Abstract
Cucumber (Cucumis sativus L.) has been a model for the study of sex differentiation over the last two decades. Cucumber sex differentiation is mainly under genetic control, but plant growth regulators can also influence or even change it. However, the effect of exogenous auxin application on cucumber sex differentiation is not well understood at the physiological level. In this study, we explored the effects of different exogenous auxin concentrations on cucumber varieties with different mutant sex-controlling genotypes and found that there was a dosage effect of exogenous indole-3-acetic acid (IAA) on the enhancement of cucumber femaleness. Several ACC synthetase (ACS) family members responded directly to exogenous IAA, increasing endogenous ethylene synthesis, and this process appeared to be independent of the previously identified sex-related ACC oxidase CsACO2. We further demonstrated that ENHANCER OF SHOOT REGENERATION 2 (ESR2) responded to exogenous auxin induction by binding to ERE cis-acting element regions in the CsACS2 promoter, directly activating CsACS2 expression and thus increasing endogenous ethylene content, which may induce femaleness. These findings reveal that exogenous auxin increases cucumber femaleness by inducing a sex-controlling gene and promoting ethylene synthesis.
Introduction
Cucumber (Cucumis sativus L.) is an economically important vegetable crop widely cultivated throughout the world. Cucumber has become a model plant for the study of sex differentiation because of its diverse sex expression and extensive studies on its physiology and genetics [1, 2]. In terms of the ratio of different sex-type flower forms produced on the individual plant, the sexual systems of cucumber include mainly monoecy, gynoecy, hermaphroditism, andromonoecy, and androecy [3, 4].
A large number of studies have confirmed that ethylene is a core regulator of cucumber sex differentiation [5, 6]. Ethylene content has been positively correlated with the number of female flowers, whereas the application of aminoethoxyvinylglycine (AVG, which inhibits ethylene biosynthesis) and Ag+ reagent (which inhibits ethylene signaling) reduce plant feminization [7–10]. Three major loci (F/f, M/m, and A/a) have been proposed to determine the sex phenotype of cucumber, and all of them encode ACC synthases, the rate-limiting enzymes in ethylene synthesis [11]. Among them, CsACS1G is responsible for gynoecy conferred by the F locus [12]. The M gene encodes CsACS2, which is expressed in the carpel region of the female flower; its inactive form (m) results in the formation of bisexual flowers [13]. The A gene encodes CsACS11, and dysfunctional Csacs11 (a) blocks the developmental pathway of female flowers, resulting in androecious plants [14]. An additional androecious mutant was identified from a mutation of the CsACO2 gene [15]. The expression of CsACO2 has no sex specificity, but it initiates carpel development by promoting ethylene synthesis synergistically with CsACS1G and CsACS11, both of which are expressed specifically in female flowers [12, 15]. CsWIP1 encodes a C2H2 family transcription factor, and its mutation results in a plant with a few bisexual flowers at the lower nodes and continuous female flowers at the upper nodes [16]. CsWIP1 is believed to arrest the development of the carpel and inhibit the expression of CsACS2 [15, 16]. A network model has been proposed to explain the interaction among the sex-controlling genes: ethylene produced by CsACS1G and/or CsACS11 suppresses the expression of the carpel suppressor CsWIP1, activating CsACS2 expression to promote carpel development and inhibit stamen development [14, 17].
In addition to genetic factors and ethylene, auxin can also affect cucumber sex differentiation [6, 18–20]. Galun (1962) reported that IAA treatment could transform male flower buds into female flower buds in cucumber [11]. Takahashi & Jaffe (1984) found that exogenous IAA treatment could increase the female flower rate in cucumber by promoting ethylene biosynthesis [8]. Later, Trebitsh et al. (1987) speculated that the effect of auxin treatment on cucumber sex differentiation might be mediated by an increase in endogenous ethylene content [21].
An effect of auxin on the regulation of endogenous ethylene biosynthesis has been reported in plant growth and development. Auxin and ethylene have overlapping functions in fruit ripening [22]. A high concentration of IAA in peach (Prunus persica L.) fruit is necessary for ethylene biosynthesis during fruit ripening [23, 24]. In plums (Prunus salicina L.), exogenous auxin can promote ethylene synthesis and fruit ripening, possibly by promoting the expression of ACS ethylene synthesis genes and ERF ethylene response genes [25, 26]. In Arabidopsis thaliana, exogenous auxin treatment can significantly increase the expression of AtACS4 and induce more ethylene [27]. These studies demonstrated the effect of exogenous auxin treatment on ethylene synthesis in different plants.
The effect of auxin application on cucumber sex differentiation has been reported for more than half a century. However, whether the enhancement of cucumber femaleness under exogenous auxin treatment depends on ethylene synthesis and which regulatory factors are involved in this crosstalk still remain to be explored. In this study, we analyzed the effect of IAA on the femaleness of several cucumber varieties with different sex expressions and found that there was a dosage effect of exogenous auxin on the enhancement of femaleness. In addition, the sex-related ACS genes responded directly to induction by exogenous IAA, increasing endogenous ethylene synthesis. This promotion appeared to be independent of the previously identified ACC oxidase ACO2. In subsequent experiments, we demonstrated that IAA treatment-induced CsESR2 could directly activate CsACS2 by binding to ERE cis-acting elements in its promoter, thereby increasing endogenous ethylene content. Therefore, this study demonstrates that exogenous auxin affects cucumber femaleness by promoting ethylene synthesis, revealing an interaction network in which ethylene acts as the core and the crosslink with the auxin pathway in cucumber sex determination.
Results
Exogenous auxin enhanced cucumber femaleness
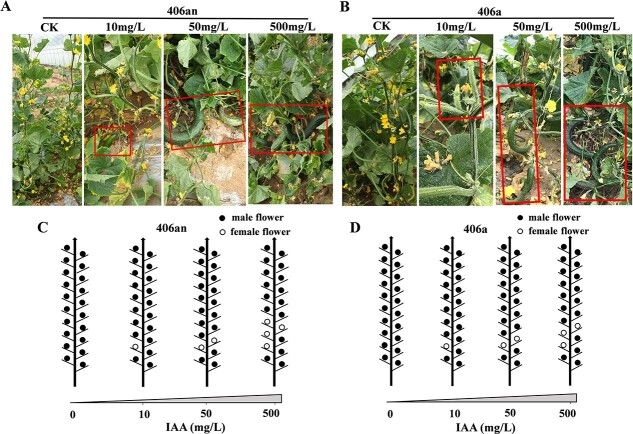
We first prepared four concentrations of IAA (0, 10, 50, and 500 mg/L) and selected five cucumber varieties with different sex types. After treatment with IAA solutions, the numbers of female flower nodes (or bisexual flowers in H38) were statistically analyzed. Numbers of female flowers and developing fruits increased in all tested lines after IAA treatment (Table 1). In the androecious lines 406an (Figure 1A) and 406a (Figure 1B), the female flower nodes were concentrated in the lower part of the plant, consistent with growth during the period of IAA treatment. As IAA concentration increased, the node numbers of female flowers increased to different degrees in all lines, and 406an (Figure 1C) and 406a (Figure 1D) plants showed the most obvious trend of female enhancement (Table 1). This result indicates that IAA treatment has a dosage-dependent effect on the process of femaleness induction. Most notably, in the androecious line 406an, approximately 3 and 4 female flower nodes were induced after 50 mg/L and 500 mg/L IAA treatment, respectively (Table 1). In addition, plant height was clearly inhibited at the 500 mg/L IAA concentration, indicating an obvious toxic effect (Figure S1).
Table 1.
Node numbers of female (bisexual) flowers in different cucumber lines after IAA treatment
| Cucumber / IAA (mg/L) | 406 | 406an | 406a | H38 | Gy14M-17 |
|---|---|---|---|---|---|
| 0 | 16.4 ± 1 | 0 | 0 | 2.5 ± 0.5 | 2.9 ± 0.7 |
| 10 | 16.9 ± 0.9 | 0.5 ± 0.5** | 0.2 ± 0.4 | 2.7 ± 0.5 | 3.5 ± 0.5* |
| 50 | 17.3 ± 0.7* | 2.2 ± 0.6** | 1.2 ± 0.4** | 3.4 ± 0.5** | 4.2 ± 0.8** |
| 500 | 18.2 ± 0.8** | 3.4 ± 0.7** | 2.3 ± 0.8** | 4.2 ± 0.6** | 4.9 ± 0.7** |
Values are mean ± SD (n=15 seedlings, *p<0.05, **p<0.01). Asterisks indicate significant differences determined by Student’s t-test.
Figure 1.
Female flowers induced by different concentrations of exogenous IAA in two cucumber androecious lines. A–B, Female flowers (in red box) of 406an and 406a induced by IAA. C–D, Schematic diagrams of the flower distribution in 406an and 406a induced by IAA. From the two-leaf stage, the seedlings were sprayed with IAA solution every five days for a total of four applications.
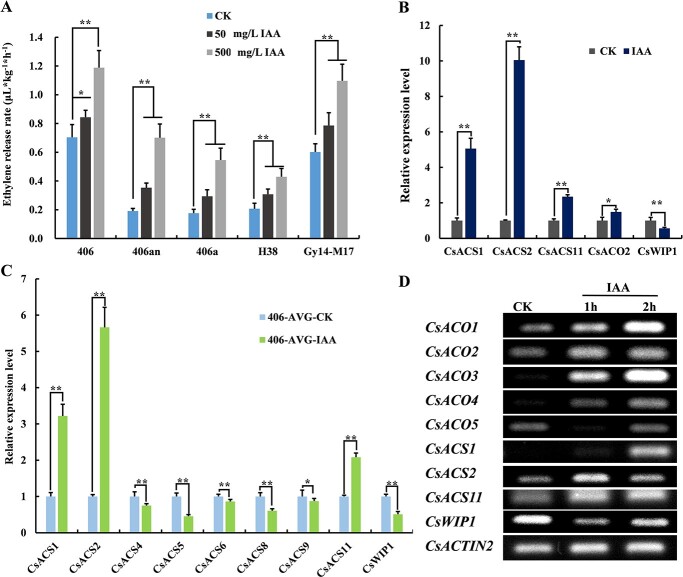
Given the femaleness enhancement observed after exogenous auxin treatment and the central role of endogenous ethylene in cucumber sex determination, we collected apical buds of different lines and measured their ethylene release rates after IAA treatment. The ethylene release rate increased significantly as IAA concentration increased in all varieties (Figure 2A). After 500 mg/L IAA treatment, the ethylene release rate of 406an showed the greatest increase, more than three times higher than that of the control. Similarly, the ethylene release rate of 406a was also increased about three-fold compared with that of the control. The increases in ethylene release rates of H38, 406, and GY14-M17 were moderate: about 100%, 80%, and 80% under the same treatment, respectively. These results showed that the change in ethylene release rate from apical buds corresponded strongly to female enhancement in different cucumber lines under exogenous auxin treatment, indicating a dosage-dependent effect of exogenous auxin on endogenous ethylene synthesis.
Figure 2.
Effect of exogenous IAA treatment on ethylene-related parameters in cucumber. A, Ethylene release rate of apical buds from 5 kinds of cucumber seedlings treated with IAA solution. Five biological replicates were performed. B, Expression of sex-controlling genes in apical buds of the 406 line treated with 50 mg/L IAA for 1 h. C, Expression of all ACS family genes in apical buds of the 406 line treated with 50 mg/L IAA for 1 h. Seedlings at the two-leaf stage were treated with AVG for 3 days and then treated with IAA. D, Expression of all ACO family genes and sex-controlling genes in apical buds of 406a after 1 h and 2 h of 50 mg/L IAA treatment. CsACTIN2 was used as the reference gene. Three biological replicates were performed in B and C. Values represent the mean and SD of all measurements, and Student’s t-test was used to determine significant differences in B and C (*p<0.05; **p<0.01).
Exogenous auxin induced expression of sex-controlling genes
The increases in ethylene release rate and femaleness may result from changes in the expression of ethylene synthesis-related and sex-controlling genes in cucumber. Therefore, after IAA treatment, we collected the apical buds of the 406 line and analyzed the expression of these genes (Figure 2B). CsACS1, CsACS2 and CsACS11 were induced after IAA treatment, and the expression change was greatest in CsACS2. The expression of CsWIP1 was significantly inhibited after IAA treatment, and the expression level of CsACO2 changed slightly.
Given the positive feedback effect of ethylene signaling in the process of cucumber sex determination [28], sex-controlling genes may indirectly respond to the endogenous ethylene induced by exogenous IAA. We first used the ethylene synthesis inhibitor AVG to interrupt endogenous ethylene synthesis, then treated with IAA solution. We analyzed all reported ACS genes in cucumber and found that only CsACS1, CsACS2, and CsACS11 were induced by exogenous IAA directly (Figure 2C), whereas other ACS genes did not respond to IAA induction. Among these genes, the induction effect on CsACS2 was the most obvious. In addition, as the suppressor of ethylene synthesis during sex determination, CsWIP1 showed significantly inhibited expression under exogenous IAA treatment.
Although the mutation of CsACO2 leads to an androecious line, we found that 406a still released ethylene (Figure 2A) and produced female flowers after IAA treatment (Table 1). We then analyzed the expression of all reported cucumber ACO genes after 1 and 2 h of IAA treatment. The expression levels of CsACO1, CsACO3, and CsACO4 increased with increasing IAA treatment time, and this effect was most obvious for CsACO3 (Figure 2D). CsACS2 and CsACS11 were induced rapidly at 1 h, and the upregulation of CsACS2 was greater. CsACS1 responded to auxin induction slowly and was upregulated at 2 h. The expression of CsWIP1 was inhibited at 1 h and then restored at 2 h (Figure 2D), in contrast to the expression pattern of CsACS2 under IAA treatment.
Identification of regulatory factors that respond to exogenous auxin
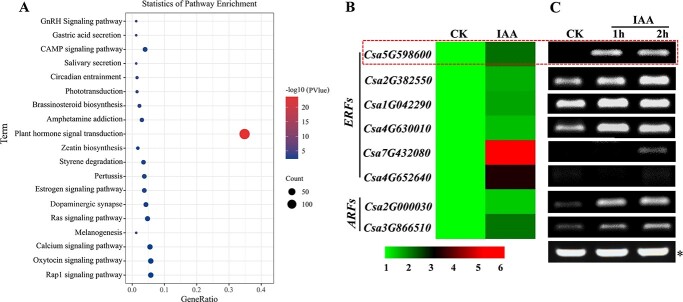
To explore the upstream factors that regulate ethylene synthesis under exogenous IAA treatment, we performed transcriptome sequencing on IAA-treated apical buds of the 406 line and identified 399 differentially expressed genes (DEGs) (Table S1). GO (Gene Ontology) term analysis showed that the DEGs were most significantly enriched in the hormone signaling response pathway (Figure 3A). We identified two auxin response factor (ARF) genes and six ethylene response factor (ERF) genes that were upregulated after IAA induction (Table S2): the ARFs Csa2G000030 and Csa3G866510 and the ERFs Csa2G382550, Csa4G630010, Csa4G652640, Csa7G432080, Csa5G598600, and Csa1G042290. Expression patterns of these candidate transcription factor genes are shown in a heat map (Figure 3B). The expression levels of these genes were also detected in the androecious line 406a after exogenous IAA treatment, and all were upregulated by the IAA treatment (Figure 3C). Csa4G630010, Csa5G598600, and Csa2G000030 were particularly highly induced after 1 h of IAA treatment.
Figure 3.
Identification of auxin-response transcription factors in the transcriptome of cucumber apical buds. A, Analysis of metabolic pathways associated with differentially expressed genes in the transcriptome. The red circle represents the plant hormone response pathway. B, Heat map of ARF and ERF transcription factor genes in DEGs. The scale from green to red indicates a gradual increase in gene expression level. C, Expression of ARF and ERF genes in the apical buds of 406a after 1 h and 2 h of 50 mg/L IAA treatments. *, the result of internal reference gene CsACTIN2. The red dotted box indicates the CsESR2 gene (Csa5G98600).
CsESR2 was involved in the female flower development of cucumber
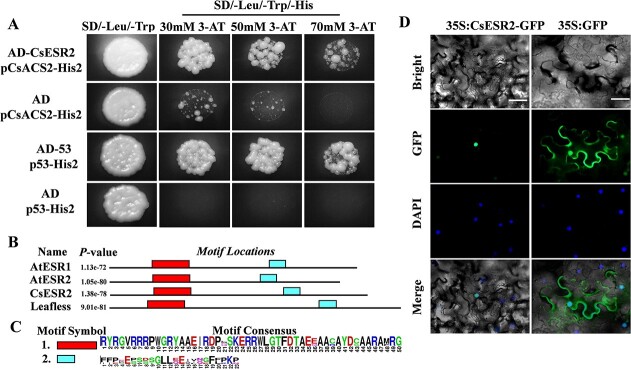
We demonstrated that sex-controlling genes (CsACS1, CsACS2, CsACS11, CsACO2, CsWIP1) could respond directly to induction by exogenous IAA, but whether the candidate ARF and ERF transcription factors could directly regulate these genes remained to be confirmed. A Y1H assay indicated that only Csa5G598600 showed binding activity to the promoter of CsACS2, whereas Csa2G000030 (ARF) and another ERF transcription factor, Csa4G630010, which were highly induced by exogenous IAA, did not bind to the promoters of these genes (Figure 4A, Figure S4). According to its gene annotation in the CuGenDB database, Csa5G598600 encodes the ethylene response factor CsESR2 in cucumber, a homolog of Arabidopsis ESR2/DRNL. Sequence alignment and motif analysis revealed that the ESR2 homologs in three species (Arabidopsis AtESR1/AtESR2, tomato LFS, and cucumber CsESR2) were highly conserved (Figure S2), especially in the AP2 domain at the N terminus and the ESR domain at the C terminus (Figure 4B, C). Subcellular localization revealed that CsESR2 had a clear nuclear localization signal (Figure 4D) and may thus play the role of a regulator, binding to the promoters of downstream genes. Cis-acting elements in the 3-kb promoter sequence of CsESR2 were predicted, and three sites of auxin response elements (−1120, −2811, −2833) were found (Table S3).
Figure 4.
Characteristics of the candidate gene CSESR2. A, The results of a Y1H assay showing direct binding between CsESR2 and the CsACS2 promoter. Vector combinations of pGADT7-53/p53-His2 and pGADT7/p53-His2 were used as the positive and negative controls, respectively. A, A series of 3-AT concentrations (30 mM, 50 mM, and 70 mM) were used for the test of yeast growth on selective medium (−Leu, −Trp, -His), and all combinations grew normally on non-selective medium (−Leu, −Trp). B, Identification of conserved motifs in the amino acid sequences of ESR homologs (AtESR1, AT1G12980; AtESR2, AT1G24590; SlESR2/LEAFLESS, SL05G013540; CsESR2, Csa5G598600). C, Conserved sequence analysis of motif1 (red rectangle) and motif2 (blue rectangle) in B. D, Subcellular localization analysis of CsESR2 in tobacco leaf epidermal cell. 35S:GFP was used as the positive control, and DAPI staining indicated the nucleus. Bar = 50 μm.
In situ hybridization was used to detect the expression pattern of CsESR2 at different stages of floral primordium development. CsESR2 was strongly expressed in the floral primordium at stages 1 and 2, during which stages specific floral tissues could not be identified (Figure 5A–C). At stage 3, with the differentiation of specific floral tissues, the expression of ESR2 could be clearly identified in the petal primordia. From stage 4 to stage 5, its expression could be detected in the primordia of carpels, stamens, and petals (Figure 5D–F). Subsequently, in female flower buds, the expression of CsESR2 was concentrated at the differentiated carpel primordium at stage 6 and was only obvious at the apex of the carpel primordium at stage 7 (Figure 5G, H). At stage 8-1, its expression was maintained at the apex of the carpel primordium and increased at the developing ovule primordium (Figure 5I). However, at stage 8-2, only the expression in the ovule area was maintained (Figure 5J). A cross-sectional view of the developing ovary showed that CsESR2 was expressed near the site of ovule and placenta formation at stage 9 in the female flower (Figure 5K).
Figure 5.
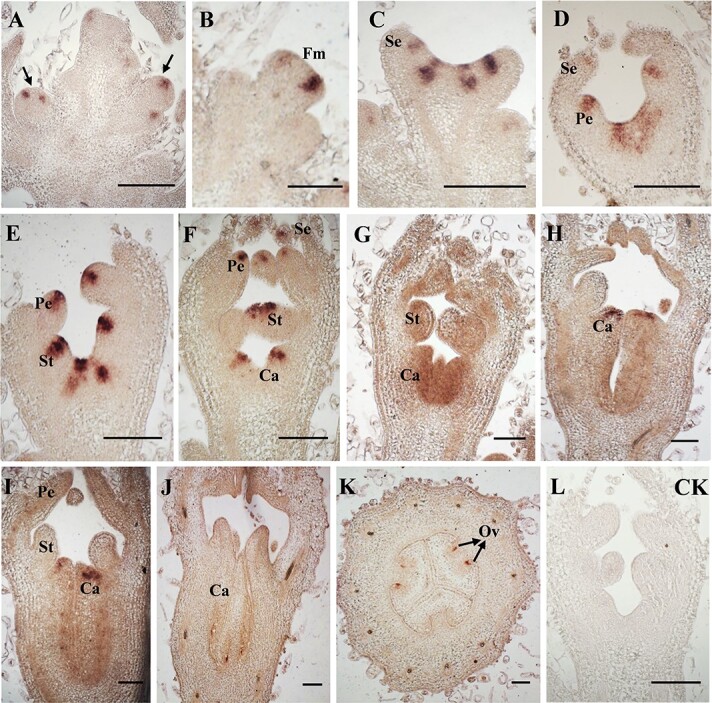
In situ hybridization of CsESR2 mRNA during different developmental stages of cucumber flower buds. A, Newly initiated floral buds (early stage 1) are indicated by arrowheads. B–H, Expression patterns of CsESR2 from stage 1 to stage 7 of flower bud differentiation. I–J, Expression patterns of CsESR2 at stages 8-1 and 8-2. K, Expression pattern of CsESR2 at stage 9. L, Negative control with no expression signal. Fm, floral meristem; Se, sepal; Pe, petal; St, stamen or stamen primordium; Ca, Carpel; Ov, ovary. Bar = 100 μm.
CsESR2 directly activated CsACS2 to participate in cucumber sex determination
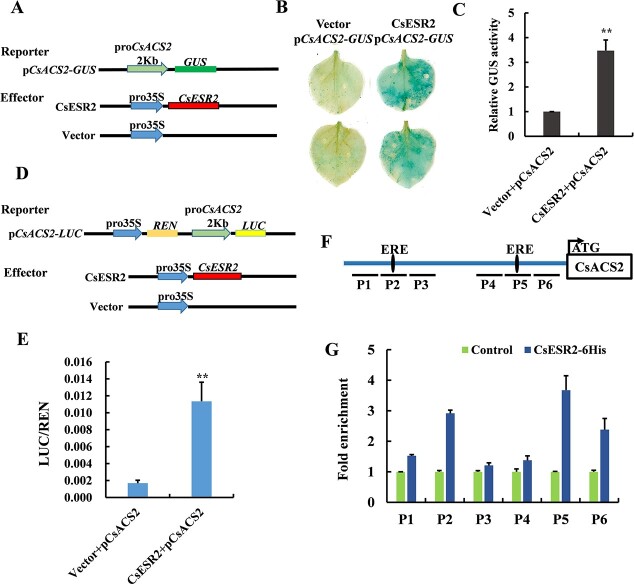
A tobacco transient transformation system was used to verify the regulatory relationship between CsESR2 and CsACS2 (Figure 6A). The effector contained the CsESR2 CDS driven by a CaMV35S promoter. The reporter construct included the 2-kb promoter sequence of CsACS2 (pCsACS2) integrated upstream of a GUS gene. A mixture of Agrobacterium solution with effector and reporter constructs was injected into tobacco leaves, and the experimental group that received CsESR2 and pCsACS2 showed stronger catalytic activity for X-Gluc substrate than the control group (Figure 6B). Quantitative analysis showed that the GUS enzymatic activity of the experimental group was about 3.5 times higher than that of the control group (Figure 6C). A dual luciferase (LUC) reporter assay was used to further verify the activation effect, and recombinant plasmids of the corresponding reporter and effector were constructed as shown in Figure 6D. The LUC enzyme activity of the experimental group (CsESR2 and pCsACS2) was more than 7 times that of the control group (empty vector and pCsACS2) (Figure 6E). This result further confirmed that CsESR2 could upregulate the expression of CsACS2.
Figure 6.
CsESR2 directly regulates the expression of CsACS2. A, Schematic diagram of the vectors used in the tobacco transient expression system. B, GUS staining was used to detect the effect of CsESR2 on the promoter activity of CsACS2. C, GUS enzyme activity assay was used to quantitatively analyze the influence of CsESR2 on the activity of the CsACS2 promoter. The data are presented as the average of ten biological replicates, and error bars represent the SD. Student’s t-test was used to determine significant differences (**p<0.01). D, Schematic diagram of the vectors used in the dual luciferase reporter system. E, Luc enzyme activity assay was used to quantitatively analyze the influence of CsESR2 on the promoter activity of CsACS2. The data are presented as the average of ten biological replicates, and error bars represent the SD. Student’s t-test was used to determine significant differences (**p<0.01). F, Six promoter regions (P1 to P6) were used in the ChIP-PCR assay. The two black ellipses represent the two ERE elements in the promoter. G, ChIP-PCR assay in transgenic tobacco leaves with 35S:CsESR2-GFP-6His construct. Uninjected transgenic tobacco leaves were used as a negative control. An “input DNA” control was used as an internal reference for normalization. Error bars represent the SD of three biological replicates.
As reported previously, two EREs (ethylene-responsive elements, core sequence AWTTCAA) at −1334 bp (the distant ERE, named D-ERE) and −154 bp (the near ERE, named N-ERE) sites were predicted in the CsACS2 promoter (Li et al., 2012). ChIP-qPCR was used to determine whether CsESR2 could bind to the EREs. Six regions of the CsACS2 promoter, including the two ERE elements and their adjacent regions (Figure 6F), were selected for enrichment detection. The enrichment signal of CsESR2 was clearly detected in the P2 and P5 regions (Figure 6G), which contained the D-ERE and N-ERE, respectively, and the enrichment level was higher in the P5 region than in the P2 region.
Discussion
Exogenous auxin enhanced cucumber femaleness in a dose-dependent manner
As early as the 1960s, Galun (1962) found that auxin treatment could transform male flower buds to female flower buds in cucumber [11]. However, the suitable conditions for exogenous auxin were not clear. Our results showed that the femaleness enhancement of cucumber seedlings increased with increasing IAA concentration, which indicated that exogenous auxin may induce female flowers in a dosage-dependent manner. Higher IAA concentrations (for example, 500 mg/L in this study) can have stronger effects on femaleness enhancement, and the androecious 406an line showed the most obvious induction effect. Consistent with these results, ethylene release rates of the treated lines rose with increasing IAA concentration, indicating that femaleness enhancement after exogenous IAA treatment may depend on endogenous ethylene synthesis in cucumber.
Cucumber sex-controlling genes can respond to regulation by exogenous auxin
After IAA treatment, ethylene biosynthesis-related genes (CsACS1, CsACS2, and CsACS11) that function in cucumber sex determination were upregulated, whereas the female suppressor gene CsWIP1 was downregulated. To exclude a feedback effect of ethylene signaling on these genes [28], we used an ethylene synthesis inhibitor to first eliminate the endogenous ethylene background and then analyzed the induction effect again (Figure 2C). Among the ACS family members, only CsACS1, CsACS2, and CsACS11 were directly induced by IAA treatment, and CsACS2 showed the strongest upregulation. As a repressor of ethylene synthesis in cucumber sex determination, CsWIP1 showed significantly lower expression under exogenous IAA treatment (Figure 2C, D), consistent with the upregulation of three ACS genes (Figure 2C, D) and the increase in ethylene release (Figure 2A). These results indicated that the antagonistic relationship between CsWIP1 expression and ethylene synthesis was still present in exogenous auxin-induced cucumber sex determination. Because the mutation in CsACO2 (in the 406a line) results in the inhibition of ethylene synthesis in sex determination [12, 15], the 406a line was used to determine the direct effect of exogenous IAA on sex-controlling genes. At 1 h of IAA treatment, CsACS2 and CsACS11 were upregulated, and the upregulation of CsACS2 was greater than that of CsACS11. In cucumber, CsACS2 and CsACS11 encode ACC synthases with different functions in sex determination. The ethylene synthesized jointly by CsACS11 and CsACO2 can promote the expression of CsACS2, which then inhibits stamen development [12, 17]. Mutations in CsACS2 and CsACS11 lead to the emergence of andromonoecious and androecious plants, respectively [14, 29]. Such functional differences between CsACS2 and CsACS11 were also found in the induction of female flowers by exogenous auxin. In this study, increased ethylene release rate and femaleness were more pronounced in the 406an line (with a mutant CsACS11 gene) than in the H38 line (with a mutant CsACS2 gene) (Figure 2A and Table 1). Therefore, we speculated that CsACS2 may be more sensitive than CsACS11 in femaleness enhancement induced by exogenous auxin.
Exogenous auxin can enhance cucumber femaleness in a CsACO2-independent manner
CsACO2 can coordinate with CsACS11 to affect female flower development through preferential ethylene production in flowers, and mutation of CsACO2 eliminated female flowers in the 406a line [12, 15]. However, when 406a plants were treated with exogenous IAA, obvious enhancement of ethylene release and femaleness were observed, indicating that other ACO family gene(s) could promote ethylene synthesis. After IAA treatment, the expression of CsACO1, CsACO3, and CsACO4 was upregulated with time, and the upregulation of CsACO3 was the most obvious. Previous studies have shown that CsACO1 and CsACO3 are partly expressed in flower buds before stage 6, and the apical buds of a CsACO2 mutant still retained some ethylene synthesis ability [15]. In this study, after exogenous IAA induction, ACO family members other than CsACO2 may have also participated in sex determination-related ethylene synthesis, thus inducing female flowers.
CsESR2 may be the link between auxin and ethylene in cucumber sex determination
It has previously been reported that crosstalk between auxin and ethylene depends on the interaction of their signaling and/or biosynthetic pathways [30]. Several ARFs and ERFs were identified in the transcriptome data (Table S2), and two ERF genes (CsESR2 and Csa4G630010) and one ARF gene (Csa2G000030) were highly induced by auxin treatment, suggesting that they may play regulatory roles in this crosstalk (Figure 3C).
Our results revealed that ethylene synthesis-related genes (CsACS2 and CsACS11) involved in sex determination responded directly to auxin induction (Figure 2C, D) and further confirmed the direct binding relationship between CsESR2 and the sex-controlling gene CsACS2. CsESR2 had high homology to AtESR2 in Arabidopsis and LFS in tomato (Figure 4B and Figure S2). AtESR2 and LFS have been shown to participate in organ formation in response to auxin induction in the meristem region [31, 32]. In Arabidopsis pistils, an auxin marker coincided with the expression signal of AtESR2 [33, 34]. The findings that CsESR2 was expressed in floral organ primordia at the early growth stage and that three auxin responsive elements were present in the CsESR2 promoter suggested that CsESR2 was likely to respond to auxin induction and participate in early floral organ development in cucumber.
CsESR2 is involved in enhancing femaleness through activation of CsACS2
Previous studies have confirmed that AtESR2 is expressed in the founder cells of lateral organs and floral organs [33]. AtESR2 is also expressed in the ovary region during late pistil development, and its mutation caused pistil dysplasia [35]. We demonstrated that CsESR2 was expressed at different stages and positions in the developing cucumber flower (Figure 5). Previous studies have shown that stage 4 is the key period for the sex determination of a flower bud, with the co-occurrence of CsACO2 and CsACS11 at stage 4 in female flower buds [15]. The expression of CsACS2 begins from the initiation of the carpel primordium at stage 5 and continues until ovary formation [37]. CsESR2 is strongly expressed at the early stage of the flower primordium, and at stage 5, its expression region overlaps with that of CsACS2 at the upper part of the carpel primordium [37]. From stage 5 to stage 8, the expression regions of CsESR2 and CsACS2 continue to overlap and shift from the upper to the lower part of the carpel primordium. In addition, both are expressed near the site of ovule and placenta formation in stage 9 of the ovary based on cross sectional analysis [37]. The earlier transcription of CsESR2 and its similar expression pattern to CsACS2 after stage 5 support the possibility that CsESR2 may regulate the expression of CsACS2 during flower development.
The direct activation of CsACS2 by CsESR2 was demonstrated in this study (Figure 4A and Figure 6). Previous studies have reported that CsERF31 binds to the D-ERE and triggers a positive feedback loop of ethylene during female flower differentiation [38]. Similarly, our ChIP-qPCR result showed that CsESR2 can bind to the promoter of CsACS2 through the ERE element regions (Figure 6F, G). This result demonstrated that CsESR2, as an upstream regulator of CsACS2, could activate its expression.
In Figure 7, we summarize the femaleness enhancement mechanisms of several materials with different sex types under exogenous auxin treatment. For monoecious material (406) with no mutations in sex-controlling genes, exogenous auxin regulates the expression of these genes to promote normal ethylene synthesis and increase the number of female flowers. For androecious materials (*, the CsACS11 mutation in the 406an mutant; **, the CsACO2 mutation in the 406a mutant), mutation of CsACS11 or CsACO2 leads to early obstruction of ethylene synthesis during sex determination. This releases CsWIP1 and inhibits CsACS2 expression [12, 15], resulting in the phenotype of all-male flowers. However, exogenous auxin can inhibit CsWIP1 expression and directly promote CsACS2 expression through CsESR2, thus bypassing the dependence on early ethylene synthesis and inducing female flowers. For andromonoecious materials (***, the CsACS2 mutation in the H38 mutant), early ethylene can promote carpel development, but late ethylene that relies on CsACS2 cannot be synthesized normally, and stamen development cannot be inhibited, resulting in bisexual flowers [12]. Under exogenous auxin treatment, the expression of CsACS11 and CsACO2 is activated, and increased ethylene synthesis in the early stage promotes carpel development; however, stamens are still not inhibited owing to the absence of late ethylene, resulting in an increased number of bisexual flowers under exogenous auxin treatment.
Figure 7.
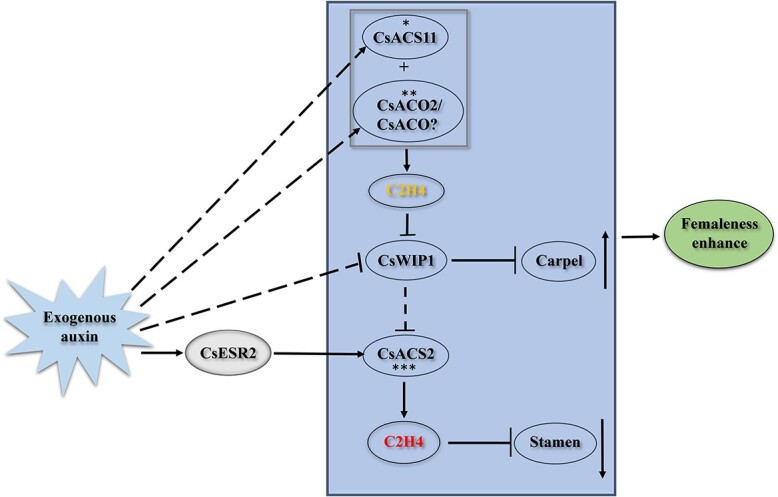
A mechanistic model of the enhancement of femaleness in materials of different sex types in response to exogenous auxin treatment. The square box contains sex-controlling genes. ➜, positive regulation;  , negative regulation; +, synergy; —, direct effect; indirect effect. *, CsACS11 mutation in 406an; **, CsACO2 mutation in 406a; ***, CsACS2 mutation in H38; CsACO?, CsACO genes induced by exogenous auxin, excluding CsACO2; yellow C2H4, ethylene in the early stage; red C2H4, ethylene in the late stage.
, negative regulation; +, synergy; —, direct effect; indirect effect. *, CsACS11 mutation in 406an; **, CsACO2 mutation in 406a; ***, CsACS2 mutation in H38; CsACO?, CsACO genes induced by exogenous auxin, excluding CsACO2; yellow C2H4, ethylene in the early stage; red C2H4, ethylene in the late stage.
In the 406a mutant, the number of female flowers increased after treatment with exogenous auxin, indicating that CsACO genes (CsACO?) other than CsACO2 may be involved in cucumber sex determination under exogenous auxin treatment. However, further experiments need to be performed to identify possible candidate CsACO genes. In conclusion, we demonstrated that CsESR2 acts as a bridge between exogenous auxin signaling and endogenous ethylene synthesis in cucumber sex determination and constructs a crosslink between the two hormones.
Materials and methods
Materials and growth conditions
The cucumber inbred lines used in the following experiments were 406 [15] (monoecious line, ffMMAA), GY14M-17 [39] (androecious line, ffMMAA), H38 [13] (CsACS2 mutant, andromonoecious line, ffmmAA), 406a [15] (CsACO2 mutant, androecious line, ffMMAA&aco2aco2) and 406an (CsACS11 mutant, androecious line, ffMMaa). The 406a and 406an mutants were identified from an EMS mutagenized mutant library of the 406 line. In 406an, a change from C to T at the 902th nucleotide of the CsACS11 CDS results in an amino acid change from tryptophan (S) to phenylalanine (F) in the conserved BOX6 motif (Figure S3). This amino acid has been shown to be highly conserved in a variety of plants [14]. Cucumber seedlings at the two-leaf stage were grown in light incubators at 28°C/16 h light and 20°C/8 h darkness. Cucumber seedlings with two fully expanded true leaves were transferred to the horticultural greenhouses at Northwest A&F University in Yangling, China.
Exogenous auxin treatment
IAA (Sigma, USA) solutions of four concentrations (0, 10, 50, and 500 mg/L) were prepared. The apical buds of cucumber seedlings at the two-leaf stage were sprayed with IAA solution, and this was repeated every 5 days for a total of four applications. For each cucumber line with a different sex type, 15 seedlings were treated with each concentration of IAA solution. This experiment was repeated in three cucumber growing seasons.
Number of female nodes in cucumber
A node with one or more blooming female flowers is considered to be a female node. The numbers of female flower nodes of 7-week-old plants treated with IAA solution were counted for the first 20 nodes (bisexual flower nodes in H38 were recorded). Fifteen plants were counted in each treatment, and the experiment was repeated in three cucumber growing seasons.
Determination of ethylene release rate
About 0.5 g of apical buds were excised from plants treated with IAA solution and placed in a 12-mL container sealed with a rubber stopper. After incubation at 28°C for 16 h, 1 mL headspace gas was withdrawn using a gas-tight syringe. The gas was analyzed using a TRACE GC Ultra gas chromatograph (Thermo Scientific, USA), and the instrument was calibrated with an ethylene gas standard before measurement. The ethylene release rate (μL/kg·h) of cucumber apical buds was calculated from the measured data. Three biological replicates were performed for each IAA-treated line.
Gene expression analysis
When 406 and 406a plants had grown to the four-leaf stage, they were treated with 50 mg/L IAA. The apical buds of 406 were collected for RNA extraction after one hour, and 406a buds were collected after one and two hours. Meanwhile, naturally growing 406 plants were treated with 1 mM AVG (Sigma, USA) in H2O containing 0.1% (V/V) Tween20 using a pipettor once a day for 3 days, and then 50 mg/L IAA was sprayed on the plants. Solution without IAA was used as the control. RNA extraction and first-strand cDNA synthesis were carried out as described previously [28]. Q311-02 SYBR qPCR Master Mix (Vazyme, China) and an ABI 7500 instrument (Thermo Scientific, USA) were used for quantitative real-time PCR (qRT-PCR). Three replicates were measured for each sample, and CsACTIN2 was used as the reference gene. Relative gene expression was calculated using the 2−ΔΔCt formula. Semi-quantitative PCR was performed for 33 cycles (94°C, 20 s; 60°C, 20 s; 72°C, 20 s), and the products were detected on a 1.5% agarose gel. CsACTIN2 was used as the reference gene to balance the added volume of different cDNA samples. The related primers are listed in Supplemental Table S4.
Transcriptome analysis
Apical buds of the 406 line at the four-leaf stage were sprayed with 50 mg/L IAA, and the tissues were harvested after 1 h. Control buds were treated with solution without IAA. At least six apical buds were mixed into one sample, and three sample replicates were analyzed by RNA-seq. RNA-seq libraries were constructed using the NEBNext Ultra Directional RNA Library Prep Kit (NEB, USA). DEGs were required to show at least a 1.5-fold expression change between the IAA-treated plants and controls with a false discovery rate (FDR) less than 0.05.
Cloning of gene sequences and subcellular localization analysis
The cDNA and genomic DNA of the 406 line were used to clone coding sequences (CDSs) and promoter sequences of the target genes, respectively. Genomic DNA of the 406 line was extracted as described previously [29]. All sequences were amplified using high-fidelity DNA polymerase (Vazyme, China) with specific primers (Supplemental Table S4). The PCR product was fused with the pMD19-T cloning vector (Takara, Japan) and identified by sequencing.
The CsESR2 cDNA was fused to the pGreenII-GFP vector for detection of subcellular localization, and all operations were performed as described previously [40]. DAPI was used to stain and label the nucleus. Specific gene primers are listed in Supplemental Table S4.
Bioinformatics analysis
Cucumber gene information in this study was obtained from the Cucurbitaceae Genome Database (CuGenDB, http://cucurbitgenomics.org/). The AtESR2 sequence of A. thaliana was obtained from the TAIR database (https://www.arabidopsis.org/), and the LFS (SlESR2) sequence of Solanum lycopersicum was obtained from the Solanaceae Genome Database (https://solgenomics.net/). Amino acid sequences of ESR2 homologs in cucumber, tomato and Arabidopsis were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Conserved motifs of ESR2 homologs were analyzed using the MEME program (http://meme-suite.org/tools/meme). Putative cis-acting elements in the CsACS2 promoter were identified using the PlantCARE database [41].
Yeast one-hybrid (Y1H) assay
Promoter sequences (2 kb) of CsACS1, CsACS2, CsACS11, CsACO2, and CsWIP1 were ligated into the pHis2 bait vector, and the CDSs of CsESR2, Csa4G630010, and Csa2G000030 were cloned into the pGADT7 prey vector. The yeast strain Y187 was used for transformation with a yeast transformation kit (Coolaber, China). Three concentrations (30, 50, and 70 mM) of 3-amino-1,2,4-triazole (3-AT) were used to inhibit background activity of the recombinant bait vector. The combination of pGADT7-53 and p53-His2 was transformed as a positive control, and the combination of pGADT7 and p53-His2 was transformed as a negative control. Specific primers are listed in Supplemental Table S4.
In situ hybridization assay
Cucumber flower buds of different developmental stages were fixed in 3.7% formalin-acetic acid-alcohol (FAA) solution and used for in situ hybridization (ISH) as described previously [12]. Sense and antisense probes were synthesized using SP6 and T7 polymerase, respectively. Probes for CsESR2 were designed using the 5′ region of the CDS sequence. Probe primers are listed in Supplemental Table S4. The stages of cucumber flower bud differentiation were divided according to Bai et al. (2004) [36].
Transient expression assay in tobacco leaves and GUS staining
The CsESR2 cDNA was fused into the pCAMBIA1305.4 vector to construct the pCAMBIA1305.4-35S:CsESR2-6xHis effector, and the pBI121-proCsACS2:GUS plasmid was used as a reporter [42]. All the vectors were transformed into the GV3101 (pSoup) Agrobacterium strain. A mixed bacterial solution of effector and reporter was injected into tobacco (N. benthamiana) leaves in a volume ratio of 9 to 1, and a bacterial mixture of the pCAMBIA1305.4 empty vector and the effector was injected as the negative control. Measurement of GUS activity was performed as described previously [43]. Each bacterial solution combination was injected independently into ten tobacco leaves. Related primers are listed in Supplemental Table S4.
Dual luciferase reporter analysis
The CsESR2 cDNA was fused into the pGreenII62-SK vector to construct the pGreenII62-SK-35S:CsESR2 effector, and the pGreenII0800-pCsACS2:LUC plasmid was used as the reporter [42]. A mixed bacterial solution of effector and reporter was injected into tobacco leaves in a volume ratio of 9 to 1, and the bacterial mixture of the pGreenII0800 empty vector and the effector was injected as the negative control. Each bacterial solution combination was injected independently into ten tobacco leaves. After 3 d, the injected leaves were removed and ground with liquid nitrogen. The samples were processed according to the instructions of the Dual-Luciferase Reporter Assay System kit (Promega, USA). The luciferase activities of firefly luciferase (LUC) and renilla luciferase (REN) in the samples were detected using an Infinite M200 PRO full-wavelength microplate reader (Tecan, Switzerland). Related primers are listed in Supplemental Table S4.
ChIP-qPCR (chromatin immunoprecipitation assay-qPCR) assay
Transgenic tobacco leaves containing the CsACS2 promoter-GUS fusion (transformed with the pBI121-proCsACS2:GUS vector [42]) were infiltrated with GV3101 Agrobacterium solution containing the pCAMBIA1305.4-35S:CsESR2-6His fusion vector (effector) or the pCAMBIA1305.4 empty vector (negative control). Positive infiltration was confirmed by observation of the GFP signal using a BX63 fluorescence microscope (Olympus, Japan). The ChIP assay was performed as described by Chen et al. (2016) [15] using an anti-6xHis antibody (Thermo Fisher Scientific, USA). After the ultrasonic treatment, 20-μL DNA samples from infiltrated leaves containing the effector or the negative control were extracted separately for “input DNA” control. The binding intensity between CsESR2 and the CsACS2 promoter fragment was examined by qPCR in triplicate, and the “input DNA” control was used to normalize the results. One microliter of the immunoprecipitated chromatin product was used as the qPCR template. The enrichment level was assessed in six regions of the CsACS2 promoter. Related primers are listed in Supplemental Table S4.
Supplementary Material
Acknowledgements
We thank Dr. Jinjing Sun from the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, for kindly providing the cucumber 406, 406a, and 406an seeds. We thank Dr. Mingxun Chen from Northwest A&F University for technical support in the ChIP-qPCR analysis. We are grateful for funding support from the National Key Research and Development Program of China (2019YFD1000300), the National Natural Science Foundation of China (32072596 and 31872111), and the Science and Technology Innovation Team of Shaanxi (2021TD-32).
Author Contributions
J.S. and Z.L. designed and supervised this study. H.N., H.W., B.Z., J.H., L.Y., X.M., and J.C. performed the research and data analysis. H.N. and J.S. prepared the manuscript with input from all co-authors.
Data availability
The data that support the results are included in this article and its supplementary materials. Other relevant materials are available from the corresponding author upon reasonable request.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
References
- 1. Barak M, Trebitsh T. A developmentally regulated GTP binding tyrosine phosphorylated protein A-like cDNA in cucumber (Cucumis sativus L.). Plant Mol Biol. 2007;65:829–37. [DOI] [PubMed] [Google Scholar]
- 2. Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. Plant Cell. 2004;16:S61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iezzoni AF, Peterson CE. Linkage of bacterial wilt resistance and sex expression in cucumber. HortScience. 1980;15:257–8. [Google Scholar]
- 4. Malepszy S, Niemirowicz-Szczytt K. Sex determination in cucumber (Cucumis sativus) as a model system for molecular biology. Plant Sci. 1991;80:39–47. [Google Scholar]
- 5. Rudich J, Halevy AH, Kedar N. Increase in femaleness of three cucurbits by treatment with Ethrel, an ethylene releasing compound. Planta. 1969;86:69–76. [DOI] [PubMed] [Google Scholar]
- 6. Yin T, Quinn JA. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am J Bot. 1995;82:1537–46. [Google Scholar]
- 7. Atsmon D, Tabbak C. Comparative effects of gibberellin, silver nitrate and aminoethoxyvinyl glycine on sexual tendency and ethylene evolution in the cucumber plant (Cucumis sativus L.). Plant Cell Physiol. 1979;20:1547–55. [Google Scholar]
- 8. Takahashi H, Jaffe MJ. Further studies of auxin and ACC induced feminization in the cucumber plant using ethylene inhibitors. Phyton. 1984;44:81–6. [PubMed] [Google Scholar]
- 9. Kahana A, Silberstein L, Kessler Net al. Expression of ACC oxidase genes differs among sex genotypes and sex phases in cucumber. Plant Mol Biol. 1999;41:517–28. [DOI] [PubMed] [Google Scholar]
- 10. Kamachi S, Sekimoto H, Kondo Net al. Cloning of a cDNA for a 1-aminocyclopropane-1-carboxylate synthase that is expressed during development of female flowers at the apices of Cucumis sativus L. Plant Cell Physiol. 1997;38:1197–206. [DOI] [PubMed] [Google Scholar]
- 11. Galun E. Study of the inheritance of sex expression in the cucumber. The interaction of major genes with modifying genetic and non-genetic factors. Genetica. 1962;32:134–63. [Google Scholar]
- 12. Zhang H, Li S, Yang Let al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene ACS1G induces female flower development in cucumber gynoecy. Plant Cell. 2021;33:306–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Z, Huang S, Liu Set al. Molecular isolation of the M gene suggests that a conserved-residue conversion induces the formation of bisexual flowers in cucumber plants. Genetics. 2009;182:1381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boualem A, Troadec C, Camps Cet al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science. 2015;350:688–91. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Sun J, Li Set al. An ACC oxidase gene essential for cucumber carpel development. Mol Plant. 2016;9:1315–27. [DOI] [PubMed] [Google Scholar]
- 16. Hu B, Li D, Liu Xet al. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017;10:1575–8. [DOI] [PubMed] [Google Scholar]
- 17. Li D, Sheng Y, Niu Het al. Gene interactions regulating sex determination in cucurbits. Front Plant Sci. 2019;10:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freidlander M, Atsmin D, Galan E. Sexual differentiation in cucumber: abscisic and gibberellic acid contents of various sex genotypes. Plant Cell Physiol. 1977;18:681–91. [Google Scholar]
- 19. Rudich J, Halevy AH, Kedar N. Ethylene evolution from cucumber plants as related to sex expression. Plant Physiol. 1972;49:998–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roy R, Saran S. Sex expression in the Cucurbitaceae. In: Biology and Utilization of the Cucurbitaceae. Cornell University Press, New York, 2019, 251–68. [Google Scholar]
- 21. Trebitsh T, Rudich J, Riov J. Auxin, biosynthesis of ethylene and sex expression in cucumber (Cucumis sativus L.). Plant Growth Regul. 1987;5:105–13. [Google Scholar]
- 22. Muday GK, Maloney GS, Lewis DR. Integration of ethylene and auxin signaling and the developmental consequences of their crosstalk. In: Ethylene in Plants. Springer, Netherlands, 2015,175–204. [Google Scholar]
- 23. Pan L, Zeng W, Niu Let al. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J Exp Bot. 2015;66:7031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tatsuki M, Nakajima N, Fujii Het al. Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J Exp Bot. 2013;64:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. El-Sharkawy I, Sherif S, Mila Iet al. Molecular characterization of seven genes encoding ethylene-responsive transcriptional factors during plum fruit development and ripening. J Exp Bot. 2009;60:907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. EI-Sharkawy I, Sherif S, Qubbaj Tet al. Stimulated auxin levels enhance plum fruit ripening, but limit shelf-life characteristics. Postharvest Biol Technol. 2016;112:215–23. [Google Scholar]
- 27. Stepanova AN, Yun J, Likhacheva AVet al. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Z, Wang S, Tao Qet al. A putative positive feedback regulation mechanism in CsACS2 expression suggests a modified model for sex determination in cucumber (Cucumis sativus L.). J Exp Bot. 2012;63:4475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li Z, Pan J, Guan Yet al. Development and fine mapping of three co-dominant SCAR markers linked to the M/m gene in the cucumber plant (Cucumis sativus L.). Theor Appl Genet. 2008;117:1253–60. [DOI] [PubMed] [Google Scholar]
- 30. Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends Plant Sci. 2012;17:181–95. [DOI] [PubMed] [Google Scholar]
- 31. Capua Y, Eshed Y. Coordination of auxin-triggered leaf initiation by tomato LEAFLESS. Proc Natl Acad Sci U S A. 2017;114:3246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chandler JW, Cole M, Jacobs Bet al. Genetic integration of DORNRÖSCHEN and DORNRÖSCHEN-LIKE reveals hierarchical interactions in auxin signalling and patterning of the Arabidopsis apical embryo. Plant Mol Biol. 2011a;75:223–36. [DOI] [PubMed] [Google Scholar]
- 33. Chandler JW, Jacobs B, Cole Met al. DORNRÖSCHEN-LIKE expression marks Arabidopsis floral organ founder cells and precedes auxin response maxima. Plant Mol Biol. 2011b;76:171–85. [DOI] [PubMed] [Google Scholar]
- 34. Larsson E, Robers CJ, Claes ARet al. Polar auxin transport is essential for medial versus lateral tissue specification and vascular-mediated valve outgrowth in Arabidopsis gynoecia. Plant Physiol. 2014;166:1998–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Durán-Medina Y, Serwatowska J, Reyes-Olalde JIet al. The AP2/ERF transcription factor DRNL modulates gynoecium development and affects its response to cytokinin. Front Plant Sci. 2017;8:1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai SL, Peng YB, Cui JXet al. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta. 2004;220:230–40. [DOI] [PubMed] [Google Scholar]
- 37. Saito S, Fujii N, Miyazawa Yet al. Correlation between development of female flower buds and expression of the CsACS2 gene in cucumber plants. J Exp Bot. 2007;58:2897–907. [DOI] [PubMed] [Google Scholar]
- 38. Pan J, Wen H, Chen Get al. A positive feedback loop mediated by CsERF31 initiates female cucumber flower development. Plant Physiol. 2021;186:1088–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li Z, Han Y, Niu Het al. Gynoecy instability in cucumber (Cucumis sativus L.) is due to unequal crossover at the copy number variation-dependent femaleness (F) locus. Hortic Res. 2020;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Niu H, Li Cet al. WUSCHEL-related homeobox1 (WOX1) regulates vein patterning and leaf size in Cucumis sativus. Hortic Res. 2020;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lescot M, Dehais P, Thijs Get al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tao Q, Niu H, Wang Zet al. Ethylene responsive factor ERF110 mediates ethylene-regulated transcription of a sex determination-related orthologous gene in two Cucumis species. J Exp Bot. 2018;69:2953–65. [DOI] [PubMed] [Google Scholar]
- 43. Li T, Jiang Z, Zhang Let al. Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 2016;88:735–48. [DOI] [PubMed] [Google Scholar]
- 44. Chandler JW. Class VIIIb APETALA2 ethylene response factors in plant development. Trends Plant Sci. 2018;23:151–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the results are included in this article and its supplementary materials. Other relevant materials are available from the corresponding author upon reasonable request.