia (promoting wound healing and barrier repair is an essential characteristic of SPMs34,35). We next assessed their ability to speed the resolution of inflammation in vivo in response to CP. CP is a chemotherapeutic drug that is broken down to acrolein (among other metabolites) which damages the urothelia during the storage phase, releasing DAMPs and triggering a massive inflammatory response. CP-induced hemorrhagic cystitis was once a very significant clinical problem, although modern use of 2-mercaptoethanesulfonate sodium (Mesna) to bind acrolein in the urine and mask its harmful effects has dramatically reduced the clinical occurrence. Despite this, CP-induced cystitis remains a very useful experimental model for bladder inflammation because of its speed and reproducibility and important findings with this model are often confirmed and expanded upon in more chronic and clinically relevant models. For example, studies of NLRP3 in CP-induced cystitis 21 led to an understanding of its role in BOO, 1 diabetes, 4 urinary tract infections,5–7 interstitial cystitis/chronic bladder pain syndrome, 8 aging, 9 and even mood disorders associated with lower urinary tract symptoms. 36
Injection of 150 mg/kg CP elicits advanced inflammatory characteristics in the bladder within 24 h and this inflammation persists for >one week. In these studies, we administered CP and 24 h later began daily administration of SPM for three days, analyzing endpoints 24 h after the last injection of SPM. This approach ensures inflammation has fully developed in the bladder before SPM exposure and thus assesses the ability of the SPM to promote resolution of inflammation (as opposed to blocking the initiation of inflammation). After assessing the activity of RvE1, MaR1, and PD1 on resolving inflammation, we examined the ability of one representative SPM, RvE1, to reverse the effect of CP on bladder function by urodynamics. Finally, we assessed the potential of RvE1 to reverse the CP-induced fibrotic response by examining changes in mRNA expression for TGF-β and collagen-1.
Materials and methods
Animals and treatment paradigm
The Institutional Animal Care and Use Committee at Duke University Medical Center approved protocols prior to beginning this work. All protocols strictly adhered to the NIH Guide for the Care and Use of Laboratory Animals. Mice (C57/BL6, female) were purchased from Envigo Inc. (Cumberland, VA) at seven to eight weeks of age and used within two to three weeks of arrival. Mice were housed in a colony room approved by the Association for Assessment and Accreditation of Laboratory Animal Care. They were given ad libitum access to food and water while being maintained at a constant temperature and humidity on a 12:12 h light–dark cycle.
Immunohistochemistry
For mice, following sacrifice by approved methods, bladders were fixed in 10% neutral buffered formalin (o/n, 4°C). They were then transferred to 70% ethanol and maintained at 4°C until processed by the Department of Surgery’s histological core facility. There they were paraffin embedded and transverse sectioned (5 µm). Sections from the caudal third of the bladder were subjected to immunohistochemistry using standard techniques. For human samples, formalin-fixed, paraffin-embedded sections (5 µm) were purchased from Zyagen Inc.(San Diego, CA) and used in an identical manner. Table 1 lists the primary antibodies and dilutions used, while the Vectastain ABC peroxidase staining kit (Vector Laboratories, Burlingame, CA, cat# PK-4000) was used for development with the antirabbit secondary antibody provided in that kit. Micrographs were captured on a Zeiss Axio Imager Microscope. All staining was repeated a minimum of three times to insure consistency.
Table 1.
Antibodies Used in This Study.
| Antigen | Host | Company | Catalog number | References |
|---|---|---|---|---|
| LgG isotype control | Rabbit | Novus Biologicals | NBP2-36463 | |
| FPR-2 | Rabbit | Novus Biologicals | NLS1878 | 37–39 |
| BLT-1 | Rabbit | LifeSpan Biosciences | LS-A1494 | |
| CHEM23 | Rabbit | LifeSpan Biosciences | LS-B12924 | |
| GPR37 | Rabbit | Abcam | Ab21834 | |
| LGR6 | Rabbit | Abcam | Ab126747 | 40–42 |
| GPR18 | Rabbit | Abcam | Ab150618 | 43 |
| Annexin-A1 | Rabbit | LifeSpan Biosciences | LS-B6711 | 44 |
Urothelial wound healing assay (scratch assay)
Urothelial cells were isolated as previously described.4,45 Briefly, following harvest bladders were submerged in phosphate-buffered saline (PBS) then inverted over the tip of an 18-guage blunt-tip needle. A purse string suture was used to close the bladder around the needle. The bladder was then inflated with PBS and the purse string pulled tight while sliding the bladder off the needle. The inflated bladders were then placed in 5 mL of 1 mg/mL collagenase P dissolved in complete media (F-12K media [HyClone Laboratories, Logan, UT] supplemented with 10% low-endotoxin, dialyzed, fetal bovine serum [HyClone Laboratories, Logan, UT], 10 µM non-essential amino acids [HyClone Laboratories, Logan, UT], 1.0 µg/mL hydrocortisone [Sigma-Aldrich, St. Louis, MO], 10 µg/mL insulin [Gibco Laboratories, Gaithersburg, Maryland], 5 µg/mL transferrin [Gibco Laboratories, Gaithersburg, MD], 6.7 ng/mL selenium [Gibco Laboratories, Gaithersburg, MD], 100 U/mL penicillin [Gibco Laboratories, Gaithersburg, MD], and 100 µg/mL streptomycin [Gibco Laboratories, Gaithersburg, MD]). The suspension was then shaken for 1 h at 37°C. Cells were then filtered through a 40-µm nylon mesh, pelleted, resuspended in complete media, counted and plated at 106 cells in 1 mL complete media in 12-well plates. Plates were incubated overnight, washed to remove debris and an additional 1 mL complete media added. Plates were then incubated an additional 48 h before beginning the scratch assay.
To perform the scratch assay, wells were marked on the bottom for reference. A scratch was then made across the reference mark using a sterile 200 µL pipette tip. The wells were then washed 2× with 1 mL complete media and a photograph then taken of the scratch with the reference mark in the field. The relevant SPM (RvE1, MaR1, or PD1, CAS# 552830-51-0, 1268720-28-0, and 660430-03-5, cat# 10007848, 10878, and 10010390, respectively, Cayman Chemicals, Ann Arbor, MI) or the equivalent volume of PBS (control) was then added to the indicated final concentration. The SPMs were purchased in 100% ethanol and were stored at −80°C until used. For use, the SPM was diluted 1:100 in complete media before being added to the cultures at the final concentration shown. Final concentrations of ethanol were <.01%. RvE1 was used to determine a dose response, whereas MaR1 and PD1 were tested only at 12.5 nM. The plates were then incubated (37°C, 95% air/5% CO2) 18 h before a second micrograph was taken at the same location. Images were imported into the NIS-Elements software (Nikon, Tokyo, Japan) and calibrated. The distance separating the leading edges of the cells on each side of the scratch was then measured and the effect of treatment calculated as % closure (100× µm of the post-treatment gap/µm of the pretreatment gap).
In vivo treatment paradigm
The treatment paradigm for in vivo experiments is shown in Figure 1. Day 0 mice were injected (i.p.) with 150 mg/kg CP monohydrate (CAS# 6055-19-2, cat# TCC2236, TCI America, Portland, OR) dissolved in saline at 20 mg/mL. On Days 1, 2, and 3, mice were injected i.p. with the indicated SPM (RvE1, MaR1, PD1; CAS#s 552830-51-0, 1268720-28-0, 660430-03-5; cat #s 10007848, 10878, 10010390, purchased from Cayman Chemical, Ann Arbor, MI) at 25 µg/kg or an equivalent volume of PBS. On Day 4, mice were assessed for the various endpoints.
Figure 1.
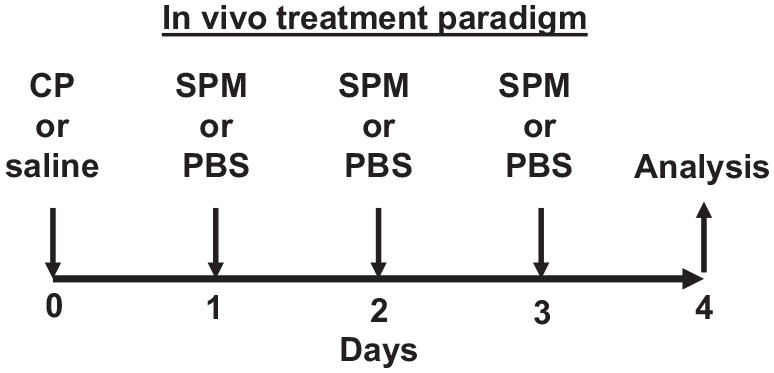
In vivo treatment paradigm used in these studies. Mice 7–8 weeks of age were injected (i.p.) with 150 mg/kg CP or saline on Day 0, followed on Days 1, 2, and 3 with i.p. injections of the various SPMs at 25 µg/kg or PBS. On Day 4, mice were analyzed for the various endpoints reported.
Evans blue dye extravasation
The extravasation of Evans blue dye was used to assess inflammation in the bladder, as previously described.1,5,21,46 On the day of assessment, Evans blue dye was dissolved in sterile saline at 10 mg/kg before being injected (intravenous [i.v.]) into the tail vein of a restrained mouse. After 1 h, the animal was sacrificed and the bladder removed and weighed. The bladder was then placed into 1 mL formamide and incubated overnight at 50°C. Absorbance (620 nm) was then assessed and a standard curve used to assess the amount of dye extracted from the bladder. This amount was then normalized to bladder weight and reported as µg dye/mg bladder tissue.
Surgery
For mice selected for urodynamic analysis, suprapubic tubes were implanted in the bladder one week prior to analysis. Briefly, animals were anesthetized with an i.p. injection of ketamine hydrochloride (50 mg/kg) and xylazine (5 mg/kg). Mice were given amikacin (10 mg/kg, s.c.) for antibiotic prophylaxis and carprofen (5 mg/kg, s.c.) for pain relief. A low, midline abdominal incision was made and the bladder externalized. Using a 6-0 silk suture with a tapered needle, a purse string suture was placed in the bladder dome. A hole was then cut in the middle of the purse string, PE-10 tubing inserted and the purse string pulled taught and tied. The PE-10 tubing had a flared intravesicular end to insure it remained securely in the bladder. The tube was then tunneled subcutaneously to the back of the neck and secured to interscapular tissue using a 6-0 silk suture. The abdominal incision was closed in two layers using a 6-0 PGA suture. Finally, the skin at the catheter exit site was closed around the catheter and the end sealed using heat.
Urodynamics
One week following catheter placement, the animals were placed in a Ballman-type restrainer (Natsume Seisakusho Co., Tokyo, Japan), following appropriate training. The sealed end of the catheter was cut and slid inside a length of PE-50 tubing. The junction between the two was adhered and sealed with cyanoacrylate adhesive. The restrainer was then placed inside a Small Animal Cystometry Lab Station (Med Associates, St. Albans, VT) where it was situated above a digital analytical balance to measure voided volume. The PE-50 tubing was attached to a syringe pump with an inline pressure transducer and sterile saline infused at a rate of 15 µL/min for 60–180 min. Near continuous readings (four per second) were recorded from both the pressure transducer and the scale using Med-CMG software (Med Associates, St. Albans, VT). Micturition cycles were allowed to stabilize (typically 30–45 min) and at least 3–9 cycles recorded before halting the infusion pump, which always took place immediately after a void. After halting the pump, the tubing was detached from the pressure transducer and attached to a 3 mL syringe. The plunger was then withdrawn and any fluid recovered (postvoid residual volume) was measured by expelling it onto the scale. Analysis of the micturition cycles (typically 5–9 per sample) took place using CMG Analysis software (version 1.06; Med Associates, St. Albans, VT). One cycle was defined as the time intravesicular pressure returned to baseline after a previous void until it returned to baseline following the next void. Voiding pressure is defined as the peak intravesical pressure occurring at the time of a void. The void volume was the amount of change on the scale associated with the voiding pressure peak. Frequency was calculated from the number of voids divided by total time for those cycles. Bladder capacity was calculated by adding the average voiding volume to the recovered postvoid residual volume.
qPCR
Urothelial cells were isolated by scraping the bladder wall, as previously described,21,47 and were placed into 1-mL ice-cold PBS until all samples were collected. Cells were pelleted and resuspended in 250 µL PBS. An equal volume of RNA later solution (ThermoFisher, Waltman, MA) was then added and the cells stored at −20°C. RNA was isolated using the Qiagen RNeasy Mini Kit (Cat# 74104, Qiagen, Hilden, Germany) and cDNA synthesized with the Applied Biosystems High Capacity Reverse Transcription kit (Cat# 4368814, ThermoFisher, Waltman, MA) using the manufacturer’s recommended protocols. cDNA was then diluted 1:10 in nuclease free water and used for qPCR analysis with PowerUP SYBR Green Master Mix (cat# A25741, ThermoFisher, Waltman, MA). The manufacturer’s recommended cycling temperatures were employed with the following primers (glyceraldehyde-3-phosphate dehydrogenase [GAPDH], f-AGGTCGGTGTGAACGGATTTG, r-TGTAGACCATGTAGTTGAGGTCA; Transforming growth factor-β1 [TGF-β1], f-CTCCCGTGGCTTCTAGTGC, r-GCCTTAGTTTGGACAGGATCTG; Collagen type I, alpha 2 [Collagen 1], f-CTGTAACATGGAAACTGGGGAAA, r-CCATAGCTGAACTGAAAACCACC). Analysis took place on a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Gene expression was normalized to GAPDH and expressed as the fold change between experimental and control tissue (ΔΔCt). 48
Statistical analysis
All parameters were assessed by ANOVA followed by Dunnett’s post hoc test comparing all to control or Student-Newman-Keul’s post hoc test, as indicated in the figure legends. All statistical analysis were performed using GraphPad InStat software (La Jolla, CA). Statistical significance was defined as p < 0.05.
Results
Receptors for numerous SPM receptors are expressed in the mouse and human bladder
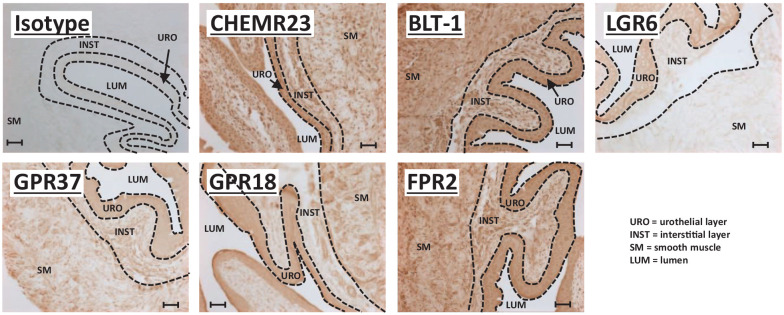
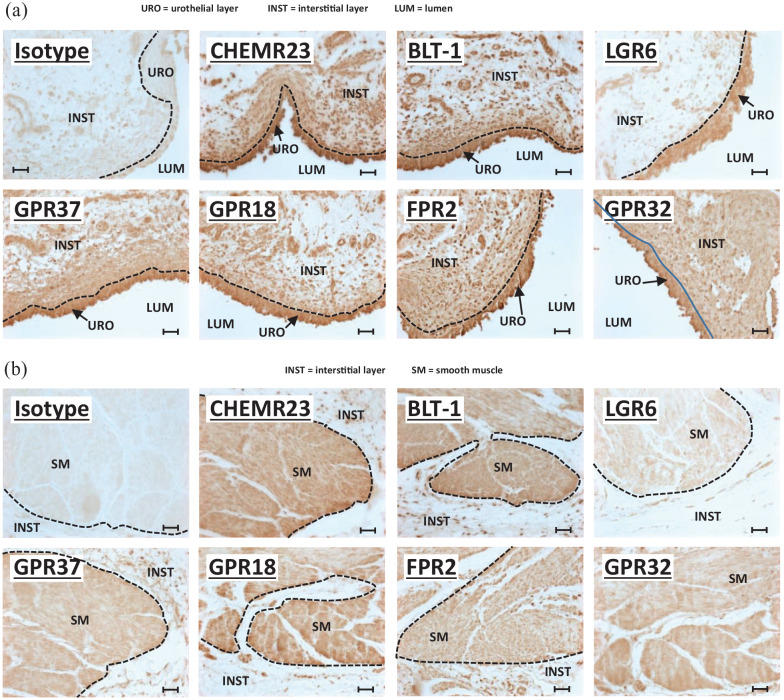
Recently, we showed the presence of various SPM receptors in the control rat bladder, some of which changed expression pattern in response to BOO. 33 Before initiating studies in the mouse, we sought to confirm expression of these SPMs in the bladder of this species and so we have performed immunocytochemistry for all the various SPM receptors on mouse bladders (Figure 2). Likewise, to determine if the investigated pathways may be relevant in humans, we have also stained sections of human bladder (Figure 3). As shown in Figure 2, all six SPM receptors (CHEMR23, BLT-1, LGR6, GPR37, GPR18, and FPR2) are expressed in the urothelia layers in control mice. The seventh, GPR32, has no rodent homolog 30 and thus was not assessed. Most of these, with the exception of LGR6, are also expressed in the smooth muscle layers. Some (BLT-1, FPR2) also appear to be expressed in the interstitial layer. Finally, CP or CP + RvE1 treatment did not significantly change the expression patterns of any of the receptors (Supplemental Figures 1 and 2). A slightly different expression pattern was apparent in human bladder. All seven SPM receptors were expressed in the urothelia (Figure 3(a)). All were also found in the bladder smooth muscle (Figure 3(b)) with the possible exception of LGR6, which was also excluded from the smooth muscle in the mouse bladder. However, unlike the rodent models, most of these receptors were also expressed in several layers of the interstitial cells immediately underlying the urothelia (Figure 3(a)). This seemed especially true for FPR2 and GPR32.
Figure 2.
SPM receptors are expressed in mouse bladder. All panels are immunohistochemistry performed on 5 µm sections of formalin-fixed, paraffin-embedded mouse bladders from untreated control mice of ≈7–11 weeks of age, as detailed in the Methods section. The primary antibody target is indicated on each panel, and all were rabbit antibodies (see Table 1). The first panel is an isotype control using a rabbit IgG isotype control from Novus Biologicals (Centennial, CO). All parameters for the slides used in this figure (incubation times, HRP product development times, etc.) were identical between all samples and for each antibody and all slides specifically used in this figure were stained on the same day. Micrographs were taken with identical settings (exposure times, light intensity, etc.). Scale bars = 50 µm. The various layers (urothelial, interstitial, and smooth muscle) are delineated with dash lines and labeled on the micrographs.
Figure 3.
SPM receptors are expressed in human bladder: (a) micrographs focused on the urothelia and underlying interstitial cells, (b) micrographs focusing on the bladder smooth muscle. All panels in (a) and (b) show immunohistochemistry performed on 5-µm sections of formalin-fixed, paraffin-embedded human bladders purchased from Zyagen Inc. (San Diego, CA). The primary antibody target is indicated on each panel and all were rabbit antibodies (see Table 1). The first panel in both (a) and (b) is an isotype control using a rabbit IgG isotype control from Novus Biologicals (Centennial, CO) (Table 1). All parameters for the slides used in this figure (incubation times, HRP product development times, etc.) were identical between all samples and for each antibody and all slides specifically used in this figure were stained on the same day. Micrographs were taken with identical settings (exposure times, light intensity etc.). Scale bars = 50 µm. The various layers (urothelial, interstitial, and smooth muscle) are delineated with dash lines and labeled on the micrographs.
RvE1, MaR1, and PD1 promote wound closure in vitro
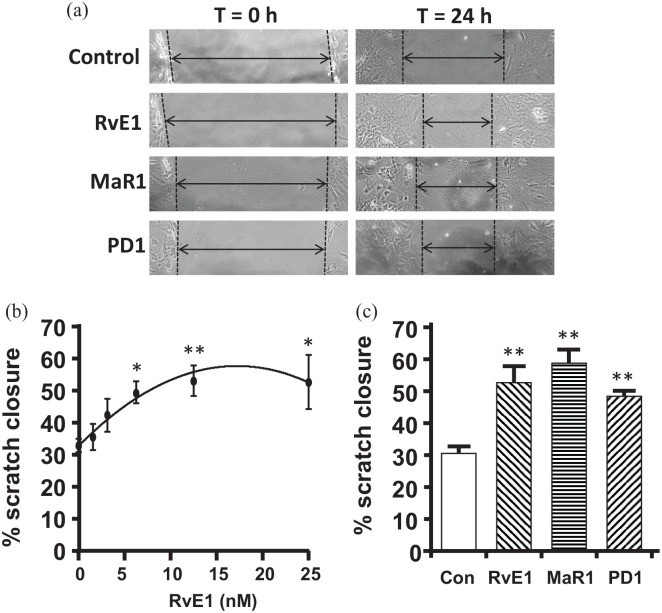
As an initial assessment of the ability of SPMs to act as resolution molecules on mouse urothelia, we examined the ability of RvE, MaR1, and PD1 to promote barrier/wound repair using a well-established in vitro scratch assay.49–51 For this, primary urothelia are cultured as a monolayer and then scratched with a p200 tip. A photograph is taken and the culture treated with the respective SPM. A second photograph is taken at the same location after 18 h and the % of closure is calculated. Figure 4(a) shows representative scratches at t = 0 h and 24 h after treatment with the respective SPM. The results are quantitated in Figure 4(b) and (c). Figure 4(b) is a dose response of Rve1 in promoting closure of the scratch. In this setting, RvE1 demonstrated an EC90 of ≈12.5 nM and an EC50 of ≈4.0 nM. We next assessed the ability of two additional SPMs, MaR1, and PD1 to promote wound repair with a similar efficacy. As shown in Figure 4(c), both Mar1 and PD1 promoted wound closure significantly above the untreated control and to levels similar to RvE1. In fact, closure levels were not significantly different between RvE1, MaR1, and PD1.
Figure 4.
RvE1, MaR1, and PD1 enhance wound closure in the in vitro scratch assay: (a) representative micrographs of the scratch wound in the monolayer of urothelia before (t = 0 h) and after (t = 24 h) treatment with the indicated SPM or an equivalent volume of PBS, as described in the methods section. (b) Dose response of RvE1. As described in the Methods section, mouse urothelia were placed in culture and allowed to form a monolayer. A scratch was then made and photographed. RvE1 was added at the indicated doses and, after an additional 18 h, the scratch photographed at the identical spot. The width of the scratch was determined at both time points and the % of the scratch closure determined. Results are the mean ± SEM. n = 10, 5, 6, 5, 5, 5, respectively. *p < 0.05, **p < 0.01, ***p < 0.001 by ANOVA and Dunnett’s post hoc test comparing all to 0 nM control. (c) Effects of RvE1, MaR1, and PD1 on scratch closure. The scratch assay was performed as described and cultures were treated with either vehicle or 12.5 nM of RvE1, MaR1, or PD1. The RvE1 value shown is the same data used in (a) but is included in (b) for ease of comparison with the other SPMs. Results are the mean ± SEM. n = 10, 5, 3, 3, respectively. **p < 0.01, ***p < 0.001 by ANOVA and Dunnett’s post hoc test comparing all to control.
RvE1, MaR1, and PD1 promote the resolution of CP-induced bladder inflammation
To determine if these three SPMs could effectively promote the resolution of bladder inflammation, we assessed their ability to reduce inflammation triggered by CP. As shown in Figure 1, mice were treated with CP on Day 0, which is well known to induce a severe inflammatory response within 24 h (Day 1). Beginning on Day 1, mice were treated daily with a given SPM (RvE1, MaR1, or PSD1) or PBS for three days. One day after the last dose (Day 4) end points were analyzed. First, inflammation was assessed using the Evans blue dye extravasation assay. As shown in Figure 5(a), treatment with CP evoked a 4.5-fold increase in Evans blue movement into the bladder tissue on Day 4. However, treatment of mice with 25 µg/kg of either RvE1, MaR1, or PD1 during the Day 1 to 3 period completely resolved this inflammation, reducing Evans blue levels back to the PBS-treated control.
Figure 5.
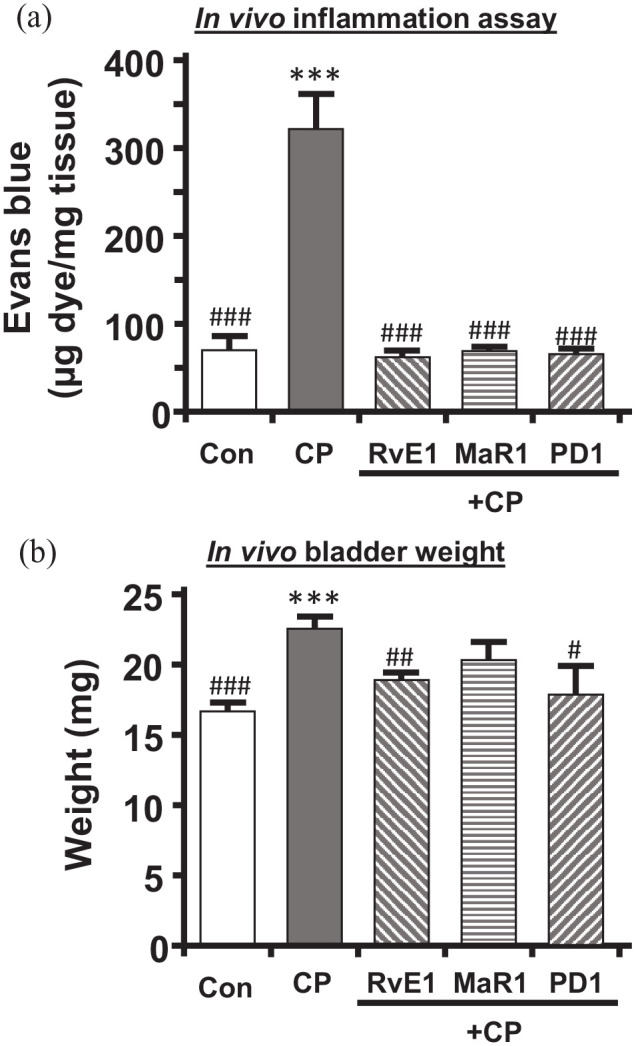
RvE1, MaR1, and PD1 reduce CP-induced in vivo bladder inflammation and weight gain: (a) bladder inflammation as measured by the Evans blue dye extravasation assay and described in the Methods section. Mice were subjected to the treatment paradigm shown in Figure 1. CP was administered at 150 mg/kg. SPMs used were RvE1, MaR1, and PD1, all at 25 µg/kg/injection. On Day 4, the mice were injected (i.v.) with Evans blue (25 mg/kg). 1 h later, bladders were removed, weighed, and placed in 1 mL formamide overnight (56°C). Absorbance (620 nm) was measured and results calculated from a standard curve. Results are the mean ± SEM. n = 10, 12, 5, 4, 4, respectively. ***p < 0.001 compared to control (Con – injected with saline and PBS only) by ANOVA and Student–Newman–Keuls. ###p < 0.001 compared to CP (CP – injected with CP and saline only) by ANOVA and Student–Newman–Keuls. (b) Bladder weights following the treatment paradigm depicted in Figure 1. At the end of the paradigm, bladders were removed for the various endpoints and weighed. Bladders from mice used for urodynamics were not included in the analysis. Results are the mean ± SEM. n = 19, 26, 15, 4, 4, respectively. ***p < 0.001 compared to control (Con – injected with saline and PBS only) by ANOVA and Student–Newman–Keuls. #p < 0.05, ##p < 0.01, ###p < 0.001 compared to CP (CP – injected with CP and saline only) by ANOVA and Student–Newman–Keuls.
Inflammation in a tissue is associated with weight gain, most commonly from edema, and this is often used as a surrogate for this endpoint.52,53 Thus, we measured bladder weights for these animals at the time of end point analysis (excluding those used for urodynamics). As shown in Figure 5(b), CP increased bladder weight by ≈35%. All three SPMs reduced bladder weight to levels not significantly different from controls, although it must be noted that the weights in response to MaR1 were also not significantly different from CP, even though they were reduced.
RvE1 restores important indices of bladder function
CP is well known to induce an overactive phenotype in the bladder which can best be defined using urodynamics. To determine if SPMs can reverse this effect, we performed urodynamics on mice treated as illustrated in Figure 1. For this study, we focused on RvE1 as a representative SPM because it is known to bind to two of the receptors identified in Figures 2 and 3 as being expressed in the bladder (ChemR23 and BLT-1). Thus, this SPM was deemed to have a higher likelihood of success in these studies. Representative urodynamic pressure tracings of the three groups analyzed (Control, CP, and CP + RvE1) are shown in Figure 6 while quantitative analyses of critical parameters are depicted in Figure 7. Additional urodynamic parameters not illustrated (e.g. voiding pressure) were not significantly different between the various groups (Figure 8).
Figure 6.
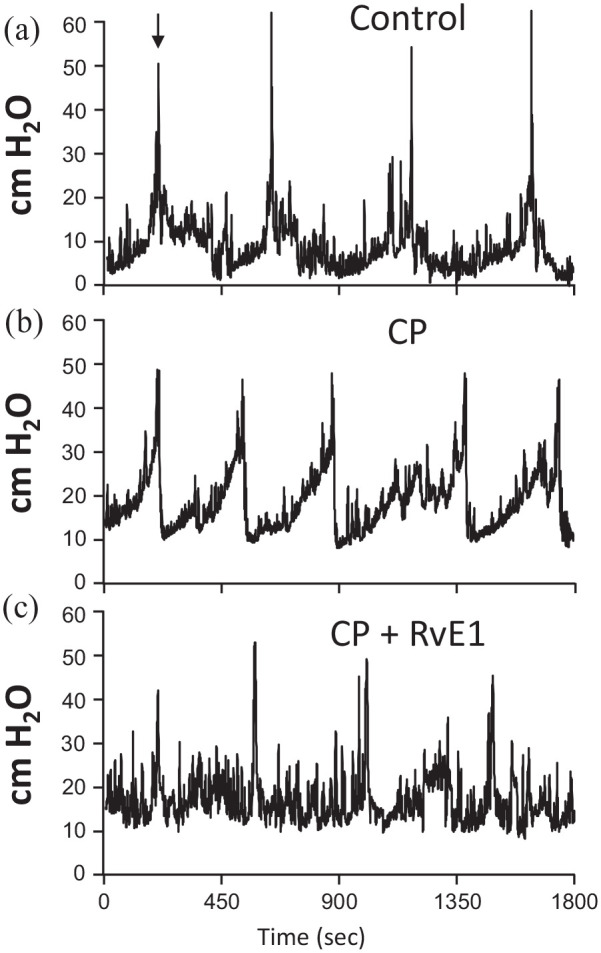
Representative urodynamic pressure tracings from control, CP-treated, and CP + RvE1–treated mice. Shown are intravesicular pressure tracings for each group through several micturition cycles. The tracing is oriented so the first peak of voiding pressure (indicated by an arrow in (a)) in each of the tracings are aligned for ease of comparison. The tracings reveal voiding pressures (the pressure at each peak which corresponds to a void – tracings from the scale that indicate voids are not shown) and the intercontraction interval (time between peaks) which is reflective of voiding frequency: (a) control untreated mice (saline and PBS only), (b) CP-treated mice (CP and PBS only), and (c) CP + RvE1 – treated mice (CP and RvE1).
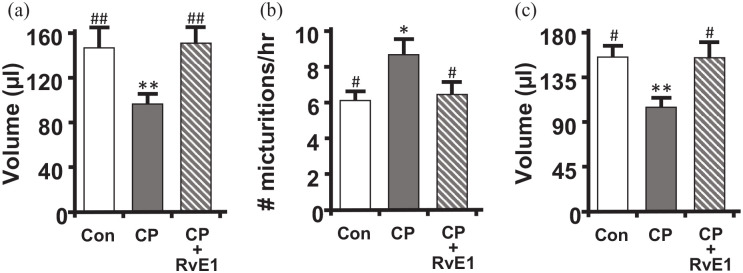
Figure 7.
RvE1 restores urodynamics parameters to control levels following CP treatment. Following the treatment paradigm shown in Figure 1, mice were subjected to urodynamics. Recordings were analyzed using Med-CMG software (Catamount, Inc., St. Albans, VT). Typically 5–10 individual micturition cycles were quantitated per animal and averaged for n = 1. (a) Average voided volume for each of the three groups. (b) Voiding frequency (number of voids divided by total time for those cycles), for each of the three groups. (c) Bladder capacity (the average voiding volume from the 5–10 individual micturition cycles analyzed for a single mouse added to the recovered postvoid residual volume of that mouse). Data are the mean ± SEM for all graphs. n = 9, 10, 9, for all, respectively. *p < 0.05, **p < 0.01 compared to control (Con – saline and PBS only treated mice) by ANOVA and Student–Newman–Keuls. #p < 0.05, ##p < 0.01 compared to CP (CP – injected with CP and saline only) by ANOVA and Student–Newman–Keuls.
Figure 8.
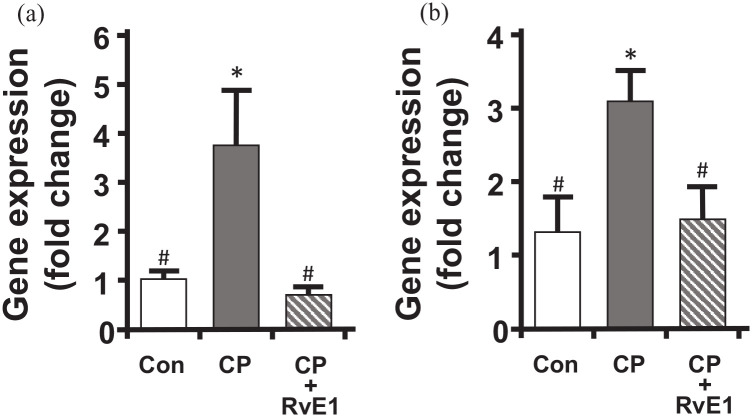
RvE1 reduces the CP-induced increase in TGF-β and Collagen 1 gene expression in urothelia. Mice were subjected to the treatment paradigm shown in Figure 1. RNA was then isolated from urothelia, reverse transcribed and subjected to qPCR using the primers and conditions reported in the Methods section. Values were normalized to GAPDH and reported as fold-change in gene expression relative to control (Con) untreated values (ΔΔCt). Data are the mean ± SEM for all graphs. n = 5, 7, 7, respectively. *p < 0.05 compared to control, #p < 0.05 compared to CP, by ANOVA and Student–Newman–Keuls.
Overactive phenotypes in rodents are characterized by decreased voiding volumes and increased voiding frequency. In this study, CP treatment caused a significant decrease in voiding volume (Figure 7(a)) and a concomitant increase in frequency (Figure 7(b)) four days after treatment. This was also associated with a significant reduction in bladder capacity at this time point (Figure 7(c)). Excitingly, RvE1 was able to reverse all of these changes and completely return these values back to control levels.
RvE1 reduces fibrotic gene expression back to control values
Inflammation in general, and in the bladder specifically, is well known to precipitate fibrosis, and there is growing evidence that the therapeutic potential of SPMs includes the regression of fibrosis.54,55 To determine if SPMs may influence fibrosis, we performed qPCR on RNA isolated from urothelia taken from control, CP-treated and CP + RvE1–treated mice. We focused on urothelia because we have previously shown 52 that this cell type is a significant producer of collagen in the bladder and responds to pro-inflammatory cytokines such as IL-1β, the product of the NLRP3 inflammasome. Here we assess changes in expression of TGF-β, which drive fibrosis in many diseases and is often known as the master regulator of fibrosis,56,57 and Collagen-1, a major component of the fibrotic tissue. As shown in Figure 6, both TGF-β and Collagen 1 levels were greatly increased by CP treatment, and this increase was restored to control levels by treatment with RvE1.
Discussion
The resolution phase of inflammation, including its pathways and mediators, is a highly understudied aspect of inflammatory disease in the bladder. This seems particularly true when one considers that the state-of-the-art thinking is that chronic inflammation is more likely a result of the failure of pro-resolving pathways than an over-stimulation of inflammation-inducing pathways 22 and chronic inflammation is a defining characteristic of many benign urological diseases. Since inflammation is also obviously critical in acute insults such as urinary tract infections and the CP-induced cystitis employed in this study, it is clear that manipulating the resolution phase could have great therapeutic potential to treat numerous bladder disorders. This is only the second paper to explore this field, define the potential pathways present and test individual SPMs for beneficial activity. In our previous manuscript, 33 we assessed the effects of one SPM, Annexin-A1, on BOO in rats and found it to be quite effective in promoting the resolution of inflammation and restoring normal bladder function. In this study, we expand that work to examine three additional SPMs, each representative of a class of the lipid-based mediators. We elaborate further on the ability of one mediator, RvE1, to facilitate the restoration of normal bladder function and fibrotic gene expression.
We began these studies by examining the repertoire of SPM receptors expressed in the mouse and human bladder. While our previous work documented expression in rat bladder 33 expression needed to be confirmed in these species. Our results show expression of all known SPM receptors in the bladder in both species (six in mice, seven in humans). Most were expressed in both the urothelial and smooth muscle, although LGR6 expression appeared to be restricted to the urothelia. The expression of such a large number of receptors suggest that numerous SPM pathways are likely to be active in the bladder, and we discuss our evidence for at least four to five active pathways below. These data also defines a repertoire of novel targets for possible pharmaceutical intervention.
Formation of a strong barrier between the underlying tissue and the stored urine is an important function of urothelia and research into many of the chronic bladder inflammatory disease have documented reductions in barrier function that are thought to play critical roles in the pathology of these diseases.58–61 Thus, efficient repair of this barrier function is critical and would help dampen the inflammatory response by limiting exposure to DAMPS in the urine. 62 One exciting characteristic of SPMs is that they promote barrier repair and wound healing.34,35 This has been most clearly defined in gut and lung epithelia, two commonly studied tissues in epithelial biology,34,63 but it is documented in many other epithelia such as the cornea epithelium in mice. 64 The ability to promote wound healing is commonly and easily assessed using a scratch assay in which a scratch is made with a pipette tip in a monolayer of epithelia. The cultures are treated, and the percent closure of the scratch assessed after a period of time (18 h in this study). To gain insight into the efficacy of SPMs on urothelial repair, we began with a dose response of RvE1 which identified an EC50 of 4.0 nM and an EC90 of 12.5 nM. This is somewhat more efficacious than previously seen with the human intestinal epithelial cell line SKCO15 (peak effects at 100 nM) 62 but similar to that seen with periodontal ligament stem cells. 65 Assessment of MaR1 and PD1 at 12.5 nM produced a similar enhancement of wound closure suggesting these SPMs had at least a similar level of efficacy to RvE1 in urothelia. It is important to note that these studies only sought to determine if these SPMs can promote wound closure, the first step in repairing a loss of barrier function and a well-established attribute of SPMs. Future work will need to assess reformation of the actual watertight barrier in vivo in pathological models characterized by barrier breakdown.
This initial work demonstrates the ability of the urothelia to respond to three additional classes of SPMs, the resolvins, the maresins and the protectins, in addition to the previously identified Annexin-A1. 33 Annexin-A1 binds to FPR2 to exert its pro-resolution activity, whereas RvE1 acts through CHEMR23 and BLT-1. 66 The positive response to RvE1 suggests that at least one, if not both, of these receptors can be activated in urothelia. Likewise, MaR1 is thought to trigger its pro-resolving activities solely through LGF625,67 and PD1 acts solely though GPR37. 25 Thus, positive results with all these ligands demonstrates that at least four, and maybe five, of the identified SPM receptors are activable in urothelia and likely to be engaged in the response to inflammatory stimuli. Most importantly, these results identify four to five novel targets which may be used to treat a multiplicity of bladder inflammatory diseases.
We next sought to determine if these SPMs were effective in vivo and could resolve inflammation in the bladder caused by CP. CP-induced cystitis is a well-used model of bladder inflammation although the exact dose and timing of analysis may vary depending on the exact situation the researcher wishes to investigate. For example, single doses from 150 to 300 mg/kg are given to mice which are then analyzed after one to four days when an acute inflammation is desired (16–24 h).68–70 In contrast, daily doses of 75 mg/kg for three or more days are common when the investigator wishes to measure chronic inflammatory effects. 70 With several doses and paradigms, studies have shown a relatively rapid return to normal after the last CP injection (3–24 days),71,72 suggesting that once the inciting stimulus is gone (i.e. CP is metabolized to acrolein and that acrolein is completely excreted in the urine), that a robust resolution program was likely engaged. Since our goal was to study the enhancement of this resolution program, we chose a paradigm of 150 mg/kg injected once, a relatively mild acute response, and analyzed inflammation four days later in which resolution was likely well-underway. Daily treatment during those four days with SPMs could then be expected to speed resolution. Indeed, all three SPMs analyzed were able to resolve inflammation back to control levels as measured by the Evans blue dye extravasation assay. They also triggered a reduction in bladder weight, the increase of which is often reported as an indirect measurement of inflammation.52,53 Thus, at least some of the pathways predicted by receptor expression and activatability in vitro, can be manipulated in vivo to enhance resolution of inflammation in this model.
Having established that all three SPMs could speed resolution of inflammation, we chose to explore RvE1 further to determine if it also restores bladder function as measured by urodynamics, the gold standard for assessing bladder function. While all three were good candidates for further exploration, RvE1 binds to two of the expressed receptors 66 and therefore seemed most likely to evoke a response. CP typically creates an overactive bladder phenotype which, in rodents, is indicated by a decreased voiding volume and increased frequency, 21 and this is exactly what we found with our current model. This change was also associated with a reduced bladder capacity. RvE1 restored all of these changes back to control levels, which is consistent with its complete elimination of inflammation.
One of the most detrimental changes in the bladder that is triggered by inflammation is fibrosis. Fibrotic alterations to the bladder are associated with irreversible bladder dysfunction, even when the inciting stimulus is removed. 73 Such is often the case with men undergoing a transurethral resection of the prostate to relieve their BOO. If fibrosis has progressed beyond a certain point (which is hard to define clinically), the restoration to normal bladder function will be suboptimal. However, SPMs are known to suppress fibrotic changes and may even reverse these effects54,55 suggesting that these classes of compounds can give hope to these patients. CP is an acute insult and so we would not expect major fibrotic changes in terms of large collagen deposition, significant distensibility changes to the bladder wall, major contractility dysfunction, and so on. However, we did measure an increase in the mRNA for TGF-β in urothelia, which is a master regulator of fibrosis,56,57 suggesting that, at the very least, in this model, the process of fibrosis has already begun. While many cell types may contribute to fibrosis, we have previously shown that the bladder urothelium is a significant source that directly responds to pro-inflammatory cytokines (IL-1β in particular) to increase secretion of collagen. 52 In addition to TGF-β, we saw an increase in Collagen 1 mRNA, further confirming this fibrotic shift. Excitingly, RvE1 completely reversed expression of both these molecules demonstrating a direct effect against the fibrotic pathways by this SPM in the bladder. It will be interesting in future studies to determine if SPMs like RvE1 can actually reverse some of the physical changes to the bladder (collagen deposition and distensibility) seen in more chronic models such as BOO. 1
Taken together, our results show that mouse and human bladders express numerous SPM receptors and that mice at least have the potential to engage several of the pro-resolution pathways – the normal involvement of which has not been studied. Overall, we have provided strong evidence that the SPMs in the bladder represent a large and widely unstudied field relevant to bladder inflammation disorders with very promising therapeutic targets and the exciting possibility of bringing about real relief to a wide variety of patients in the future.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211067465 for Specialized pro-resolution mediators in the bladder: Receptor expression and recovery of bladder function from cystitis by Francis M Hughes, Armand Allkanjari, Michael R Odom, Huixia Jin and J Todd Purves in Experimental Biology and Medicine
Acknowledgments
The authors would like to thank Yasheng Gao and the Light Microscopy Core Facility at Duke University for their help obtaining images. They would also like to thank Julie Fuller and the Substrate Services Core and Research Support Services (SCRSS) in the Department of Surgery at Duke University for their help with histological embedding and sectioning. Finally, they would like to thank Dr. Brent Stanfield for his advice regarding PCR and Dr. Shyni Varghese and her laboratory for the kind use of the qPCR thermocycler. Both Drs. Stanfield and Varghese are in the Department of Orthopedics at Duke University.
Footnotes
Authors’ Contributions: Each author has met the EBM authorship requirements. FMH and JTP conceived the project and designed experiments; FMH, AA, MRO, and HJ performed experiments; FMH analyzed data; FMH, AA, MRO, and JTP interpreted results of experiments; FMH prepared figures; FMH drafted manuscript; FMH, AA, MRO, and JTP edited and revised manuscript. FMH and JTP approved final version of manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Institutional Animal Care and Use Committee at Duke University Medical Center approved protocols prior to beginning this work. All protocols strictly adhered to the NIH Guide for the Care and Use of Laboratory Animals.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases to JTP (grant no. R01DK117890) and MRO (grant no. K12DK100024). Additional funds were provided by the Duke University School of Medicine.
ORCID iD: Francis M Hughes Jr  https://orcid.org/0000-0003-3776-3653
https://orcid.org/0000-0003-3776-3653
Supplemental material: Supplemental material for this article is available online.
References
- 1. Hughes FM, Jr, Hill HM, Wood CM, Edmondson AT, Dumas A, Foo WC, Oelsen JM, Rac G, Purves JT. The NLRP3 inflammasome mediates inflammation produced by bladder outlet obstruction. J Urol 2016;195:1598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panigrahy R, Singh B, Das SK. Diabetic uropathy and bladder dysfunctions. Diabetes Metab Syndr 2017;11:81–2 [DOI] [PubMed] [Google Scholar]
- 3. Wittig L, Carlson KV, Andrews JM, Crump RT, Baverstock RJ. Diabetic bladder dysfunction: a review. Urology 2019;123:1–6 [DOI] [PubMed] [Google Scholar]
- 4. Hughes FM, Jr, Hirshman NA, Inouye BM, Jin H, Stanton EW, Yun CE, Davis LG, Routh JC, Purves JT. NLRP3 promotes diabetic bladder dysfunction and changes in symptom-specific bladder innervation. Diabetes 2019;68:430–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hughes FM, Jr, Kennis JG, Youssef MN, Lowe DW, Shaner BE, Purves JT. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome mediates inflammation and voiding dysfunction in a lipopolysaccharide-induced rat model of cystitis. J Clin Cell Immunol 2016;7:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hamilton C, Tan L, Miethke T, Anand PK. Immunity to uropathogens: the emerging roles of inflammasomes. Nat Rev Urol 2017;14:284–95 [DOI] [PubMed] [Google Scholar]
- 7. Verma V, Gupta S, Kumar P, Yadav S, Dhanda RS, Gaind R, Arora R, Frimodt-Moller N, Yadav M. Involvement of NLRP3 and NLRC4 inflammasome in uropathogenic E. Coli mediated urinary tract infections. Front Microbiol 2019;10:2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tudrej KB, Piecha T, Kozlowska-Wojciechowska M. Role of NLRP3 inflammasome in the development of bladder pain syndrome interstitial cystitis. Ther Adv Urol 2019;11:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, He PL, Yang J, Yang YF, Wang K, Amend B, Stenzl A, Zhang YM, Wang ZL, Xing SS, Luo X. NLRP3/IL1beta inflammasome associated with the aging bladder triggers bladder dysfunction in female rats. Mol Med Rep 2019;19:2960–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 2002;10:417–26 [DOI] [PubMed] [Google Scholar]
- 11. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov 2020;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 2020;20:95–112 [DOI] [PubMed] [Google Scholar]
- 13. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Ann R Pathol 2020;15:493–518 [DOI] [PubMed] [Google Scholar]
- 14. Paik S, Kim JK, Silwal P, Sasakawa C, Jo EK. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell Mol Immunol 2021;18:1141–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol 2021;22:550–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–7 [DOI] [PubMed] [Google Scholar]
- 17. Huizinga GP, Singer BH, Singer K. The collision of meta-inflammation and SARS-CoV-2 pandemic infection. Endocrinology 2020;161:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onyango IG, Jauregui GV, Carna M, Bennett JP, Jr, Stokin GB. Neuroinflammation in Alzheimer’s disease. Biomedicines 2021;9:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pessentheiner AR, Ducasa GM, Gordts P. Proteoglycans in obesity-associated metabolic dysfunction and meta-inflammation. Front Immunol 2020;11:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shapiro H, Lutaty A, Ariel A. Macrophages, meta-inflammation, and immuno-metabolism. Sci World J 2011;11:2509–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hughes FM, Jr, Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, Nietert PJ, Spruill LS, Purves JT. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. Am J Physiol Renal Physiol 2014;306:F299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnig C, Frossard N, Levy BD. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther 2018;186:98–113 [DOI] [PubMed] [Google Scholar]
- 23. Serhan CN, Hamberg M, Samuelsson B. Trihydroxytetraenes: a novel series of compounds formed from arachidonic acid in human leukocytes. Biochem Biophys Res Commun 1984;118:943–9 [DOI] [PubMed] [Google Scholar]
- 24. Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA 1984;81:5335–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 2020;64:443–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brennan E, Kantharidis P, Cooper ME, Godson C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol 2021;17:725–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fattori V, Zaninelli TH, Rasquel-Oliveira FS, Casagrande R, Verri WA, Jr. Specialized pro-resolving lipid mediators: a new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol Res 2020;151:104549. [DOI] [PubMed] [Google Scholar]
- 28. Ge YJ, Liao QW, Xu YC, Zhao Q, Wu BL, Ye RD. Anti-inflammatory signaling through G protein-coupled receptors. Acta Pharmacol Sin 2020;41:1531–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mastromarino M, Lacivita E, Colabufo NA, Leopoldo M. G-protein coupled receptors involved in the resolution of inflammation: ligands and therapeutic perspectives. Mini Rev Med Chem 2020;20:2090–103 [DOI] [PubMed] [Google Scholar]
- 30. Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol 2014;171:3551–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Monastyrskaya K, Babiychuk EB, Draeger A, Burkhard FC. Down-regulation of annexin A1 in the urothelium decreases cell survival after bacterial toxin exposure. J Urol 2013;190:325–33 [DOI] [PubMed] [Google Scholar]
- 32. Zeng J, Ekman M, Jiang C, Uvelius B, Sward K. Non-uniform changes in membrane receptors in the rat urinary bladder following outlet obstruction. Eur J Pharmacol 2015;762:82–8 [DOI] [PubMed] [Google Scholar]
- 33. Hughes FM, Jr, Harper SN, Nose BD, Allkanjari A, Sheng MT, Jin H, Purves JT. Specialized pro-resolution mediators in the bladder; Annexin-A1 normalizes inflammation and bladder dysfunction during bladder outlet obstruction. Am J Physiol Renal Physiol 2021;321:F443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duvall MG, Bruggemann TR, Levy BD. Bronchoprotective mechanisms for specialized pro-resolving mediators in the resolution of lung inflammation. Mol Aspects Med 2017;58:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang RX, Colgan SP. Special pro-resolving mediator (SPM) actions in regulating gastro-intestinal inflammation and gut mucosal immune responses. Mol Aspects Med 2017;58:93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hughes FM, Jr, Hirshman NA, Malick HA, White SW, Jin H, Harper SN, Purves JT. A possible mechanism underlying mood disorders associated with LUTS: chronic bladder outlet obstruction causes NLRP3-dependent inflammation in the hippocampus and depressive behavior in rats. Neurourol Urodyn 2020;39:1700–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abdelmoaty S, Wigerblad G, Bas DB, Codeluppi S, Fernandez-Zafra T, El-Awady el S, Moustafa Y, Abdelhamid Ael-D, Brodin E, Svensson CI. Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PLoS ONE 2013;8:e75543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen K, Liu M, Liu Y, Yoshimura T, Shen W, Le Y, Durum S, Gong W, Wang C, Gao JL, Murphy PM, Wang JM. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J Clin Invest 2013;123:1694–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh D, Qi R, Jordan JL, San Mateo L, Kao CC. The human antimicrobial peptide LL-37, but not the mouse ortholog, mCRAMP, can stimulate signaling by poly(I:C) through a FPRL1-dependent pathway. J Biol Chem 2013;288:8258–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jank BJ, Kadletz L, Dunkler D, Haas M, Schnoell J, Kenner L, Heiduschka G. Epithelial stem cell marker LGR6 expression identifies a low-risk subgroup in human papillomavirus positive oropharyngeal squamous cell carcinoma. Oral Oncol 2020;105:104657 [DOI] [PubMed] [Google Scholar]
- 41. Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, McElreavey K, Sarkisian T, Grateau G, Alnemri ES, Amselem S. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc Natl Acad Sci USA 2008;105:1614–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu V, Auchman M, Mollica PA, Sachs PC, Bruno RD. ALDH1A1 positive cells are a unique component of the tonsillar crypt niche and are lost along with NGFR positive stem cells during tumourigenesis. Pathology 2018;50:524–9 [DOI] [PubMed] [Google Scholar]
- 43. Baranowska-Kuczko M, Kozlowska H, Kloza M, Sadowska O, Kozlowski M, Kusaczuk M, Kasacka I, Malinowska B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: modification by hypertension and the potential pharmacological opportunities. J Hypertens 2020;38:896–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jannaway M, Torrens C, Warner JA, Sampson AP. Resolvin E1, resolvin D1 and resolvin D2 inhibit constriction of rat thoracic aorta and human pulmonary artery induced by the thromboxane mimetic U46619. Br J Pharmacol 2018;175:1100–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kloskowski T, Uzarska M, Gurtowska N, Olkowska J, Joachimiak R, Bajek A, Gagat M, Grzanka A, Bodnar M, Marszalek A, Drewa T. How to isolate urothelial cells? Comparison of four different methods and literature review. Hum Cell 2014;27:85–93 [DOI] [PubMed] [Google Scholar]
- 46. Inouye BM, Jr, Hughes FM, Jin H, Lütolf R, Potnis K, Routh JC, Rouse C, Foo W-C, Purves JT. Diabetic bladder dysfunction is associated with bladder inflammation triggered through hyperglycemia not polyuria. Res Rep Urol 2018;10:219–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hughes FM, Jr, Corn AG, Nimmich AR, Pratt-Thomas JD, Purves JT. Cyclophosphamide Induces an early wave of acrolein-independent apoptosis in the urothelium. Adv Biosci Biotechnol 2013;4:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
- 49. Hopkins AM, Bruewer M, Brown GT, Pineda AA, Ha JJ, Winfree LM, Walsh SV, Babbin BA, Nusrat A. Epithelial cell spreading induced by hepatocyte growth factor influences paxillin protein synthesis and posttranslational modification. Am J Physiol Gastrointest Liver Physiol 2004;287:G886–98 [DOI] [PubMed] [Google Scholar]
- 50. Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, Parkos CA, Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol 2007;179:8112–21 [DOI] [PubMed] [Google Scholar]
- 51. Leoni G, Nusrat A. Annexin A1: shifting the balance towards resolution and repair. Biol Chem 2016;397:971–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hughes FM, Jr, Sexton SJ, Jin H, Govada V, Purves JT. Bladder fibrosis during outlet obstruction is triggered through the NLRP3 inflammasome and the production of IL-1beta. Am J Physiol-Renal 2017;313:F603–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kanno Y, Mitsui T, Kitta T, Moriya K, Tsukiyama T, Hatakeyama S, Nonomura K. The inflammatory cytokine IL-1beta is involved in bladder remodeling after bladder outlet obstruction in mice. Neurourol Urodyn 2016;35:377–81 [DOI] [PubMed] [Google Scholar]
- 54. Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018;155:282–302.e8 [DOI] [PubMed] [Google Scholar]
- 55. Musso G, Gambino R, Cassader M, Paschetta E, Sircana A. Specialized proresolving mediators: enhancing nonalcoholic steatohepatitis and fibrosis resolution. Trends Pharmacol Sci 2018;39:387–401 [DOI] [PubMed] [Google Scholar]
- 56. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–38 [DOI] [PubMed] [Google Scholar]
- 57. Stewart AG, Thomas B, Koff J. TGF-beta: master regulator of inflammation and fibrosis. Respirology 2018;23:1096–7 [DOI] [PubMed] [Google Scholar]
- 58. Hurst RE, Greenwood-Van Meerveld B, Wisniewski AB, VanGordon S, Lin H, Kropp BP, Towner RA. Increased bladder permeability in interstitial cystitis/painful bladder syndrome. Transl Androl Urol 2015;4:563–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Romih R, Korosec P, Jezernik K, Sedmak B, Trsinar B, Deng FM, Liang FX, Sun TT. Inverse expression of uroplakins and inducible nitric oxide synthase in the urothelium of patients with bladder outlet obstruction. BJU Int 2003;91:507–12 [DOI] [PubMed] [Google Scholar]
- 60. Niemczyk G, Czarzasta K, Radziszewski P, Wlodarski P, Cudnoch-Jedrzejewska A. Pathophysiological effect of bladder outlet obstruction on the urothelium. Ultrastruct Pathol 2018;42:317–22 [DOI] [PubMed] [Google Scholar]
- 61. Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 2007;4:46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Quiros M, Feier D, Birkl D, Agarwal R, Zhou DW, Garcia AJ, Parkos CA, Nusrat A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc Natl Acad Sci USA 2020;117:9477–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daneshgari F, Leiter EH, Liu G, Reeder J. Animal models of diabetic uropathy. J Urol 2009;182:S8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem 2005;280:15267–78 [DOI] [PubMed] [Google Scholar]
- 65. Albuquerque-Souza E, Schulte F, Chen T, Hardt M, Hasturk H, Van Dyke TE, Holzhausen M, Kantarci A. Maresin-1 and Resolvin E1 promote regenerative properties of periodontal ligament stem cells under inflammatory conditions. Front Immunol 2020;11:585530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pirault J, Back M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front Pharmacol 2018;9:1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest 2019;129:5294–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kawasaki S, Soga M, Sakurai Y, Nanchi I, Yamamoto M, Imai S, Takahashi T, Tsuno N, Asaki T, Morioka Y, Fujita M. Selective blockade of transient receptor potential vanilloid 4 reduces cyclophosphamide-induced bladder pain in mice. Eur J Pharmacol 2021;899:174040. [DOI] [PubMed] [Google Scholar]
- 69. Fujita M, Kasai E, Omachi S, Sakaguchi G, Shinohara S. A novel method for assessing bladder-related pain reveals the involvement of nerve growth factor in pain associated with cyclophosphamide-induced chronic cystitis in mice. Eur J Pain 2016;20:79–91 [DOI] [PubMed] [Google Scholar]
- 70. Tooke K, Girard B, Vizzard MA. Functional effects of blocking VEGF/VEGFR2 signaling in the rat urinary bladder in acute and chronic CYP-induced cystitis. Am J Physiol Renal Physiol 2019;317:F43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Choi SH, Byun Y, Lee G. Expressions of uroplakins in the mouse urinary bladder with cyclophosphamide-induced cystitis. J Korean Med Sci 2009;24:684–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Narla ST, Bushnell DS, Schaefer CM, Nouraie M, Bates CM. Keratinocyte growth factor reduces injury and leads to early recovery from cyclophosphamide bladder injury. Am J Pathol 2020;190:108–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kim SJ, Kim J, Na YG, Kim KH. Irreversible bladder remodeling induced by fibrosis. Int Neurourol J 2021;25:S3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211067465 for Specialized pro-resolution mediators in the bladder: Receptor expression and recovery of bladder function from cystitis by Francis M Hughes, Armand Allkanjari, Michael R Odom, Huixia Jin and J Todd Purves in Experimental Biology and Medicine